Abstract
This study was aimed at identifying the signalling pathways involved in the activation of volume-regulatory mechanisms of human cervical cancer cells.
Osmotic swelling of human cervical cancer cells induced a substantial increase in intracellular Ca2+ ([Ca2+]i) by the activation of Ca2+ entry across the cell membrane, as well as Ca2+ release from intracellular stores. This Ca2+ signalling was critical for the normal regulatory volume decrease (RVD) response.
The activation of swelling-activated ion and taurine transport was significantly inhibited by tyrosine kinase inhibitors (genistein and tyrphostin AG 1478) and potentiated by the tyrosine phosphatase inhibitor Na3VO4. However, the Src family of tyrosine kinases was not involved in regulation of the swelling-activated Cl− channel.
Cell swelling triggered mitogen-activated protein (MAP) kinase cascades leading to the activation of extracellular signal-regulated kinase 1 and 2 (ERK1/ERK2) and p38 kinase. The volume-responsive ERK1/ERK2 signalling pathway linked with the activation of K+ and Cl− channels, and taurine transport. However, the volume-regulatory mechanism was independent of the activation of p38 MAP kinase.
The phosphorylated ERK1/ERK2 expression following a hypotonic shock was up-regulated by protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and down-regulated by PKC inhibitor staurosporine. The response of ERK activation to hypotonicity also required Ca2+ entry and depended on tyrosine kinase and mitogen-activated/ERK-activating kinase (MEK) activity.
Considering the results overall, osmotic swelling promotes the activation of tyrosine kinase and ERK1/ERK2 and raises intracellular Ca2+, all of which play a crucial role in the volume-regulatory mechanism of human cervical cancer cells.
Cells have to avoid excessive changes of cell volume, which jeopardize structural integrity and constancy of the intracellular milieu. Homeostasis of cell volume does not simply mean maintaining a constant volume, but rather serves as the integration of events in regulating cell function, including epithelial transport, metabolism, cell proliferation, migration and cell death (reviewed in Lang et al. 1998; Hoffmann & Dunham, 1995). Cells defend themselves against hypotonic stress by losing solutes together with osmotically obligated water, a process termed regulatory volume decrease (RVD). The principal solutes lost in RVD are K+, Cl− and a variety of largely uncharged or zwitterionic organic solutes, such as taurine (Kirk, 1997). In most cell types, the predominant pathway for RVD is the opening of separate K+ and Cl− channels (Okada, 1997). Another important pathway for RVD is the K+-Cl− cotransporter (KCC), which transports K+ and Cl− stoichiometrically in either direction across plasma membranes and is observed predominantly in erythrocytes (Ellory et al. 1998), neurons and some epithelial cells (Hoffmann & Dunham, 1995).
We have demonstrated previously that volume-regulatory ion channels and the cotransporter work synergistically for volume regulation of human cervical cancer cells (Shen et al. 2000a). More importantly, the volume-regulatory transport systems for KCl and organic osmolyte efflux are strongly up-regulated during the process of malignant transformation of human cervical epithelial cells (Shen et al. 1996, 2000a; Chou et al. 1995, 1997). The cell cycle progression of cervical cancer cells is also accompanied by differential activities of the swelling-activated Cl− channel and taurine transport (Shen et al. 2000b, 2001). In addition, alterations in osmosensing signalling pathways are apparent during human cervical carcinogenesis (Shen et al. 1998, 1999; Chou et al. 1998). For example, phospholipase C (PLC) signalling with downstream activation of PKCα is involved in the cell volume regulation of cervical cancer cells. On the other hand, different PKC isoforms that are not related to upstream PLC regulation are involved in the RVD of human papilloma virus-immortalized and normal cervical epithelial cells.
The mechanisms underlying the activation of volume-regulatory ion channels and transporters are not fully understood. The signalling pathways linking the osmosensor(s) to the RVD effectors also remain ill-defined. This study was designed to investigate the signalling roles of Ca2+, protein tyrosine phosphorylation and MAP kinases in the regulation of swelling-activated ion and taurine transport pathways in human cervical cancer cells. There were four specific aims: (1) to clarify the role of Ca2+ signalling in volume regulation of human cervical cancer cells; (2) to assess the involvement of tyrosine phosphorylation in the RVD response and in the activation of swelling-activated ion and taurine transport; (3) to determine whether signal cascades of MAP kinases are provoked in swollen cervical cancer cells and, if so, whether these signalling pathways are linked with cell volume regulation; (4) to characterize the interactions between the pathways involved in the response to osmotic stress that have been identified in human cervical cancer cells.
METHODS
Cell culture
SiHa cells, a human cervical cancer cell line, were obtained from the American Type Culture Collection (Rockville, MD, USA). SiHa cells from passage 15-35 were used, and maintained at 37 °C in a CO2-air (5 %-95 %) atmosphere and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Lab., Grand Island, NY, USA) supplemented with 10 % fetal calf serum (FCS; Gibco), 80 i.u. ml−1 penicillin and 80 μg ml−1 streptomycin (Sigma-Aldrich Company Ltd, Dorset, UK).
Chemicals and solutions
The following primary antibodies were purchased from New England Biolabs Inc. (Beverly, MA, USA): anti-phospho-ERK1/ ERK2 (Thr202/Tyr204), anti-ERK1/ERK2, anti-phospho-p38 MAP kinase (Thr180/Tyr182) and anti-p38 MAP kinase. The secondary antibody, goat anti-rabbit IgG conjugated to horseradish peroxidase, and PD 98059, a specific inhibitor of mitogen-activated/ERK-activating kinase (MEK), were also obtained from New England Biolabs Inc. SB 202198 and PP2, the specific inhibitors for p38 MAP kinase and Src tyrosine kinase respectively, were purchased from Calbiochem (CN Biosciences, Nottingham, UK). Tyrphostin AG 1478 was obtained from Alexis (Alexis Corporation Ltd, Nottingham, UK). Active and inactive peptides of the Src family (EPQ(pY)EEIPIA and EPQYEEIPIA) were synthesized by Princeton Biomolecules (Columbus, OH, USA). All other chemicals were obtained from Sigma-Aldrich Company Ltd (Dorset, UK). The osmolarity of solutions was measured using a vapour pressure osmometer (Wescor 5500, Schlag Instruments, Gladbach, Germany). The isotonic medium contained (in mm): NaCl 100, KCl 5, MgCl2 1, CaCl2 1.5, glucose 10, Hepes 10 and mannitol 70, titrated to pH 7.4 with NaOH (300 ± 3 mosmol kg−1, n = 5). The components of the hypotonic medium were the same as those of the isotonic medium except that mannitol was omitted, resulting in a 23 % hypotonicity (230 ± 3 mosmol kg−1). To measure the activity of the swelling-activated Cl− channel, the KCl was replaced by CsCl in the media and the pipette solutions contained (mm): CsCl 40, caesium aspartate 100, MgCl2 1, CaCl2 1.93, EGTA 5, ATP 2, GTP 0.5, Hepes 5. In this pipette solution, the free Ca2+ concentration was buffered at 100 nm. In some experiments, the free Ca2+ concentration was buffered to near zero by the following components of pipette solution (mm): CsCl 40, caesium aspartate 100, MgCl2 1, BAPTA 10, ATP 2, GTP 0.5, Hepes 5. All pipette solutions were adjusted to pH 7.2 with CsOH. The solvent for various chemicals was DMSO. The final DMSO concentration in all experiments was less than 0.05 %. This DMSO concentration has no effect on electrophysiological recordings, flux and cell volume measurements.
