Abstract
The involvement of the motor cortex during human walking was evaluated using transcranial magnetic stimulation (TMS) of the motor cortex at a variety of intensities. Recordings of EMG activity in tibialis anterior (TA) and soleus muscles during walking were rectified and averaged.
TMS of low intensity (below threshold for a motor-evoked potential, MEP) produced a suppression of ongoing EMG activity during walking. The average latency for this suppression was 40.0 ± 1.0 ms. At slightly higher intensities of stimulation there was a facilitation of the EMG activity with an average latency of 29.5 ± 1.0 ms. As the intensity of the stimulation was increased the facilitation increased in size and eventually a MEP was clear in individual sweeps.
In three subjects TMS was replaced by electrical stimulation over the motor cortex. Just below MEP threshold there was a clear facilitation at short latency (≈28 ms). As the intensity of the electrical stimulation was reduced the size of the facilitation decreased until it eventually disappeared. We did not observe a suppression of the EMG activity similar to that produced by TMS in any of the subjects.
The present study demonstrates that motoneuronal activity during walking can be suppressed by activation of intracortical inhibitory circuits. This illustrates for the first time that activity in the motor cortex is directly involved in the control of the muscles during human walking.
During human walking, cells in the motor cortex that project to lower limb motoneurones are more excitable than at rest or during a tonic contraction (Petersen et al. 1998; see also Schubert et al. 1997; Capaday et al. 1999). In the studies by Schubert et al. (1997) and Capaday et al. (1999) changes in the size of the motor-evoked potential (MEP) were used as an indicator of the level of excitability in the motor cortex. Since large MEPs, particularly in the dorsiflexors, were observed during walking it was suggested that transmission in the corticospinal pathway was as significant during walking as during voluntary tonic contraction. However, transcranial magnetic stimulation (TMS) of the motor cortex activates not only cells with monosynaptic connections to the motoneurones but also pathways with polysynaptic connections (Burke et al. 1993; Nielsen et al. 1993; Nielsen & Petersen, 1995). Changes in the size of the TMS-induced MEP alone therefore reflect not only cortical excitability changes, but also changes at a subcortical level.
With H reflex testing a sufficient time resolution can be obtained to allow better identification of the various pathways activated by the magnetic stimulus. The first observable effect of TMS on the H reflex is a facilitation, which is probably due to activation of the corticomotoneuronal pathway by the magnetic stimulus (Nielsen et al. 1993). Petersen et al. (1998) used this paradigm to investigate transmission in the corticomotoneuronal pathway during walking. The short latency facilitation of the H reflex was produced by a magnetic stimulus of lower intensity during walking than at rest. In contrast, there was no difference in the threshold of the short latency facilitation produced by electrical stimulation of the motor cortex during walking and at rest. These results provided evidence that the excitability in the motor cortex is higher during walking compared to that at rest (Petersen et al. 1998).
The aim of the present study was to determine whether the increased cortical excitability directly relates to the activation of spinal motoneurones during human walking. We hypothesized that activation of inhibitory mechanisms effective only within the cortex (i.e. intracortical inhibition) should decrease cortical excitability and thus reduce cortical output. Such a reduction ought to be reflected as a suppression of the ongoing EMG activity if cortical cells make any contribution to the overall activation of the motoneurones. The principle of this idea was introduced by Davey et al. (1994) who demonstrated a suppression of the voluntary EMG activity in upper limb muscles following subthreshold TMS. Control experiments supported the idea that the observed suppression was due to the removal of cortical output by the magnetic stimulus.
Thus, if a magnetic stimulus with no direct effects on the motoneurones is capable of suppressing EMG activity during walking, then this would suggest that activity in cortical cells is transmitted to spinal motoneurones during walking in humans.
Part of this work has been presented previously in abstract form (Petersen et al. 2000).
METHODS
The experimental protocol was approved by the local ethics committee (County of Copenhagen) and was in accordance with the Declaration of Helsinki. The 19 subjects (20-37 years old) participating were all volunteers and gave written informed consent to the experimental procedures. None of the subjects had any history of neurological disease.
