Gastrointestinal smooth muscles contract rhythmically in the absence of neuronal or hormonal stimulation and each contraction is associated with a long-lasting wave of depolarization, termed a slow wave. Although the contractions result from the entry of Ca2+ into smooth muscle cells, following opening of L-type calcium channels, each component of the slow wave appears to be initiated by specialized non-contractile cells within the gut wall known as interstitial cells of Cajal (ICC).
Sub-populations of ICC are distributed through the gut wall. In most regions of the muscular wall of the gut, a network of ICC lies in the myenteric region of the gut, ICC-MY. In the gastric antrum ICC-MY generate the pacemaker potentials which initiate slow waves in the adjacent muscle layers (Dickens et al. 1999). A second population of ICC has an intramuscular distribution, ICC-IM. These are distributed through the muscle layers. In the small intestine ICC-IM are concentrated in a deep muscular plexus, ICC-DMP. There they play a key role in the transmission of nervous information from enteric postganglionic axons to smooth muscle cells (Ward et al. 2000). In the stomach ICC-IM are more widely distributed through the bundles of circular smooth muscle cells. In addition to their role in the transmission of neuronal information to the stomach, ICC-IM augment the waves of passive depolarization generated by ICC-MY to reinforce the depolarization so that the membrane potential of circular smooth muscle cells is dragged through a window where smooth muscle L-type calcium channels open (Dickens et al. 2001). Many of these views about the role of ICC have come from experiments carried out on mutant mice, W/WV strain, which lack specific populations of ICC in different regions of the gastrointestinal tract. Thus in the small intestine of W/WV mice, ICC-MY are largely absent but ICC-DMP are present. In these tissues where the pacemaker ICC are absent, the tissues fail to generate slow waves (Ward et al. 1994; Huizinga et al. 1995).
On the other hand since ICC-DMP are present, the responses to both inhibitory and excitatory nerve stimulation are present (Ward et al. 1994). In the stomachs of W/WV mice the pattern of ICC loss is reversed. Thus in the antral region of the W/WV stomachs, where pacemaker ICC-MY are present, rhythmical waves of depolarization are detected but as ICC-IM are absent these waves of depolarization are not augmented (Dickens et al. 2001) and neuronal responses are much reduced (Ward et al. 2000).
Each of these opinions about the three distinct roles of ICC in the control of gastrointestinal motility has been derived from experiments involving small laboratory animals. In these animals the organization of the gut wall is relatively simple. The wall consists of a thin outer layer of longitudinal smooth muscle cells. This overlies the network of pacemaker ICC-MY. The inner circular muscle layer is made up of a single layer of bundles of smooth muscle. Thus pacemaker currents generated by ICC-MY can readily depolarize both smooth muscle layers. However, in larger animals the organization of the muscle wall is more complex. In particular the circular layer is much thicker, being made up of several bundles of circular smooth muscle (Fig. 1). This being the case it was difficult to envisage how pacemaker currents, generated by the thin ICC-MY plexus, could initiate waves of depolarization in the deeper muscle layers. The paper by Horiguchi et al. (2001) in this issue of The Journal of Physiology provides a possible explanation for this puzzle and suggests that a separate population of ICC play a role in conducting electrical information from ICC-MY deep into the distant circular muscle bundles. The paper gives histological evidence that a population of ICC, ICC-SEP, lies within the septa between the circular muscle bundles.
Figure 1. Diagram showing functional organization of ICC in the canine gastric antrum.
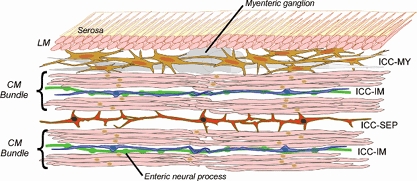
The antral wall contains two layers of smooth muscle cells: the outer longitudinal muscle layer (LM) lacking ICC and the inner circular muscle layer (CM) in which individual smooth muscle cells are organized into bundles. In this layer ICC-IM are distributed through individual CM bundles. A network of ICC lies between the LM and CM (ICC-MY). In CM bundles, ICC-IM function both to augment depolarizations reaching them from ICC-MY and as essential intermediaries in the transmission of information from enteric neural processes to nearby smooth muscle cells. ICC-SEP form functional cables which transmit information from pacemaker ICC-MY to deeper CM bundles present in the stomachs of larger animals. Although not demonstrated as yet, it may well be that ICC-SEP and ICC-MY are directly connected; alternatively ICC-SEP may be electrically excited by activity in CM bundles lying closer to the ICC-MY pacemaker network.
Electrophysiological data are presented which indicate that when the normal pathway from ICC-MY is sectioned, electrical stimulation of the cut ends of the muscle bundles can initiate slow waves over considerable distances. In the absence of stimulation, the muscle bundles isolated from ICC-MY can generate rhythmical activity but do so at low frequencies. Thus a distinct population of ICC, ICC-SEP, exists which can transfer pacemaker depolarizations from ICC-MY deep into the distant bundles of circular muscle. Although ICC-SEP have the potential to generate pacemaker activity they are not normally the dominant pacemaker centre.
As an analogy with the generation of pacemaker activity in the heart, the plexus of ICC-MY, like the sino-atrial node, is the dominant pacemaker centre. ICC-SEP, like Purkinje fibres, have the potential to generate pacemaker activity, but normally function to convey electrical activity from the dominant pacemaker region to more distant tissues.
References
- Dickens EJ, Edwards FR, Hirst GDS. Journal of Physiology. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi KG, Semple SA, Sanders KM, Ward SM. Journal of Physiology. 2001;537:237–250. doi: 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, et al. Nature. 373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Ward SM, Becket EA, Wang X, et al. Journal of Neuroscience. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, et al. Journal of Physiology. 1994;480:835–846. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]