Fluorescence measurements of [Ca2+]i with fura-2
For [Ca2+]i measurement, cells were detached from culture flasks by treatment with phosphate-buffered Ca2+-free saline containing 1 mm EDTA and 0.01 % trypsin. In some preliminary experiments, attached cells on a glass cover slip were loaded and measured, giving the same results as cells in suspension but much smaller and noisier signals. We therefore used cell suspension in subsequent experiments, in which the hypotonic solution was made by direct dilution but [Ca2+]o was kept constant at 1.5 mm. To load the cells with 3 μm fura-2/AM, the cell suspension of 106 cells ml−1 was incubated in DMEM culture medium at room temperature for 30 min and then at 37 °C for 10 min. After loading, cells were washed twice with phosphate-buffered saline and re-suspended in the isotonic solution. Dye fluorescence was measured in a fluorimeter at room temperature (F-2000, spectrophotometer, Hitachi, Tokyo, Japan). Excitation wavelength was alternated between 340 nm (I340) and 380 nm (I380) and fluorescence intensity was monitored at 510 nm. [Ca2+]i was calculated from the I340/I380 ratio using the equation proposed by Grynkiewicz et al. (1985):
where Kd is the dissociation constant for fura-2 in the cytosol (250 nm) and Fmin and Rmin are the 380 nm fluorescence intensity and I340/I380 ratio at low [Ca2+]i, respectively. Fmax and Rmax are the 380 nm fluorescence intensity and I340/I380 ratio at high [Ca2+]i, and R is the I340/I380 ratio recorded during experiments. Calibration measurements of Fmin and Rmin were performed after incubating cells for 10 min in nominally Ca2+-free isotonic solution containing 3 mm EGTA. Cells were then superfused with isotonic solution containing 1 μm thapsigargin, 5 μm ionomycin and 10 mm Ca2+ to evaluate Fmax and Rmax.
Electrophysiological measurements
The whole-cell mode of the patch-clamp technique was used to measure membrane currents at room temperature (22-25 °C) as previously described (Chou et al. 1998). When the pipettes were connected to the input stage of an Axopatch-200A amplifier (Axon Instruments, Burlingame, CA, USA), their DC resistance varied between 3 and 5 MΩ. A Ag-AgCl wire was used as reference electrode. The current-voltage relationship and time course of swelling-activated Cl− current were obtained from either a ramp or a step protocol. The ramp protocol consisted of: a step to -80 mV for 0.4 s followed by a 1.3 s linear voltage ramp to +80 mV, after which the potential was stepped back to the holding potential of -20 mV. This voltage protocol was repeated every 15 s from a holding potential of -20 mV. Currents were sampled at 2 ms intervals (1024 points per record, filtered at 200 Hz). The step protocol consisted of a 1 s voltage step, applied every 15 s from a holding potential of -20 mV to test potentials from -80 to +80 mV with an increment of 20 mV. Currents were sampled at 1 ms intervals. Current densities were determined by normalizing the whole-cell current to the membrane capacitance. Data from electrophysiological experiments were digitized and analysed using pCLAMP software (Version 6.0.3, Axon Co., Foster City, CA, USA). For patch clamp studies, the inhibitors (PD 98059, SB 202190, PP2) and the Src tyrosine kinase activator peptide and its inactive counterpart were present in the pipette solution, such that cells were subject to intracellular dialysis. Each compound was therefore present at least 5 min prior to any current measurements. In addition, some patch clamp experiments were also performed in which PD 98059, SB 202190 and PP2 were added to the bath solution instead of pipette solution during current measurements.
Functional [3H]taurine efflux assays
[3H]Taurine efflux experiments were carried out at room temperature as described in detail elsewhere (Shen et al. 2000a). In brief, SiHa cells, grown on six-well plates, were pre-incubated with isotonic culture medium loaded with 1 μCi ml−1[3H]taurine for 2 h at 37 °C. After pre-incubation, the loading medium was aspirated and cells were washed rapidly seven times with phosphate-buffered saline. After washing, appropriate efflux medium with or without drugs tested was added to the cells for a 30 min pre-incubation. After pre-incubation, 1 ml aliquots of efflux medium were taken and replaced at indicated time intervals and saved for counting. The drugs tested were present throughout the pre-incubation and flux measurement periods. Release of [3H]taurine from preloaded cells was measured from the efflux medium at indicated time intervals within a 25 min duration. Cells were finally lysed with 0.5 m NaOH to release the remaining [3H]taurine. The radioactivity present in the efflux samples and in cell lysates was then determined by liquid scintillation. [3H]Taurine efflux rate constants were estimated from the negative slope of the graph of ln[Xi(t)/Xi(t = 0)]vs. time (t), where Xi(t = 0) denotes the total amount of [3H]taurine inside the cells at the beginning of the efflux time course and Xi(t) denotes the amount of [3H]taurine inside the cells at the time point t. Following hypotonic shock, [3H]taurine efflux occurs in two phases: an initial fast loss within 5 min and followed by a slower loss in the subsequent time course (Shen et al. 2001). Therefore, the [3H]taurine efflux rate constant in the hypotonic solution was calculated from the negative slope of the semi-logarithm graph in the initial 5 min after exposure to hypotonicity. The swelling-activated [3H]taurine efflux was defined as the difference of efflux rate constant between hypotonic solution and isotonic solution.
Functional K+ (86Rb+) efflux assays
86Rb+ has been shown to be a valid K+ congener for K+ transport and therefore 86Rb+ efflux assays were used to study the activity of K+ transport in various experimental conditions. Efflux experiments were carried out as described in detail elsewhere (Shen et al. 2000a). In brief, unidirectional K+ efflux was measured in cells grown on six-well plates using 86Rb+ as a tracer. Cells were pre-incubated with isotonic culture medium loaded with 2 μCi ml−186Rb+ for 2 h at 37 °C. After pre-incubation, the loading medium was aspirated and cells were washed rapidly seven times with phosphate-buffered saline. After washing, appropriate efflux medium (containing 0.1 mm ouabain and 0.01 mm bumetanide to inhibit the Na+-K+ pump and Na+-K+-2Cl− cotransporter, respectively) was added to the cells. Release of 86Rb+ from preloaded cells was measured from the efflux medium at indicated time intervals within a 25 min duration. Cells were finally lysed with 50 % trichloroacetic acid to release the remaining cellular 86Rb+. 86Rb+ efflux rate constants were estimated by the same method as used above to calculate [3H]taurine efflux. In some experiments to study the activity of KCl cotransport, the Cl− dependence of 86Rb+ efflux was examined by substituting NO3− for Cl− both in the washing medium and the efflux medium.
Transient transfection with dominant negative raf-1 mutant
To provide genetic evidence that the signal pathways of ERK1/ERK2 were involved in the activation of the swelling-activated Cl− channel, transient transfection was used. Using the calcium phosphate coprecipitation method (Chen & Okayama, 1987), SiHa cells cultured in six-well plates were cotransfected with green fluorescence protein (GFP, 100 ng per well; Clontech, USA) together with pRSVC4Braf-1 (500 ng per well) or with empty vector (500 ng per well). The pRSVC4Braf-1 encoding the dominant-negative mutant of raf-1 (Chang et al. 1999) was a gift from Dr H. S. Liu, Department of Immunology and Microbiology, National Cheng Kung University, Taiwan. Using this ratio (GFP:cDNA of interest = 1:5), more than 85 % of the GFP-positive cells also expressed the vector of interest, as determined in separate experiments by immunostaining the epitope tag in the dominant-negative raf-1 mutant. Electrophysiological measurements were done 48 h after transfection.
Western immunoblotting
SiHa cells, grown in 100 mm dishes with 90 % confluence were starved in serum-free DMEM for 24 h before experiments. After serum starvation, the culture medium was replaced by isotonic or hypotonic solution at room temperature. After stimulation with hypotonic solution, cultures were washed quickly with ice-cold phosphate-buffered saline and harvested on ice with protein sample buffer (10 % glycerol, 62.5 mm Tris-base, 2 % SDS, and 2.5 % β-mercaptoethanol). Then, lysates were collected and centrifuged at 20 000 g for 20 min at 4 °C. Protein concentrations were determined with a Bio-Rad protein assay. Equal amounts of proteins were separated by 10 % SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride (PVDF; Stratagene, La Jolla, CA, USA) membranes. Non-specific binding was blocked with 5 % (w/v) non-fat dried milk in TBS-T (20 mm Tris, pH 7.5, 137 mm NaCl, 0.2 % Tween-20) for 1 h at room temperature. The blots were incubated with primary antibodies at 1/3000 dilution in TBS-T overnight at 4 °C, washed with TBS-T four times (10 min each time), and then incubated with goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase at 1/5000 dilution for 1 h at room temperature. Following washing three times with TBS-T, the membrane was developed with enhanced chemiluminescence according to the manufacturer's instructions (Amersham Pharmacia Biotech, Bucks, UK).
