Abstract
The formation of bubbles is the basis for injury to divers after decompression, a condition known as decompression illness. In the present study we investigated the effect of endurance training in the rat on decompression-induced bubble formation.
A total of 52 adult female Sprague-Dawley rats (300-370 g) were randomly assigned to one of two experimental groups: training or sedentary control. Trained rats exercised on a treadmill for 1.5 h per day for 1 day, or for 2 or 6 weeks (5 days per week) at exercise intervals that alternated between 8 min at 85-90 % of maximal oxygen uptake (V̇O2,max) and 2 min at 50-60 % of V̇O2,max. Rats were compressed (simulated dive) in a decompression chamber in pairs, one sedentary and one trained, at a rate of 200 kPa min−1 to a pressure of 700 kPa, and maintained for 45 min breathing air. At the end of the exposure period, rats were decompressed linearly to the ‘surface’ (100 kPa) at a rate of 50 kPa min−1. Immediately after reaching the ‘surface’ (100 kPa) the animals were anaesthetized and the right ventricle was insonated using Doppler ultrasound.
Intensity-controlled interval training significantly increased V̇O2,max by 12 and 60 % after 2 and 6 weeks, respectively. At 6 weeks, left and right ventricular weights were 14 and 17 % higher, respectively, in trained compared to control rats. No effect of training was observed on skeletal muscle weight. Bubble formation was significantly reduced in trained rats after both 2 and 6 weeks. However, the same effect was seen after a single bout of aerobic exercise lasting 1.5 h on the day prior to decompression. All of the rats that exercised for 1.5 h and 2 weeks, and most of those that trained for 6 weeks, survived the protocol, whereas most sedentary rats died within 60 min post-decompression.
This study shows that aerobic exercise protects rats from severe decompression and death. This may be a result of less bubbling in the trained animals. The data showed that the increase in aerobic capacity per se was not the main mechanism, but rather an acute effect that was most notable 20 h after a single, or the last, exercise bout, with less effect after 48 h.
The formation of bubbles is the basis for injury to divers after decompression, a condition known as decompression illness (DCI). While many cases can be treated successfully, severe injury to the central nervous system is possible and can lead to permanent disability (Francis & Gorman, 1993). There is a concern that diving can lead to permanent damage to the central nervous system even in the absence of DCI, and bubble formation has been implicated in this (Hope et al. 1994).
Vascular bubbles have been used as an indicator of decompression stress (Nishi, 1990). Even though bubbles can be present without overt clinical symptoms (Behnke, 1951), the occurrence of many bubbles is clearly linked to a high risk of DCI (Nishi, 1990). It is generally accepted that gas bubbles grow from pre-existing nuclei (Yount & Strauss, 1982). The nature of such nuclei is not well defined, but they are probably small (≈1 μm in diameter) gas-filled bubble precursors attached to the blood vessel endothelium (Harvey et al. 1944). Broome et al. (1995) demonstrated that exercise training reduced significantly the incidence of neurological DCI in the pig, and Rattner et al. (1979) showed that 14 and 28 days of treadmill training for 1 h per day, reduced the symptoms of DCI and increased survival after decompression, when compared to sedentary rats. This indicates that exercise can either influence bubble formation or can change the reaction of the organism to the bubbles. Unfortunately, interpretation of, and comparisons between, previous studies is difficult due to a common weakness in not controlling the exercise intensity and/or documenting the cardiovascular effects of the endurance training. Recently, we have established a model for training of rats (Wisloff et al. 2001a) that gives a robust improvement in maximal oxygen uptake (V̇O2,max) and cardiac hypertrophy, which we now use to determine the effect of aerobic endurance exercise and training on bubble formation and DCI in the rat.
