Abstract
We have investigated voltage-dependent outward K+ currents of dentate granule cells (DGCs) in acute brain slices from young and adult rats using nucleated and outside-out patch recordings.
In adult DGCs, the outward current pattern was dominated by a transient K+ current component. One portion of this current (∼60 %) was blocked by micromolar concentrations of tetraethylammonium (TEA; IC50 42 μm) and BDS-I, a specific blocker of Kv3.4 subunits (2.5 μm). A second component was insensitive to tetraethylammonium (10 mm) and BDS-I. The transient outward current could be completely blocked by 4-aminopyridine (IC50 296 μm).
The TEA- and BDS-I-sensitive and the TEA-resistant current components were isolated pharmacologically. The current component that was blocked by BDS-I and TEA showed a depolarized threshold of activation (∼-30 mV) reminiscent of Kv3.4 subunits, while the current component resistant to TEA activated at more hyperpolarized potentials (∼-60 mV).
In nucleated patches obtained by placing the patch pipette adjacent to the apical dendrite, only small Na+ currents and small BDS-I-sensitive transient currents were detected. Nucleated patches obtained from either the cell soma (see above) or the axon hillock showed significantly larger amplitude Na+ currents as well as larger BDS-I-sensitive currents, indicating that this current was predominantly localized within the axosomatic compartment. This result was in good agreement with the distribution of Kv3.4 protein as determined by immunohistochemistry.
Current-clamp as well as mock action potential-clamp experiments revealed that the BDS-sensitive current component contributes to action potential repolarization.
A comparison of the two age groups (4-10 days and 60-100 days) revealed a marked developmental up-regulation of the BDS-I-sensitive component. These functional changes are paralleled by a developmental increase in Kv3.4 mRNA expression determined by quantitative real-time RT-PCR, as well as a pronounced up-regulation of Kv3.4 on the protein level determined by immunohistochemistry.
These functional and molecular results argue that Kv3.4 channels located predominantly in the axosomatic compartment underlie a transient K+ current in adult DGCs, and that these channels are functionally important for regulating spike repolarization. The marked developmental regulation suggests an important role of Kv3.4 in neuronal maturation.
Voltage-dependent K+ channels are key regulators of membrane excitability. The diversity of subunits underlying K+ currents is remarkable and allows formation of channels with very different biophysical and pharmacological properties (Coetzee et al. 1999). Transient K+ currents form a subgroup of voltage-dependent K+ currents characterized by their rapid activation and inactivation upon depolarization (Hille, 1992; Coetzee et al. 1999). The presence of such currents profoundly affects membrane excitability in several ways. The rapid kinetics of transient K+ currents make them well suited to contribute to action potential repolarization (Kang et al. 2000). In addition, the activation of transient K+ currents during action potentials provides transient hyperpolarizing drive that prolongs the interspike interval and may permit some neurons to discharge at slow rates (Connor & Stevens, 1971a, b; Song et al. 1998; Kanold & Manis, 1999; Kang et al. 2000). Furthermore, transient K+ currents in dendrites confer active electrical properties to these compartments (Hoffmann et al. 1997; Johnston et al. 1999; Magee & Carruth, 1999; Korngreen & Sakmann, 2000).
Hitherto, several K+ channel subunits have been cloned that give rise to transient K+ channels in expression systems. Amongst these are Kv4.1, Kv4.2 and Kv4.3 of the Shal family, Kv3.3 and Kv3.4 of the Shaw family, and Kv1.4 of the Shaker-related subfamily. In addition, Kv1.1 may form rapidly inactivating potassium channels when accessory β subunits are present (Heinemann et al. 1994; Stephens et al. 1996; Rhodes et al. 1997; Salinas et al. 1997). These channel subunits differ in their pharmacology and kinetic characteristics. Furthermore, these channel subunits are differentially modulated by second-messenger systems (Hoffman & Johnston, 1998) or by oxidation (Ruppersberg et al. 1991). This diversity may be further enhanced by the Kv8.1 (or Kv2.3) subunits (Castellano et al. 1997; Chiara et al. 1999) that block functional expression of Kv3.4 (Hugnot et al. 1996). Some of these channel subunits show a striking differential pattern of expression in different hippocampal cell types (Martina et al. 1998) and subcellular compartments (Sheng et al. 1992; Tsaur et al. 1992; Sheng et al. 1993, 1994; Veh et al. 1995; Grosse et al. 2000). The distinct properties of different pore-forming channel subunits may permit the generation of transient K+ currents with specific characteristics appropriate for their cellular localization.
During neuronal plasticity (Mackler et al. 1992) or ontogenetic maturation (Ribera & Spitzer, 1992), the variation of K+ channel subunit expression may represent a key mechanism by which neuronal excitability and synaptic integration are regulated. However, parallel molecular and functional data that shed light on how differential expression of Kv channel subunits affects K+ current properties and thereby discharge behaviour are scarce (Maletic-Savatic et al. 1995; Murakoshi & Trimmer, 1999; Seifert et al. 1999; Grosse et al. 2000). We have therefore investigated transient K+ channels in hippocampal neurons at the functional, mRNA and protein level. Our functional and molecular results argue that Kv3.4 is up-regulated during development and contributes significantly to the transient K+ current in adult dentate granule cells (DGCs). We show that this current is predominantly located in the basal portion of DGCs and contributes to spike repolarization.
METHODS
Slice preparation and maintenance
Hippocampal slices were prepared using a vibratome from Wistar rats aged 4-10 days (young group) and 60-100 days (adult group), which were killed by decapitation after deep anaesthesia with halothane. All procedures were carried out according to the guidelines laid down by the University of Bonn Animal Care and Use Committee. Slices (300 μm) were then transferred to the stage of an upright microscope and visualized using infrared video microscopy and differential interference contrast (DIC) optics (Martina et al. 1998). For most recordings, the bath solution contained (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2.0 CaCl2, 1.0 MgCl2 and 25 dextrose. For pharmacological experiments, we used Hepes-buffered bath solution (mm): 125 NaCl, 2.5 KCl, 10 Hepes, 2.0 CaCl2, 1.0 MgCl2, 25 dextrose and 20 sucrose. Application of Hepes-buffered bath solution was carried out for up to 10 min.
Recording from nucleated and outside-out patches
Glass microelectrodes were pulled from borosilicate glass (2.0 mm diameter, wall thickness 420 μm), and coated with Sylgard. For outside-out and nucleated patch recordings (pipette resistance 3-6 MΩ), the pipette solution contained (mm): 20 KCl, 120 potassium gluconate, 2.0 MgCl2, 2 Na2ATP, 2 glutathione-SH, 10 EGTA and 10 Hepes, pH adjusted to 7.0 with KOH. In some experiments, the concentration of glutathione-SH was increased to 5 mm or glutathione-SH was omitted altogether.
A liquid junction potential of -11 mV was experimentally determined as described for the solutions used in outside-out and nucleated patch recordings (Neher, 1992) and command voltages were corrected accordingly. Outside-out or nucleated patches were obtained at room temperature (21-24 °C) using a patch-clamp amplifier (Axopatch 200B, Axon Instruments). After establishing the whole-cell configuration in voltage-clamp mode, we immediately switched to current-clamp mode and resting membrane potential was measured. The membrane potential proved to be more depolarized in young compared to old animals (-55.8 ± 1.5 mV, n = 58 and -82.1 ± 1.0 mV, n = 63 in young and adult animals, respectively). The patch pipette was subsequently slowly withdrawn under visual control and continuous monitoring of seal resistance. In the case of nucleated patches, negative pressure (150 ± 10 mbar) was applied, resulting in the formation of a large patch engulfing the cell nucleus (Martina et al. 1998). The diameter of nucleated patches was estimated visually from video images, resulting in an average patch area of 454.3 ± 18.9 μm2 (mean ±s.e.m., n = 15). In all recordings, the capacitance compensation circuitry of the patch-clamp amplifier was employed to reduce capacitive transients. Traces are averages of 10-20 sweeps (outside-out patch recordings) or five sweeps (nucleated patch recordings) that were leak-subtracted online (P/4 protocol) with an inter-sweep holding potential of -80 mV (4 s).