Measurements of cell volume
Cell volume was measured at room temperature as described previously (Shen et al. 1999; Ross & Cahalan, 1995). Briefly, cells grown in tissue culture flasks were harvested by trypsinization. The 3 × 106 cells were suspended in 5 ml isotonic solution for 10 min. Then, 500 μl aliquots were transferred and allowed to achieve cell attachment in Petri dishes for approximately 60 min. A 2 ml bath, which was continuously superfused at a rate of 2 ml min−1 with isotonic solution or hypotonic solution with or without the chemical tested at room temperature, was then applied. Cells were viewed with magnification up to ×400 by an Olympus IX70 inverted microscope that was equipped with Hoffman modulation optics (Olympus, Tokyo, Japan). In order to monitor the change of cell size, the microscope was coupled to a video camera system and the images were recorded in real time and stored on a video cassette recorder (National Inc., Tokyo, Japan). Images were then analysed by the public domain NIH image program (available on the Internet via http://rsb.info.nih.gov/nih-image). The majority of cells observed were spheroid and the relative volume change (V/Vo) was calculated from the cross-sectional surface area at the beginning (So) of the experiment and during (S) the experiments from the relation: V/Vo= (S/So)3/2 (Ross & Cahalam, 1995). The validity of this approach to measuring cell volume has been demonstrated in mouse thymocytes (Ross & Cahalan, 1995), renal A6 cells (Urbach et al. 1999) and lymphocytes (Lepple-Wienhues et al. 1998). Data were presented as the ratio of starting volume (V/Vo), as a function of time.
Statistics
All values in the present study were reported as means ±s.e.m. (standard error of the mean). ANOVA and Student's paired or unpaired t test were used for statistical analyses. Differences between values were considered significant when P < 0.05.
RESULTS
Ca2+ signalling in swollen human cervical cancer cells
In the first series of experiments we studied Ca2+ signalling in response to hypotonicity. Superfusion of SiHa cells with the hypotonic solution elicited a rapid rise in [Ca2+]i from the basal level of 120 ± 15 nm to a peak of 1050 ± 80 nm (n = 10 experiments). This initial steep rise in [Ca2+] was followed by a decay to finally reach a plateau of 325 ± 40 nm (Fig. 1A and B). To determine the source responsible for elevating [Ca2+]i we examined the effect of Ca2+ channel antagonists and trivalent metal cations on the Ca2+ influx. Ca2+ influx has been reported to be blocked effectively by trivalent metal cations in a wide range of cell types (Ross & Cahalan, 1995). Figure 1C displays recordings of [Ca2+]i with increasing concentrations of the trivalent metal cation Gd3+. Gd3+ shows a concentration-dependent inhibition of the initial peak rise in [Ca2+]i induced by hypotonicity. Gd3+ at 1 μm inhibited the peak rise in [Ca2+]i from 1050 ± 80 nm to 450 ± 20 nm (n = 10 experiments). At 10 μm, Gd3+ further suppressed the peak level to 180 ± 20 nm (n = 10 experiments). However, Gd3+ at either 1 or 10 μm had the same small effect on the plateau level of [Ca2+]i. Nifedipine, a Ca2+ channel inhibitor, also showed a similar inhibition of the [Ca2+]i rise evoked by hypotonicity (Fig. 1D). In the presence of 50 μm nifedipine, hypotonicity induced a progressive rise in [Ca2+]i, reaching a plateau of 310 ± 30 nm (n = 6) 200 s after superfusing with hypotonic solution. These results suggest that the initial steep rise in [Ca2+]i results mainly from Ca2+ influx through a Ca2+-permeable channel which is sensitive to Gd3+ and nifedipine. This notion is further substantiated by experiments where extracellular Ca2+ is altered. In the absence of extracellular Ca2+, the basal [Ca2+]i was 45 ± 5 nm and hypotonicity induced a progressive increase in [Ca2+]i to reach a plateau of 106 ± 5 nm (Fig. 2A, n = 10 experiments, P < 0.05, paired t test). More importantly, the pattern of [Ca2+]i rising in the absence of extracellular Ca2+ is very similar to that in the presence of Gd3+ or nifedipine.
Figure 1. Ca2+ signalling in swollen SiHa cells.

A, a representative recording of the changes in intracellular Ca2+ concentration ([Ca2+]i) evoked by a hypotonic solution containing 1.5 mm Ca2+ ([Ca2+]o). B, summary of the changes in [Ca2+]i evoked by hypotonic solution containing 1.5 mm Ca2+. Each column represents mean ±s.e.m. (n = 10). *P < 0.01, **P < 0.001, paired t test, compared with the isotonic condition. C and D, swelling-activated elevation of [Ca2+]i is blocked by Gd3+ or nifedipine. ISO, isotonic solution, 300 mosmol kg−1; HYPO, hypotonic solution, 230 mosmol kg−1.
Figure 2. Swelling activation of intracellular Ca2+ depends on both Ca2+ influx and release from internal stores.

A, a representative recording from 10 different experiments to show the changes in [Ca2+]i evoked by hypotonic solution in the absence of extracellular Ca2+, plus 1.5 mm EGTA ([Ca2+]o∼0 mm). B and C, representative recordings from six different experiments to show the effects of thapsigargin on [Ca2+]i in the absence or presence of extracellular Ca2+ ([Ca2+]o).
Depletion of internal Ca2+ stores by 2 μm thapsigargin in the absence of extracellular Ca2+ prevents any further increase in [Ca2+]i by hypotonicity (Fig. 2B, a representative recording from six different experiments). When cells were depleted to empty the internal Ca2+ stores and then exposed to a Ca2+-containing hypotonic solution, [Ca2+]i was significantly increased, as expected (Fig. 2C, a representative recording from six different experiments). These results indicate that hypotonic stress increases [Ca2+]i by activation of Ca2+-permeable channels in cell membranes as well as Ca2+ release from intracellular stores.
We studied the significance of Ca2+ signalling in volume regulation further. In Fig. 3A, whole-cell voltage-clamp recordings were obtained from SiHa cells in which the pipette solution contained 1.93 mm CaCl2 and 5 mm EGTA with 1.5 mm CaCl2 in the extracellular medium. Membrane currents recorded during the step protocol applied to SiHa cells in isotonic solutions were small and time independent. Application of a hypotonic solution induced cell swelling, which was accompanied by an activation of large outwardly rectifying currents. At potentials more positive than +60 mV, the currents showed time-dependent inactivation, which became more pronounced at higher membrane potentials. However, more than 90 % of the swelling-activated Cl− current was suppressed when [Ca2+]i was buffered to near zero by 10 mm BAPTA in the pipette solution, as well as the absence of extracellular Ca2+ (Fig. 3B). A similar effect was noted in the swelling-activated taurine and K+ efflux. Incubating SiHa cells with 50 μm BAPTA-AM for 30 min prior to the Ca2+-free hypotonic stimulus markedly decreased the swelling-activated taurine efflux (Fig. 3C, n = 6 for each group). Under the same conditions, the swelling-activated K+ (86Rb+) transport also significantly decreased by 40 % (Fig. 3D, n = 6 for each group). Therefore, Ca2+ signalling is critical in the regulation of swelling-activated ion and taurine transport.
Figure 3. Effect of Ca2+ signalling on the activation of the swelling-activated Cl− channel and K+ and taurine transport.

A, current traces (step protocol) were recorded in isotonic and hypotonic solutions, in which the pipette solution contained 1.93 mm CaCl2 and 5 mm EGTA, and with 1.5 mm CaCl2 in the extracellular medium (control group). Horizontal lines represent zero current levels. Current-voltage relationships were obtained from traces in isotonic and hypotonic solutions. • and ○ are hypotonic and isotonic currents, respectively. Each point represents mean ±s.e.m. (n = 5). B, current traces were recorded in isotonic and hypotonic solutions with [Ca2+]i buffered near 0 nm. For these experiments, the pipette solution contained 10 mm BAPTA and the external solution was free of Ca2+, plus 1.5 mm EGTA. Current-voltage relationships were obtained from traces in isotonic and hypotonic solutions. • and ○ are hypotonic and isotonic currents, respectively. Each point represents mean ±s.e.m. (n = 5). The step protocol for A and B consisted of a 1 s voltage step, applied every 15 s from a holding potential of -20 mV to test potentials from -80 to +80 mV with an increment of 20 mV. C and D, Ca2+ dependence of swelling-activated taurine and K+ efflux. In these experiments, SiHa cells were pre-incubated with 50 μm BAPTA-AM for 30 min and then efflux was measured in Ca2+-free medium containing 1.5 mm EGTA. Each column represents mean ±s.e.m. (n = 6). *P < 0.05, **P < 0.01, compared with control group, unpaired t test.