METHODS
Study population
A total of 52 adult female Sprague-Dawley rats (Møllegaards, Denmark) of 300-370 g were maintained six in each cage, and randomly assigned to either a training or a sedentary control group. Light was controlled on a 12 h dark and 12 h light cycle. The temperature was maintained at 22.5 ± 1.4 °C, and the humidity at 55.6 ± 4.0 %. Animals were fed a pelleted rodent diet ad libitum and had free access to water. None of the rats were excluded from the study because they avoided running. The experimental procedures conform to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, and the protocol was approved by the Norwegian Council for Animal Research.
Maximal oxygen uptake
Oxygen uptake and the respiratory exchange ratio were measured during treadmill exercise as described previously (Wisloff et al. 2001a). To train the rats, they were placed onto a 70 cm-long treadmill that had a stainless-steel grid at the end of the lane. This grid supplied an electrical stimulus (0.25 mA, 1 stimulus of 200 ms duration every second) to keep the rats running on the lane. All rats avoided the electrical grid, only touching it 2 ± 1 times per training session. The treadmill was placed into an 11 l metabolic chamber. Ambient air was pumped through the metabolic chamber at a flow rate of 4.5 l min−1, and samples of extracted air (200 ml min−1) were directed to an oxygen analyser that was based on a paramagnetic oxygen transducer (Servomex type 1155, Servomex, UK), and a carbon dioxide analyser (LAIR 12, M&C Instruments, The Netherlands). Maximal oxygen uptake (V̇O2,max) and work economy were measured as described previously (Wisloff et al. 2001a). Briefly, after a 15 min warm-up at 40-50 % of V̇O2,max, treadmill speed was increased by 0.03 m s−1 every 2 min until a levelling-off of oxygen uptake despite increased workload was observed. Based on a previous study (Wisloff et al. 2001a), all treadmill tests and training were performed using 25 deg inclination (corresponding to ≈47 % slope) of the treadmill during the rat's dark cycle. Work economy was defined as oxygen cost at a given submaximal running velocity.
Training procedure
The rats were assigned to one of four groups, as described in Table 1. Trained rats exercised on the treadmill for 1.5 h per day, 5 days per week, and at the start of every week V̇O2,max was measured and workloads adjusted accordingly. In training rats, exercise intervals alternated between 8 min at 85-90 % of V̇O2,max and 2 min at 50-60 %. Before the first interval, each rat performed a 20 min warm-up at 40-50 % of V̇O2,max. In sedentary rats, treadmill-running skill was maintained by treadmill running for 15 min at 0 deg inclination at 0.15 m s−1, 3 days per week. After each training or test session, each rat was rewarded with 0.5 g of chocolate (Crispo, Nidar Bergene, Norway). Sedentary rats were given the same amount. From previous studies (Wisloff et al. 2001a, b) we know that it takes about 2 weeks of training to induce a physiologically significant improvement in V̇O2,max (of about 10 %), and that 6 weeks of training produces a maximal adaptation of V̇O2,max in our training model. We therefore wanted to determine the effect of training on bubble formation and survival at these time points. In addition, a group of rats was included to determine whether a single bout of exercise could have some protective effect. As there was a trend towards heavier body weights in sedentary rats after 2 weeks, another set of weight-matched sedentary rats was studied, which also served as controls for the acutely trained rats (group III).
Table 1.
Overview of group assignment and the number of rats used in each protocol
| Group | Protocol | n | Time of compression/decompression |
|---|---|---|---|
| I | Training and sedentary, 2 weeks | 14 | 20 h post-training |
| II | Training and sedentary, 6 weeks | 14 | 20 h post-training |
| III | Exercise and sedentary, 1.5 h | 12 | 20 h post-training |
| IV | Training and sedentary, 6 weeks | 12 | 48 h post-training |
n, total number of rats, half in each group; time of compression/decompression, time of compression/decompression after last training session.