The drugs tetraethylammonium (TEA) and 4-aminopyridine (4-AP) at different concentrations were bath-applied. The toxins BDS-I and α-dendrotoxin (DTX) were applied via a multi-barrelled superfusion pipette placed at 50-100 μm from the patch. In toxin experiments, 0.1 % bovine serum albumin was added to the Hepes-buffered bath solution. All substances were purchased from Sigma, except BDS-I (Alomone Labs, Israel) and tert-butyl hydroperoxide (t-BHP) (Fluka).
Current-clamp recordings
Current-clamp recordings were carried out in the whole-cell configuration of the patch-clamp technique (Axopatch 200B, Axon Instruments; mode switch set to I Clamp normal), with extracellular solutions of the same composition as used for outside-out and nucleated patches. In the intracellular solutions, 10 mm EGTA was substituted for 0.5 mm EGTA. d-Aminophosphonovalerate (APV, 50 μm), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and bicuculline (20 μm) were added to the extracellular solution to block synaptic transmission. TTX was not used in these experiments. Voltage signals were recorded using an Axopatch 200B amplifier in current-clamp mode.
Data analysis
The voltage dependence of activation and inactivation was characterized using standard protocols. The conductance was calculated as:
| (1) |
where VK is the K+ reversal potential, V the command potential and I(V) the peak current amplitude. G(V) was then fitted with the following Boltzmann equation:
| (2) |
where Gmax is the maximum K+ conductance, V½ is the voltage where G(V) is the half of Gmax and k is the slope of the relationship between channel activation/inactivation and membrane voltage.
Time constants (τ) of decay and recovery from inactivation were extracted by fitting with a single exponential function of the form:
| (3) |
where I(t) is the current amplitude at the time point t and A0 is a constant offset.
Concentration-response curves were fitted with a modified Hill equation according to:
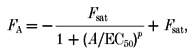 |
(4) |
where FA is the current fraction blocked by the antagonist concentration A, Fsat the current fraction blocked at saturating concentrations of A, IC50 the agonist concentration at half-maximal current inhibition and p the Hill coefficient. In all experiments, statistical significance was proven with Student's t two-tailed test with the level of significance set to P < 0.05.
Harvesting of dentate granule cells for isolation of mRNA
The dentate gyrus was dissected from hippocampal slices (400 μm), treated enzymatically with 1.5 mg ml−1 pronase from Streptomyces griseus (Sigma) and gently triturated mechanically. Granule cells were allowed to settle to the bottom of a Petri dish and harvested with a glass pipette under visual control. Granule cells could be separated from putative interneurons by their considerably smaller size. To avoid contamination, harvesting was carried out under continuous perfusion with an autoclaved extracellular solution. From each animal, 45-60 granule cells were pooled and mRNA was isolated using the Dynabeads mRNA Direct Micro Kit (Dynal, Oslo, Norway) according to the manufacturer's protocol. The mRNA isolate was aliquoted into 18 tubes to allow three simultaneous analyses of five target genes and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
RT-PCR analysis of potassium channel transcripts
The so-called ‘TaqMan’ approach is based on the 5′-nuclease activity of the Taq polymerase by cleavage of a fluorogenic hybridization probe labelled with two fluorescent dyes (Holland et al. 1991; Livak et al. 1995). Cleavage of the sequence-specific probe results in the release of a reporter dye and an emission increase is observed at the reporter dye wavelength. In the PE Biosystems PRISM 7700 Sequence Detection System which we have used for mRNA quantification, the increase in fluorescence intensity is monitored online in each tube during the PCR run. For data analysis, the fluorescence signals are normalized to an internal reference (Rn), yielding a normalized value ΔRn. The threshold cycle (Ct) is generally set where ΔRn reaches 10 times standard deviation of the baseline. The Ct is used for quantification of the starting copy number of the target mRNA. Relative quantification, the so-called ΔΔCt-method (Fink et al. 1998), was applied to determine the amounts of the different Kv subunits. Using the ΔΔCt-method, the number of target mRNA copies is normalized to an endogenous reference. In the present study, the housekeeping gene GAPDH was chosen as a reference gene because this gene does not show a developmental variation in expression in the CNS (Prime et al. 2000). Specific PCR primers and TaqMan oligonucleotides were designed for the rat Kv1.4, Kv3.3, Kv3.4, Kv4.2, Kv4.3 and GAPDH cDNAs using Primer Express software (PE Biosystems, Foster City, CA, USA; see Table 1). The TaqMan EZ RT-PCR Kit (PE Biosystems, Foster City, CA, USA) was used for a one-tube, single-enzyme RT-PCR according to the manufacturer's protocol, but in a total reaction volume of 12.5 μl. mRNA isolated and aliquoted as described served as template for RT-PCR. Reaction conditions were optimized at 5.0 mm manganese (II) acetate and 300 μm of each deoxyribonucleoside triphosphate (dNTP), 100 nm of fluorogenic probe, 1 × TaqMan EZ buffer, 0.1 U μl−1 rTth DNA polymerase, 0.01 U μl−1 AmpErase UNG, and primer concentrations as outlined in Table 1. Cycling conditions were 50 °C for 2 min, 60 °C for 20 min for the RT step, followed by 95 °C for 5 min, and a two-step PCR with 60 cycles of 94 °C for 15 s and 59 °C for 60 s. The normalized values of K+ channel mRNA quantification were related to the average value obtained in the young group for each PCR run.
Table 1.
Sequences, amplicon sizes and concentration ratios of primers and probes with labelling dyes which were used for RT-PCR
| Gene | Forward (f)/reverse (r) primers | Hybridization probe | Primer conc.(f/r) (nM) | Amplicon size(bp) |
|---|---|---|---|---|
| Kv1.4 | (f)5′TTTGCAGAGGCAGATGAACCT3′ | F5′CACCCATTTCCAAAGCATTCCAGATGC3′T | 50/300 | 73 |
| (r)5′TGGTTACCACAGCCCACCA3′ | ||||
| Kv3.3 | (f)5′TTCATCCACATCAGCAACAAGAC3′ | F5′TGACGCAGGCCTCTCCAATCCCT3′T | 300/300 | 80 |
| (r)5′TCCACATTGGTGATATTCTCCG3′ | ||||
| Kv3.4 | (f)5′GCTGCCCTGATACGTTGGA3′ | F5′TTTGTCAAGAACCTGCTCAACATCATCGACT3′T | 300/300 | 70 |
| (r)5′AAGGGCAAGATGGCCACA3′ | ||||
| Kv4.2 | (f)5′CTTCAGTCGGATCTACCACCAAA3′ | F5′CCAACGAGCGGACAAACGAAGGG3′T | 50/300 | 68 |
| (r)5′CCAGCCTCGCTTTCTTCTGT3′ | ||||
| Kv4.3 | (f)5′AGCGCTACTCCGTGGCTTT3′ | F5′TCTGCCTGGACACTGCGTGTGTCA3′T | 50/300 | 71 |
| (r)5′GAGGAGGTACTCCACCGTGAAG3′ | ||||
| GAPDH | (f)5′TGCCAAGTATGATGACATCAAGAAG3′ | F5′TGGTGAAGCAGGCGGCCGAG3′T | 50/300 | 72 |
| (r)5′TAGCCCAGGATGCCCTTTAGT3′ |
For all hybridization probes, F indicates a reporter dye (FAM) and T a quencher dye (TAMRA).