Role of protein tyrosine phosphorylation in regulating cell volume
We investigated subsequently whether protein tyrosine phosphorylation was involved in the activation of volume-regulatory ion and taurine transport pathways. As depicted in Fig. 4A and B, the membrane currents under isotonic conditions recorded during the ramp protocol applied to SiHa cells were small. Application of hypotonic solutions induced cell swelling detected by visual inspection, which was accompanied by an activation of large outwardly rectifying currents. Genistein, an inhibitor of tyrosine kinase, blocked the swelling-activated Cl− channel in a dose-dependent manner. For example, 50 μm genistein inhibited reversibly about 50-60 % of the swelling-activated Cl− current. Further, 100 μm genistein inhibited about 90 % of the swelling-activated Cl− current (Fig. 4A and B). Tyrphostin AG 1478, another tyrosine kinase inhibitor, also blocked the swelling-activated Cl− current dose-dependently (Fig. 4C). Conversely, 100 μm Na3VO4, an inhibitor of tyrosine phosphatase, potentiated the swelling-activated Cl− current by 35 ± 3 % (n = 4), measured at +80 mV, Fig. 4D). These results suggest that the activation of swelling-activated Cl− channels depends on protein tyrosine phosphorylation.
Figure 4. Regulation of the swelling-activated Cl− current in human cervical cancer SiHa cells by protein tyrosine phosphorylation, which is independent of the Src-like family.

A, time course of membrane currents activated at a membrane potential (MP) of +80 mV. Data points were obtained from the voltage ramp protocol that was applied every 15 s. The labelled points correspond to the current traces recorded in B. Thick horizontal bar, application of hypotonic solution or genistein (GN); thin horizontal line, zero current level. B, representative recordings of swelling-activated Cl− currents from ramp protocol. Trace 1, isotonic membrane current; trace 2, hypotonic membrane current; traces 3 and 4, currents recorded after perfusion with hypotonic solution in the presence of 50 μm and 100 μm genistein, respectively. C, dose-response curves for the inhibition of the swelling-activated Cl− channel by genistein and tyrphostin AG 1478. Each point represents mean ±s.e.m. (n = 4). D, time course of membrane Cl− currents activated by hypotonicity and subsequently potentiated by Na3VO4, a tyrosine phosphatase inhibitor. Data points were obtained from the voltage ramp protocol that was applied every 15 s and recorded at a membrane potential (MP) of +80 mV. Thick horizontal bar, application of hypotonic solution or Na3VO4 (100 μm); thin horizontal line, zero current level. E, the intracellular applications of active peptide (5 mm EPQ(pY)EEIPIA) or inactive peptide (5 mm EPQYEEIPIA) of the Src-like family showed no effect on the activity of the swelling-activated Cl− channel. Each column represents mean ±s.e.m. and numbers of cells examined are indicated in parentheses. F, a representative recording from six different experiments to show the time course of membrane Cl− currents activated by hypotonicity and not affected by PP2, a cell-permeable inhibitor of Src tyrosine kinase. Data points were obtained from the voltage ramp protocol that was applied every 15 s and recorded at a membrane potential (MP) of +80 mV. Thick horizontal bar, application of hypotonic solution or PP2 (0.5 μm); thin horizontal line, zero current level.
The tyrosine kinase p56lck, a Src-like tyrosine kinase, has been proposed to mediate the activation of the swelling-activated Cl− channel and RVD in lymphocytes (Lepple-Wienhues et al. 1998). Accordingly, we performed experiments in which cells were intracellularly perfused with a pipette solution containing Src-activating peptide (EPQ(pY)EEIPIA) or inactive peptide (EPQYEEIPIA). This active peptide stimulated the Src family of tyrosine kinase by binding to the SH2-domain and has been used to demonstrate the regulation of NMDA channels and the induction of long-term potentiation by Src tyrosine kinase (Yu et al. 1997; Huang & Hsu, 1999). Intracellular application of 1 or 5 mm EPQ(pY)EEIPIA or EPQYEEIPIA did not result in a pronounced increase in isotonic current within 15 min after the whole-cell configuration. The activity of the swelling-activated Cl− channel was also independent of the presence of 1 or 5 mm active or inactive peptide (Fig. 4E). PP2 is a membrane-permeable, specific Src tyrosine kinase inhibitor with an IC50 of 5-10 nm (Salazar & Rozengurt, 1999). We have tested concentrations ranging from 10 to 500 nm, which had no effect on the activity of the swelling-activated Cl− channel when this drug was included in the pipette solution or applied in the bath solution. As depicted in Fig. 4F, the activity of swelling-activated Cl− channel was not changed when 0.5 μm PP2 was superfused for 5 min. Therefore we suggest that the Src-like family of tyrosine kinases are not involved in the regulation of the swelling-activated Cl− channel.
Figure 5A–D shows the time course of K+ (86Rb+) and taurine efflux from SiHa cells in the presence or absence of modulators of tyrosine phosphorylation. The K+ (86Rb+) efflux in the isotonic medium was low and stable either in the presence or absence of modulators of tyrosine phosphorylation. Exposure to the hypotonic solution markedly increased K+ (86Rb+) efflux both in the Cl−-containing medium and NO3−-containing (Cl−-free) medium (Fig. 5A). Analysis of the data confirmed that cell swelling induced both Cl−-dependent and -independent K+ (86Rb+) efflux, presumably mediated by K+-Cl− cotransport and K+ channels, respectively (Shen et al. 2000a, 2001). Under hypotonic challenge, both the Cl−-dependent and -independent K+ (86Rb+) efflux were blocked significantly by inhibitors of tyrosine kinase, genistein (50 and 100 μm) and tyrphostin AG 1478 (0.5 μm), whereas these K+ efflux components were potentiated by the inhibitor of tyrosine phosphatase, 100 μm Na3VO4 (Fig. 5A and B).
Figure 5. Effects of tyrosine phosphorylation on swelling-activated K+ and taurine transport in human cervical cancer SiHa cells.

A, time course of K+ efflux from SiHa cells exposed to Cl− or NO3− (Cl−-free) media. Graph shows logarithm of fraction of original intracellular K+ remaining as a function of time. The thick horizontal line indicates the application of hypotonic solution (HYPO, 230 mosmol kg−1). SiHa cells were pre-incubated with 0.05 % DMSO (control group) or inhibitors (50 and 100 μm genistein (GN), 0.5 μm tyrphostin AG 1478, 100 μm Na3VO4) for 30 min at room temperature and then exposed to flux medium in the presence or absence of inhibitors. B, summary of swelling-activated K+ efflux in Cl−- or NO3−-containing solution, calculated from A and expressed as the fraction of control group. To study the activity of KCl cotransport, the Cl− dependence of K+ (86Rb+) efflux was examined by substituting NO3− for Cl− both in the washing medium and in the efflux medium. The Cl−-dependent K+ efflux is defined as the difference of efflux rate constant between Cl− and NO3− media and this efflux represents the activity of K+-Cl− cotransport. C, time course of taurine efflux from SiHa cells exposed to isotonic and hypotonic media. Graph shows logarithm of fraction of original intracellular [3H]taurine remaining as a function of time. The thick horizontal line indicates the application of hypotonic solution (HYPO, 230 mosmol kg−1). SiHa cells were pre-incubated with 0.05 % DMSO (control group) or inhibitors (50 or 100 μm genistein, 0.5 μm tyrphostin AG 1478, 100 μm Na3VO4) for 30 min at room temperature and then exposed to flux medium in the presence or absence of inhibitors. D, summary of swelling-activated [3H]taurine efflux calculated from C and expressed as the fraction of control group. Each value in A–D represents mean ±s.e.m. (n = 6). *P < 0.05, **P < 0.01 compared with control group, unpaired t test. E, time course of volume changes following perfusion with hypotonic (230 mosmol kg−1) solution, or hypotonic solution plus 50 μm genistein (GN) or 0.5 μm tyrphostin AG 1478. SiHa cells were pre-incubated with 0.05 % DMSO (control group) or drugs (100 μm genistein, 0.5 μm tyrphostin AG 1478) for 30 min at room temperature prior to being exposed to hypotonic solution. The y-axis depicts the cell volume at the indicated times divided by the cell volume at zero time (V/Vo). Each point represents mean ±s.e.m. (n = 50 cells). *P < 0.05, **P < 0.01, unpaired t test, comparing the final cell volume ratio with the control group.