Rats were compressed (simulated dive) in a decompression chamber in pairs, one sedentary and one trained, at a rate of 200 kPa min−1 to a pressure of 700 kPa, and maintained at that pressure for 45 min breathing air. At the end of the exposure period, rats were decompressed linearly to the ‘surface’ (100 kPa) at a rate of 50 kPa min−1. Immediately after ‘surfacing’, the animals were anaesthetized with midazolam (Dormicum ‘Roche’)/fentanyl/ fluanison (Hypnorm) (0.1 ml (100 g)−1s.c.), and the right ventricle was insonated using a GE Vingmed Vivid 5 scanner, with a 10 MHz transducer. Bubbles could be seen in the right ventricle and the pulmonary artery as bright spots. Data were stored and played back in slow motion for analysis. If there are only a few bubbles they are difficult to detect in the images due to the high heart rate in rats, but they can be seen clearly on slow-motion playback. When many bubbles are present, there is a pressure increase in the right heart, providing better images of the right ventricle as it dilates. Images were graded according to a previously described method (Eftedal & Brubakk, 1997) with the observer blinded to the group allocation of the rat. To facilitate the ultrasonic scanner method, Doppler was used to locate the relatively small vessels and confirm the presence of bubbles. Surviving rats were killed after 60 min. After death, the heart and the soleus muscle (predominantly slow-twitch, type I fibres) and extensor digitorum longus muscle (predominantly fast-twitch, type II fibres) were removed and weighed.
Statistics
Data are expressed as means ±s.d., or as the median and range. We chose to use non-parametric tests since each protocol produced data from only a few rats. The Friedman test, applying appropriate procedures for multiple comparisons (Conover, 1999), was used to determine changes in oxygen uptake, work economy and body weight throughout the experimental period. A Mann-Whitney U test was used to evaluate differences in bubble formation, whereas the Gehan generalized Wilcoxon test was used to evaluate differences in survival between groups. P < 0.05 was considered as statistically significant.
RESULTS
Aerobic endurance training significantly increased V̇O2,max by 12 and 60 % after 2 and 6 weeks, respectively (Fig. 1A). Work economy, measured as oxygen uptake at a given submaximal running speed, was improved by 7 ± 3.4 % (2 weeks) and 16 ± 4.2 % (6 weeks; Fig. 1B).
Figure 1. Cardiovascular adaptations.
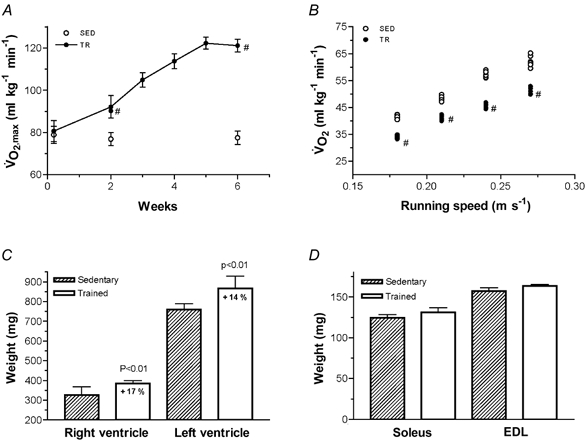
A, maximal oxygen uptake (V̇O2,max) in trained (filled symbols) and sedentary control (open symbols) rats. B, work economy after 6 weeks of training. C, weight of the right and left ventricles after 6 weeks. D, weight of the soleus and extensor digitorum longus (EDL) muscles after 6 weeks. Each of the data points represents the mean ±s.d. from seven rats (A, C and D), or are presented individually (B). # Differences between groups, P < 0.01.
Consistent with previous experiments, the present training protocols induced marked adaptive cardiac hypertrophy. At 6 weeks, left and right ventricular weights were 14 and 17 % higher, respectively, in trained animals than in sedentary rats (Fig. 1C). No significant training effect on left (training: 805 ± 51 mg; sedentary: 753 ± 40 mg) or right (training: 352 ± 34 mg; sedentary: 329 ± 41 mg) ventricle weights was observed after 2 weeks. No effect of training was observed in skeletal muscle weight after 2 weeks (data not shown) or at 6 weeks (Fig. 1D).