Immunohistochemistry
Brains of Wistar rats aged 4 days (n = 2), 5 days (n = 2), 6 days (n = 2), 8 days (n = 2) and 10 days (n = 2) for the young group and aged between 60 and 100 days (n = 9) for the adult group were fixed in a solution of 4 % paraformaldehyde (Sigma) and 15 % picric acid (Sigma) in 0.1 m phosphate buffer, pH 7.4, for 24 h at 4 °C. After pretreatment with 30 % saccharose, 18 μm cryostat sections were prepared and processed according to established protocols (Veh et al. 1995). In short, cryostat sections were pretreated with a solution of 0.3 % Triton X-100 (Sigma), 10 % normal goat serum (Vector/Camon) and 0.05 % phenylhydrazine (Merck), for 30 min at room temperature. After incubation with the primary antibody (affinity-purified anti-Kv3.4 1:2000; Veh et al. 1995) for 36 h at 4 °C, sections were treated with secondary antibody (biotinylated goat anti-rabbit IgG, 1:2000, Vector/Camon) for 24 h at 4 °C and with an avidin-biotin complex (ABC, Vectastain Elite, 1:1000, Vector/Camon) for 6 h at room temperature. Peroxidase activity was detected with 1.4 mm 3,3′-diaminobenzidine (Sigma), 10 mm imidazole, 0.3 % nickelous ammonium sulphate and 0.015 % H2O2 in 50 mm Tris-HCl, pH 7.6, for 5 min at room temperature.
RESULTS
Voltage-dependent activation and inactivation of the transient K+ current in adult granule cells
We have first obtained outside-out and nucleated patch-clamp recordings from visually identified granule neurons in adult (P60-P100) rats. The transient K+ current was isolated by a subtraction method similar to that described previously (Numann et al. 1987; Beck et al. 1992). Traces in which the transient K+ current had been inactivated by a 50 ms delay (-20 mV, Fig. 1Ab) were subtracted from equivalent current traces evoked without a delay (Fig. 1Aa). The resulting traces showed an isolated current component with a rapid decay during a 150 ms command pulse (Fig. 1Ac). Steady-state voltage-dependent inactivation of this transient K+ current component was investigated by varying a 500 ms conditioning prepulse from -120 to +60 mV followed by a constant test pulse to +40 mV (Fig. 1B). The amplitude of the inactivating component was calculated by subtracting the current at the end of the test pulse from the peak current during the test pulse. The voltage-dependent activation and inactivation curves were constructed from the normalized peak conductances (eqn (1)) and fitted with an appropriate Boltzmann equation (eqn (2)) shown superimposed on the data points (Fig. 1C; for values see Table 2).
Figure 1. Transient K+ currents in adult DGCs in outside-out and nucleated patches.
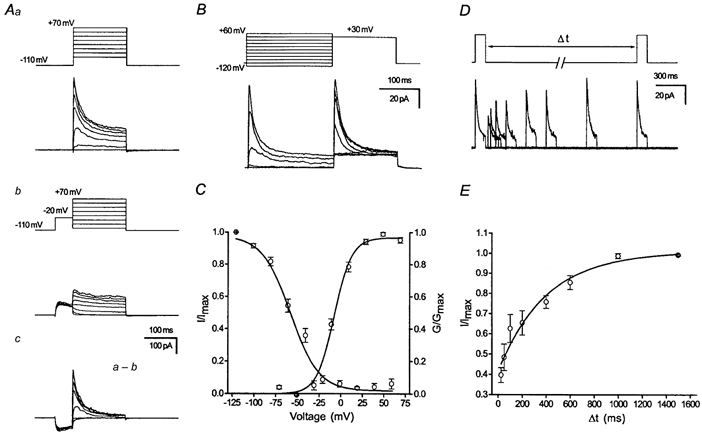
Aa, voltage-dependent activation behaviour measured with voltage commands ranging from -70 to +70 mV (150 ms, nucleated patches). b, current traces in which the transient K+ current has been inactivated with a brief delay at -20 mV (50 ms, see inset). c, transient K+ current isolated by digital subtraction of current traces in which the transient K+ current had been inactivated by a 50 ms delay (b) from corresponding current traces without such a delay (a). Traces are ensemble averages of 5-10 sweeps. B, steady-state inactivation of the transient current was determined by varying the voltage of a conditioning pulse from -120 to +60 mV and measuring the inactivation during a subsequent command pulse (see inset, outside-out patches). C, steady-state activation and inactivation curves. Data points for the steady-state activation curve were obtained by calculating the conductance from peak current values of the isolated transient K+ current (Ac), and plotting normalized and averaged values vs. the command voltage. Data points for the steady-state inactivation were obtained by subtracting current amplitudes at the end of the command pulse from the peak current values, thus obtaining the amplitude of the inactivating component. These values were normalized and averaged and plotted vs. the voltage of the conditioning pulse. Boltzmann functions were fitted to the data points of steady-state activation and inactivation using a least-squares algorithm and are shown superimposed on the data points. D, double-pulse experiments were used to analyse recovery from inactivation of transient K+ currents obtained from the two age groups (data pooled from outside-out and nucleated patches). The voltage protocol is shown above the current traces. Currents were elicited by two 150 ms voltage steps (+40 mV) from a holding potential of -110 mV and the time between the voltage steps was varied from 25 to 1500 ms. E, time course of recovery from inactivation. The transient current component was isolated by subtracting current values at the end of the 150 ms pulse from the peak values. The current amplitude was normalized to the maximal amplitude of the transient current and was then plotted vs. the interpulse interval. The data points were fitted with a monoexponential equation shown superimposed. The recovery time constants averaged across individual experiments are given in Table 2.
Table 2.
Comparison of functional properties of transient K+ current in young and adult DGCs
| Activation curve | Inactivation curve | Recovery from inactivation | ||||
|---|---|---|---|---|---|---|
| Young | Adult | Young | Adult | Young | Adult | |
| V1/2(mV) | 3.3 ± 1.5 | −7.6 ± 1.6 | −64.9 ± 4.0 | −53.0 ± 4.2 | — | — |
| k | 11.9 | 10.1 | −23.7 | −17.6 | — | — |
| τrec(ms) | — | — | — | — | 93.2 ± 12.4 | 400.2 ± 109* |
| n | 5 | 5 | 11 | 12 | 7 | 5 |
V1/2 of activation and inactivation: the voltage of half-maximal activation and inactivation, respectively. k: slope factor (see Methods). τrec: time constant of recovery from inactivation. Asterisk indicates significant differences (P < 0.05).
Recovery from inactivation was analysed with double pulses (150 ms, +40 mV) spaced at variable intervals (Δt: 20 ms to 1.5 s; Fig. 1D, upper panel). The transient K+ current recovered from inactivation within 1.5 s without large changes in the sustained component (Fig. 1D and E). Recovery from inactivation could be fitted with monoexponential equations (eqn (3)), which is shown superimposed on the data points in Fig. 1E.
Granule cells in adult animals express a TEA- and BDS-I-sensitive transient K+ current
In view of the fact that the transient K+ channel dominates the outward current pattern in adult DGCs, we have attempted to obtain a first idea of the molecular correlates underlying the transient K+ current. Since cloned transient K+ channels show differential sensitivity to common K+ channel blockers (Coetzee et al. 1999), we have first determined the sensitivity of transient currents in adult DGCs to 4-AP and TEA in nucleated patch recordings. The transient current isolated by subtraction as in Fig. 1Ac was sensitive to low concentrations of 4-AP as in other preparations, with a virtually complete block of transient K+ currents at 10 mm (Fig. 2Ac, 99 % block). The concentration-response curve could be fitted with a modified Hill function as given in eqn (4) (Fig. 2Ac) with an IC50 of 296 μm (Hill coefficient 0.794, n = 8-10). Surprisingly, however, TEA, which has only rarely been shown to affect transient K+ currents, also blocked a substantial portion of the transient K+ current in adult rats (64 % block at 10 mm; Fig. 2Ac). Fitting with a Hill function yielded an IC50 of 42 μm (Hill coefficient 1.290, n = 4-6). Because cloned Kv3.3 and Kv3.4 channels are sensitive to TEA, while Kv4 or Kv1.4 channels are not, the former channels are good candidates which might underlie the TEA-sensitive transient K+ current in adult granule cells (Schröter et al. 1991; Rettig et al. 1992; Coetzee et al. 1999).
Figure 2. Pharmacology of the transient K+ current in adult DGCs.
