Abstract
The aim of this study was to examine the effect of carbohydrate (CHO) ingestion on changes in ATP and phosphocreatine (PCr) concentrations in different muscle fibre types during prolonged running and relate those changes to the degree of glycogen depletion.
Five male subjects performed two runs at 70 % maximum oxygen uptake (
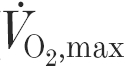 ), 1 week apart. Each subject ingested 8 ml (kg body mass (BM))−1 of either a placebo (Con trial) or a 5.5 % CHO solution (CHO trial) immediately before each run and 2 ml (kg BM)−1 every 20 min thereafter. In the Con trial, the subjects ran to exhaustion (97.0 ± 6.7 min). In the CHO trial, the run was terminated at the time coinciding with exhaustion in the Con trial. Muscle samples were obtained from the vastus lateralis before and after each trial.
), 1 week apart. Each subject ingested 8 ml (kg body mass (BM))−1 of either a placebo (Con trial) or a 5.5 % CHO solution (CHO trial) immediately before each run and 2 ml (kg BM)−1 every 20 min thereafter. In the Con trial, the subjects ran to exhaustion (97.0 ± 6.7 min). In the CHO trial, the run was terminated at the time coinciding with exhaustion in the Con trial. Muscle samples were obtained from the vastus lateralis before and after each trial.Carbohydrate ingestion did not affect ATP concentrations. However, it attenuated the decline in PCr concentration by 46 % in type I fibres (CHO: 20 ± 8 mmol (kg dry matter (DM))−1; Con: 34 ± 6 mmol (kg DM)−1; P < 0.05) and by 36 % in type II fibres (CHO: 30 ± 5 mmol (kg DM)−1; Con: 48 ± 6 mmol (kg DM)−1; P < 0.05).
A 56 % reduction in glycogen utilisation in type I fibres was observed in CHO compared with Con (117 ± 39 vs. 240 ± 32 mmol glucosyl units (kg DM)−1, respectively; P < 0.01), but no difference was observed in type II fibres.
It is proposed that CHO ingestion during exhaustive running attenuates the decline in oxidative ATP resynthesis in type I fibres, as indicated by sparing of both PCr and glycogen breakdown. The CHO-induced sparing of PCr, but not glycogen, in type II fibres may reflect differential recruitment and/or role of PCr between fibre types.
Early studies showed that fatigue during prolonged exercise of an intensity between 65 and 85 % maximum oxygen uptake (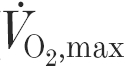 ) was associated with glycogen depletion in active skeletal muscle (Hermansen et al. 1967; Saltin & Karlsson, 1971). Although the precise mechanism by which glycogen depletion causes fatigue is still unclear, it has been hypothesised that it could be related to a decrease in the rate of oxidative adenosine triphosphate (ATP) resynthesis (Broberg & Sahlin, 1989). The close relationship between skeletal muscle glycogen depletion and fatigue development during prolonged, submaximal exercise is supported by the observation that carbohydrate (CHO) ingestion delays the onset of fatigue during this type of exercise (Tsintzas & Williams, 1998). Although it is well established that CHO ingestion increases blood glucose uptake and oxidation in skeletal muscle (Coyle et al. 1986; McConell et al. 1994; Jeukendrup et al. 1999), the exact mechanism underlying this ergogenic effect is not clear. Carbohydrate ingestion during exercise has been shown to attenuate the decline in mixed muscle hexo-monophosphates and tricarboxylic acid cycle intermediates, and offset the accumulation of inosine monophosphate (IMP) (Spencer et al. 1991), all of which are indicative of oxidative metabolism being better maintained.
) was associated with glycogen depletion in active skeletal muscle (Hermansen et al. 1967; Saltin & Karlsson, 1971). Although the precise mechanism by which glycogen depletion causes fatigue is still unclear, it has been hypothesised that it could be related to a decrease in the rate of oxidative adenosine triphosphate (ATP) resynthesis (Broberg & Sahlin, 1989). The close relationship between skeletal muscle glycogen depletion and fatigue development during prolonged, submaximal exercise is supported by the observation that carbohydrate (CHO) ingestion delays the onset of fatigue during this type of exercise (Tsintzas & Williams, 1998). Although it is well established that CHO ingestion increases blood glucose uptake and oxidation in skeletal muscle (Coyle et al. 1986; McConell et al. 1994; Jeukendrup et al. 1999), the exact mechanism underlying this ergogenic effect is not clear. Carbohydrate ingestion during exercise has been shown to attenuate the decline in mixed muscle hexo-monophosphates and tricarboxylic acid cycle intermediates, and offset the accumulation of inosine monophosphate (IMP) (Spencer et al. 1991), all of which are indicative of oxidative metabolism being better maintained.