Electromyographic (EMG) activity was recorded from the left or right tibialis anterior (TA) and soleus muscles using surface Ag-AgCl disc electrodes placed over the belly of the muscle. The signals were amplified (×1000-5000), band-pass filtered (25-1000 Hz) then digitized and sampled (2 kHz) to a computer using a micro1401 interface (Cambridge Electronic Design Ltd, Cambridge, UK). The rectified EMG signal was averaged (triggered from the magnetic stimulus) and monitored on an oscilloscope for on-line inspection of the effect of the stimulation.
A pressure-sensitive trigger placed under the heel of the subject's shoe (same side as the EMG recordings) was used to trigger the sampling to the computer. Delays of various lengths from the time of heel contact could be set on the computer for sampling and stimulation. One delay was examined in each subject. The exact duration of the delay depended on the stride length chosen by the subject for a walking speed of 4 km h−1. In experiments examining the effect of TMS on TA EMG activity the delay ranged from 650 to 900 ms, which corresponds to the first half of the swing phase. In experiments examining the effect of TMS on soleus EMG activity the delay range was 300-400 ms (corresponding to mid-stance). Sweeps of 400 ms in length were recorded around the time of stimulation (100 ms prior to the stimulus and 300 ms after). Between 60 and 200 sweeps were collected.
Cortical stimulation
In most experiments we used a Magstim Rapid Rate stimulator (Magstim Company Ltd, Dyfed, UK) for magnetic stimulation of the motor cortex; occasionally a Magstim 200 stimulator was used. At the beginning of each experiment the coil (figure-of-eight, loop diameter 9 cm; model 8106) was placed with the current running in the posterior-anterior direction and in an optimal location for evoking a motor response (MEP) in the muscle under investigation. Overall this was slightly lateral to the vertex (contralateral to the side under investigation). The coil was held in a fixed position in relation to the head by a specially designed harness (Balgrist Tec, Zurich, Switzerland; see Schubert et al. 1997; Petersen et al. 1998). Additionally, the position of the coil was regularly inspected throughout the experiments in relation to markers drawn on the scalp.
At a rate of 0.5 Hz the signal from the pressure-sensitive resistor under the heel of the subject's shoe triggered recording of EMG activity. The trigger signal was used to activate the magnetic stimulator. Background EMG activity was recorded randomly alternating with the EMG activity following stimulation. With this protocol the subjects received a stimulus approximately once every three steps.
In three experiments TMS was replaced by electrical stimulation (Digitimer 180A, Digitimer Ltd, Welwyn Garden City, UK) of the motor cortex. The electrodes (Ag-AgCl cup electrodes, diameter 0.5 cm) were placed with the cathode 5 cm anterior to the vertex and the anode 2 cm lateral to the vertex (contralateral to the side of investigation). When setting up the experiments we verified that the electrically induced MEP had a latency 1-2 ms shorter than the magnetically induced MEP. This latency difference is explained by a different site of activation of the corticospinal tract cells by magnetic and electrical stimulation (Nielsen et al. 1993, 1995; see also Edgley et al. 1990).
In the majority of studies making use of magnetic and/or electrical cortical stimulation, the MEP threshold has been defined as the intensity at which a MEP was clearly distinguishable from the background activity in 50 % of the trials (e.g. Kujirai et al. 1993; Liepert et al. 1998). In the present study we have used a large number of sweeps to investigate the effect of TMS. The average of such a large number of trials reveals effects of TMS that are not obvious in single trials. To facilitate comparisons with other studies we use the term MEP threshold in the usual way to denote the intensity at which a MEP was observed in 50 % of trials, whereas we use the term facilitation threshold to denote the intensity at which a clear facilitation (this could be a small MEP, but mostly it is below the traditional MEP threshold) was observed in the average of all trials. The MEP threshold was determined during walking at the appropriate time in the step cycle (i.e. at the same delay from heel contact that was selected for the experiment).
Analysis
The average of sweeps recorded for steps with stimulation was superimposed on the average of sweeps recorded for steps without stimulation. The onset and end of the facilitation and suppression were estimated by visual inspection of the recordings. For quantification of the amount of suppression, the mean level of EMG activity was measured between two cursors placed at the onset and end of the suppression (see Fig. 1C and Fig. 2B). This mean was expressed as a percentage of the mean level of background EMG activity measured in the same time window but for the average of sweeps with no stimulation. The suppression was only measured for averages where no facilitation was observed. Student's paired t test was performed on the group data to reveal the statistical significance of the suppression.