The data showed that 2 weeks of endurance training decreased bubble formation and increased survival (Table 2). As seen in Table 2, there was a trend towards a higher body weight in sedentary rats after 2 weeks. To avoid the bias caused by differences in body weight, a weight-matched sedentary group was studied (see sedentary rats in Table 2, group III).
Table 2.
Effects of exercise on bubble formation and survival
| 2 week (group I) | 6 week (group II) | Acute (group III) | 6 week (group IV) | |||||
|---|---|---|---|---|---|---|---|---|
| TR | SED | TR | SED | TR | SED | TR | SED | |
| Weight (g) | 313 ± 11 | 327 ± 20 | 353 ± 10 | 353 ± 11 | 309 ± 15 | 310 ± 5 | 351 ± 10 | 349 ± 3 |
| Scan grade | 1.0 (0–4) * | 5.0 (3–5) | 3.0 (1–5) * | 5.0 (4–5) | 0.5 (0–2) * | 5.0 (4–5) | 4.0 (0–5) * | 5.0 (—) |
| Survival time (min) | > 60 (—) * | 16 (4–60) | 60 (16–60) * | 16 (4–20) | > 60 (—) * | 15 (7–60) | 20 (4–60) * | 6 (2–17) |
Significant differences between the groups, P < 0.03. Note that rats that are registered as dead after 60 min were actually still alive at that point, but were killed after 60 min. Body weights are presented as means ±s.d.; the rest of the data are presented as median (range). Decompression time was 12 min for all groups. For groups I–III the compression/decompression protocol was performed 20 h after the last training session, whereas the protocol in group IV was performed 48 h post-training. TR, trained; SED, sedentary.
To test whether an even higher aerobic capacity (Fig. 1) could reduce bubble formation further, we trained another group of rats for 6 weeks (group II). These results are presented in Table 2.
The resulting data show that 6 weeks of endurance training reduced bubble formation and increased the survival rate. However, even though these animals had a markedly higher aerobic capacity (Fig. 1), bubble formation was higher (P < 0.01) than in rats that had been trained for 2 weeks, and a trend towards higher mortality was also observed. As 2 weeks of endurance training had a similar or better effect on bubble formation and survival, we tested whether a single bout of exercise on the day prior to the dive could give some protective effect. These data are presented in Table 2 (group III). Surprisingly, rats performing a single bout of exercise lasting for 1.5 h, with 20 h rest before the dive, had significantly less bubble formation compared to sedentary rats. From this, we speculated that there was an acute exercise effect and we therefore trained a new set of rats for 6 weeks (group IV) to achieve a maximal training effect on V̇O2,max in our training model, and let them rest for 48 h after the last training session before performing the compression/decompression protocol. These results are presented in Table 2 (group IV).
The data show that training still has a statistically significant effect on bubble formation and the rate of survival. However, there was a trend towards a less protective effect than after 20 h for both bubble formation (P = 0.06) and survival (P = 0.08). Despite some significant differences in the scan data amongst groups, we examined further this effect by combining data from all rats that dived 20 h following exercise (groups I, II and III) and compared them to the rats that dived 48 h following exercise (group IV); a significant difference for both bubble scores (P < 0.008) and the rate of survival (P < 0.001) was noted.
DISCUSSION
The present study demonstrates two previously unreported findings, that high-intensity aerobic exercise protects rats from severe decompression and death, and that a single bout of exercise is as effective as a prolonged daily training regime.