However, human skeletal muscles are composed of at least two major fibre types, which differ in their physiological, metabolic and contractile characteristics (Essen et al. 1975; Schiaffino & Reggiani, 1996). In a previous study, using a quantitative, biochemical method, we examined the effect of CHO ingestion on exercise capacity and metabolite changes in pools of muscle fibres (Tsintzas et al. 1996a). The main findings from that study were firstly, glycogen depletion occurred exclusively in type I fibres during exercise in the absence of CHO ingestion, and secondly, when CHO was ingested, muscle glycogen utilisation was reduced in type I fibres and the subjects exercised 27 % longer than in the control trial. Based on the results of that study, we hypothesised that CHO ingestion offset the development of fatigue by maintaining oxidative ATP resynthesis principally in type I fibres (Tsintzas et al. 1996a). However, without direct determination of ATP and phosphocreatine (PCr) concentrations in different muscle fibre types, it was not possible to accept this hypothesis. The only study to date in which PCr concentrations in different fibre types were determined during submaximal exercise did not employ a CHO treatment (Sahlin et al. 1997). In that study it was proposed that the decline in PCr observed in both type I and type II muscle fibres may result from glycogen depletion and hence contribute to fatigue through an increase in the concentrations of muscle adenosine diphosphate (ADP) and inorganic phosphate (Pi). However, the glycogen concentrations in different fibre types were not determined in the study by Sahlin et al. (1997) and, thus, the above hypothesis was not confirmed.
The aim of this study was to examine the effect of CHO supplementation on changes in ATP and PCr concentrations in type I and type II muscle fibres during prolonged, exhaustive running in humans and relate those changes to the degree of glycogen depletion. It was hypothesised that if CHO ingestion delays the onset of fatigue by contributing to oxidative ATP production specifically in type I fibres, then it should attenuate the decline in PCr in this fibre type.
METHODS
Subjects
Five male recreational runners gave written informed consent and volunteered to participate in this study. All procedures used conformed with the Declaration of Helsinki and were approved by the University of Loughborough Ethical Advisory Committee. The mean age, height, mass, 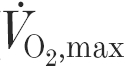 and maximum heart rate of the subjects were 25.2 ± 1.0 years (mean ± s.e.m.), 180 ± 1 cm, 76.0 ± 2.5 kg, 57.4 ± 1.5 ml kg−1 min−1 and 195 ± 6 beats min−1, respectively. The subjects were highly motivated and experienced volunteers who had participated in other experiments and were familiar with the sensation and symptoms of fatigue during prolonged exhaustive exercise.
and maximum heart rate of the subjects were 25.2 ± 1.0 years (mean ± s.e.m.), 180 ± 1 cm, 76.0 ± 2.5 kg, 57.4 ± 1.5 ml kg−1 min−1 and 195 ± 6 beats min−1, respectively. The subjects were highly motivated and experienced volunteers who had participated in other experiments and were familiar with the sensation and symptoms of fatigue during prolonged exhaustive exercise.
Preliminary measurements
Following familiarisation with treadmill running and experimental procedures, the subjects undertook two preliminary tests to determine (i) the relationship between running speed and oxygen uptake (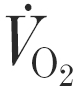 ) using a 16 min incremental submaximal test, and (ii) the
) using a 16 min incremental submaximal test, and (ii) the 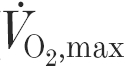 using an uphill incremental treadmill running test to exhaustion (Taylor et al. 1955). One week before the first experimental trial, subjects undertook a 60 min treadmill run at 70 %
using an uphill incremental treadmill running test to exhaustion (Taylor et al. 1955). One week before the first experimental trial, subjects undertook a 60 min treadmill run at 70 %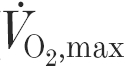 in order to confirm the relative exercise intensity and fully familiarise themselves with prolonged treadmill running and the measurements used during the experimental trials.
in order to confirm the relative exercise intensity and fully familiarise themselves with prolonged treadmill running and the measurements used during the experimental trials.
Experimental design
All subjects completed two runs at 70 %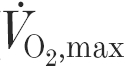 on a motorised treadmill 7 days apart. On each occasion, subjects ingested 8 ml (kg body mass (BM))−1 of either a placebo solution containing artificial sweetener (Con trial) or a 5.5 % CHO solution (CHO trial) immediately before each run and 2 ml (kg BM)−1 every 20 min thereafter. The CHO solution was an isotonic, 5.5 % solution, which consisted of glucose (1.7 %), fructose (1.1 %), maltose (0.6 %), higher saccharides (1.9 %) and electrolytes (sodium: 61 mg (100 ml)−1; potassium: 10 mg (100 ml)−1). In the Con trial, subjects ran to exhaustion, whereas in the CHO trial the run was terminated at the time coinciding with exhaustion in the Con trial. The subjects ingested a total of 1097 ± 57 ml of fluid during the Con trial and 1131 ± 74 ml during the CHO trial. In the latter trial, this fluid ingestion resulted in the consumption of 62.2 ± 4.1 g of CHO. The subjects were asked to refrain from heavy exercise for 3 days before each trial and record their normal diet for 3 days preceding the first trial. They were then asked to replicate exactly the same diet for the same period of time before the second trial. The subjects were also asked to refrain from alcohol, caffeine and tobacco consumption for 2 days before each main trial.