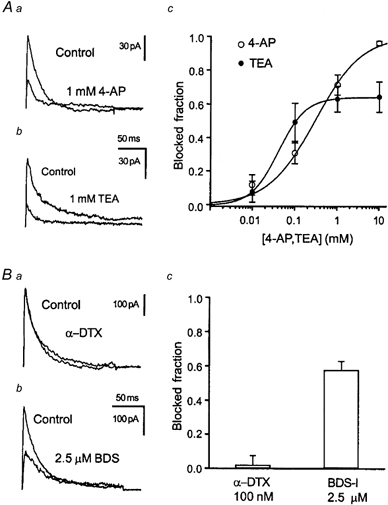
Aa and b, representative current traces of the isolated transient K+ current in the absence and presence of 1 mm 4-AP (a) or TEA (b) obtained from nucleated patches. The transient K+ current was isolated by subtraction as in Fig. 1 at command pulse potentials of +30 mV. Ac, inhibition of the peak transient K+ current by 4-AP (○) or TEA (•), plotted against concentration, for nucleated patches from adult DGCs. Data points were fitted with a modified Hill function (see Methods) shown superimposed (IC50 of 296 μm for 4-AP and 42 μm for TEA). Thus, adult DGCs express a large fraction of TEA-sensitive transient K+ current. Ba, application of 0.1 μmα-DTX does not alter outward currents in adult DGCs (not shown), or transient K+ currents isolated by subtraction. b, in contrast, application of the anemone toxin BDS-I (2.5 μm), a specific antagonist for K+ currents carried by the Kv3.4 subunit, blocks transient but not sustained (not shown) outward currents. c, summary of α-DTX and BDS-I effects on the transient K+ current.
In order to further test this hypothesis, we have applied to nucleated patches the subunit-selective anemone toxin BDS-I, which has been shown to specifically block Kv3.4, but not other K+ channels of the Kv1, Kv2, Kv3 or Kv4 subfamilies (Diochot et al. 1998). At concentrations of 2.5 μm, BDS-I blocked around 60 % of the isolated transient K+ channel without affecting sustained components (Fig. 3Bb and c, n = 4). In addition to Kv3.3 and 3.4, Kv1.1 may form rapidly inactivating, TEA-sensitive K+ channels when accessory β subunits are present (Heinemann et al. 1994; Stephens et al. 1996; Rhodes et al. 1997; Salinas et al. 1997). Channels containing the Kv1.1 subunit, however, should be highly sensitive to the mamba toxin α-DTX. We therefore tested for a possible presence of Kv1 subunits using α-DTX in adult DGCs, and were unable to demonstrate reduction of the transient K+ current with up to 100 nmα-DTX (Fig. 2Ba and Bc, n = 4). Taken together, these pharmacological data are strongly reminiscent of properties demonstrated for cloned Kv3.4 channels.
Figure 3. As transient K+ currents were pharmacologically heterogeneous in adult DGCs, the activation behaviour of pharmacologically dissected transient K+ current components was determined.
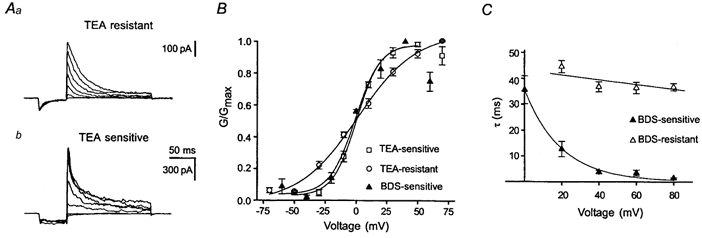
Aa, the TEA-resistant component corresponds to the residual transient K+ current after application of 1 mm TEA. b, the TEA-sensitive current component was determined by subtracting transient K+ current traces in the presence of TEA from corresponding control traces. B, the activation behaviour of TEA- and BDS-I-sensitive, as well as TEA-resistant components was determined as in Fig. 1. Note the more depolarized threshold of activation for the TEA- or BDS-I-sensitive current component. C, inactivation time constants were determined by applying a monoexponential fit to the decaying phase of the pharmacologically dissected transient K+ components (Aa and b). Whereas the inactivation rate at different voltages remained quite constant in the BDS-resistant component (▵), the inactivation rate increased considerably with increasing depolarization in the BDS-sensitive current component (▴).
Compared to A-type K+ channels in other types of native neurons (Zbicz & Weight, 1985; Bourque, 1988) the A-type current in DGCs seemed to be independent of Ca2+ influx. Under our recording conditions with 10 mm EGTA in the recording pipette, removing Ca2+ from the external solution (n = 3) or blocking voltage-dependent Ca2+ currents with 100 μm Cd2+ (n = 13) did not affect the outward current pattern.
Voltage dependence of BDS-I-sensitive and -insensitive transient K+ currents
Since our pharmacological data indicated that the transient K+ current in adult DGCs consists of a BDS-I- and TEA-sensitive and -insensitive component, we have subsequently investigated the activation behaviour of these components separately (Fig. 3Aa and b). Clearly, both the BDS- and the TEA-sensitive transient current component showed a considerably more depolarized threshold of activation than the TEA-resistant component (Fig. 3B). In addition the BDS-sensitive and to a lesser extent the TEA-sensitive component show rectification and a negative slope conductance at voltages more depolarized than +40 mV, similar to the behaviour of Kv3.4 subunits (Rettig et al. 1992). A further kinetic difference between BDS/TEA-sensitive and -resistant components was apparent in the isolated components (Fig. 3Aa and b). Whereas the inactivation rate at different voltages remained quite constant in the BDS-resistant component, the inactivation rate increased considerably with increasing depolarization in the BDS-sensitive current component (Fig. 3C). Thus, the activation behaviour and the voltage dependence of the inactivation rate of the TEA/BDS-sensitive transient K+ current are similar to the characteristic properties of cloned Kv3.4 subunits (Rudy et al. 1991; Schröter et al. 1991; Vega Saenz de Miera et al. 1992).
Differential subcellular localization of the BDS-sensitive K+ current
In previous studies, immunohistochemistry has suggested that the Kv3.4 protein may be located primarily within DGC axons and the axon initial segment (Veh et al. 1995; see also Fig. 8), with a lower expression on the cell soma. We have therefore tested whether the Kv3.4-like, BDS-I-sensitive K+ current we have described shows a similar subcellular localization. To this end, we have compared the recordings in Fig. 2 to recordings in which nucleated patches were obtained by placing the recording electrode either immediately adjacent to the apical dendrite (Fig. 4Aa, inset: ‘apical configuration’) or the axon (Fig. 4Ab, inset: ‘basal configuration’). We have then recorded voltage-dependent Na+ currents in these configurations to assess the contribution of axonal membrane areas to these patches (Fig. 4Aa and b). In ‘basal’ nucleated patches (n = 9), as well as in nucleated patches obtained after placing the pipette centrally onto the DGC soma (configuration as in Fig. 2, n = 8), large voltage-dependent Na+ channels were observed that were around fourfold and twofold larger than in ‘apical’ patches (n = 8, Fig. 4Ac). These data suggest that the ‘basal’ configuration results in formation of a nucleated patch including large portions of axonal membrane, while nucleated patches obtained in the ‘apical’ configuration would contain mainly somatodendritic membrane. We have used this experimental approach to test for polarity of expression of the BDS-sensitive K+ current. Clearly, transient K+ currents elicited in ‘apical’ patches (Fig. 4Ba, n = 7) were significantly less sensitive to BDS-I than those in ‘basal’ patches (Fig. 4Bb and c, n = 6) as well as those in the recordings shown in Fig. 2. The amplitude of the transient K+ current proved to be slightly larger in basal (364.5 ± 54.5 pA) vs. apical (281.5 ± 29.4 pA) nucleated patches.
Figure 8. Kv3.4 immunoreactivity in the rat hippocampus during development.