Similarly, taurine efflux from SiHa cells in the isotonic medium was low and stable either in the presence or absence of modulators of tyrosine phosphorylation. Exposure to the hypotonic solution markedly increased the taurine efflux (Fig. 5C). The swelling-activated taurine efflux was blocked significantly by inhibitors of tyrosine kinase, genistein and tyrphostin AG 1478 (Fig. 5C and D). Pretreatment of SiHa cells with 50 or 100 μm genistein or 0.5 μm tyrphostin AG 1478 for 30 min decreased significantly the swelling-activated taurine efflux by 55 ± 8 % (n = 6, P < 0.05), 14 ± 5 % (n = 6, P < 0.01) and 12 ± 1 % (n = 6, P < 0.01), respectively. On the other hand, 100 μm Na3VO4, an inhibitor of tyrosine phosphatase, potentiated the swelling-activated taurine efflux by 35 ± 9 % (n = 6, P < 0.05, Fig. 5C and D). Taken together, these results confirm that tyrosine phosphorylation is involved in the regulation of swelling-activated ion and taurine transport.
To study the effects of tyrosine kinase on the RVD process, SiHa cells were pre-incubated with 50 μm genistein or 0.5 μm tyrphostin AG 1478 for 30 min prior to perfusion with hypotonic solutions. As shown in Fig. 5E, the typical volume response of SiHa cells to hypotonic stress can be divided into three phases: (1) an initial and rapid osmotic swelling, reaching a peak cell volume 38 ± 4 % (n = 50) greater than the original cell size at about 2 min; (2) a rapid shrinkage in the following 3 min; (3) a more gradual decrease of cell volume to finally reach a plateau amounting to 8 ± 2 % greater than the original cell volume at about 10 min. In contrast to the typical RVD process, 50 μm genistein increased initial osmotic swelling, attenuated the shrinkage phase, and inhibited the gradual decrease in cell volume. Another tyrosine kinase inhibitor, tyrphostin AG 1478, also showed similar inhibitory effects on the RVD process (Fig. 5E). Overall, these results suggest that protein tyrosine phosphorylation plays an important role in RVD regulation by affecting the activity of swelling-activated taurine and ion transport.
Hypotonicity stimulates the activation of ERK1/ERK2 and p38 MAP kinase
The above observations prompt the question of which downstream cascades link with the activation of volume-sensitive tyrosine kinase. A well-documented pathway triggered by tyrosine kinases, either receptor or non-receptor kinases, is the MAP kinase transduction cascade. Accordingly, we subsequently investigated the roles of two major MAP kinases in cell volume regulation: (1) ERK1/ ERK2 and (2) the cytokine-suppressive anti-inflammatory drug-binding protein p38 MAP kinase (Cobb, 1999).
Figure 6 depicts the phosphorylation of ERK1/ERK2 and p38 MAP kinase following hypotonic shock. The phosphorylated ERK1/ERK2 was almost undetectable after serum starvation for 24 h. Hypotonicity induced a phosphorylation of ERK1/ERK2, which appeared within 3 min of exposure to hypotonic stimulation, reached maximal levels at about 20 min and decayed at 30 min (Fig. 6A). Analysed by densitometry, the band intensity of phosphorylated ERK2 was always higher than that of phosphorylated ERK1. However, the level of total ERK1/ERK2 proteins remained steady and was not affected by osmotic change (data not shown). Osmotic swelling of SiHa cells also leads to significantly increased activity of p38 MAP kinase. The phosphorylated form of p38 reached a maximal value after 20 min of hypotonic stimulation, which was about 8-fold more than that in isotonic conditions (Fig. 6B).
Figure 6. Time courses of ERK1/ERK2 (A) and p38 (B) activation in response to hypotonicity.

Serum-starved SiHa cells were exposed to either isotonic solution (ISO, 300 mosmol kg−1) for 20 min or hypotonic solution (HYPO, 230 mosmol kg−1) for the time periods indicated. Whole-cell extracts were separated by SDS-PAGE (50 μg lane−1), transferred to PVDF, and immunoblotted with anti-phospho-ERK1/ERK2 (Thr202/Tyr204) or anti-phospho-p38 MAP kinase (Thr180/Tyr182). Phosphorylated ERK1/ERK2 or p38 levels were analysed by scanning densitometry and the results were expressed as arbitrary units. Each column represents mean ±s.e.m. (n = 3 independent experiments). p-ERK1, phosphorylated ERK1; p-ERK2, phosphorylated ERK2; p-p38, phosphorylated p38 MAP kinase; P, positive control for phosphorylated p38 MAP kinase.
Activation of ERK1/ERK2 is involved in cell volume regulation
We subsequently studied whether the osmotic activation of MAP kinases is an important step in the RVD response. To investigate the involvement of ERK1/ERK2 in the activation of the swelling-activated Cl− channel, we applied 1, 10 and 50 μm PD 98059, a specific MEK inhibitor, directly in the pipette solution. Under this treatment, SiHa cells presented a background isotonic current density, averaging -6.0 ± 2.0 pA pF−1 at -80 mV and 10 ± 1.4 pA pF−1 at +80 mV (n = 12), both of which were similar to those of control cells. For PD 98059-treated cells, hypotonicity also induced a fast activating and outward rectifying current which reversed at a potential close to the theoretical equilibrium potential for Cl−, indicating that the swelling-activated currents were carried mainly by Cl− (Fig. 7B).
Figure 7. ERK1/ERK2 is involved in the activation of swelling-activated Cl− currents.

Representative recordings of swelling-activated Cl− currents from ramp protocol in the control group (A, wild type) or in the presence of 1, 10 and 50 μm PD 98059 in the pipette solution (B). PD 98059 is a specific inhibitor of mitogen-activated/ERK-activating kinase (MEK). C, transfection with dominant-negative raf-1 mutants inhibited the activation of swelling-activated Cl− currents. ISO, basal membrane current recorded in the isotonic (300 mosmol kg−1) solution; HYPO, currents recorded after perfusion with hypotonic (230 mosmol kg−1) solution. D, normalized currents activated by hypotonicity measured at +80 mV in the wild type (Control), in the presence of 1, 10 and 50 μm PD 98059 or in the transfection of dominant-negative raf-1 mutants. Each column represents mean ±s.e.m. and numbers of cells examined are indicated above each column. *P < 0.05, **P < 0.01 by unpaired t test.
To compare values for the channel activity, we normalized the swelling-activated Cl− current, which is defined as the difference in current density between isotonic and hypotonic solutions and is expressed per unit of membrane capacitance. As shown in Fig. 7B and D, PD 98059 suppressed the activity of the swelling-activated Cl− channel in a dose-dependent manner. For control cells, the normalized swelling-activated Cl− current was 109 ± 5 pA pF−1 (n = 20) at +80 mV. For PD 98059-treated cells, the current density at +80 mV was significantly decreased to 59 ± 4 pA pF−1 (n = 6, P < 0.05, unpaired t test) and 37 ± 2 pA pF−1 (n = 10, P < 0.01) for 10 μm and 50 μm, respectively. Because PD 98059 was cell-permeable, we also did experiments in which this drug was added in the bath solution. The results (n = 6) were similar to those with intracellular dialysis with PD 98059. Importantly, transfection with dominant-negative raf-1 mutants inhibited significantly the normalized swelling-activated Cl− current to 24 ± 4 pA pF−1 (n = 12, P < 0.01) at +80 mV (Fig. 7C and D). Transfection with empty vector (n = 6) showed no effect on the activation of the swelling-activated Cl− channel. Therefore, interference with ERK activation by either specific MEK inhibitor or a dominant-negative raf-1 mutant profoundly suppressed the activity of the swelling-activated Cl− channel.