on a motorised treadmill 7 days apart. On each occasion, subjects ingested 8 ml (kg body mass (BM))−1 of either a placebo solution containing artificial sweetener (Con trial) or a 5.5 % CHO solution (CHO trial) immediately before each run and 2 ml (kg BM)−1 every 20 min thereafter. The CHO solution was an isotonic, 5.5 % solution, which consisted of glucose (1.7 %), fructose (1.1 %), maltose (0.6 %), higher saccharides (1.9 %) and electrolytes (sodium: 61 mg (100 ml)−1; potassium: 10 mg (100 ml)−1). In the Con trial, subjects ran to exhaustion, whereas in the CHO trial the run was terminated at the time coinciding with exhaustion in the Con trial. The subjects ingested a total of 1097 ± 57 ml of fluid during the Con trial and 1131 ± 74 ml during the CHO trial. In the latter trial, this fluid ingestion resulted in the consumption of 62.2 ± 4.1 g of CHO. The subjects were asked to refrain from heavy exercise for 3 days before each trial and record their normal diet for 3 days preceding the first trial. They were then asked to replicate exactly the same diet for the same period of time before the second trial. The subjects were also asked to refrain from alcohol, caffeine and tobacco consumption for 2 days before each main trial.
The experiment was conducted using a single-blind design, with the Con trial occurring first. A random order of trials was not employed because the main aim of this study was to investigate the metabolic environment within the muscle at that point in time during the CHO trial that coincided with exhaustion in the Con trial. It should be noted that in our previous studies which employed a cross-over design an order effect was not observed and the ingestion of the same 5.5 % CHO solution was associated with both a delay in the onset of fatigue (Tsintzas et al. 1996b) and a decrease in the rate of muscle glycogen utilisation and lactate accumulation (Tsintzas et al. 1995).
Protocol
On the day of the experiment each subject arrived in the laboratory following a 12 h overnight fast. An indwelling catheter (venflon, 18 G, Ohmeda, Hatfield, Herts, UK) was inserted in an ante-cubital vein while the subject lay on an examination couch. The catheter was kept patent by infusion with sterile saline. A resting muscle sample was then obtained from the vastus lateralis muscle. The subject was then asked to assume an upright position and a 10 ml resting venous blood sample was obtained immediately before a warm-up which consisted of a 5 min run at 60 %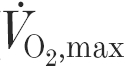 . The treadmill was then stopped for 10 min and the subject consumed 8 ml (kg BM)−1 of the assigned fluid while standing on the treadmill. Immediately after this 10 min period, the speed of the treadmill was increased and each subject ran at a speed equivalent to 70 %
. The treadmill was then stopped for 10 min and the subject consumed 8 ml (kg BM)−1 of the assigned fluid while standing on the treadmill. Immediately after this 10 min period, the speed of the treadmill was increased and each subject ran at a speed equivalent to 70 %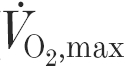 . A 1 min expired air sample followed by a 10 ml venous blood sample were taken at 10 and 20 min and every 20 min of the run thereafter. Following the expired air and blood sampling, 2 ml (kg BM)−1 of the assigned fluid was ingested by the subjects, using plastic volumetric syringes to avoid any spillage and ensure that the correct volume was ingested. Heart rate was recorded continuously during each run by means of short-range telemetry (Polar Electro sports testers PE 3000, Finland). The last expired air and blood samples were collected during the final minute of the run (exhaustion point). Immediately upon the completion of the first run (Con trial), a second muscle sample was obtained after the subject had been transferred quickly to an examination couch alongside the treadmill. During the second trial (CHO trial), exactly the same procedures were followed. However, the treadmill was stopped at the same point in time at which fatigue occurred in the Con trial and the subject was quickly transferred to an adjacent couch where a second muscle sample was obtained from the vastus lateralis. In all trials, the dry bulb temperature within the laboratory was maintained at ∼19 °C.
. A 1 min expired air sample followed by a 10 ml venous blood sample were taken at 10 and 20 min and every 20 min of the run thereafter. Following the expired air and blood sampling, 2 ml (kg BM)−1 of the assigned fluid was ingested by the subjects, using plastic volumetric syringes to avoid any spillage and ensure that the correct volume was ingested. Heart rate was recorded continuously during each run by means of short-range telemetry (Polar Electro sports testers PE 3000, Finland). The last expired air and blood samples were collected during the final minute of the run (exhaustion point). Immediately upon the completion of the first run (Con trial), a second muscle sample was obtained after the subject had been transferred quickly to an examination couch alongside the treadmill. During the second trial (CHO trial), exactly the same procedures were followed. However, the treadmill was stopped at the same point in time at which fatigue occurred in the Con trial and the subject was quickly transferred to an adjacent couch where a second muscle sample was obtained from the vastus lateralis. In all trials, the dry bulb temperature within the laboratory was maintained at ∼19 °C.
Collection and analysis of expired air and blood samples
Expired air samples were collected using the Douglas bag method and analysed for 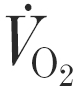 , carbon dioxide production (
, carbon dioxide production (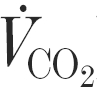 ) and respiratory exchange ratio (RER) using methods previously described (Tsintzas et al. 1995). Venous blood samples were collected into lithium-heparinised tubes except for a 4–5 ml aliquot which was placed into a non-heparinised tube to obtain serum. Whole blood lactate and glucose, and serum insulin concentrations were obtained using methods previously described (Tsintzas et al. 1995).
