Abstract
We transiently introduced the human GABAA receptor ε subunit cDNA into a human embryonic kidney (HEK) cell line stably expressing α1β3γ2 receptors (WSS-1 cells) to establish whether the subunit competes with the γ2 subunit for assembly into receptors. GABA-evoked currents were recorded using the patch-clamp technique from cells transfected with cDNA encoding green fluorescent protein (GFP) alone or in combination with the ε subunit cDNA.
The ε subunit did not change the potency of GABA: the GABA EC50 was 34 ± 6 μm in control WSS-1 cells and 37 ± 6 μm in cells expressing the ε subunit. The introduction of the ε subunit reduced the peak current amplitude activated by GABA (1 mm) from 1.8 ± 0.2 nA in control cells to 0.9 ± 0.2 nA in cells expressing the ε subunit (P < 0.05).
The ε subunit caused the appearance of leak currents recorded in the absence of GABA. Outside-out patches excised from ε subunit-containing WSS-1 cells exhibited spontaneously opening GABAA channels not seen in patches excised from control GFP-expressing WSS-1 cells. Introduction of the ε subunit did not alter the GABA-evoked single-channel cord conductance.
The anaesthetic 2,6-diisopropylphenol (propofol, 3 μm) and the benzodiazepine flunitrazepam (1 μm) potentiated GABA-evoked currents recorded from control cells labelled with GFP. The ε subunit reduced potentiation by both agents 48–96 h after transfection.
The introduction of the ε subunit had no effect on the ability of propofol (3–30 μm) relative to GABA (1 mm) to activate GABAA receptors in WSS-1 cells. High concentrations of propofol (> 100 μm) produced a more marked desensitization of GABAA receptor activity in WSS-1 cells transfected with cDNA for the ε subunit than in control cells.
There was no difference in the potency of Zn2+ as an inhibitor of currents recorded from control cells (IC50 = 165 ± 34 μm) or cells expressing the ε subunit (IC50 = 179 ± 11 μm).
GABA-activated currents recorded both from control cells and cells expressing the ε subunit reversed in sign at the Cl− equilibrium potential and exhibited outward rectification.
The introduction of the ε subunit changes the functional properties of GABAA receptors in WSS-1 cells. The resulting receptors have a unique combination of properties indicative of the co-assembly of α, β, γ and ε subunits.
GABAA receptors are heterogeneous by virtue of the diversity of genes that encode their subunits, α1–6, β1–3, γ1–3, δ, ε, π and θ (Bonnert et al. 1999; Mehta & Ticku, 1999). With five subunits making up each receptor-Cl− channel complex (Nayeem et al. 1994) the theoretical number of unique combinations is large. The actual number is limited by the failure of many subunits to form functional receptors. An additional limitation on receptor diversity in the brain is imposed by regional segregation of subunit expression (McKernan & Whiting, 1996).
Recombinant α and β subunits form functional receptors when expressed in cell lines, but most mature neurons predominantly express αβγ receptors. The γ2 subunit confers increased single channel conductance, benzodiazepine sensitivity and reduced sensitivity to blockade by Zn2+ (Pritchett et al. 1989; Draguhn et al. 1990; Smart et al. 1991; Angelotti & Macdonald, 1993). In some neurons subunits other than γ2 (i.e. δ or ε) may combine with α and β subunits producing receptors with unique properties (Saxena & Macdonald, 1994; Davies et al. 1997a). The γ2, δ and ε subunits have overlapping distributions and in some cases all three transcripts are expressed in the same cell (Brooks-Kayal et al. 1998, 1999). This could result in the existence of multiple receptor subtypes in a single cell as in the case of αβδ and αβγ receptors in cerebellar granule cells (Nusser et al. 1998). Alternatively, γ2, δ and/or ε subunits may co-assemble within the same receptor complex. If so, it is important to know if any of the co-assembled subunits has a dominant effect on receptor function.
Both kindling- and pilocarpine-induced seizures change the properties of GABAA receptors expressed by rodent dentate granule cells (Buhl et al. 1996; Brooks-Kayal et al. 1998). Following seizures GABAA receptors exhibit an increased sensitivity to being blocked by Zn2+ and a reduced sensitivity to the potentiating effects of anxiolytic benzodiazepines. These functional changes are associated with increases in the levels of ε and δ subunit mRNAs without a significant change in the level of the γ2 transcript (Brooks-Kayal et al. 1998). The loss of γ-subunit properties could be explained in three ways. (1) Receptors appear that lack γ, δ and ε subunits. (2) The γ2 subunit and ε or δ subunits compete for occupation of the receptor. After seizures this competition is won by ε and/or δ subunits. (3) Receptors form that contain γ2 subunits and either ε or δ subunits, their properties being dominated by the ε or δ subunit.
In this study we mimicked the induction of ε subunit expression in neurons by transiently transfecting ε subunit cDNA into WSS-1 cells (HEK cells already stably expressing α1β3γ2 receptors) (Wong et al. 1992; Davies et al. 2000). We used the patch-clamp technique to examine whether the γ2 and ε subunits compete for control of GABAA receptor function. Receptors containing αβ, αβγ and αβε subunits can be distinguished by their distinctive functional properties. The well-described anaesthetic potentiation of αβ and αβγ GABAA receptor function is markedly reduced in αβε receptors (Davies et al. 1997a). Furthermore, αβγ receptors, but not αβ or αβε receptors, are sensitive to benzodiazepines. The αβ, αβγ and αβε receptors can also be distinguished by their differential sensitivities to Zn2+, an inhibitor of GABAA receptor function, and by their current-voltage relationships (Draguhn et al. 1990; Davies et al. 1997a; Whiting et al. 1997). Using these properties we provide evidence for the existence of αβγε receptors.