A reduction in the incidence of DCI after 2 and 6 weeks of endurance training is in line with previous reports in mice (Rattner et al. 1979) and in pigs (Broome et al. 1995). Furthermore, in a study on divers, Lagrue et al. (1978) noted that there was less bubble formation in divers who were in good physical shape. It is not known why training reduces bubble formation. However, there are some indications that increased aerobic fitness can increase the rate of nitrogen elimination, and this might reduce the amount of nitrogen available for diffusion into bubbles. Dick et al. (1984) observed that gas elimination was faster in one of their subjects after a period of training than after a period with little activity. Rønning (1999) showed that there was a linear relationship between V̇O2,max and the rate of nitrogen elimination; however, this study involved few subjects and needs verification. If indeed the mechanism for the positive training effect is the increased rate of nitrogen elimination, then it is most likely that this happens in the large muscle groups, possibly as a result of increased capillary density, and thereby increased blood flow to skeletal muscles both at rest and during exercise. This has been shown both in rat models (Armstrong & Laughlin, 1984; Sexton et al. 1988) and in man (Sinoway et al. 1987). However, increased blood flow will also result in a faster uptake of nitrogen, and any reduced bubble formation due to faster washout will depend upon the dive duration and decompression rate. If the muscle is largely saturated after the dive, as in the present study, rats with similarly faster washout of gas may experience reduced bubbling. However, it seems unlikely that only 1.5 h of exercise prior to the dive improves a subject's capillary density and nitrogen elimination capacity. The time course of 20 h is too long for any commonly known acute exercise effects such as, for example, hyperaemia, or elevated lactic acid, corticosteroid or catecholamine levels to persist (Åstrand & Rodahl, 1986).
Rather than altering nitrogen elimination, exercise may alter the population of nuclei from which bubbles form. Gas nuclei are thought to exist adhered to blood vessel walls (Harvey et al. 1944). Endothelium is considered to be mostly hydrophilic, but a study by Hills (1992) demonstrated that the endothelium of veins and the aorta is hydrophobic. He postulated that this hydrophobicity is caused by surface-active substances coating the surface of the endothelium. Bubbles can be stable more or less indefinitely on a hydrophobic surface (Libermann, 1957). Thus, any process that influences the surface properties of the endothelium may affect bubble formation in the vascular system. It is known that the production of NO from endothelial cells increases within hours following an increase in flow and shear stress, as occurs during exercise (Buga et al. 1991). Recently it has also been shown that veins and venules do not normally exhibit nitric oxide (NO) production, but NO is produced on stimulation (Riezebos et al. 1994; Vallance, 2000). As it is known that NO inhibits leucocyte adhesion and platelet adhesion and aggregation (Bult, 1996), it is conceivable that an increase in NO also will reduce the hydrophobicity of the endothelial wall, reducing the number of nuclei adhering to the surface. Such a mechanism could conceivably influence vascular bubble formation by reducing the stability of bubble nuclei. One could also speculate whether exercise depletes bubble precursors (nuclei). The proposed 10-100 h time course for regeneration of a depleted nucleus population (Yount & Strauss, 1982) might explain the difference in bubble formation between dives that are endured 20 and 48 h post-exercise.
Conclusions
This study has demonstrated that a single bout of aerobic exercise prevents bubble formation in the rat and, thereby, reduces the risk of serious injury or death. Further work is needed to elucidate the mechanisms underlying this training-induced reduction in bubble formation, and to determine the importance of this finding in man.
Acknowledgments
This study was supported by the Norwegian Petroleum Directorate, Norsk Hydro, Esso Norge and Statoil under the ‘dive contingency contract’ (no. 4600002328) with Norwegian Underwater Intervention (NUI).