) and respiratory exchange ratio (RER) using methods previously described (Tsintzas et al. 1995). Venous blood samples were collected into lithium-heparinised tubes except for a 4–5 ml aliquot which was placed into a non-heparinised tube to obtain serum. Whole blood lactate and glucose, and serum insulin concentrations were obtained using methods previously described (Tsintzas et al. 1995).
Muscle sample collection and analysis
All muscle samples were obtained from the vastus lateralis using the needle biopsy technique with suction being applied (Bergstrom, 1962). Each sample was taken through a separate skin incision (3–5 mm long). All incisions were made under local anaesthetic (2–3 ml of 1 % lidocaine (lignocaine)) using a surgical blade before the start of exercise while the subject lay on an examination couch. After being removed from the leg, the biopsy needle was immediately immersed in liquid nitrogen. Muscle samples were then removed from the needle and stored in liquid nitrogen until they were freeze dried, after which they were stored at −70 °C. At a later date, one part of the freeze-dried muscle sample was dissected free of visible blood and connective tissue, powdered and washed twice with 40 % petroleum ether to remove fat. Muscle metabolites (glucose-6-phosphate (G-6-P), lactate, ATP and PCr) were extracted and determined enzymatically (Lowry & Passonneau, 1972; Harris et al. 1974). A portion of the freeze-dried muscle powder was also used for the determination of muscle glycogen. The muscle powder was digested in 0.5 mm NaOH and neutralised with HCl-citrate buffer, pH 4.9. The glycogen present in the supernatant was hydrolysed with α-amyloglucosidase and analysed for glucosyl units using an enzymatic method (Harris et al. 1974).
The remaining piece of freeze-dried muscle sample was also washed twice with 40 % petroleum ether to remove fat and was used for the determination of glycogen, ATP, PCr and G-6-P in pools of type I and type II muscle fibres. Sixty to 80 fragments of single muscle fibres were dissected from the muscle piece using low-power microscopy (× 10 magnification). The ends of the individual fibre fragments were then cut off and stained in triplicate for myofibrillar ATPase in order to identify type I (slow-twitch) and type II (fast-twitch) fibres using a modification of the method of Brooke & Kaiser (1970). The remaining part of each fibre was then stored at −70 °C. Only those fibres that were identified as the same fibre type following the staining procedure were considered for further analysis. At a later date, 8–10 fibres of each type were pooled and weighed on a microbalance (Perkin-Elmer AD-2), the total weight ranging from 10 to 40 μg. Two sets of fibres were pooled for each fibre type. Each pool of fibres was weighed twice, and an average of the two readings was used in subsequent calculations. The microbalance was calibrated by determining the weight of DNA fragments using first the microbalance and subsequently a spectrophotometer. The microbalance was found to be both accurate (difference of microbalance from spectrophotometrically determined weight: 4.8 ± 1.0 %, r = 0.99, n = 32) and precise (coefficient of variation: 0.4 %) within the weighing range of 10–40 μg.
Glycogen was then extracted from one set of pooled fibres by adding 20 μl KOH (1 mm). Following this, the samples were agitated on a vortex mixer and then warmed to 50 °C for 15 min. This resulted in the complete digestion of the fibres. The extract was then neutralised by adding HCl (0.25 mm) and analysed for glucose using a modification of the method of Harris et al. (1974). PCr, ATP and G-6-P were extracted from the second set of pooled fibres by adding 100 μl perchloric acid (0.5 mm) and EDTA (1 mm). An aliquot of the supernatant was then neutralised by adding KHCO3 (2.2 mm) and assayed fluorimetrically using a modified version of the method of Harris et al. (1974) involving microvolumes. Briefly, following addition of extract to a triethanolamine buffer (1 mm), measurements of PCr, ATP and G-6-P were made by the serial addition of glucose-6-phosphate dehydrogenase (1 mg ml−1; 1:2 H2O), hexokinase (2 mg ml−1; 1:1 H2O) and creatine phosphokinase (15 mg ml−1; 0.5 % NaHCO3, 0.1 % bovine serum albumin).
Statistical analysis
Analysis of variance (ANOVA) for repeated measures on two factors (experimental treatment and sampling time) was used to assess overall differences between physiological and metabolic responses to both trials. When a significant difference was obtained, a Neuman-Keuls post hoc test was used to locate any difference. Changes in glycogen and PCr (Δ values) obtained during the trials were analysed using Student's paired t test. Statistical significance was accepted at a 5 % level. Results are presented as means ± s.e.m.