METHODS
Cell culture and transfection
HEK cells (ATCC CRL-1573) were grown in medium comprising Dulbecco's modified Eagle's medium supplemented with 10 % calf serum, 100 i.u. ml−1 penicillin, and 100 μg ml−1 streptomycin. WSS-1 cells (ATCC, CRL-2029) were grown in HEK medium supplemented with 400 μg ml−1 geneticin (G-418), used to positively select cells that express GABAA receptors. Resistance to the antibiotic is conferred by the vector containing cDNA encoding the rat α1 subunit (Wong et al. 1992). Cells were maintained for 1 week in a humid environment of 5 % CO2, 95 % air at 37 oC before subculturing. Once the cells approached confluence they were suspended and seeded into 35 mm diameter dishes for transfection as described previously (Davies et al. 1997b). Cells were transfected in HEK medium using the calcium phosphate precipitation method. WSS-1 cells were transiently transfected with either green fluorescent protein (GFP) cDNA or the human ε subunit and GFP cDNAs (both in pCDM8). HEK cells were transiently transfected with human α1, β3, γ2 and ε (all in pCDM8) cDNAs to produce recombinant α1β3, α1β3γ2, α1β3ε, GABAA receptors. After transfection cells were incubated (5 % CO2, 95 % air at 37 oC) for 24 h, washed and incubated for a further 24–144 h before experimentation.
Electrophysiology
The whole-cell configuration of the patch-clamp technique was used to record GABA-activated currents from voltage-clamped HEK or WSS-1 cells. GABA (100 μm) was applied by pressure (70 kPa, 0.04 Hz) ejection from modified patch pipettes; other compounds were applied by perfusion into the recording chamber. In experiments investigating the modulation of GABA-evoked currents by anaesthetics and flunitrazepam the duration of GABA application was sufficient to activate ∼10 % of the maximum GABA (100 μm)-activated current. GABA or propofol were applied by prolonged (1 s) pressure ejection from low resistance micropipettes in order to determine their concentration dependence as GABAA receptor agonists as described previously (Adodra & Hales, 1995). The recording chamber was continuously perfused (5 ml min−1) with an extracellular solution comprising (mm): NaCl, 140; KCl, 4.7; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; Hepes-NaOH, 10 (pH 7.4). The electrode solution contained (mm): KCl, 140; MgCl2, 2.0; EGTA, 11; ATP (Mg2+ salt) 0.1; Hepes-KOH, 10 (pH 7.4). Junction potentials were nulled with an open electrode in the recording chamber prior to each experiment. The liquid junction potential was trivial (1.7 mV) and its inappropriate compensation was ignored. Cells were voltage clamped at −60 mV except in those experiments in which the relationship between holding potential and current amplitude was examined. Experiments were performed at room temperature (20–24 oC).
Acquisition and analysis of data
GABA-evoked currents were monitored by an Axopatch-200A amplifier, then low-pass filtered with a cut-off frequency of 2 kHz, recorded on chart paper (Gould, Brush 2200) and simultaneously digitized, using a DigiData 1200 interface (Axon Instruments, Burlingame, CA, USA), for acquisition onto the hard drive of a personal computer. Currents were averaged, superimposed and measured using pCLAMP software (Axon Instruments). Graphs of concentration-response relationships were fitted using the logistic function as described previously (Adodra & Hales, 1995). To test the possibility that α1β3γ2 and α1β3ε exist in WSS-1 cells expressing ε subunits we plotted curves generated by adding two logistic equations using the parameters determined when these receptor subtypes were expressed alone. All data are expressed as the arithmetic mean ± s.e.m.
Drugs and reagents used
γ-Aminobutyric acid (GABA) was from Sigma (St Louis, MO, USA). 2,6-Diisopropylphenol (propofol) was from Aldrich (Milwaukee, WI, USA). Stock solutions of flunitrazepam and propofol, in ethanol, were diluted to achieve a final ethanol concentration < 0.1 %. This concentration of ethanol had no effect on GABA (100 μm)-activated currents. Tissue culture reagents were purchased from Gibco-BRL (Gaithersburg, MD, USA) and all other reagents were from Sigma.
RESULTS
Introduction of the ε subunit alters GABAa receptor function
We examined whether the human ε subunit alters GABAA receptor function upon transient introduction of its cDNA into WSS-1 cells (HEK cells that stably express recombinant α1 and γ2 subunits and an endogenous β3 subunit) (Wong et al. 1992; Davies et al. 2000). Cells were transfected with cDNAs encoding the ε subunit and green fluorescent protein (GFP), then located using fluorescence microscopy. Control WSS-1 cells were transfected with GFP cDNA alone. We focused on pharmacological properties conferred specifically by γ or ε subunits as a means to verify the incorporation of ε subunits into functional GABAA receptors. These properties are benzodiazepine sensitivity and resistance to anaesthetic modulation of GABA-evoked currents, respectively (Pritchett et al. 1989; Davies et al. 1997a). GABA-activated currents recorded from control WSS-1 cells 24–144 h post-transfection showed no significant change in sensitivity to the anaesthetic agent propofol (3 μm) after transfection with GFP cDNA (Fig. 1A and B). The inclusion of ε subunit cDNA, in contrast, caused a decline in the modulatory effect of propofol. The potentiation of GABA-activated currents evoked by propofol 48 h after transfection was greatly reduced (Fig. 1A), indicating that the ε subunit was incorporated into functional GABAA receptors of WSS-1 cells. Consistent with the eventual dilution and degradation of ε subunit cDNA, the modulation of GABA-activated currents by propofol showed signs of recovery 120 h after transfection and was indistinguishable from control after 144 h. While the potentiation of GABA-evoked currents by propofol (3 μm) was markedly reduced after ε subunit expression, there was a significant increase in current elicited by the anaesthetic in the absence of GABA (Fig. 1A, P < 0.005, n = 11). From Fig. 3 it is clear that this effect cannot be entirely accounted for by direct GABAA receptor activation by propofol (3 μm) and may represent the ability of this agent to potentiate the spontaneous activity of ε subunit-containing channels. The data for propofol (3 μm) modulation of GABA-evoked currents recorded from WSS-1 cells transfected with GFP cDNA alone or in combination with the ε subunit cDNA 48–96 h after transfection are shown in Table 1. The levels of propofol-evoked potentiation of GABA-evoked currents mediated by recombinant α1β3, α1β3ε and α1β3γ2 receptors transiently expressed in HEK cells are included for comparison (Table 1).