Figure 1. The effect of TMS on TA EMG activity during walking.
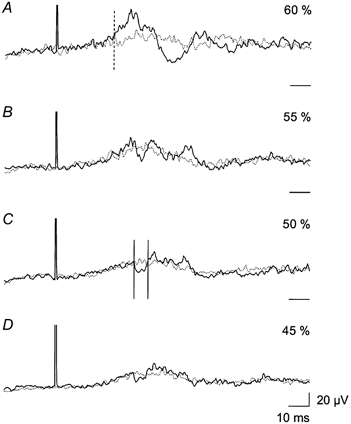
Each trace is an average of 200 sweeps of the rectified EMG activity in the TA. The average was triggered from the magnetic stimulus and obtained in the early part of the swing phase (700 ms after heel contact). Two traces are superimposed; the continuous line is the average of sweeps with magnetic stimulation, whilst the dotted line is the average EMG activity without stimulation. Sweeps with and without magnetic stimulation were sampled randomly. Four different intensities of magnetic stimulation were used (expressed as a percentage of the maximum stimulator output): A, 60 %; B, 55 %; C, 50 %; and D, 45 %. The vertical dashed line in A shows the onset of the facilitation. The vertical continuous lines in C indicate the onset and end of the suppression; this time window was used for quantification of the amount of suppression. The threshold for evoking a MEP in this subject was 65 %. Horizontal time scale bars on the right indicate the baseline (0 μV) for each trace.
Figure 2. The effect of TMS on soleus EMG activity during walking.
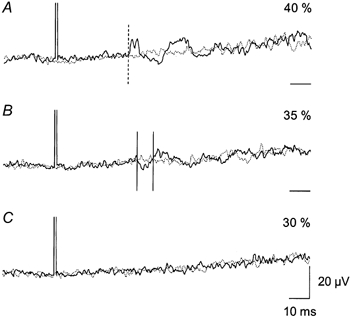
Same set-up as in Fig. 1. Each trace is the average of 150 sweeps of the rectified soleus EMG activity triggered 300 ms after heel contact. Continuous lines are EMG activity with magnetic stimulation and dotted lines are EMG activity without magnetic stimulation. The intensity of the magnetic stimulation is expressed as a percentage of maximum stimulator output: A, 40 %; B, 35 %; and C, 30 %. The MEP threshold was 50 % maximum stimulator output. The onset of facilitation is indicated in A by the vertical dashed line. The time window for measurement of the suppression is illustrated by the two vertical continuous lines in B. Time scale bars indicate the baseline (0 μV) for each trace.
RESULTS
In 12 of 19 subjects investigated, application of TMS to the motor cortex produced a distinct suppression of the rectified averaged EMG activity during walking without the appearance of facilitation at a shorter latency. Data obtained for TA from one of these subjects are shown in Fig. 1. The magnetic stimulus was applied in the early part of the swing phase, 700 ms after heel contact (onset of TA EMG activity was around 650 ms). At an intensity of TMS just subthreshold for a MEP (0.9 times MEP threshold, ≈55 % maximum stimulator output) a facilitation of the EMG activity of short latency was observed (28.0 and 32.5 ms; Fig. 1A and B, respectively). This facilitation was only visible in the averages, not in single trials. The short latency facilitation was followed by a suppression at a latency of 39.0 ms and a second period of facilitation at a latency of 45.0 ms. At weaker intensities of TMS (0.8 times MEP threshold, ≈50 % maximum stimulator output) the short latency facilitation disappeared, whereas the later suppression could still be observed (Fig. 1C). At 0.7 times MEP threshold the suppression was still apparent in this subject (Fig. 1D).