Inhibiting the ERK1/ERK2 cascade with PD 98059 also blocked swelling-activated taurine and K+ transport. Pre-incubation with 10 and 50 μm PD 98059 for 30 min at room temperature significantly decreased the swelling-activated taurine efflux by 32 ± 5 % (n = 6, P < 0.05, unpaired t test) and 65 ± 6 % (n = 6, P < 0.01), respectively (Fig. 8A). For K+ transport, 50 μm PD 98059 significantly reduced the swelling-activated K+ efflux by 30 ± 3 % (n = 6, P < 0.05, Fig. 8B) in the Cl− flux medium. In NO3− flux medium, PD 98059 also suppressed significantly the swelling-activated K+ efflux by 35 ± 5 % (n = 6, P < 0.05). However, the Cl−-dependent K+ efflux was similar in control and PD 98059-treated cells (Fig. 8B). We have demonstrated previously that swelling SiHa cells induces both Cl−-dependent and -independent K+ (86Rb+) efflux, which is presumably mediated by K+-Cl− cotransport (KCC) and a K+ channel, respectively (Shen et al. 2000a). Therefore, osmotic induction of the ERK1/ERK2 cascade is also involved in the activation of the swelling-activated K+ channel.
Figure 8. ERK1/ERK2 is involved in the activation of swelling-activated taurine and K+ transport and the RVD response.

A, [3H]taurine efflux rate in hypotonic solution. B, swelling-activated K+ efflux in Cl− or NO3− media. SiHa cells were pre-incubated with 0.05 % DMSO (control group) or 10 and 50 μm PD 98059 for 30 min at room temperature and then exposed to hypotonic solution in the presence or absence of 50 μm PD 98059. The Cl−-dependent K+ efflux is defined as the efflux difference between Cl− and NO3− media. Each column represents mean ±s.e.m. (n = 6). *P < 0.05, **P < 0.01 by unpaired t test. C, time course of volume changes following perfusion with hypotonic (230 mosmol kg−1) solution, or hypotonic solution plus 50 μm PD 98059. SiHa cells was pre-incubated with 0.05 % DMSO (control group) or 50 μm PD 98059 for 30 min at room temperature prior to being exposed to hypotonic solution. The y-axis depicts the cell volume at the indicated times divided by the cell volume at zero time (V/Vo). Each point represents mean ±s.e.m. (n = 50 cells). *P < 0.05, unpaired t test, comparing the final cell volume ratio with the control group.
The RVD process is also sensitive to blockade of the ERK1/ERK2 cascade by PD 98059 (Fig. 8C). When pre-incubated with 50 μm PD 98059 at room temperature for 30 min prior to hypotonic exposure, SiHa cells swelled with time and reached a peak volume 50 % above the original cell volume at about 4 min. A slow decrease in cell volume followed and a plateau of 30 % above the original cell size was finally reached at about 10-15 min. Taken together, these results indicate that hypotonicity activates the ERK1/ERK2 signal pathway, which links with the activation of swelling-activated ion channels and taurine transport, and thereby involves the regulation of cell volume.
Role of p38 MAP kinase in volume regulation
SB 202190, a specific and membrane-permeable inhibitor of p38 MAP kinase, was used to determine the possible involvement of p38 MAP kinase in cell volume regulation. As shown in Fig. 9A, 1 μm SB 202190 completely abolished p38 activity in the hypotonic solution. We then tested drug concentrations of 1, 5 and 10 μm on volume-regulatory ion and taurine transport. However, SB 202190 concentrations of up to 10 μm showed no effect on the activation of swelling-activated taurine transport (Fig. 9B), K+ transport (Fig. 9C) and Cl− channels (Fig. 9D). The process of RVD was also insensitive to SB 202190, up to 10 μm (Fig. 9E). Therefore, hypotonicity-induced activation of p38 MAP kinase does not serve as a regulator in the swelling-activated ion and taurine transport pathways.
Figure 9. The volume-regulatory mechanism is independent of the swelling-activation of p38 MAP kinase.

A, phosphorylation of p38 MAP kinase (p-p38) in isotonic (ISO) or hypotonic (HYPO) solution with or without the specific p38 inhibitor SB 202190 (1 μm). B and C, summary of swelling-activated [3H]taurine and K+ (86Rb+) efflux with or without 10 μm SB 202190. Each column represents mean ±s.e.m. (n = 6). D, a representative recording from six different experiments which shows the time course of membrane Cl− currents recorded at +80 mV or -80 mV. Data points were obtained from the voltage ramp protocol that was applied every 15 s. Thick horizontal bar, application of hypotonic solution or 10 μm SB 202190; thin horizontal line, zero current level. E, time course of volume changes following perfusion with hypotonic (230 mosmol kg−1) solution, or hypotonic solution plus 10 μm SB 202190. The y-axis depicts the cell volume at the indicated times divided by the cell volume at zero time (V/Vo). Each point represents mean ±s.e.m. (n = 50 cells). For immunoblotting, flux measurement and cell volume measurement, SB 202190 was applied 30 min before and during stimulation with hypotonic solution.
Cross-talk between signal pathways following hypotonic shock
Finally, we investigated possible interactions between the signalling pathways that have been identified as being activated by hypotonic shock in human cervical cancer cells. According to the above results, levels of phosphorylated ERK1/ERK2 reached maximal activity 20 min after hypotonic stimulation (Fig. 6A). Therefore, the activity of phosphorylated ERK1/ERK2 at that indicated time point (20 min) was used as the control and the effects of protein kinase C (PKC), tyrosine kinases and Ca2+ signalling were examined on osmotically induced ERK1/ERK2 activation. As depicted in Fig. 10, the PKC activator phorbol 12-myristate 13-acetate (PMA, 1 μm) strongly enhanced the hypotonicity-induced activity of ERK1/ERK2, whereas the PKC inhibitor staurosporine (1 μm) showed an inhibitory effect. Depletion of extracellular Ca2+ also decreased the phosphorylation of ERK1/ERK2 significantly, indicating that hypotonicity-induced ERK activation requires a Ca2+ influx. The tyrosine kinase inhibitor genistein (50 μm) markedly depressed the phosphorylation of ERK1/ERK2 when it was applied 15 min before and during stimulation with hypotonic solution. Similarly, the MEK inhibitor PD 98059 (50 μm) also inhibited ERK1/ERK2 activation in the hypotonic solution (Fig. 10). These results suggest that the hypotonicity-induced ERK1/ERK2 activation depends on the activation of PKC, tyrosine kinase and Ca2+ influx.
Figure 10. Activation of ERK1/ERK2 in response to hypotonicity is regulated by PKC, Ca2+ influx, tyrosine kinase and MEK.
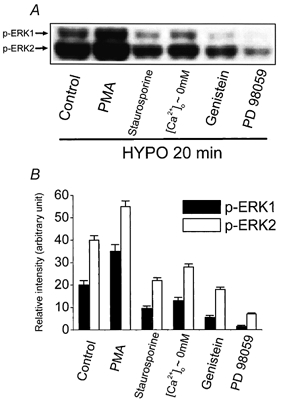
The activity of phosphorylated ERK1/ERK2 20 min after exposure to hypotonic solution (HYPO, 230 mosmol kg−1) was used as the control. PMA (1 μm), staurosporine (1 μm), [Ca2+]o∼0 mm, genistein (50 μm) or PD 98059 (50 μm) was applied 15 min before and during stimulation with hypotonic solution. Whole-cell extracts were separated by SDS-PAGE (50 μg lane−1), transferred to PVDF, and immunoblotted with anti-phospho-ERK1/ERK2 (Thr202/Tyr204; A). Phosphorylated ERK1/ERK2 (p-ERK1/p-ERK2) was analysed by scanning densitometry and the results were expressed as arbitrary units (B). Each column in panel B represents mean ±s.e.m. (n = 3 independent experiments). [Ca2+]o∼0 mm, the absence of extracellular Ca2+, plus 1.5 mm EGTA; PMA, phorbol 12-myristate 13-acetate.
