Abstract
Intracellular recordings were made from neurones in rat hypothalamic tissue slices, primarily in the preoptic area and anterior hypothalamus, a thermoregulatory region that integrates central and peripheral thermal information. The present study compared morphologies and local synaptic inputs of warm-sensitive and temperature-insensitive neurones.
Warm-sensitive neurones oriented their dendrites perpendicular to the third ventricle, with medial dendrites directed toward the periventricular region and lateral dendrites directed toward the medial forebrain bundle. In contrast, temperature-insensitive neurones generally oriented their dendrites parallel to the third ventricle.
Both warm-sensitive and temperature-insensitive neurones displayed excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs). In most cases, EPSP and IPSP frequencies were not affected by temperature changes, suggesting that temperature-insensitive neurones are responsible for most local synapses within this hypothalamic network.
Two additional neuronal groups were identified: silent neurones having no spontaneous firing rates and EPSP-driven neurones having action potentials that are primarily dependent on excitatory synaptic input from nearby neurones. Silent neurones had the most extensive dendritic trees, and these branched in all directions. In contrast, EPSP-driven neurones had the fewest dendrites, and usually the dendrites were oriented in only one direction (either medially or laterally), suggesting that these neurones receive more selective synaptic input.
Although neurones in the preoptic area and anterior hypothalamus (PO/AH) are important in regulating body temperature, little is known about their morphologies and synaptic interactions. In vivo and in vitro electrophysiological studies find that the majority of PO/AH neurones are relatively insensitive to temperature; however, 30 % of the neurones are classified as warm sensitive because their firing rates significantly increase during increases in hypothalamic temperature (Boulant, 1996). Unlike temperature-insensitive neurones, most warm-sensitive neurones respond not only to hypothalamic temperature, but also to skin or spinal temperatures (Boulant & Hardy, 1974). PO/AH warm-sensitive neurones, therefore, appear be the integrators of central and peripheral thermal information.
Peripheral thermal information ascends over spinohypothalamic and spinothalamic tracts (Burstein et al. 1991; Dado et al. 1994). These afferent signals enter hypothalamic nuclei both from a lateral direction via the medial forebrain bundle and from a medial direction via the periventricular stratum (Palkovits & Zaborszky, 1979; Cliffer et al. 1991). Since PO/AH warm-sensitive neurones receive much of this afferent input, it might be expected a priori that these neurones orient their dendrites medially and laterally toward the ascending pathways. No previous study has identified the morphologies of warm-sensitive and temperature-insensitive neurones in the mammalian hypothalamus. Hence, one objective of the current study was to fill warm-sensitive and temperature-insensitive hypothalamic neurones with intracellular dyes to determine their dendritic configurations.
A second objective was to characterize the synaptic inputs that different PO/AH neurones receive from nearby neurones. A popular neuronal model (Hammel, 1965) suggests that temperature-sensitive and -insensitive neurones send antagonistic synaptic inputs to various thermoregulatory effector neurones controlling heat loss and heat production. This model proposes, for example, that heat loss effector neurones are excited by nearby warm-sensitive neurones and inhibited by temperature-insensitive neurones. The present study examined PO/AH neuronal networks by determining the effect of temperature on the frequency of excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs). During warming, the frequency of postsynaptic potentials determined by warm-sensitive neurones should increase, while the frequency of postsynaptic potentials determined by temperature-insensitive neurones should remain constant. Accordingly, the types of neurones that constitute local synaptic networks may be revealed by analysing the effect of temperature on EPSP and IPSP frequencies.
METHODS
Preparation of tissue slices for electrophysiological recording
Tissue slices were prepared from 200-350 g, male Sprague-Dawley rats. Each rat was quickly decapitated with a guillotine, according to procedures approved by NIH and the Laboratory Animal Care and Use Committees of the Ohio State University, Beth Israel Hospital and Harvard Medical School. The brain was removed and a hypothalamic tissue block was sectioned with a Vibratome. Tissue slices were cut (300-400 μm thick) in either coronal (Kelso et al. 1982) or horizontal planes (Dean & Boulant, 1988). Slices were incubated in a recording chamber, perfused (1-2 ml min−1) with oxygenated (95 % O2-5 % CO2) nutrient medium, consisting of (mm): 124 NaCl, 26 NaHCO3, 10 glucose, 5 KCl, 2.4 CaCl2, 1.3 MgSO4 and 1.24 KH2PO4. This medium is identical to that used in most hypothalamic slice studies of thermosensitive neurones, and the 6.24 mm potassium concentration may result in a high level of synaptic activity. After a 2 h incubation period, whole-cell patch recordings were made using 2 μm tip (3-5 MΩ) glass pipettes filled with a solution containing (mm): 130 potassium gluconate, 10 EGTA, 10 Hepes, 2 ATP, 1 CaCl2 and 1 MgCl2 (pH 7.2-7.3, 295 mosmol l−1). To determine neuronal morphologies, either 0.5 % lucifer yellow or 0.5 % biocytin was added to this electrode solution. At these concentrations, the intracellular dyes clearly filled neuronal dendrites; however, small diameter axons were not distinguished. As previously described (Griffin & Boulant, 1995), the ground electrode was maintained in a constant temperature bath, connected to the recording chamber by a filter paper bridge.