References
- Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. American Journal of Physiology. 1984;246:H59–68. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- Åstrand PO, Rodahl K. Textbook of Work Physiology. Singapore: McGraw-Hill; 1986. pp. 295–522. [Google Scholar]
- Behnke A. Decompression sickness following exposure to high pressures. In: Fulton JF, editor. Decompression Sickness. London: Saunders; 1951. pp. 53–89. [Google Scholar]
- Broome J, Dutka A, McNamee G. Exercise conditioning reduces the risk of neurologic decompression illness in swine. Undersea and Hyperbaric Medicine. 1995;22:73–85. [PubMed] [Google Scholar]
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Bult H. Nitric oxide and atherosclerosis: possible implications for therapy. Molecular Medicine Today. 1996;2:510–518. doi: 10.1016/s1357-4310(97)81455-4. [DOI] [PubMed] [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. New York: Wiley; 1999. [Google Scholar]
- Dick AP, Vann RD, Mebane GY, Feezor MD. Decompression induced nitrogen elimination. Undersea and Biomedical Research. 1984;11:369–380. [PubMed] [Google Scholar]
- Eftedal O, Brubakk A. Agreement between trained and untrained observers in grading intravascular bubble signals in ultrasonic images. Undersea and Hyperbaric Medicine. 1997;24:293–299. [PubMed] [Google Scholar]
- Francis T, Gorman D. Pathogenesis of the decompression disorders. In: Elliot DH, Bennett PB, editors. The Physiology and Medicine of Diving. London: Saunders; 1993. pp. 454–480. [Google Scholar]
- Harvey EN, Whiteley AH, McElroy WD, Pease DC, Barnes DK. Bubble formation in animals II. Gas nuclei and their distribution in blood and tissues. Journal of Cellular and Comparative Physiology. 1944;24:23–34. [Google Scholar]
- Hills BA. A hydrophobic oligolamellar lining to the vascular lumen in some organs. Undersea Biomedical Research. 1992;19:107–120. [PubMed] [Google Scholar]
- Hope A, Lund T, Elliot D, Halsey M, Wiig L. Long Term Health Effects of Diving. Bergen: Nutec-report; 1994. [Google Scholar]
- Lagrue D, Lepechon J, Kisman K, Masurel G, Guillerm R. Etude comparative de la methode de decompression du Ministre Francais du Travail et de la methode de decompression dite du surface de l'U. S. Navy pour la plongee a lu air. Médecine et de Physiologie Subaquatiques et Hyperbares. 1978;68:353–357. [Google Scholar]
- Libermann L. Air bubbles in water. Journal of Applied Physiology. 1957;28:205–211. [Google Scholar]
- Nishi R. Doppler evaluation of decompression tables. In: Lin YC, Shida KK, editors. Man in the Sea. Flagstaff, AZ, USA: Best; 1990. pp. 297–316. [Google Scholar]
- Rattner B, Gruenau S, Altland P. Cross-adaptive effects of cold, hypoxia, or physical training on decompression sickness in mice. Journal of Applied Physiology. 1979;47:412–417. doi: 10.1152/jappl.1979.47.2.412. [DOI] [PubMed] [Google Scholar]
- Riezebos J, Watts IS, Vallance PJ. Endothelin receptors mediating functional responses in human small arteries and veins. British Journal of Pharmacology. 1994;111:609–615. doi: 10.1111/j.1476-5381.1994.tb14780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønning P. MSc Thesis. Trondheim, Norway: Norwegian University of Science and Technology; 1999. Respiratorisk nitrogeneliminasjon hos unge menn med ulik aerob kapasitet; pp. 1–69. [Google Scholar]
- Sexton WL, Korthuis RJ, Laughlin MH. High-intensity exercise training increases vascular transport capacity of rat hindquarters. American Journal of Physiology. 1988;254:H274–278. doi: 10.1152/ajpheart.1988.254.2.H274. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. Journal of Applied Physiology. 1987;62:1063–1067. doi: 10.1152/jappl.1987.62.3.1063. [DOI] [PubMed] [Google Scholar]
- Vallance P. Nitric oxide synthesised from l-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovascular Research. 2000;45:143–147. doi: 10.1016/s0008-6363(99)00315-6. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO2,max and cardiac hypertrophy. American Journal of Physiology – Heart and Circulatory Physiology. 2001a;280:H1301–1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovascular Research. 2001b;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- Yount D, Strauss R. On the evolution, generation and regeneration of gas cavitation nuclei. Journal of the Acoustical Society of America. 1982;65:1431–1439. [Google Scholar]