In the 12 subjects in whom it was possible to evoke a suppression of the TA EMG activity without short latency facilitation the average latency of the suppression was 40.0 ± 1.0 ms (mean ±s.e.m.). The latency for the facilitation in the same 12 subjects seen at stronger intensities of stimulation was on average 29.5 ± 1.0 ms. The average difference in stimulator output (per cent of maximum output) used to induce these effects was 8.6 ± 1.7 % (mean ±s.e.m.). In terms of threshold, the facilitation appeared at a mean intensity of 0.91 times MEP threshold, whereas the suppression could be evoked with a mean intensity of 0.77 times MEP threshold. On average, the TA EMG activity was suppressed by 20.3 ± 1.8 % (P < 0.001) of the background EMG activity. In the remaining 7 of the 19 subjects a suppression was not seen without short latency facilitation at any of the intensities of TMS used.
In 6 of 7 subjects, in whom the effect of TMS on soleus EMG activity was investigated, TMS reduced the soleus EMG activity by 16.6 ± 1.9 % (P < 0.05) when there was no short latency facilitation. Data from one of these subjects are illustrated in Fig. 2. TMS was applied 300 ms after heel contact. At 40 % maximum stimulator output (0.8 times MEP threshold) a facilitation at a latency of 36.0 ms was observed (Fig. 2A). At intensities of 30 and 35 % of maximum stimulator output (0.7 and 0.6 times MEP threshold) only suppression was seen, but at a slightly longer latency (40.5 ms).
It is generally accepted that TMS at lower intensities activates corticospinal cells indirectly whereas electrical stimulation activates the cell axons directly (Edgley et al. 1990). Comparison of the effects induced by magnetic and electrical stimulation of the motor cortex can thus provide important information about the level (spinal or cortical) at which a given effect takes place. We therefore repeated the experiments but replaced TMS with electrical stimulation over the motor cortex in three subjects. Figure 3 illustrates the results from one of these subjects. The suppression could be induced without short latency facilitation when using magnetic stimulation (middle traces in Fig. 3A). In Fig. 3B the effect of electrical transcranial stimulation is shown. At an intensity just below MEP threshold an increase in EMG activity was seen at a short latency (28.0 ms, upper trace). This difference in the latency of the facilitation between electrical and magnetic stimulation (27.5 vs. 29.5 ms, respectively) probably reflects a difference in the site of stimulation, i.e. magnetic stimulation activates the cortical cells indirectly whereas electrical stimulation activates the axons of the corticospinal cells (Nielsen et al. 1993, 1995). As the intensity of the electrical stimulation was reduced the facilitation became smaller and disappeared at an intensity of around 8 % maximum stimulator output. With electrical stimulation it was not possible to produce a suppression of the EMG activity with the same characteristics as the suppression produced by TMS of the motor cortex in any of the subjects investigated.
Figure 3. The effect of TMS vs. electrical transcranial stimulation on TA EMG activity during walking.
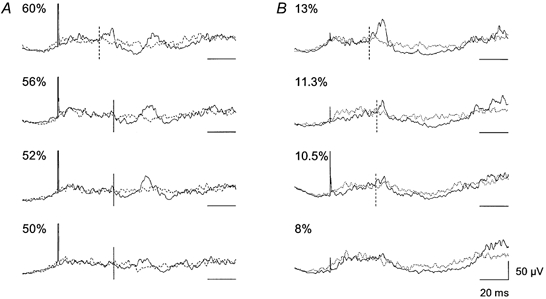
Continuous lines represent averages of sweeps (n = 100) with stimulation; TMS in A and transcranial electrical stimulation in B. Dotted lines represent averages (n = 100) of background EMG activity. The intensity of the stimulation as a percentage of the maximum stimulator output is indicated for each trace. Horizontal scale bars indicate the baseline level (0 μV) for each trace. The vertical dashed lines show the onset of the facilitation and the vertical continuous lines show the onset of the suppression.
DISCUSSION
The results from the present study demonstrate that ongoing EMG activity during walking can be suppressed by weak magnetic stimulation of the motor cortex. The main characteristics of this suppression were similar to those of the suppression of EMG activity in upper limb muscles reported by Davey et al. (1994). The onset of the suppression was approximately 10 ms later than the earlier occurring facilitation, which was only seen at stimulation intensities close to MEP threshold. In the majority of the subjects investigated the suppression was seen independently of the early facilitation at intensities well below MEP threshold. In the remaining subjects the early facilitation obscured the suppression, probably because in these subjects the facilitation and the suppression had similar thresholds to the magnetic stimulus.