RESULTS
Running times, percentage 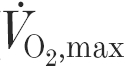 , RER and heart rate
, RER and heart rate
Running time to exhaustion was 97.0 ± 6.7 min in the Con trial. The subjects performed the same amount of exercise during the second (CHO) trial. In agreement with our previous study (Tsintzas et al. 1996a), none of the subjects was exhausted at the same point in time at which fatigue occurred in the Con trial. The mean percentage 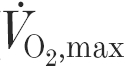 sustained during the Con and CHO trials was 70.4 ± 2.1 and 69.5 ± 1.6 %, respectively (n.s.). The mean RER value was not different between the Con and CHO trials (0.94 ± 0.01 vs. 0.96 ± 0.02, respectively). However, the RER tended to be higher during the first hour of exercise in the CHO trial when compared with Con, with the difference reaching statistical significance at 40 min of exercise (0.97 ± 0.03 vs. 0.93 ± 0.01, respectively, P < 0.05). The mean heart rate in the Con and CHO trials was 173 ± 8 and 170 ± 5 beats min−1, respectively (n.s.).
sustained during the Con and CHO trials was 70.4 ± 2.1 and 69.5 ± 1.6 %, respectively (n.s.). The mean RER value was not different between the Con and CHO trials (0.94 ± 0.01 vs. 0.96 ± 0.02, respectively). However, the RER tended to be higher during the first hour of exercise in the CHO trial when compared with Con, with the difference reaching statistical significance at 40 min of exercise (0.97 ± 0.03 vs. 0.93 ± 0.01, respectively, P < 0.05). The mean heart rate in the Con and CHO trials was 173 ± 8 and 170 ± 5 beats min−1, respectively (n.s.).
Blood metabolites
A higher blood glucose concentration was observed at 10 min of exercise in the CHO trial compared with the Con trial (5.2 ± 0.2 vs. 3.9 ± 0.1 mmol l−1, respectively; P < 0.01). No difference in blood glucose concentration was found between the two trials at any other sampling points. The serum insulin concentration was higher at 10 and 20 min of exercise in the CHO trial when compared with Con (19.9 ± 3.6 vs. 6.1 ± 0.5 mU l−1 at 10 min and 13.5 ± 0.9 vs. 5.8 ± 0.6 mU l−1 at 20 min, respectively; P < 0.01). There was no difference in serum insulin concentrations between the two trials at any other sampling points. There was no difference in blood lactate concentration between trials during the first 60 min of exercise. However, the blood lactate concentration was lower at the end of the run in the CHO trial when compared with Con (2.7 ± 0.7 vs. 3.6 ± 0.1 mmol l−1, respectively; P < 0.05).
Mixed muscle metabolites
Mixed muscle glycogen, lactate and PCr concentrations at rest were the same in both trials (Table 1). However, at the end of exercise, the muscle glycogen concentration in the CHO trial was higher than in the Con trial (P < 0.05). This reflected a 27 % reduction in mixed muscle glycogen utilisation in the CHO trial. A lower accumulation of muscle lactate was found at the end of the run in the CHO trial (P < 0.05; Table 1). Carbohydrate ingestion also resulted in a higher muscle PCr concentration at the end of the run compared with Con (P < 0.01; Table 1). There were no differences in mixed muscle ATP and G-6-P concentrations between trials.
Table 1.
Mixed muscle metabolites at rest (Rest) and immediately after (End) submaximal running with (CHO) and without (Con) carbohydrate supplementation
| Con | CHO | |||
|---|---|---|---|---|
| Rest | End | Rest | End | |
| [ATP] | 23.9 ± 0.8 | 20.2 ± 1.6 | 23.1 ± 0.8 | 23.1 ± 2.1 |
| [PCr] | 75.1 ± 1.3 | 34.1 ± 3.5 | 77.3 ± 3.1 | 51.5 ± 5.9 * |
| [Glycogen] | 369. ± 31 | 132. ± 17 | 372. ± 27 | 200 ± 14 ** |
| [G-6-P] | 1.3 ± 0.2 | 2.7 ± 0.5 | 1.2 ± 0.4 | 2.4 ± 0.6 |
| [Lactate] | 5.0 ± 0.6 | 19.8 ± 6.4 | 6.3 ± 0.6 | 6.7 ± 0.6 ** |
Values are means ± s.e.m. (n = 5), given in mmol glucosyl units (kg dry matter (DM))-1 for glycogen and mmol (kg DM)-1 for other metabolites.
* Significantly different from Con (P < 0.01)
** significantly different from Con (P < 0.05).
Type I and type II muscle fibre metabolites
In both Con and CHO trials, resting glycogen concentrations in type II fibres were higher than in type I fibres (Table 2). At the end of the run, the glycogen concentration in type I fibres was higher in the CHO trial than in Con (Table 2). Consequently, a 56 ± 9 % reduction in glycogen utilisation in type I fibres was observed in the CHO trial compared with Con (Fig. 1). In contrast, no difference in glycogen concentration in type II fibres was observed at the end of the run between the two trials. Furthermore, there was no difference in glycogen utilisation in type II fibres between trials (Fig. 1). Resting PCr concentrations in type II fibres were higher than in type I fibres within each trial (Table 2). However, there was no difference in resting PCr concentrations between trials. At the end of the run, PCr concentrations in type I and type II fibres were higher in the CHO trial compared with Con (Table 2). Carbohydrate ingestion attenuated the decline in PCr concentration by 46 ± 17 % in type I fibres and by 36 ± 9 % in type II fibres (Fig. 2). A positive relationship was observed between Δ[PCr] and Δ[glycogen] in type I fibres (Con: r = 0.95, P < 0.01; CHO: r = 0.90, P < 0.02; Fig. 3). No such correlation was found in type II fibres (r = −0.13 and r = 0.41, respectively). There were no differences in ATP and G-6-P concentrations in type I and type II muscle fibres between trials (Table 2).