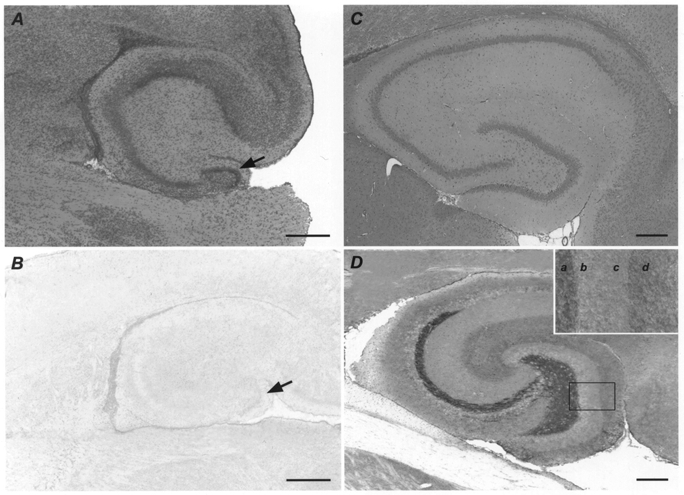
A, haematoxylin and eosin stain in the hippocampus of a young rat (P4). B, no specific Kv3.4 immunoreaction product is detectable in the hippocampus of a young rat (P4). Arrows in A and B indicate the dentate gyrus granule cell layer. C, haematoxylin and eosin stain in the hippocampus of an adult rat (P66). D, in the hippocampus of an adult rat (P66), strong immunostaining for Kv3.4 is observed in the mossy fibre system. The dentate gyrus granule cell layer and inner molecular layer are weakly labelled, whereas moderate staining is found in the outer molecular layer. The inset in D presents a higher magnification of the indicated dentate gyrus segment. a, hilus and polymorphic layer; b, granule cell layer; c, inner molecular layer; d, outer molecular layer. Scale bars in A and B represent 400 μm; scale bars in C and D represent 1 mm.
Figure 4. Subcellular localization of the Kv3.4-like, BDS-I-sensitive K+ current.
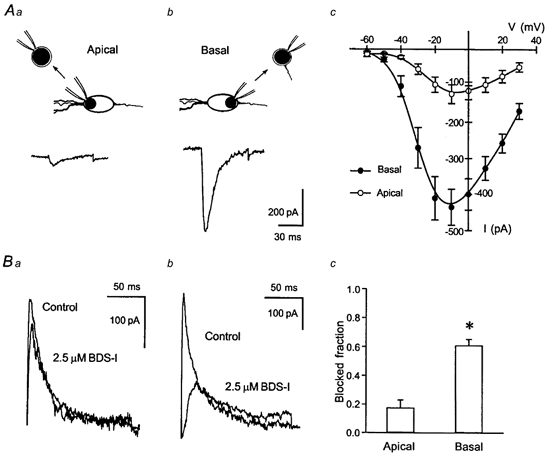
Aa-c, nucleated patches were obtained following placement of the electrode either immediately adjacent to the apical dendrite (a, inset) or onto the axon hillock (b, inset). Recording of voltage-dependent Na+ currents in both configurations showed that ‘basal’ nucleated patches (b, n = 9) contained a considerably higher density of Na+ channels than ‘apical’ patches (a, n = 8), suggesting that ‘basal’ patches contained significantly larger amounts of the axon hillock than ‘apical’ patches (c). Ba-c, polarity of expression of the Kv3.4-like, BDS-sensitive K+ current. Clearly, transient K+ currents elicited in ‘apical’ patches (a, n = 7) were significantly less sensitive to BDS-I than in ‘basal’ patches (b and c, n = 6).
Functional role of the transient K+ channel in adult DGCs
After demonstrating the pharmacological properties and subcellular localization of the Kv3.4-like transient K+ current, we have attempted to clarify the functional role of this current component. We have performed a series of experiments using mock action potentials as a voltage command in nucleated patches. A brief action potential-like depolarization caused a transient outward current that peaked during the repolarization phase of the mock action potential. A slower decay of the outward current was observed after complete repolarization of the patch membrane (Fig. 5Aa and b). A low concentrations of TEA (100 μm) and 2.5 μm BDS blocked a substantial fraction of the transient outward current (Fig. 5Aa and b). These data demonstrate that the BDS/TEA-sensitive K+ current component is rapidly activated during brief action potential-like depolarizations, and that the maximal amplitude of this component is observed during the repolarization phase of mock action potentials. Our results are consistent with a role of the BDS-sensitive transient K+ current in action potential repolarization. To further test this idea, we performed current-clamp experiments using the whole-cell configuration of the patch-clamp technique in the slice preparation (Fig. 5Ba and b). These experiments proved difficult to interpret with respect to the repetitive firing properties of neurons because both TEA and BDS-I showed effects other than block of the transient K+ current. In particular, we could demonstrate blocking effects of BDS-I on voltage-dependent Na+ currents at higher (5 μm) concentrations (unpublished data). Therefore, we have limited our analysis to single action potential waveforms generated by brief current injection. We have analysed only such action potentials in which no changes in action potential amplitude could be observed following perfusion of TEA or BDS-I. The repolarization proved to be clearly slower both following application of 100 μm TEA and following application of 2.5 μm BDS-I (Fig. 5Bc). These results suggest that the transient, BDS-I-sensitive K+ current is involved in action potential repolarization in adult dentate granule cells.
Figure 5. Activation of the TEA- and BDS-I-sensitive current during mock action potentials, and influence on action potential characteristics in adult DGCs.
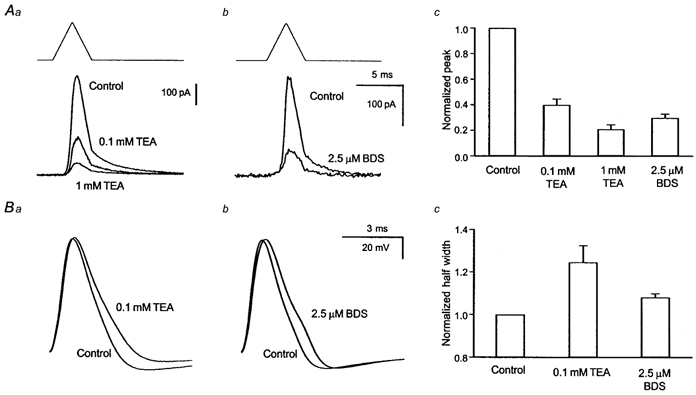
Aa-c, application of mock action potentials (upstroke and downstroke 2 ms, +30 mV peak voltage) in nucleated patches resulted in a transient outward current with a peak during the repolarization phase of the mock action potential. TEA at 100 μm (a) as well as 2.5 μm BDS-I (b) substantially blocked the peak outward current (c). Ba-c, current-clamp recordings in the whole-cell configuration from adult DGCs show differences in action potential morphology elicited by brief current injections (50 pA) following application of either 100 μm TEA (a) or 2.5 μm BDS-I (b). The half-width of action potentials was significantly increased by application of either substance (c).
Developmental increase in the current density of transient but not sustained K+ currents
It has been shown previously that some K+ channel mRNAs, amongst them Kv3.4, show developmental increases in abundance in hippocampal neurons (Kues & Wunder, 1992). In order to obtain a first estimate of possible developmental variations in transient and sustained K+ currents in the somatic membrane of DGCs, we have employed nucleated patch-clamp recordings from visually identified granule neurons in young (P4-P10) and adult (P60-P100) rats (Fig. 6). The constant surface area of such patches from recording to recording permits accurate quantification of current density (see Methods). The outward currents recorded from adult DGCs (Fig. 6Ab, n = 8) showed a considerably larger peak K+ current compared to young DGCs (Fig. 6Aa and Ac, n = 14, P < 0.05). When the transient K+ current component was inactivated by a brief 50 ms delay at -20 mV prior to the test pulse (Fig. 6Ba and b), the remaining sustained K+ current did not show significant developmental variation (Fig. 6Bc). Subtracting traces in which the transient K+ current was inactivated by a delay from equivalent current traces evoked without a delay yielded the transient K+ current component in isolation (Fig. 6Ca and b; Thompson, 1982; Numann et al. 1987; Beck et al. 1992). Clearly, the developmental increase in peak amplitude was due to a significant developmental increase in the amplitude of the transient K+ current (P < 0.05, Fig. 6Cc). When the voltage dependence of activation and inactivation was analysed as in Fig. 1, no significant differences in the properties of voltage-dependent inactivation were detected, whereas the voltage of half-maximal activation was shifted around 10 mV in a hyperpolarizing direction in adult compared to young rats (see Table 2). The time constants of recovery were significantly slower in adult rats compared to young animals (see Table 2).