Figure 1. Transient expression of the ε subunit alters the pharmacology of stably expressed αβγ GABAA receptor.
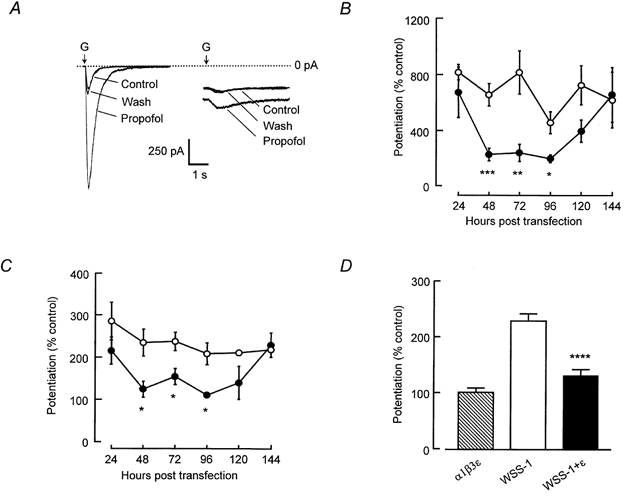
A, left panel: sub-maximal GABA-evoked currents (equivalent in amplitude to currents activated by GABA EC10) recorded from GFP-expressing WSS-1 cells were potentiated by propofol (3 μm). Dotted line represents zero current. A, right panel: WSS-1 cells expressing ε subunits exhibited an inward current in the absence of drug application. The application of propofol caused an increase in inward current in the absence of GABA application but had little effect on GABA-activated currents recorded from the WSS-1 cell expressing ε subunits. B, bath application of propofol (3 μm) potentiated sub-maximal currents activated by brief applications of GABA (100 μm) to WSS-1 cells 24–144 h after transient transfection with GFP cDNA (○). After 48 h post-transfection, GABA-evoked currents recorded from cells expressing ε subunits (•) exhibited a significantly reduced modulation by propofol. The modulation of GABA-evoked currents by propofol was indistinguishable from control levels by 144 h post-transfection of WSS-1 cells with ε cDNA. C, GABA-evoked currents, recorded from GFP-expressing WSS-1 cells, were potentiated by flunitrazepam (1 μm) indicating the presence of α and γ subunits (○). The positive modulatory affects of flunitrazepam were abolished from 48 to 96 h after the transfection of WSS-1 cells with ε subunit cDNA (•). D, the flunitrazepam-evoked potentiation of GABA-activated currents recorded from WSS-1 cells 48–96 h post-transfection was significantly reduced (P < 0.0005) in cells expressing the ε subunit. Data, represented as mean ± s.e.m., were recorded from at least three different cells at each time point, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0005.
Figure 3. Propofol directly activates GABAA receptors in WSS-1 cells transfected with GFP cDNA alone or in combination with the ε subunit cDNA.

A, propofol causes a concentration-dependent activation of GABAA receptors recorded from GFP-expressing WSS-1 cells. Surge currents occur on cessation of the 1 s application of propofol at 100 μm. Bar represents a 1 s application of propofol. Dotted line indicates zero current. B, WSS-1 cells transfected with cDNA encoding the ε subunit exhibited leak currents. Examples are shown of increasing concentrations of propofol applied for 1 s (continuous line). Surge current relative to peak propofol-evoked current is smaller in ε subunit-expressing cells compared to control cells (A). C, graph showing the concentration-dependent relationship for the direct activation of GABAA receptors by a 1 s application of propofol. Propofol-evoked current amplitudes are expressed relative to currents activated in the same cells by a 1 s application of GABA (1 mm). Propofol (3–30 μm) evoked a similar current, relative to 1 mm GABA, in both control (○) and ε subunit-expressing WSS-1 cells (•). At higher concentrations of propofol (100 and 300 μm) WSS-1 cells transfected with ε subunit cDNA exhibited a marked reduction of peak propofol-evoked current compared to GFP-transfected cells. Peak current amplitudes were measured during the application of propofol. Data are expressed as mean ± s.e.m. from at least 3 cells 48–96 h post-transfection. *P < 0.01.
Table 1.
Comparison of the effects of GABA, propofol and Zn2+ on recombinant GABAA receptors
| Subunit combinations | GABA EC50 (μM) | GABA Hill slope | Propofol (3 μM) (% control IGABA) | Zn2+ IC50 (μM) | Zn2+ Hill slope | Current ratio (60/-60 mV) |
|---|---|---|---|---|---|---|
| α1β3 | 9. ± 1 | 0.8 ± 0.1 | 706. ± 173 | 0.22 ± 0.04 | 0.9 ± 0.1 | 1.9 ± 0.4 |
| α1β3ɛ | 9. ± 4 | 0.7 ± 0.2 | 163 ± 19a | 62 ± 12c | 0.6 ± 0.1 | 0.7 ± 0.1d |
| α1β3γ | NT | NT | 767. ± 188 | 180. ± 28 | 0.7 ± 0.1 | 1.1 ± 0.1 |
| WSS-1 | 34. ± 6 | 1.3 ± 0.2 | 655. ± 77 | 165. ± 34 | 0.7 ± 0.1 | 1.2 ± 0.1 |
| WSS-1 +ɛ | 37. ± 6 | 1.0 ± 0.1 | 222 ± 27b | 179. ± 11 | 0.8 ± 0.1 | 1.2 ± 0.1 |
Data, recorded from at least four cells, are expressed as means ± s.e.m. The EC50 values and Hill slopes were obtained from logistic fits to GABA and Zn2+ concentration–response relationships.
The propofol-evoked potentiation of GABA-activated currents was significantly smaller in recordings from cells expressing α1β3ɛ than in recordings from cells expressing either α1β3 (P < 0.0005) or α1β3γ2 (P < 0.0001).
The ± subunit caused a significant reduction (P < 0.0001) in propofol's potentiation of GABA-activated currents recorded from WSS-1 cells stably expressing α1, β3 and γ2 subunits.