DISCUSSION
This study addresses the question of the signalling pathways involved in the activation of volume-regulatory mechanisms of human cervical cancer cells. We have demonstrated that the tyrosine kinase inhibitors genistein and tyrphostin AG 1478 show a dose-dependent inhibition of swelling-activated ion and taurine transport. Conversely, the tyrosine phosphatase inhibitor Na3VO4 potentiates the activity of this transport. These observations point to a link between protein tyrosine phosphorylation and the volume-regulatory mechanisms in human cervical cancer cells. Tyrosine phosphorylation is also involved in the volume-regulatory mechanism of bovine endothelial cells (Voets et al. 1998) and human intestine 407 cells (Tilly et al. 1993). However, it is still not very clear which specific tyrosine kinase is responsible for the volume-regulatory mechanisms. The tyrosine kinase p56lck, a Src-like tyrosine kinase, has been proposed to mediate the activation of the swelling-activated Cl− channel and RVD in lymphocytes (Lepple-Wienhues et al. 1998). However, the present study shows that the activation of the swelling-activated Cl− channel is independent of the activity of Src tyrosine kinase in human cervical cancer cells.
Ca2+ is essential for the normal RVD response in human cervical cancer cells. Osmotic swelling of human cervical cancer cells induces a substantial increase in [Ca2+]i to which both external and internal sources contribute, and which is critical for taurine transport and K+ and Cl− channel activity. The hypotonicity-induced Ca2+ influx is through a Ca2+-permeable channel sensitive to Gd3+ and nifedipine, suggesting Ca2+ entry is via stretch-activated cation channels (Okada et al. 1990; McCarty & O'Neil, 1992; Ross & Cahalan, 1995). Increased [Ca2+]i on osmotic swelling has been described in a variety of cell types (for review, see McCarty & O'Neil, 1992), whilst [Ca2+]i apparently remains constant in Ehrlich ascites cells, retinal pigment epithelium, collecting duct cells and lymphocytes in some species (for review, see Lang et al. 1998).
The present study also demonstrates that cell swelling triggers MAP kinase cascades leading to the activation of ERK1/ERK2 and p38 MAP kinase. An important question is whether the cascades of ERK1/ERK2 and p38 MAP kinase are involved in the activation of volume-regulatory mechanisms. Our results clearly show that hypotonicity activates the ERK1/ERK2 signal pathway which links with the activation of swelling-activated K+ and Cl− channels and taurine transport and therefore is involved in cell volume regulation. While PD 98059 (50 μm) can inhibit at least 80 % of ERK1/ERK2 MAP kinase activity, the effects of this compound on swelling-activated ion and taurine transport are less pronounced. It is unlikely that ERK signalling is the only pathway which controls the RVD response. Nevertheless, our observation that transfection with raf-1 mutant to abolish ERK1/ERK2 activity inhibits 80 % of the activity of the swelling-activated Cl− channel supports our assertion that this pathway plays an important role in RVD signalling.
There are interactions between multiple pathways in the signalling sequences. For example, Ca2+ influx is an essential step in eliciting the ERK1/ERK2 signalling in response to hypotonic shock. The expression of swelling-activated ERK1/ERK2 is up-regulated by the PKC activator PMA and down-regulated by the PKC inhibitor staurosporine. PKC is critically involved in the volume regulation of human cervical cancer cells. Hypotonic stress provokes the translocation of PKC-α from cytosol to cell membrane, which has been demonstrated by immunofluorescence staining and Western blotting (Chou et al. 1998). Inhibiting PKC with staurosporine, H7 or PKC pseudosubstrate completely blocked the activation of the swelling-activated Cl− channel in human cervical cancer cells (Chou et al. 1998). Moreover, the RVD process of human cervical cancer cells was accelerated by the PKC activator PMA and abolished by the PKC inhibitor staurosporine or H7 (Shen et al. 1998).
The importance of PKC in volume regulation varies in different cell types. For example, inhibiting PKC with either the PKC pseudosubstrate calphostin C or staurosporine completely blocked the activation of the swelling-activated Cl− channel in bicarbonate-secreting pancreatic duct cells (Verdon et al. 1995). On the other hand, activation of PKC by PMA inhibited the activity of the channel in rat brain endothelial cells (von Weikersthal et al. 1999). However, stimulation of PKC by PMA did not affect the activity of the swelling-activated Cl− channel in endothelial cells from bovine pulmonary artery (Szücs et al. 1996).
In human cervical cancer cells, the osmotic activation of MAP kinases depends on tyrosine kinase and MEK activity because such an activation is significantly blocked by the tyrosine kinase inhibitor genistein and a specific inhibitor of MEK, PD 98059. These results also point to the relationship of tyrosine kinases to MAP kinases. The tyrosine kinase inhibitor used in this study does not act directly on MEK (Cox et al. 1996); however, it inhibited the activation of MAP kinase, suggesting that tyrosine kinases act upstream of MEK.
In addition to their involvement in volume regulation, what other possible functional significance is there for the activation ERK1/ERK2 cascades? An increased expression of the intermediate early genes c-fos and c-jun has been observed in swollen hepatoma cells (Schliess et al. 1995) and cardiomyocytes (Sadoshima et al. 1996), suggesting a possible role for ERK1/ERK2 in transcription regulation, maintaining cellular homoeostasis and/or long-term survival. In hepatocytes, a relationship has been found between ERK1/ERK2 activation and the rapid swelling-induced excretion of taurocholate into bile (Noéet al. 1996). However, for epithelial cells, such as human cervical cancer cells, a definite physiological function for hypotonicity-provoked ERK1/ERK2 activation remains to be established.
ERK1 and ERK2 are MAP kinases, a group of serine/ threonine kinases that are activated by dual-specificity protein kinases through phosphorylation on both threonine and tyrosine residues. ERK1 and ERK2 are activated by diverse extracellular stimuli and affect many important cellular processes via protein phosphorylation of specific targets. Several stimulators may lead to ERK1/ERK2 activation, such as growth factors, hormones and stress (Seger & Krebs, 1995; Cobb, 1999). The commonest pathway leading to ERK1/ERK2 activation is the signalling cascade initiated by tyrosine kinase-containing receptors, which involves activation of the small G-protein p21ras (Seger & Krebs, 1995). Activation of ERK1/ERK2 by hypotonicity has been reported in some mammalian cell types, including astrocytes (Crepel et al. 1998), human intestine 407 cells (van der Wijk et al. 1998), hepatoma cells (Schliess et al. 1995), cardiac myocytes (Sadoshima et al. 1996) and C6 glioma cells (Sinning et al. 1997), as well as for yeast (Davenport et al. 1995). However, very little is known about the role of ERK1/ERK2 in the regulation of cell volume. In astrocytes, activation of tyrosine and MAP kinases by swelling was proposed as a critical step in the opening of the swelling-activated Cl− channel (Crepel et al. 1998). However, human intestine 407 cells apparently differ from human cervical cancer cells. In that cell type, the swelling activation of ERK1/ERK2, which was downstream of the Ras/Raf pathway, was independent of PKC, Ca2+ influx and PI-3 kinase (van der Wijk et al. 1998). In addition, the activation of ERK1/ERK2 seemed not to be involved in the volume control of human intestine 407 cells.
The osmotic induction of the p38 MAP kinase signalling cascade has been reported in renal epithelial A6 cells (Niisato et al. 1999) and intestine 407 cells (Tilly et al. 1996), whereas p38 MAP kinase was not activated by hypotonicity in cardiac myocytes (Sadoshima et al. 1996). The present study shows that osmotic swelling of human cervical cancer cells leads to a significant increase in the phosphorylation of p38 MAP kinase. However, the volume-regulatory mechanism is independent of p38 MAP kinase, since blockade of the p38 MAP kinase cascade by a specific and potent inhibitor SB 202190 does not inhibit the swelling-activated Cl− channel or K+ and taurine transport. Instead of being involved in the rapid adjustment of cell volume, the p38 MAP kinase cascade may play another role in cellular function, such as transduction of osmotic-stress signals from the cell surface to the nucleus and subsequent cellular repair processes. Further investigation will be required to determine the specific target for osmotically sensitive p38 MAP kinase cascades.