Table 2.
Type I and type II muscle fibre metabolites at rest (Rest) and immediately after (End) submaximal running with (CHO) and without (Con) carbohydrate supplementation
| Rest | End | ||||
|---|---|---|---|---|---|
| Type I | Type II | Type I | Type II | ||
| [ATP] | Con | 21.6 ± 0.8 | 23.6 ± 1.3 | 22.3 ± 1.8 | 23.9 ± 2.0 |
| CHO | 23.2 ± 1.3 | 22.6 ± 2.8 | 23.2 ± 1.2 | 23.6 ± 1.2 | |
| [PCr] | Con | 66 ± 3 † | 80. ± 2 | 32. ± 6 | 32. ± 6 |
| CHO | 67 ± 4 † | 78. ± 5 | 47 ± 4 ** | 48 ± 4 ** | |
| [Glycogen] | Con | 304 ± 34 † | 403. ± 44 | 64. ± 2 | 175 ± 33 ‡ |
| CHO | 290 ± 29 † | 423. ± 48 | 173 ± 13 ** | 221. ± 26 | |
| [G-6-P] | Con | 0.6 ± 0.1 | 1.2 ± 0.6 | 1.5 ± 1.2 | 1.3 ± 0.8 |
| CHO | 0.8 ± 0.2 | 1.8 ± 0.6 | 0.3 ± 0.1 | 0.5 ± 0.2 | |
Values are means ± s.e.m. (n = 5), given in mmol glucosyl units (kg DM)-1 for glycogen and mmol (kg DM)-1 for other metabolites.
** Significantly different from Con (P < 0.05)
† significantly different from ‘Rest’ type II within the same treatment (P < 0.01)
‡ significantly different from ‘End’ type I within the same treatment (P < 0.05).
Figure 1. Changes (Δ values) in type I and type II muscle fibre glycogen concentrations during submaximal running with (CHO) and without (Con) carbohydrate supplementation.
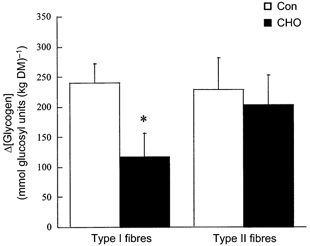
Values are means ± s.e.m.; n = 5. * Significantly different from Con (P < 0.01).
Figure 2. Changes (Δ values) in type I and type II muscle fibre PCr concentrations during submaximal running with (CHO) and without (Con) carbohydrate supplementation.
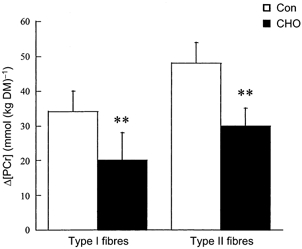
Values are means ± s.e.m.; n = 5. ** Significantly different from Con (P < 0.05).
Figure 3. Relationship between changes (Δ values) in PCr and glycogen concentrations in type I (A) and type II (B) muscle fibres during submaximal running.
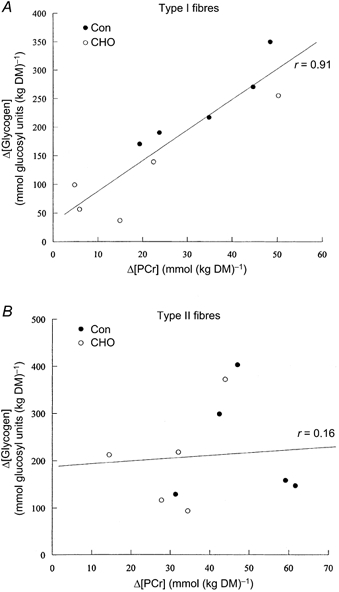
Data pooled from both Con and CHO trials (n = 10).
DISCUSSION
The present study is the first to investigate the effect of CHO ingestion on changes in ATP and PCr concentrations in type I and type II muscle fibres during prolonged, submaximal exercise and relate those changes to the degree of glycogen depletion. The main finding from this study was that carbohydrate ingestion attenuated the decline in PCr in type I and type II fibres, but spared glycogen in only type I fibres. Since selective glycogen depletion in type I fibres was observed at the point of fatigue in the absence of CHO ingestion, these data appear to support our hypothesis that CHO ingestion offsets fatigue development during prolonged exercise by better maintaining oxidative ATP resynthesis principally in type I fibres. However, it cannot be ignored that CHO ingestion also attenuated the decline in PCr concentrations in type II fibres. Since glycogen depletion in this fibre type was not evident at the end of exercise, and glycogen utilisation was unaffected by CHO ingestion, a number of factors other than an attenuation in the decline in oxidative ATP production might account for the CHO-induced sparing of PCr in type II fibres.