The 99 % confidence interval for the logistic fit to the Zn2+ concentration–response relationship for α1β3ɛ receptors does not overlap with those of α1β3 or α1β3γ2 receptors.
The ratio of the amplitudes of GABA-activated currents recorded at 60 mV and −60 mV was significantly smaller for α1β3ɛ receptors than for either α1β3 (Davies et al. 1997a) or α1β3γ2 receptors (P < 0.05).
The benzodiazepine receptor agonist flunitrazepam (1 μm) potentiated GABA-activated currents recorded from control cells 24–144 h after transfection (Fig. 1C). By contrast, cells transfected with ε subunit cDNA exhibited a significant decline in flunitrazepam sensitivity 48 h after transfection. By 120 h after transfection the flunitrazepam-evoked potentiation was beginning to recover and, as for propofol-potentiation, was indistinguishable from control at 144 h. Together, these data demonstrate that expression of the ε subunit can displace γ subunit-dependent pharmacology in cells stably expressing αβγ receptors.
The mean flunitrazepam-elicited modulation of GABA-evoked currents recorded from WSS-1 cells expressing the ε subunit 48–96 h after transfection (131 ± 11 % of control, n = 11) was significantly reduced (P < 0.00005) compared to that seen in control WSS-1 cells (228 ± 13 % of control, n = 14, Fig. 1D). Recombinant α1β3ε receptors lack flunitrazepam sensitivity (101 ± 8 % of control, n = 4). The low-level residual flunitrazepam potentiation seen in WSS-1 cells expressing the ε subunit could be caused by the formation of mixed α1β3ε/α1β3γ2 receptors, alternatively α1β3γ2ε receptors with a modest sensitivity to flunitrazepam may form. If mixed α1β3ε/α1β3γ2 receptors do exist in WSS-1 cells expressing ε subunits then on average 24 % of receptors would have to be α1β3γ2 to enable flunitrazepam to potentiate GABA-activated currents to 131 % of control.
The concentration dependence of GABAA receptor activation was examined by pressure applying GABA (1 μm to 1 mm) for 1 s. Currents were recorded from fluorescent cells between 48 and 96 h after transfection. The introduction of the ε subunit had no effect on the potency of GABA (Fig. 2C). GABA activated currents recorded from control WSS-1 cells and cells transfected with the ε subunit cDNA with EC50 values of 34 ± 6 μm and 36 ± 6 μm, respectively (Table 1). The introduction of the ε subunit significantly reduced the peak amplitude of the current activated by GABA (1 mm) from 1.8 ± 0.2 nA (n = 11) in control cells to 0.9 ± 0.2 nA (n = 13) in cells expressing the ε subunit (P < 0.05). WSS-1 cells expressing ε subunits may contain a mixture of α1β3ε and α1β3γ2 receptors. Alternatively they may contain α1β3γ2ε receptors. For comparison HEK cells lacking GABAA receptors were transiently transfected with cDNAs encoding α1, β3 and ε subunits. These cells exhibited a GABA EC50 that was markedly different from GABA EC50 values determined for control WSS-1 cells and WSS-1 cells expressing the ε subunit (Table 1). We used parameters derived from fits of GABA concentration-response relationships in control WSS-1 cells and HEK cells expressing α1β3ε receptors to determine whether a mixed α1β3ε/α1β3γ2 receptor population could cause the relationship seen in WSS-1 cells expressing the ε subunit. As discussed above, on the basis of the low level of flunitrazepam sensitivity of WSS-1 cells after transfection with ε subunit cDNA we generated a theoretical GABA concentration-response curve assuming 24 % of the receptors were α1β3γ2 and 76 % were α1β3ε. The fact that the theoretical curve is markedly different from the experimental data suggests that WSS-1 cells transfected with ε subunit cDNA do not express mixed α1β3ε/α1β3γ2 receptors. Regardless of the ratio of α1β3ε to α1β3γ2 receptors used it was not possible to accurately represent the observed GABA concentration-response relationship.
Figure 2. Activation of GABAA receptors expressed by WSS-1 cells transfected with GFP cDNA alone or in combination with ε subunit cDNA.

A, currents activated by increasing concentrations of GABA, applied for 1 s, recorded from WSS-1 cells 48 to 96 h after transfection with GFP cDNA alone. Continuous line represents the 1 s application of GABA. Dotted line represents zero current. B, GABA-evoked currents recorded from WSS-1 cells expressing the ε subunit. Note the presence of a leak current in the absence of GABA not seen in control recordings (A). C, plot of the concentration-response relationship for GABA-evoked currents observed 48–96 h after transfection of WSS-1 cells with GFP cDNA alone (○) or in combination with ε subunit cDNA (•). From the logistic fit (Adodra & Hales, 1995) the EC50 values and Hill coefficients for activation by GABA are listed in Table 1. The dotted line is a theoretical curve generated by the sum of two logistic equations showing the expected GABA concentration-response relationship for mixed α1β3ε/α1β3γ2 receptors in the ratio 0.76:0.24. This ratio is required to explain the nominal flunitrazepam sensitivity of GABAA receptors in WSS-1 cells expressing ε subunits (see Results). The other parameters used to generate the theoretical curve are from Table 1. Data points represent the mean of at least three cells ± s.e.m. Inset, bar graph showing the leak current expressed as a mean percentage ± s.e.m. of peak current amplitude activated in the same cells by 1 s applications of GABA (100 μm). Spontaneous inward current is only observed in WSS-1 cells transfected with the ε subunit (filled bar).
Leak currents routinely appeared upon achieving the whole-cell configuration in WSS-1 cells 48–96 h after transfection with ε subunit cDNA (Fig. 1–3 and Fig. 6). The mean amplitude of the leak currents was −198 ± 46 pA (n = 13). Such spontaneous currents were not seen in control WSS-1 cells at similar times after transfection with GFP cDNA alone (Fig. 2C). The mean amplitude of leak currents recorded from control cells was −5 ± 13 pA (n = 8). This observation is consistent with the ε subunit causing the appearance of spontaneously active GABAA receptors (Neelands et al. 1999). In support of this hypothesis, picrotoxin (50 μm, data not shown) and Zn2+ (100 μm, Fig. 6A) inhibited the spontaneous current recorded from ε subunit-expressing WSS-1 cells.