The regulatory mechanisms of RVD in human cervical cancer cells could be summarized as follows: osmotic swelling induces Ca2+ influx through a channel sensitive to Gd3+ and nifedipine. The resultant Ca2+ influx is followed by Ca2+ release from intracellular stores. Then, a Ca2+-activated K+ channel is activated by hypotonicity-induced Ca2+ signalling (Shen et al. 2001). In parallel, osmotic swelling activates the volume-sensitive Cl− channel, which also depends on raised Ca2+ intracellularly. Osmotic swelling induces PKC-α translocation, which is critical for the activation of the volume-sensitive Cl− channel (Chou et al. 1998). Tyrosine kinases and the downstream MAP kinases are also activated by hypotonic stress, but the volume-responsive p38 MAP kinase is not involved in the volume regulation. The phosphorylation of ERK1/ERK2 MAP kinase following a hypotonic shock is regulated by Ca2+ influx and PKC. Ion channels and cotransporter work synergistically for volume regulation and thus K+, Cl− and uncharged organic osmolytes (e.g. taurine) are released (Shen et al. 2000a). The efflux of KCl and taurine drives water exit, thereby achieving RVD.
Acknowledgments
This work was partly supported by The Wellcome Trust and National Science Council, Taiwan (NSC 89-2314-B-006-0112 and NSC 90-2314-B-006-032). Meng-Ru Shen holds a Swire Scholarship supported by John Swire & Sons Ltd. We thank Dr K. S. Hsu and Dr H. S. Liu at National Cheng Kung University Taiwan for kindly providing the Src-activating peptides and plasmids for dominant-negative mutants of ERK1/ERK2, respectively.
References
- Chang MY, Won SJ, Yang BC, Jan MS, Liu HS. Selective activation of Ha-ras(val12) oncogene increases susceptibility of NIH/3T3 cells to TNF-alpha. Experimental Cell Research. 1999;248:589–598. doi: 10.1006/excr.1999.4436. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Molecular and Cellular Biology. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CY, Shen MR, Chen TM, Huang KE. Volume-activated taurine transport is differentially activated in human cervical cancer HT-3 cells, but not activated in HPV-immortalized Z 183A and normal cervical epithelial cells. Clinical and Experimental Pharmacology and Physiology. 1997;24:935–939. doi: 10.1111/j.1440-1681.1997.tb02722.x. [DOI] [PubMed] [Google Scholar]
- Chou CY, Shen MR, Hsu KS, Huang HY, Lin HC. Involvement of PKC-α in regulatory volume decrease responses and activation of volume-sensitive chloride channels in human cervical cancer HT-3 cells. Journal of Physiology. 1998;512:435–448. doi: 10.1111/j.1469-7793.1998.435be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CY, Shen MR, Wu SN. Volume-sensitive chloride channels associated with human cervical carcinogenesis. Cancer Research. 1995;55:6077–6083. [PubMed] [Google Scholar]
- Cobb MH. MAP kinase pathways. Progress in Biophysics and Molecular Biology. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Cox ME, Ely CM, Catling AD, Weber MJ, Parsons SJ. Tyrosine kinases are required for catecholamine secretion and mitogen-activated protein kinase activation in bovine adrenal chromaffin cells. Journal of Neurochemistry. 1996;66:1103–1112. doi: 10.1046/j.1471-4159.1996.66031103.x. [DOI] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly ME, Macvicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. Journal of Neuroscience. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. Journal of Biological Chemistry. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Ellory JC, Gibson JS, Stewart GW. Pathophysiology of abnormal cell volume in human red cells. Contribution of Nephrolology. 1998;123:220–239. doi: 10.1159/000059915. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. International Review of Cytology. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. Journal of Physiology. 1999;520:783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. Swelling-activated organic osmolyte channels. Journal of Membrane Biology. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiological Reviews. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. Journal of Cell Biology. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiological Reviews. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Niisato N, Post M, Van Driessche W, Marunaka Y. Cell swelling activates stress-activated protein kinases, p38 MAP kinase and JNK, in renal epithelial A6 cells. Biochemical and Biophysical Research Communications. 1999;266:547–550. doi: 10.1006/bbrc.1999.1843. [DOI] [PubMed] [Google Scholar]
- Noé B, Schliess F, Wettstein M, Heinrich S, Häussinger D. Regulation of taurocholate excretion by a hypo-osmolarity-activated signal transduction pathway in rat liver. Gastroenterology. 1996;110:858–865. doi: 10.1053/gast.1996.v110.pm8608896. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hazama A, Yuan WL. Stretch-induced activation of Ca2+-permeable ion channels is involved in the volume regulation of hypotonically swollen epithelial cells. Neuroscience Research. 1990;12:S5–13. doi: 10.1016/0921-8696(90)90004-m. [DOI] [PubMed] [Google Scholar]
- Ross PE, Cahalan MD. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. Journal of General Physiology. 1995;106:415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO Journal. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- Salazar EP, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. Journal of Biological Chemistry. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- Schliess F, Schreiber R, Haussinger D. Activation of extracellular signal-regulated kinases Erk-1 and Erk-2 by cell swelling in H4IIE hepatoma cells. Biochemical Journal. 1995;309:13–17. doi: 10.1042/bj3090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB Journal. 1995;9:726–735. [PubMed] [Google Scholar]
- Shen MR, Chou CY, Ellory JC. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflügers Archiv. 2000a;440:751–760. doi: 10.1007/s004240000338. [DOI] [PubMed] [Google Scholar]
- Shen MR, Chou CY, Ellory JC. Swelling-activated taurine and K+ transport in human cervical cancer cells: association with cell cycle progression. Pflügers Archiv. 2001;441:787–795. doi: 10.1007/s004240000476. [DOI] [PubMed] [Google Scholar]
- Shen MR, Chou CY, Hsu KF, Hsu KS, Wu ML. Modulation of volume-sensitive Cl− channel and cell volume by actin filaments and microtubules in human cervical cancer HT-3 cells. Acta Physiologica Scandinavica. 1999;167:215–225. doi: 10.1046/j.1365-201x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Shen MR, Chou CY, Wu ML, Huang KE. Differential osmosensing signalling pathways and G-protein involvement in human cervical cells with different tumor potential. Cellular Signalling. 1998;10:113–120. doi: 10.1016/s0898-6568(97)00115-0. [DOI] [PubMed] [Google Scholar]
- Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. Journal of Physiology. 2000b;529:385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MR, Wu SN, Chou CY. Volume-sensitive chloride channels in the primary culture cells of human cervical carcinoma. Biochimica et Biophysica Acta. 1996;1315:138–144. doi: 10.1016/0925-4439(95)00115-8. [DOI] [PubMed] [Google Scholar]
- Sinning R, Schliess F, Kubitz R, Haussinger D. Osmosignalling in C6 glioma cells. FEBS Letters. 1997;400:163–167. doi: 10.1016/s0014-5793(96)01376-2. [DOI] [PubMed] [Google Scholar]
- Szücs G, Heinke S, De Greef C, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. The volume-activated chloride current in endothelial cells from bovine pulmonary artery is not modulated by phosphorylation. Pflügers Archiv. 1996;431:540–548. doi: 10.1007/BF02191901. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Gaestel M, Engel K, Edixhoven MJ, de Jonge HR. Hypo-osmotic cell swelling activates the p38 MAP kinase signalling cascade. FEBS Letters. 1996;395:133–136. doi: 10.1016/0014-5793(96)01028-9. [DOI] [PubMed] [Google Scholar]
- Tilly BC, van den Berghe N, Tertoolen LG, Edixhoven MJ, de Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. Journal of Biological Chemistry. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Urbach V, Leguen I, O'Kelly I, Harvey BJ. Mechanosensitive calcium entry and mobilization in renal A6 cells. Journal of Membrane Biology. 1999;168:29–37. doi: 10.1007/s002329900495. [DOI] [PubMed] [Google Scholar]
- van der Wijk T, Dorrestijn J, Narumiya S, Maassen JA, de Jonge HR, Tilly BC. Osmotic swelling-induced activation of the extracellular-signal-regulated protein kinases Erk-1 and Erk-2 in intestine 407 cells involves the Ras/Raf-signalling pathway. Biochemical Journal. 1998;331:863–869. doi: 10.1042/bj3310863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon B, Winpenny JP, Whitfield KJ, Argent BE, Gray MA. Volume-activated chloride currents in pancreatic duct cells. Journal of Membrane Biology. 1995;147:173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. Journal of Physiology. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weikersthal SF, Barrand MA, Hladky SB. Functional and molecular characterization of a volume-sensitive chloride current in rat brain endothelial cells. Journal of Physiology. 1999;516:75–84. doi: 10.1111/j.1469-7793.1999.075aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, II, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]