Figure 6. Transient K+ current amplitude in DGCs increases during development.
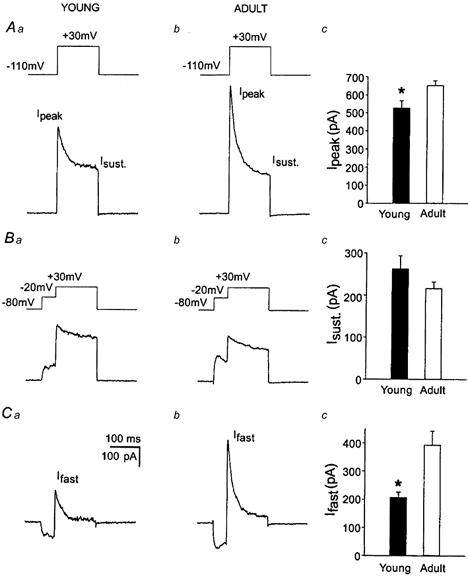
Current amplitudes were determined from nucleated patches because of the reproducibility of patch size (see Methods). Aa and b, representative current traces obtained from nucleated patches of young and adult rats using command pulses to +30 mV (150 ms) following a hyperpolarizing prepulse to -120 mV (200 ms). Ba and b, current traces in which the transient K+ current has been inactivated with a brief delay at -20 mV (50 ms, see inset). Ac and Bc, the peak current Ipeak proved larger in adult rats than in young rats, while the sustained current Isust remained stable. Ca-c, the increase in peak current was due to an increase in the amplitude of the transient K+ current shown following isolation by digital subtraction (isolation as in Fig. 1).
Developmental changes in transient K+ current properties
Next, we asked whether a TEA-sensitive transient K+ current is also present in young dentate granule cells. The transient current isolated by subtraction as in Fig. 1Ac was sensitive to low concentrations of 4-AP with an IC50 of 804 μm (Hill coefficient 0.744, n = 4-6, Fig. 7A and B) that was not significantly different from adult rats. In contrast to adult rats, however, the transient K+ current showed no sensitivity to the Kv3.4-selective antagonist BDS-I (2.5 μm, n = 8, Fig. 7Ac) and little TEA sensitivity (21 % reduction with 10 mm TEA, Fig. 7A and B). Fitting the concentration dependence of TEA block with a Hill function yielded an IC50 of 23 μm (Hill coefficient 0.905, n = 6-8). The average density of TEA-sensitive and -insensitive current components could be calculated from the fraction of these components of the total current and the transient current density as determined in nucleated patches. This analysis yielded a dramatic increase of the mean TEA-sensitive current density during development (from 0.096 to 0.556 pA μm−2), while the TEA-insensitive current component remained stable (from 0.362 to 0.313 pA μm−2). The results suggest that the increase in transient current amplitude is due to the increase of a Kv3.4-like transient K+ current.
Figure 7. Pharmacological characteristics of transient K+ currents in immature DGCs.
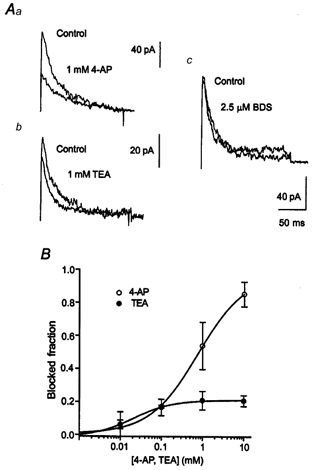
A, superimposed representative current traces of the transient K+ current in the absence and presence of 1 mm 4-AP or TEA obtained from nucleated patches in young rats (a and b). The currents were completely insensitive to BDS-I (c). The transient K+ current was isolated by subtraction as in Fig. 1 at command pulse potentials of +30 mV. B, inhibition of the peak transient K+ current by 4-AP (○) or TEA (•), plotted against concentration, for nucleated patches from young rats. Data points were fitted with a modified Hill function (see Methods) shown superimposed (IC50 of 804 μm for 4-AP and 23 μm for TEA).
Immunohistochemical analysis of Kv3.4 distribution during development
In order to compare the distinctive subcellular localization and developmental regulation of the Kv3.4-like current component with the subcellular distribution of Kv3.4 protein, we performed an immunohistochemical analysis of Kv3.4 in the two different age groups under investigation. Without exception, no specific Kv3.4 immunoreaction product was detectable in the hippocampi of the young group (P4-P10, n = 10, Fig. 8B). In contrast, a distinctive pattern of immunostaining was observed in all hippocampi of the adult group, with the strongest labelling in the mossy fibre system (P60-P100, n = 9, Fig. 8D), possibly in part due to the densely packed axonal membranes in this fibre tract. The dentate gyrus granule cell layer and inner molecular layer were weakly immunoreactive, whereas moderate staining was observed in the outer molecular layer. Our findings in the adult group correspond well to an earlier immunohistochemical analysis of Kv3.4 in the adult rat hippocampus (Veh et al. 1995). In summary, there is a significant up-regulation of Kv3.4 on the protein level from young to adult rats that parallels the increase of Kv3.4-like K+ current at the functional level. In addition, the preferential subcellular localization of Kv3.4 immunoreaction product within the mossy fibre system is in good agreement with the preferential location of the Kv3.4-like current in patch-clamp experiments (see Fig. 4).
Molecular analysis of transient K+ channel transcripts using real-time quantitative PCR
In order to probe the molecular basis of the developmental changes in transient K+ channel properties, we quantitatively investigated the expression of K+ channel subunits known to give rise to transient K+ channels in expression systems. The increase of fluorescence intensity reflecting the specific amplification is shown in an exemplary fashion for the amplification of Kv3.4 mRNA (Fig. 9A). Quantification of K+ channel mRNAs after normalization for GAPDH according to the ΔΔCt-method (Fink et al. 1998) showed significant changes in mRNA abundance during ontogenesis (Fig. 9B). In order to minimize errors due to inter-run variation, amplification of mRNA isolated from two young and two adult animals was always performed simultaneously on one plate. There proved to be a variation in mRNA abundance for all genes from run to run. However, for Kv1.4, Kv3.4 and Kv4.2 (n = 6 adult and 6 young rats), mRNA abundance was considerably greater in adult compared to young rats in each run. Significant differences between the young and adult groups were calculated with the mRNA abundances normalized with respect to values obtained in young animals. Under these conditions, inter-group differences were significant for Kv1.4, Kv3.4 and Kv4.2 (Fig. 9B, P < 0.05).
Figure 9. Young and adult DGCs show a differential expression pattern of K+ channel mRNAs known to give rise to transient K+ currents.