Figure 6. The potency of inhibition by Zn2+ and the GABA-evoked current-voltage relationship are similar in control cells and cells expressing the ε subunit.

A, examples of superimposed GABA-evoked currents recorded before and after the application of Zn2+ (100 μm). The dotted line represents zero current. In the recording from cells expressing the ε subunit, Zn2+ application caused inhibition of both the GABA-evoked current and the spontaneous current (*). In both cases GABA (100 μm) was applied by pressure ejection for 20 ms. B, graph illustrating the concentration dependence of the inhibition of GABA-evoked currents recorded from WSS-1 cells expressing GFP cDNA alone (○) or in combination with ε subunit cDNA (•). The IC50 values and Hill slopes for the Zn2+ inhibition of GABA-evoked currents recorded from control and ε subunit-expressing cells are listed in Table 1. The dotted line is a theoretical curve generated by the sum of two logistic equations showing the expected Zn2+ concentration-response relationship for mixed α1β3ε/α1β3γ2 receptors in the ratio 0.76:0.24. This ratio is required to explain the nominal flunitrazepam sensitivity of GABAA receptors in WSS-1 cells expressing ε subunits (see Results). The other parameters used to generate the theoretical curve are from Table 1. Data points are averages recorded from at least three cells, vertical bars are ± s.e.m. C, graph showing the relationship between holding potential and the amplitudes of currents recorded from control cells (○) or cells expressing the ε subunit (•). Data were recorded from three cells. The data points were fitted with a polynomial function. Vertical bars represent ± s.e.m.
Propofol and other general anaesthetic modulators of GABA responses are able to directly activate GABAA receptors in the absence of GABA (Hales & Lambert, 1991) regardless of whether the receptors contain either γ or ε subunits (Jones et al. 1995; Davies et al. 1997a,b). The introduction of the ε subunit had no discernible effect on the ability of propofol (3–30 μm) relative to GABA (1 mm) to activate GABAA receptors in WSS-1 cells (Fig. 3). High concentrations of propofol (100 μm and 300 μm) produced a more marked reduction of GABAA receptor activity in ε subunit-expressing WSS-1 cells than in control cells. This may be due to an increase in receptor desensitization (Whiting et al. 1997) and/or increased GABAA receptor blockade by propofol (Adodra & Hales, 1995).
Spontaneous and GABA-activated channels
Spontaneous single channel events were apparent in recordings from outside-out patches excised from WSS-1 cells expressing ε subunits (n = 3, Fig. 4). These events varied substantially in their durations and had two different cord conductances, 13 ± 0.3 and 36 ± 3 pS, measured at −80 mV (Fig. 4B). No spontaneous events were observed in recordings from outside-out patches excised from control cells (n = 5, Fig. 4A). GABA (1 μm) activated channels in patches excised from WSS-1 cells expressing GFP with or without the ε subunit (Fig. 5). At least two conductance levels were apparent in these recordings. The most frequent conductance activated by GABA in all patches tested was the larger of the two conductance levels, 28 ± 1 pS (n = 5) recorded from control patches and 30 ± 4 pS (n = 3) recorded from patches excised from WSS-1 cells expressing the ε subunit (Fig. 5). In all cases the application of GABA (1 μm) revealed the presence of multiple channels in outside-out patches preventing us from undertaking kinetic analyses of these events. In future studies ultra-rapid GABA application will be employed to examine differences in gating kinetics between channels of control cells and cells expressing the ε subunit.
Figure 4. Spontaneous channel events recorded from outside-out patches excised from cells expressing the ε subunit.

A, recording from an outside-out patch, voltage clamped at −80 mV, excised from a control WSS-1 cell transfected with GFP cDNA shows very little channel activity in the absence of GABA. B, spontaneous channel opening of ε subunit-containing receptors held at −80 mV recorded from an outside-out patch excised from a WSS-1 cell transfected with ε and GFP cDNAs. Two sub-conductance states are clearly seen in the upper trace with the smaller of the two being the most frequent. C, all-points histogram of currents recorded from an outside-out patch excised from a control GFP-positive WSS-1 cell (same patch as in A). The data were well fitted by a single Gaussian function representing the distribution of current around the baseline (0 pA). The existence of GABAA receptors in this patch was subsequently verified by applying GABA (1 μm) (see Fig. 5A). D, all-points histogram of current recorded from an outside-out patch excised from an ε subunit-expressing WSS-1 cell (same patch as in B). A closed state and two conductance states, 12 and 30 pS, were measured by fitting the sum of three Gaussian functions to the data.
Figure 5. GABA-activated channels recorded from outside-out patches excised from control cells and cells expressing the ε subunit.

A, GABA (1 μm) was bath-applied to an outside-out patch excised from a GFP-positive WSS-1 cell. At least two GABA-activated channels are present in this patch, recorded at −80 mV. B, GABA-activated single channels recorded from an outside-out patch excised from a WSS-1 cell expressing the ε subunit. C, current-voltage relationship of GABA-evoked channel activity recorded from outside-out patches excised from control WSS-1 cells. Data were fitted using a linear function from which a reversal potential of −0.5 mV was calculated. The mean cord conductance of the main state was 28 ± 1 pS (n = 3). D, the relationship between voltage and the amplitude of GABA (1 μm)-activated single channel currents recorded from outside-out patches excised from WSS-1 cells expressing ε subunits. The mean cord conductance of the main state was 30 ± 4 pS (n = 3).
αβγε receptors have unique functional properties
The inhibition of αβ GABAA receptor function by Zn2+ is strongly influenced by incorporation of γ or ε subunits (Draguhn et al. 1990; Whiting et al. 1997; Fisher & Macdonald, 1998; Neelands et al. 1999). Zn2+ caused a concentration-dependent inhibition of GABA-evoked currents recorded from HEK cells expressing α1β3 receptors (Table 1). Zn2+ was less potent as an inhibitor of α1β3ε receptors and had the lowest potency as an inhibitor of α1β3γ2 receptors (Table 1).