In the Con trial of this study, the ATP concentrations in type I and type II muscle fibres at the point of fatigue were maintained at their pre-exercise levels. This is consistent with previous studies which demonstrated no decline in ATP concentration in either fibre type during prolonged, submaximal exercise without CHO ingestion (Norman et al. 1987; Ball-Bernett et al. 1991). Although carbohydrate ingestion did not affect ATP concentrations in either fibre type, it attenuated the decline in PCr in type I fibres by 46 %. The extent of PCr decline during prolonged, constant intensity exercise, which leads to muscle glycogen depletion, reflects the extent of the inability of the working muscles to maintain oxidative ATP production (Hultman et al. 1967; Sahlin et al. 1997). The strong positive correlation observed between changes in PCr and glycogen concentrations in type I, but not in type II, fibres in both trials of this study further supports the presence of a close functional link between oxidative ATP production and PCr degradation in type I fibres. Thus, the sparing of PCr in the absence of a decline in ATP concentration was likely to reflect a better maintained rephosphorylation of ADP by aerobic processes during exercise, and thus, reduced accumulation of ADP, AMP and Pi. In support of this conclusion, CHO ingestion during submaximal exercise has been shown to attenuate the accumulation of mixed muscle IMP (a marker of adenine nucleotide loss) in the working muscles compared with placebo ingestion (Spencer et al. 1991). Inasmuch as glycogen depletion in type I fibres limited energy production in this fibre type, a CHO-induced maintenance of rephosphorylation of ADP by aerobic processes may explain the delay in the onset of fatigue possibly through a reduction in the accumulation of ADP and Pi (Yamashita et al. 1994; Fryer et al. 1995).
Since both Pi and AMP are thought to be important post-transformational regulators of glycogen phosphorylase, the rate-limiting enzyme in glycogen breakdown (Chasiotis, 1983; Ren & Hultman, 1989), a reduction in their accumulation during exercise with CHO ingestion could explain the decrease in muscle glycogen utilisation in type I fibres. Interestingly, these responses to CHO ingestion are similar to those observed during exercise when the plasma free fatty acid (FFA) concentration was elevated following intralipid and heparin infusion (Dyck et al. 1993, 1996). In those studies, a sparing of muscle glycogen and PCr was observed and was associated with an attenuated accumulation of free ADP and AMP during exercise. Taken together, these results suggest that an increase in exogenous substrate provision to skeletal muscle in the form of either glucose or FFA reduces intramuscular glycogen breakdown by increasing the drive for mitochondrial oxidative phosphorylation.
It cannot be ignored, however, that CHO ingestion attenuated the decline in PCr in type II fibres. A change in the pattern of recruitment following CHO ingestion might explain this observation of PCr sparing in this fibre type. From previous studies (Gollnick et al. 1974; Vollestad & Blom, 1985; Ball-Bernett et al. 1991; Tsintzas et al. 1995, 1996a), it appears that relatively little glycogen is utilised in type II fibres during the first hour of submaximal exercise. In contrast, a substantial breakdown of glycogen occurs in type II fibres towards the end of exercise, at a time when an increase in the recruitment of type II fibres seems to occur to compensate for loss of recruitment of type I fibres as a result of glycogen depletion. It seems plausible therefore that the slower rate of glycogen depletion in type I fibres in the CHO trial of the present study might have resulted in a concomitant delay in the recruitment of type II fibres, thereby leading to a reduction in PCr breakdown in the latter fibre type.
It is also possible that the decline in PCr during muscular contraction might serve a different purpose in different fibre types. It has been suggested that the dominant function of PCr in slow-twitch muscles might be to act as an energy transport system, e.g. connecting sites of ATP production to sites of ATP utilisation (Wallimann et al. 1992; Veksler et al. 1995). In contrast, in fast-twitch muscles PCr might simply act to buffer excess accumulation of ADP, especially during transient periods of high energy utilisation (Wallimann et al. 1992; Veksler et al. 1995), e.g. towards the end of exercise, at a time when an increase in the recruitment of type II fibres seems to occur to compensate for the depletion of glycogen in type I fibres. Thus, a differential role of PCr across fibre types might also explain the apparent dissociation between the responses of muscle glycogen and PCr in type II fibres to CHO ingestion.
In summary, this is the first study to provide evidence that CHO ingestion during prolonged submaximal exercise attenuates the decline in oxidative ATP production (as indicated by sparing of PCr and glycogen breakdown) in type I skeletal muscle fibres. Since glycogen depletion in type II fibres was not evident at fatigue, and the rate of glycogen utilisation was unaffected by CHO ingestion, the CHO-induced sparing of PCr in this fibre type may reflect differential recruitment and/or role of PCr between fibre types. Therefore, further investigation is required to evaluate the implications of these findings for the development of fatigue and the inability to maintain oxidative ATP production during submaximal exercise.
Acknowledgments
The authors wish to acknowledge the support of this study by GlaxoSmithKline.