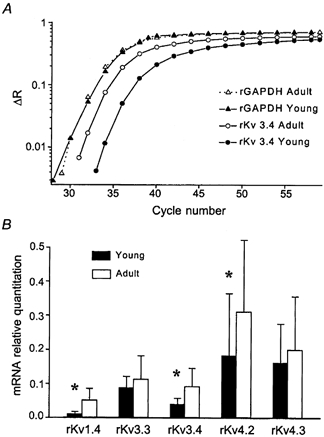
A, quantitative determination of K+ channel subunit mRNAs was carried out using the TaqMan approach. The increase of fluorescence intensity reflecting the specific amplification is shown in an exemplary fashion for the amplification of Kv3.4 and GAPDH mRNA. For data analysis the fluorescent signals are normalized to an internal, passive reference dye (Rn), yielding a normalized value ΔRn. Whereas GAPDH expression is almost identical between immature and adult animals, the mRNA levels for rKv3.4 in young animals are considerably lower than in adult animals. B, quantification of K+ channel mRNA abundance after normalization to GAPDH according to the ΔΔCt-method (Fink et al. 1998) showed changes in mRNA abundance during ontogenesis. In order to exclude errors due to inter-run differences, amplification of mRNA isolated from two young and two adult animals was always performed on one plate. Significant differences between the young and adult groups were then calculated with the mRNA abundances normalized with respect to values obtained in young animals. Under these conditions, significant inter-group differences could be detected for mRNA levels of Kv1.4, Kv3.4 and Kv4.2 (n = 6 adult and 6 young rats). Note that the ΔΔCt values shown are not normalized to values in young animals; for this reason, significant changes are less apparent.
DISCUSSION
Voltage-dependent K+ channels are key determinants of neuronal excitability. Expression of a subgroup of K+ channel subunits gives rise to K+ currents that show a rapid activation and inactivation during a voltage step. The biophysical characteristics of such transient K+ currents make them ideally suited to provide transient hyperpolarizing drive during and after brief depolarizations that can contribute to temporal and spatial integration of synaptic input (Schoppa & Westbrook, 1999) and patterning of action potentials (Turrigiano et al. 1995; Kanold & Manis, 1999; Shibata et al. 2000). Therefore, it is of considerable importance to determine which subunits underlie transient K+ current components in native neurons (Martina et al. 1998; Song et al. 1998; Kanold & Manis, 1999; Malin & Nerbonne, 2000; Shibata et al. 2000) and how expression of transient K+ currents might be modified during development or plasticity (Tsaur et al. 1992).
Properties of a Kv3.4-like K+ current in adult granule cells
In adult DGCs, the major portion of the transient K+ current showed pharmacological and biophysical properties which were very similar to cloned Kv3.4 channels. Similar to this current component, cloned Kv3.4 subunits give rise to currents that are sensitive to low concentrations of TEA and 4-AP with IC50 values as low as 90 and 500 μm (Schröter et al. 1991; Vega Saenz de Miera et al. 1992), and to micromolar concentrations of BDS-I, an anemone toxin selective for Kv3.4 (Diochot et al. 1998). It is improbable that Kv1.1 coexpressed with accessory β subunits underlies the rapidly inactivating, TEA-sensitive K+ current because we could not observe the sensitivity to α-dendrotoxin described for such channels (Heinemann et al. 1994; Stephens et al. 1996; Rhodes et al. 1997; Salinas et al. 1997). In addition to the pharmacology, the TEA/BDS-I-sensitive K+ current in DGCs showed several key characteristics of cloned Kv3.4 channels, namely a depolarized threshold of activation, a markedly voltage-dependent rate of inactivation and a pronounced rectification at positive membrane potentials (Rudy et al. 1991; Schröter et al. 1991; Vega Saenz de Miera et al. 1992). Finally, the developmental up-regulation of the Kv3.4-like current in DGCs closely parallels an up-regulation of Kv3.4 at both the mRNA and protein level.
Subcellular localization of the Kv3.4-like current and Kv3.4 protein
Our parallel functional and immunohistochemical investigations allowed us to compare the localization of the BDS-sensitive K+ current with the localization of Kv3.4 protein. Kv3.4 protein is found mainly within the mossy fibre system, with lower but detectable levels of Kv3.4 immunoreactivity within the somatodendritic compartment of DGCs (see also Veh et al. 1995). Our recordings from nucleated patches encompassing mainly axosomatic vs. somatodendritic membrane areas also suggest a similarly polar expression for the BDS-I-sensitive, Kv3.4-like current with a predominant localization in the axosomatic compartment.
In contrast to the axosomatic compartment, mossy fibre terminals contain a dendrotoxin- and TEA-sensitive transient K+ channel suggestive of heteromultimers containing Kv1.4 subunits (Geiger & Jonas, 2000), consistent with the prominent expression of Kv1.4 mRNA in adult DGCs and the localization of Kv1.4 protein (Sheng et al. 1992; Tsaur et al. 1992; Veh et al. 1995; Cooper et al. 1998). Subcellular segregation has also been demonstrated for the TEA-insensitive Kv4.2 and 4.3 subunits, both of which are located predominantly in somatodendritic compartments (Sheng et al. 1992; Tsaur et al. 1992; Maletic-Savatic et al. 1995). This finding agrees well with the low portion of TEA-insensitive transient K+ current (36 %) in these cells despite the prominent expression of Kv4.2 and 4.3 mRNA. Taken together, these findings suggest a high degree of compartmentalization of different Kv channel proteins in DGCs. Within the hippocampus, the prominent expression of Kv3.4 sets DGCs apart from other neuron types such as CA1 and CA3 pyramidal neurons in CA1 or CA3, which do not express Kv3.4 mRNA (Weiser et al. 1994) or protein (see Fig. 8). Thus, DGCs and the mossy fibre system are important model neurons for studying the functional role of Kv3.4 expression in native systems.
Developmental changes in transient K+ current components
The dentate gyrus is a structure which undergoes profound postnatal maturation (Bayer, 1982; Altman & Bayer, 1990; Kuhn et al. 1996). We have found a pronounced up-regulation of transient K+ current amplitude during development, similar to hippocampal neurons (Ficker & Heinemann, 1992; Spigelman et al. 1992; Wu & Barish, 1992), or cerebellar granule neurons in culture (Shibata et al. 2000). Developmental up-regulation of transient K+ currents is not ubiquitous, since some neuron types lose a transient K+ current during ontogenesis (Mienville et al. 1999). The developmental increase of transient K+ current in DGCs was due to an increase in a Kv3.4-like current component and was paralleled by an up-regulation of Kv3.4 both at the protein and at the mRNA level, as demonstrated previously by in situ hybridization (Kues & Wunder, 1992). The up-regulation of Kv3.4 subunits may be highly significant because the presence of a single Kv3.4 subunit is thought to be sufficient to impart rapid inactivation characteristics to a heteromultimeric channel composed of different members of the Shaw family. In addition, such heteromultimers may show severalfold larger currents than Kv3.4 homomers (Weiser et al. 1994), thus amplifying considerably the functional effects of Kv3.4 up-regulation. Taken together, the molecular and functional data suggest that up-regulation of Kv3.4 subunit expression underlies the developmental increase in transient K+ current in adult DGCs.
Functional implications of subunit changes in somatic K+ channel subunits
There are several important functional implications of the up-regulation of overall transient K+ current density, and in particular the BDS-I-sensitive transient K+ current. Using mock action potentials as a voltage command in nucleated patch recordings, we were able to show that the BDS-I- and TEA-sensitive K+ current component is prominently activated during the repolarizing phase of mock action potentials. We could further demonstrate in whole-cell current-clamp recordings that application of either BDS-I or low concentrations of TEA (100 μm) prolongs the repolarization phase of action potentials. These results suggest that the Kv3.4-like current in adult DGCs contributes to action potential repolarization, consistent with observations in globus pallidus neurons (Tkatch et al. 1999). Due to the depolarized threshold of activation, this current may not be involved in determining the latency to the first action potential, unlike A-type K+ currents operating in the subthreshold range (Connor & Stevens, 1971a; Malin & Nerbonne, 2000). Nevertheless, during recovery from inactivation, Kv3.4 channels may reopen, thus contributing also to membrane excitability in the subthreshold range following action potentials (Ruppersberg et al. 1991). The lower density of transient K+ channels, as well as inactivation of these channels by the depolarized resting membrane potential of immature DGCs (≈-55 mV), suggests a different functional role in this age group.
Acknowledgments
This research was supported by a University of Bonn Medical Center grant ‘BONFOR’, the German-Israel collaborative research program of the MOS and the BMBF and a grant of the Deutsche Forschungsgemeinschaft (DFG EL 122/7). We thank Professors P. Jonas and C. Steinhäuser for valuable comments on the manuscript. We thank Professor R. Veh for providing us with an antibody directed against Kv3.4.