We examined whether the modulation by Zn2+ of GABA-activated currents recorded from WSS-1 cells 48–96 h after transfection was influenced by the incorporation of the ε subunit (Fig. 6A and B). Zn2+ (1 μm to 3 mm) caused a concentration-dependent inhibition of GABA (100 μm)-activated currents recorded from GFP-expressing WSS-1 cells, with an IC50 of 165 ± 34 μm (Fig. 6B, Table 1). Surprisingly, GABA-activated currents recorded from WSS-1 cells expressing ε subunits had similar sensitivity to Zn2+ (IC50 of 179 ± 11 μm). This observation was surprising in view of the fact that Zn2+ is more effective as an inhibitor of αβε receptors compared to αβγ receptors (Table 1). It demonstrates that receptors can form with ε-like anaesthetic pharmacology and a much-reduced Zn2+ sensitivity. The small flunitrazepam modulation of GABAA receptors in WSS-1 cells after transfection with ε subunit cDNA (Fig. 2B) indicates that if α1β3γ2 receptors are present they would on average make up only 24 % of the total receptor population. We generated a theoretical Zn2+ concentration-response relationship assuming 24 %α1β3γ2 receptors and 76 %α1β3ε receptors, using the parameters listed in Table 1. The theoretical curve does not fit the data points obtained from WSS-1 cells expressing ε subunits. In fact, regardless of the ratio of α1β3ε to α1β3γ2 receptors used, the theoretical curves always failed to adequately describe the experimental observations. The low Zn2+ sensitivity of GABA-evoked currents argues against the presence of both αβε and αβ receptors in WSS-1 cells transfected with ε subunit cDNA. Taken together the data suggest the occurrence of receptors containing α, β, γ and ε subunits.
A lack of αβε receptors in WSS-1 cells transfected with ε subunit cDNA is also suggested by the relationship between current amplitude and holding potential (Fig. 6C). Currents mediated by αβε receptors exhibit either a linear relationship to voltage (Davies et al. 1997a) or inward rectification (Neelands et al. 1999), properties that distinguish them from the outward rectification seen in recordings from cells expressing either αβ or αβγ receptors. This can be appreciated by examining the ratio of GABA-evoked current amplitudes recorded at +60 and −60 mV (Table 1). Recombinant αβ or αβγ receptors exhibit +60/-60 mV current ratios of > 1, while currents recorded from cells expressing αβε receptors have +60/-60 mV current ratios < 1 (Table 1). GABA-activated currents recorded from control WSS-1 cells and cells expressing the ε subunit were indistinguishable, both exhibiting outward rectification. The absence of a linear current-voltage relationship in ε subunit-expressing cells demonstrates a lack of αβε receptors and, taken together with the Zn2+ data, suggests that αβγε receptors are formed with a distinctive combination of functional properties.
DISCUSSION
The ε subunit has greater amino acid sequence similarity to the γ subunits than to any of the other classes of GABAA receptor polypeptides. Furthermore, the ε and γ subunits both require the presence of α and β subunits to incorporate within functional receptors (Davies et al. 1997a). Therefore we initially hypothesized that ε and γ subunits compete for a common site in the GABAA receptor complex. In this study we have demonstrated that expression of the ε subunit does indeed modify the functional properties of α1β3γ2 receptors stably expressed in WSS-1 cells. However, rather than the ε subunit simply displacing the γ2 subunit from the receptor, the distinctive functional properties of the resulting receptors suggest that an αβγε receptor combination is formed.
WSS-1 cells stably express GABAA receptors that are modulated by benzodiazepines, loreclezole and intravenous general anaesthetics (Davies et al. 2000). The transient introduction of cDNA encoding GFP had no effect on the modulation of GABA-activated currents by the intravenous anaesthetic propofol, or the benzodiazepine flunitrazepam. By contrast, GABA-activated currents recorded from WSS-1 cells transiently transfected with GFP and ε subunit cDNAs became resistant to benzodiazepine modulation 48–96 h after transfection, and had a greatly diminished modulation by propofol. These properties suggest that neither αβ nor αβγ receptors are present at detectable levels. Instead, the resistance to benzodiazepine and anaesthetic potentiation suggests that either αβε or αβγε receptors are expressed. We were able to distinguish between these two possibilities by examining the GABA concentration-response relationship and by using Zn2+, a potent non-competitive inhibitor of GABAA receptors that lack γ subunits (Draguhn et al. 1990; Krishek et al. 1998). The GABA EC50 was not altered upon incorporation of the ε subunit into WSS-1 cell GABAA receptors despite the fact that α1β3ε receptors are more potently activated by GABA than are α1β3γ2 receptors in WSS-1 cells (Table 1). This observation demonstrates that there are a lack of α1β3ε receptors in WSS-1 cells expressing ε subunits and supports the existence of α1β3γ2ε receptors with similar GABA sensitivity to that of α1β3γ2 receptors.
The potencies of Zn2+ as an inhibitor of GABA-activated currents recorded from control WSS-1 cells and cells transfected with ε subunit cDNA were similar despite the fact that αβε receptors are more potently blocked by Zn2+ than are αβγ receptors (Table 1). This indicates the formation of αβγε receptors with some ε-like properties and a high resistance to Zn2+. It is not yet known whether the inhibition of ε subunit-containing receptors by Zn2+ is competitive as in the case of αβδ receptors or non-competitive as in the case of αβ and αβγ receptors (Draguhn et al. 1990; Krishek et al. 1998).
Studying the voltage dependence of GABA-activated currents recorded from WSS-1 cells expressing ε subunits provided further evidence against the existence of mixed αβε/αβγ receptor populations and, therefore, in favour of the formation of αβγε receptors. Currents mediated by αβε receptors exhibit either a linear relationship to voltage (Davies et al. 1997a) or inward rectification (Neelands et al. 1999), properties that clearly distinguish them from both αβ and αβγ receptors (Table 1). Currents recorded from control WSS-1 cells and cells expressing the ε subunit displayed similar relationships to voltage, exhibiting outward rectification indistinguishable from that seen in recordings from cells expressing recombinant αβγ receptors.