References
- Ball-Bernett M, Greenand HJ, Houston ME. Energy metabolism in human slow and fast twitch fibres during prolonged cycle exercise. Journal of Physiology. 1991;437:257–267. doi: 10.1113/jphysiol.1991.sp018594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1962;14(suppl. 68):1–100. [Google Scholar]
- Broberg S, Sahlin K. Adenine nucleotide degradation in human skeletal muscle during prolonged exercise. Journal of Applied Physiology. 1989;67:116–122. doi: 10.1152/jappl.1989.67.1.116. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fibre types: how many and what kind? Archives of Neurology. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Chasiotis D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiologica Scandinavica Supplementum. 1983;518:1–68. [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilisation during prolonged strenuous exercise when fed carbohydrate. Journal of Applied Physiology. 1986;61:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Peters SJ, Wendling PS, Chesley A, Hultman E, Spriet LL. Regulation of muscle glycogen phosphorylase activity during intense aerobic cycling with elevated FFA. American Journal of Physiology. 1996;270:E116–125. doi: 10.1152/ajpendo.1996.270.1.E116. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Putman CT, Heigenhauser GJF, Hultman E, Spriet LL. Regulation of fat-carbohydrate interaction in skeletal muscle during intense aerobic cycling. American Journal of Physiology. 1993;265:E852–859. doi: 10.1152/ajpendo.1993.265.6.E852. [DOI] [PubMed] [Google Scholar]
- Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiologica Scandinavica. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. Journal of Physiology. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiologica Scandinavica. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Hultman E, Bergstrom J, Anderson NM. Breakdown and resynthesis of phosphorylcreatine and adenosine triphosphate in connection with muscular work in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1967;19:56–66. doi: 10.3109/00365516709093481. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Raben A, Gijsen A, Stegen JHCH, Brouns F, Saris WHM, Wagenmakers A. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. Journal of Physiology. 1999;515:579–589. doi: 10.1111/j.1469-7793.1999.579ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. pp. 189–193. [Google Scholar]
- McConell G, Fabris S, Proietto J, Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise. Journal of Applied Physiology. 1994;77:1537–1541. doi: 10.1152/jappl.1994.77.3.1537. [DOI] [PubMed] [Google Scholar]
- Norman B, Solleui A, Kaijser L, Jansson E. ATP breakdown products in human skeletal muscle during prolonged exercise to exhaustion. Clinical Physiology. 1987;7:503–509. doi: 10.1111/j.1475-097x.1987.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Ren JM, Hultman E. Regulation of glycogenolysis in human skeletal muscle. Journal of Applied Physiology. 1989;67:2243–2248. doi: 10.1152/jappl.1989.67.6.2243. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibres of human muscle after sustained submaximal exercise. American Journal of Physiology. 1997;273:C172–178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- Saltin B, Karlsson J. Muscle glycogen utilisation during work of different intensities. In: Pernow B, Saltin B, editors. Muscle Metabolism During Exercise. New York: Plenum Press; 1971. pp. 289–299. [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiological Reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Spencer MK, Yan Z, Katz A. Carbohydrate supplementation attenuates IMP accumulation in human muscle during prolonged exercise. American Journal of Physiology. 1991;261:C71–76. doi: 10.1152/ajpcell.1991.261.1.C71. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Buskirk ER, Henschell A. Maximum oxygen uptake as an objective measure of cardio-respiratory performance. Journal of Applied Physiology. 1955;8:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Tsintzas K, Williams C. Human muscle glycogen metabolism during exercise. Effect of carbohydrate supplementation. Sports Medicine. 1998;25:7–23. doi: 10.2165/00007256-199825010-00002. [DOI] [PubMed] [Google Scholar]
- Tsintzas O-K, Williams C, Boobis L, Greenhaff P. Carbohydrate ingestion and glycogen utilization in different muscle fibre types in man. Journal of Physiology. 1995;489:243–250. doi: 10.1113/jphysiol.1995.sp021046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsintzas OK, Williams C, Boobis L, Greenhaff P. Carbohydrate ingestion and single muscle fiber glycogen metabolism during prolonged running in men. Journal of Applied Physiology. 1996a;81:801–809. doi: 10.1152/jappl.1996.81.2.801. [DOI] [PubMed] [Google Scholar]
- Tsintzas OK, Williams C, Wilson W, Burrin J. Influence of carbohydrate supplementation early in exercise on endurance running capacity. Medicine and Science in Sports and Exercise. 1996b;28:1373–1379. doi: 10.1097/00005768-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Veksler IV, Kuznetsov AV, Anflous K, Mateo P, Van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. Journal of Biological Chemistry. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Vollestad NK, Blom PCS. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiologica Scandinavica. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Sata M, Sugiura S, Momomura S, Serizawa T, Iizuka M. ADP inhibits the sliding velocity of fluorescent actin filaments on cardiac and skeletal myosins. Circulatory Research. 1994;74:1027–1033. doi: 10.1161/01.res.74.6.1027. [DOI] [PubMed] [Google Scholar]