References
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. Journal of Comparative Neurology. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Beck H, Ficker E, Heinemann U. Properties of two voltage-activated potassium currents in acutely isolated juvenile rat dentate gyrus granule cells. Journal of Neurophysiology. 1992;68:2086–2099. doi: 10.1152/jn.1992.68.6.2086. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. Journal of Physiology. 1988;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano A, Chiara MD, Mellstrom B, Molina A, Monje F, Naranjo JR, Lopez-Barneo J. Identification and functional characterization of a K+ channel α-subunit with regulatory properties specific to brain. Journal of Neuroscience. 1997;17:4652–4661. doi: 10.1523/JNEUROSCI.17-12-04652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara MD, Monje F, Castellano A, Lopez-Barneo J. A small domain in the N terminus of the regulatory α-subunit Kv2. 3 modulates Kv2.1 potassium channel gating. Journal of Neuroscience. 1999;19:6865–6873. doi: 10.1523/JNEUROSCI.19-16-06865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz DM, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Prediction of repetitive firing behavior from voltage clamp data on an isolated neurone soma. Journal of Physiology. 1971a;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward current in gastropod neural somata. Journal of Physiology. 1971b;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1. 4-containing A-type potassium channels near excitatory synapses in the hippocampus. Journal of Neuroscience. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Schweitz H, Beress L, Lazdunski M. Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3. 4. Journal of Biological Chemistry. 1998;273:6744–6749. doi: 10.1074/jbc.273.12.6744. [DOI] [PubMed] [Google Scholar]
- Ficker E, Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. Journal of Physiology. 1992;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink L, Seeger W, Ermert L, Hänze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nature Medicine. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Grosse G, Draguhn A, Hohne L, Tapp R, Veh RW, Ahnert-Hilger G. Expression of Kv1 potassium channels in mouse hippocampal primary cultures: development and activity-dependent regulation. Journal of Neuroscience. 2000;20:1869–1882. doi: 10.1523/JNEUROSCI.20-05-01869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Rettig J, Lorra C, Pongs O. Potassium channel β-subunits confer A-type channel characteristics on delayed rectifiers. Pflügers Archiv. 1994;426(suppl.):R61. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates; 1992. [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. Journal of Neuroscience. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proceedings of the National Academy of Sciences of the USA. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugnot JP, Salinas M, Lesage F, Guillemare E, De Weille J, Heurteaux C, Mattei MG, Lazdunski M. Kv8. 1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels. EMBO Journal. 1996;15:3322–3331. [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Current Opinion in Neurobiology. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. Journal of Neurophysiology. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. Journal of Neuroscience. 1999;19:2195–2208. doi: 10.1523/JNEUROSCI.19-06-02195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. Journal of Physiology. 2000;525:621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kues WA, Wunder F. Heterogeneous expression patterns of mammalian potassium channel genes in developing and adult rat brain. European Journal of Neuroscience. 1992;4:1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. Journal of Neuroscience. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods and Applications. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- Mackler SA, Brooks BP, Eberwine JH. Stimulus-induced coordinate changes in mRNA abundance in single postsynaptic hippocampal CA1 neurons. Neuron. 1992;9:539–548. doi: 10.1016/0896-6273(92)90191-f. [DOI] [PubMed] [Google Scholar]
- Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1999;82:1895–1901. doi: 10.1152/jn.1999.82.4.1895. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. Journal of Neuroscience. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4. 2W362F: molecular dissection of IA. Journal of Neuroscience. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. Journal of Neuroscience. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville JM, Maric I, Maric D, Clay JR. Loss of IA expression and increased excitability in postnatal rat Cajal-Retzius cells. Journal of Neurophysiology. 1999;82:1303–1310. doi: 10.1152/jn.1999.82.3.1303. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Trimmer JS. Identification of the Kv2. 1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. Journal of Neuroscience. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Numann RE, Wadman WJ, Schwindt W. Outward currents of single hippocampal cells obtained from the adult guinea-pig. Journal of Physiology. 1987;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime G, Horn G, Sutor B. Time-related changes in connexin mRNA abundance in the rat neocortex during postnatal development. Developmental Brain Research. 2000;119:111–125. doi: 10.1016/s0165-3806(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schröter, K H, Ruppersberg JP, Veh R, Pongs O. Characterization of a Shaw-related potassium channel family in rat brain. EMBO Journal. 1992;11:2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 β-subunits with Kv1 α-subunits in mammalian brain K+ channel complexes. Journal of Neuroscience. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera AB, Spitzer NC. Developmental regulation of potassium channels and the impact on neuronal differentiation. Ion Channels. 1992;3:1–38. doi: 10.1007/978-1-4615-3328-3_1. [DOI] [PubMed] [Google Scholar]
- Rudy B, Sen K, Vega-Saenz De Miera E, Lau D, Ried T, Ward DC. Cloning of a human cDNA expressing a high voltage-activating, TEA-sensitive, type-A K+ channel which maps to chromosome 1 band p21. Journal of Neuroscience Research. 1991;29:401–412. doi: 10.1002/jnr.490290316. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP, Frank R, Pongs O, Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991;353:657–660. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991;352:711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Salinas M, De Weille J, Guillemare E, Lazdunski M, Hugnot JP. Modes of regulation of shab K+ channel activity by the Kv8. 1 subunit. Journal of Biological Chemistry. 1997;272:8774–8780. doi: 10.1074/jbc.272.13.8774. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nature Neuroscience. 1999;2:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- Schröter, K-H, Ruppersberg JP, Wunder F, Rettig J, Stocker M, Pongs O. Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain. FEBS Letters. 1991;278:211–216. doi: 10.1016/0014-5793(91)80119-n. [DOI] [PubMed] [Google Scholar]
- Seifert G, Kuprijanova E, Zhou M, Steinhauser C. Developmental changes in the expression of Shaker- and Shab-related K+ channels in neurons of the rat trigeminal ganglion. Brain Research. Molecular Brain Research. 1999;74:55–68. doi: 10.1016/s0169-328x(99)00268-5. [DOI] [PubMed] [Google Scholar]
- Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993;365:73–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur M-L, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur M-L, Jan Y-N, Jan L-Y. Contrasting subcellular localization of the Kv1. 2 K+ channel subunit in different neurons of rat brain. Journal of Neuroscience. 1994;14:2408–2417. doi: 10.1523/JNEUROSCI.14-04-02408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. Journal of Neuroscience. 2000;20:4145–4155. doi: 10.1523/JNEUROSCI.20-11-04145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4. 2 and Kv4.1 subunits. Journal of Neuroscience. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Zhang L, Carlen PL. Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: membrane excitability and K+ currents. Journal of Neurophysiology. 1992;68:55–69. doi: 10.1152/jn.1992.68.1.55. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Cockett MI, Nawoschik SP, Schecter LE, Owen DG. The modulation of the rate of inactivation of the mKv1. 1 K+ channel by the β subunit, Kv β 1 and lack of effect of a Kv β 1 N-terminal peptide. FEBS Letters. 1996;378:250–252. doi: 10.1016/0014-5793(95)01469-1. [DOI] [PubMed] [Google Scholar]
- Thompson SH. Aminopyridine block of transient potassium current. Journal of General Physiology. 1982;80:1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Kv3. 4 K+ channels may be responsible for spike broadening during burst firing in globus pallidus neurons. Socety for Neuroscience Abstracts. 1999;179:17. [Google Scholar]
- Tsaur ML, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, Lemasson G, Marder E. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. Journal of Neuroscience. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega Saenz De Miera E, Moreno H, Fruhling D, Kentros C, Rudy B. Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel. Proceedings of the Royal Society. 1992;248:9–18. doi: 10.1098/rspb.1992.0036. B. [DOI] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. European Journal of Neuroscience. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz De Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. Journal of Neuroscience. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R-L, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. Journal of Neuroscience. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbicz KL, Weight FF. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. Journal of Neurophysiology. 1985;53:1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