It is not known whether GABAA receptors containing both ε and γ subunits exist in vivo. Immunoprecipitation studies, in which subunit-specific antibodies were used to identify combined subunits, support the coexistence of more than one type of γ subunit in some receptors (McKernan & Whiting, 1996). In view of the amino acid sequence similarities between ε and γ subunits it would not be surprising if similar studies in the future identify receptors in specific brain regions into which both subunits assemble. Immunobiochemical studies provide evidence for (Mertens et al. 1993) and against (Quirk et al. 1995; Araujo et al. 1998) the existence of neuronal receptors containing both δ and γ subunits. The distinctive properties of recombinant receptors expressed by cells transfected with α, β, γ2 and δ subunits suggest that receptors containing all four subunits can form (Saxena & Macdonald, 1994; Krishek et al. 1996). Such receptors may be confined to a small subset of neurons making their functional or biochemical detection problematic. The occurrence of receptors containing γ and δ or ε subunits may become more widespread following seizures as a result of increased levels of ε and δ subunit expression (Brooks-Kayal et al. 1998). GABAA receptors in dentate granule cells become more Zn2+ sensitive and less benzodiazepine sensitive following pilocarpine-induced seizures. This cannot be entirely explained on the basis of the formation of αβγε receptors alone, since these have a similar Zn2+ sensitivity to αβγ receptors. However, it is possible that mixed ε and δ subunit-containing receptor populations may exist in neurons following seizures.
Functional and biochemical evidence supports the existence of GABAA receptors with stoichiometries of 2α:2β:1γ and 2α:1β:2γ subunits (Backus et al. 1993; Im et al. 1995; Chang et al. 1996; Farrar et al. 1999). Studies involving site-directed mutagenesis of these subunits support the hypothesis that GABA binds to residues within adjacent α and β subunits while benzodiazepines bind to amino acids on adjacent α and γ subunits (Sigel & Buhr, 1997; Horenstein et al. 2001). It is not clear how the additional inclusion of the ε subunit would affect subunit stoichiometry. The modest benzodiazepine sensitivity of α1β3γ2ε receptors may indicate the existence of an interface between α and γ subunits. The ε subunit differs in amino acid sequence from both α and β subunits within the putative GABA binding domains. The lack of effect of the ε subunit on the GABA EC50 may indicate that there is the same number of α/β interfaces in α1β3γ2 and α1β3γ2ε receptors. These data could be explained in the light of the current dogma regarding GABA and benzodiazepine binding by WSS-1 receptors having the stoichiometry of 2α:1β:2γ, changing to 2α:1β:1γ:1ε in cells expressing ε subunits. Alternatively if the WSS-1 cell receptor has a stoichiometry of 2α:2β:1γ then the ε subunit must displace α and/or β subunits to create receptors of unknown stoichiometry. It is not clear how this could occur without altering the GABA EC50. We are currently using cDNA constructs expressing concatenated subunits to further examine this issue.
Although receptors in cells expressing α, β, γ and ε subunits exhibit greatly reduced potentiation of GABA-activated currents by propofol, the anaesthetic was able to directly activate the receptor in the absence of GABA. Anaesthetics are also able to directly activate αβε receptors (Davies et al. 1997a). There was no significant difference in the ability of propofol (3–30 μm) relative to GABA to directly activate GABAA receptors in ε subunit- containing and control WSS-1 cells. In both cases currents activated by high concentrations of propofol (100 and 300 μm) were associated with rebound or ‘surge’ currents upon cessation of propofol application. The surge current appears to be caused by propofol unbinding from a low affinity inhibitory site on the β subunit (Adodra & Hales, 1995; Davies et al. 1997b). Compared to control WSS-1 cells, ε subunit-expressing cells had smaller propofol-evoked current amplitudes when higher concentrations of the anaesthetic were applied (P < 0.05 for 100 μm propofol). The reduced peak current amplitudes could be caused by increased receptor blockade or desensitization induced by propofol. Increased GABAA receptor desensitization by GABA has previously been reported for αβε compared to αβγ receptors (Whiting et al. 1997).
Both αβγε and αβε receptors exhibit spontaneous channel openings in the absence of GABA. These events were evident from the picrotoxin- and Zn2+-sensitive leak currents that routinely developed upon achieving the whole-cell configuration in recordings from WSS-1 cells expressing ε subunits. Spontaneous single channel activity was also directly observed in recordings from outside-out patches excised from WSS-1 cells expressing the ε subunit. Neither leak currents nor spontaneous channels were seen in whole-cell or outside-out patch recordings from control WSS-1 cells. GABA activated single channels in patches excised from either control WSS-1 cells or cells expressing the ε subunit. The main state conductance of GABA-activated αβγε channels was similar to the conductance of single channels recorded from outside-out patches excised from cells expressing either αβγ or αβε receptors, but larger than that observed in recordings of αβ single channels (Angelotti & Macdonald, 1993; Neelands et al. 1999). This similarity between ε- and γ2-containing receptors suggests that the amino acids responsible for controlling channel conductance are homologous in the two subunits.
The prolonged (> 20 s) bath application of a low concentration of propofol (3 μm) to WSS-1 cells containing ε subunits caused the appearance of a current that was significantly larger than that seen in similar recordings from control WSS-1 cells. By contrast currents activated by brief (1 s) local application of propofol (3 μm) to WSS-1 cells with or without the ε subunit were not significantly different in amplitude. One explanation for these data is that propofol is able to potentiate spontaneous channel openings. This effect may be delayed by a requirement for the channels to enter the open state. In order to examine the possibility that propofol modulates the kinetics of spontaneous channels it will be necessary to directly compare spontaneous single channel openings in the absence and presence of propofol at a concentration below that required for direct activation.