The suppression of the EMG activity can be explained in at least two ways. Either the magnetic stimulus activated descending pathways with indirect inhibitory effects on the motoneurones (e.g. activation of spinal inhibitory interneurones) or alternatively cortical cells with a net inhibitory effect (i.e. intracortical inhibitory cells) on the cortical output cells were activated by the magnetic stimulus.
The former possibility is not very likely for several reasons. Firstly, the intensity of TMS at which the depression was observed was too weak to produce any facilitation in the active TA, although this muscle generally has the lowest threshold for TMS (Brouwer & Ashby, 1992; Capaday et al. 1999). This makes it unlikely that these weak stimuli evoked any descending activity. In addition, direct recordings from the epidural space have shown that TMS at intensities relative to MEP threshold comparable to those used in the present study do not produce any descending volleys in the corticospinal tract (Di Lazzaro et al. 1998b). Finally, only TMS produced the EMG depression, whereas electrical cortical stimulation failed to do so, although the two stimuli were adjusted to activate descending pathways to the same extent, as judged from the size and threshold of facilitatory effects in the TA EMG. Subcortical circuits must therefore be assumed to have been activated to a similar extent by the two stimuli. A more likely explanation for the failure of electrical cortical stimulation to evoke depression is therefore that the cortical cells have a different sensitivity to activation by the two types of stimulation. Many studies have now shown that intracortical circuits have a lower threshold for TMS than corticospinal tract cells (e.g. Kujirai et al. 1993; Di Lazzaro et al. 1998a). Transcranial electrical stimulation on the other hand tends to activate the axons of the corticospinal cells at a low threshold, and only activates intracortical circuits at stronger intensities (Edgley et al. 1990; Nielsen et al. 1995; Di Lazzaro et al. 1998a; see also Burke et al. 1993). Thus the most likely explanation for the EMG depression is that the weak magnetic stimulation activates intracortical inhibitory circuits, which by decreasing the excitability of cortical cells reduces the output from the motor cortex during walking. Previous studies support this as subthreshold magnetic stimulation applied to the motor cortex activates cortical interneurones with inhibitory projections to excitatory corticospinal cells (Kujirai et al. 1993; Burke et al. 1993; for further references see review by Rothwell, 1997).
Conclusions
Because only magnetic (and not electrical) stimulation could produce a suppression of EMG activity during walking, the effect of the magnetic stimulation is probably restricted to the cortical level (i.e. intracortical inhibition). We suggest that the suppression of the EMG activity following weak magnetic stimulation over the motor cortex is due to less excitation of the motoneurones from cortical cells as a result of a reduction in the activity of these cells caused by the magnetic stimulus. This demonstrates that the motor cortex is directly involved in the continuing activation of the lower limb motoneurones during human walking.
Acknowledgments
The study was supported by The Danish Sports Research Council, The Danish Multiple Sclerosis Foundation and The European Commission, Training and Mobility of Researchers (ERBFMMACT980464/65).
References
- Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Experimental Brain Research. 1992;89:649–654. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. Journal of Physiology. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. Journal of Neurophysiology. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. Journal of Physiology. 1994;477:223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazarro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalography and Clinical Neurophysiology. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. Journal of Physiology. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Experimental Brain Research. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. Journal of Physiology. 1995;486:779–788. doi: 10.1113/jphysiol.1995.sp020853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. Journal of Physiology. 1995;484:791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. Journal of Physiology. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Nielsen J. The effect of transcranial magnetic stimulation on the soleus H reflex during human walking. Journal of Physiology. 1998;513:599–610. doi: 10.1111/j.1469-7793.1998.599bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Hansen NL, Jensen HS, Butler JE, Ledebt A, Fisher R, Marchand-Pauvert V, Nielsen JB. Suppression of locomotor EMG activity by transcranial magnetic stimulation of the motor cortex in man. Journal of Physiology. 2000;525:43P. doi: 10.1111/j.1469-7793.2001.00651.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. Journal of Neuroscience Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Experimental Brain Research. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]