Interestingly, recent reports suggest that specific single amino acid substitutions in GABAAα and/or β subunits result in receptors with spontaneous channel activity, together with a decrease in sensitivity of the channel to several GABA receptor modulators including benzodiazepines and anaesthetics (Thompson et al. 1999; Findlay et al. 2000). Findlay and colleagues suggest that their tryptophan mutations at serine 270 in the α2 subunit or 265 in the β1 subunit decrease the free energy of the open state thus increasing the tendency of the channel to open in the absence of agonist. As would be expected from this hypothesis the mutant receptors show an increased sensitivity to activation by GABA. The mutation may mimic the effect of positive allosteric modulators such as benzodiazepines and anaesthetics making the actions of these agents redundant. Spontaneous openings induced by the ε subunit may decrease anaesthetic and benzodiazepine modulation through a similar mechanism. However, it is unlikely that such a simple scheme accounts for the resistance to modulation induced by the ε subunit, because there is little increase in the sensitivity of αβε (Davies et al. 1997a) and αβγε receptors to activation by agonists.
The peak GABA-evoked current amplitude seen in recordings from ε subunit-expressing WSS-1 cells was approximately 60 % of that seen in control WSS-1 cell recordings. This can be explained by the fact that spontaneous channel activity accounts for approximately 40 % of the GABA-evoked current amplitude in ε subunit-expressing cells. Therefore the total number of αβγε and αβγ receptors in transfected and untransfected cells remains unchanged.
By transiently introducing ε subunit cDNA into cells stably expressing αβγ receptors we have mimicked the induction of ε subunit expression in dentate granule neurons seen after pilocarpine-induced seizures in rats (Brooks-Kayal et al. 1998). Although the level of ε subunit mRNA increases relative to other GABAA mRNAs in the pilocarpine model of epilepsy the level of γ2 subunit mRNA is unchanged. The incorporation of the ε subunit into functional receptors may provide a mechanism for changing receptor properties without requiring either a reduction of γ2 transcript or the omission of γ2 subunits from functional receptors.
Acknowledgments
We are grateful to Megan Dankovich for her expert technical assistance. Research support was provided by the National Institute of Health grants GM58037 (T.G.H.) and NS34702 (E.F.K.).
References
- Adodra S, Hales TG. Potentiation, activation and blockade of GABAA receptors of clonal murine hypothalamic GT1–7 neurones by propofol. British Journal of Pharmacology. 1995;115:953–960. doi: 10.1111/j.1476-5381.1995.tb15903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: α1β1and α1β1γ2S subunits produce unique ion channels with dissimilar single-channel properties. Journal of Neuroscience. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. European Journal of Pharmacology. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H, Benson JA. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. NeuroReport. 1993;5:285–288. doi: 10.1097/00001756-199312000-00026. [DOI] [PubMed] [Google Scholar]
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. θ, a novel gamma-aminobutyric acid type A receptor subunit. Proceedings of the National Academy of Sciences of the USA. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Lin DD, Rikhter TY, Holloway KL, Coulter DA. Human neuronal gamma-aminobutyric acidA receptors: coordinated subunit mRNA expression and functional correlates in individual dentate granule cells. Journal of Neuroscience. 1999;19:8312–8318. doi: 10.1523/JNEUROSCI.19-19-08312.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nature Medicine. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. Journal of Neuroscience. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997a;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- Davies PA, Hoffmann EB, Carlisle HJ, Tyndale RF, Hales TG. The influence of an endogenous β3 subunit on recombinant GABAA receptor assembly and pharmacology in WSS-1 cells and transiently transfected HEK-293 cells. Neuropharmacology. 2000;39:611–620. doi: 10.1016/s0028-3908(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Activation by general anesthetics of rat β3GABAA subunit homomers expressed in human embryonic kidney 293 cells. British Journal of Pharmacology. 1997b;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. Journal of Biological Chemistry. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Ueno S, Harrison NL, Harris RA. Allosteric modulation in spontaneously active mutant gamma-aminobutyric acid(A) receptors in frogs. Neuroscience Letters. 2000;293:155–158. doi: 10.1016/s0304-3940(00)01503-2. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. The role of an α subtype M2-M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. Journal of Neuroscience. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. British Journal of Pharmacology. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nature Neuroscience. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- Im WB, Pregenzer JF, Binder JA, Dillon GH, Alberts GL. Chloride channel expression with the tandem construct of α6-β2 GABAA receptor subunit requires a monomeric subunit of α6 or γ2. Journal of Biological Chemistry. 1995;270:26063–26066. doi: 10.1074/jbc.270.44.26063. [DOI] [PubMed] [Google Scholar]
- Jones MV, Harrison NL, Pritchett DB, Hales TG. Modulation of the GABAA receptor by propofol is independent of the γ subunit. Journal of Pharmacology and Experimental Therapeutics. 1995;274:962–968. [PubMed] [Google Scholar]
- Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. Journal of Physiology. 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. Journal of Physiology. 1998;507:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends in Neurosciences. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by α5- and δ-subunit-specific immunopurification. Journal of Biological Chemistry. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Nayeem N, Green TP, Martin IL, Barnard EA. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. Journal of Neurochemistry. 1994;62:815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Fisher JL, Bianchi M, Macdonald RL. Spontaneous and gamma-aminobutyric acid (GABA)-activated GABAA receptor channels formed by ε subunit-containing isoforms. Molecular Pharmacology. 1999;55:168–178. doi: 10.1124/mol.55.1.168. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of delta-subunit containing GABAA receptors from rat brain. European Journal of Pharmacology. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. Journal of Neuroscience. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding of GABAA receptors. Trends in Pharmacological Sciences. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. British Journal of Pharmacology. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Smith MZ, Wingrove PB, Whiting PJ, Wafford KA. Mutation at the putative GABA(A) ion-channel gate reveals changes in allosteric modulation. British Journal of Pharmacology. 1999;127:1349–1358. doi: 10.1038/sj.bjp.0702687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O'Donnell R, Rigby MR, Sirinathsinghji DJ, Marshall G, Thompson SA, Wafford KA. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. Journal of Neuroscience. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, Sei Y, Skolnick P. Stable expression of type I γ-aminobutyric acidA/benzodiazepine receptors in a transfected cell line. Molecular Pharmacology. 1992;42:996–1003. [PubMed] [Google Scholar]