Recording intracellular activity
Intracellular recordings were made using whole-cell patch-clamp techniques (Konnerth, 1990). Membrane potential, postsynaptic potentials and action potentials were displayed on an oscilloscope and tape recorded, along with temperature and integrated firing rate. The liquid junction potential (Barry & Lynch, 1991) was determined to be 12.0 mV, and this was subtracted from all recorded potentials (Griffin & Boulant, 1995). Acceptable recordings consisted of action potential amplitudes of at least 60 mV (from resting membrane potential) at neutral temperatures (i.e. 34-37 °C) and stable membrane potential recordings for at least 20 min.
Examples of EPSPs and IPSPs are shown in Fig. 1, which contains intracellular recordings from two PO/AH neurones. Criteria for acceptable recordings of postsynaptic potentials were similar to those previously published (Burgoon & Boulant, 1998). Identified EPSPs and IPSPs had an initial, rapid change in membrane potential (at least 1 mV greater than background noise) followed by a slower return to baseline potential. Frequencies of EPSPs and IPSPs were determined at three different temperatures, grouped as cool (31-33 °C), neutral (34-37 °C) and warm (38-40 °C). At each temperature, the number of postsynaptic potentials was counted over 1 s intervals for 20-30 s. From these data, frequency means and standard errors of the mean were obtained and plotted as a function of temperature. As shown in Fig. 2B, to facilitate counting postsynaptic potentials for some neurones, slight hyperpolarizing current was briefly applied to temporarily suppress action potentials. In some cases, IPSP reversal potentials were determined by holding the membrane potential at progressively more hyperpolarized levels.
Figure 1. Examples of EPSPs (*) and IPSPs (†) in recordings from two types of PO/AH neurones.
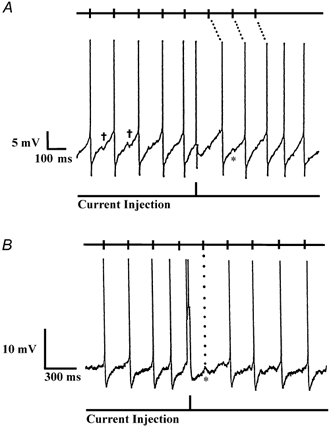
All action potentials have been truncated. Halfway through each record, a 2 ms 200 pA depolarizing current pulse produced a premature action potential. Tick marks at the top of each record show the predicted times of action potentials, based on the firing pattern before the current pulse. A, spontaneously firing, warm-sensitive neurone displaying slow, depolarizing prepotentials preceding each action potential. After the premature action potential at the current pulse, all subsequent action potentials were shifted in time, suggesting that the firing pattern was intrinsically generated. B, EPSP-driven neurone whose firing pattern was not altered by the current injection. Note the EPSPs prior to each action potential and the prominent EPSP without an action potential at the first tick mark (dotted line) after the current pulse.
Figure 2. Tetrodotoxin (TTX) strongly attenuates postsynaptic potentials in a preoptic EPSP-driven neurone recorded in a coronal tissue slice.
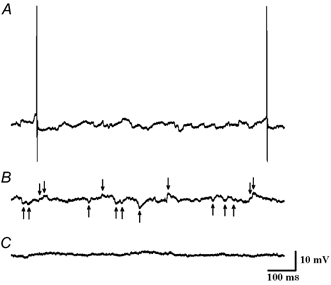
A, with no holding current, EPSPs and IPSPs are present, and most action potentials are preceded by EPSPs. Resting membrane potential, -50.0 mV; firing rate, 1.1 imp s−1; thermosensitivity, 0.2 imp s−1°C−1. B, action potentials are eliminated by brief injection of -12 pA holding current which slightly hyperpolarizes the membrane potential (3.5 mV). Arrows identify EPSPs (↓) and IPSPs (↑) that meet criteria mentioned in the text. C, with no holding current, perfusion with 0.5 μm TTX suppresses postsynaptic potentials and action potentials.
The perfusion medium temperature was controlled by a thermoelectric assembly, and tissue temperature was recorded by a thermocouple located in the medium below the slices (Kelso et al. 1982). Tissue slices were maintained at a constant thermoneutral temperature, except when thermosensitivity was periodically tested. The thermoelectric assembly allowed the slices to be quickly warmed or cooled 3-5 °C in order to determine the effect of temperature on the frequency of action potentials and postsynaptic potentials. Integrated firing rate or action potential frequency (impulses per second; imp s−1) was measured with a ratemeter and recorded with temperature on a polygraph and computer. Neuronal firing rate was plotted as a function of temperature, and the regression coefficient (imp s−1°C−1) defined each neuron's thermosensitivity. Criteria for thermosensitivity were the same as in previous studies (Boulant & Hardy, 1974; Boulant & Dean, 1986). Warm-sensitive neurones had thermosensitivities of 0.8 imp s−1°C−1 or greater, and temperature-insensitive neurones had lesser thermosensitivities. If a neurone did not have a spontaneous firing rate but could be stimulated (with depolarizing current) to produce action potentials, it was classified as a silent neurone.
As shown in Fig. 1A, most spontaneously firing neurones displayed slow, depolarizing prepotentials that preceded the action potentials. Prepotentials were observed in both warm-sensitive and temperature-insensitive neurones, and these same neurones often displayed brief IPSPs and EPSPs superimposed on the slower prepotentials. A different type of neurone is shown in Fig. 1B, where slow depolarizing prepotentials were not prominent, but instead, action potentials occurred after brief EPSPs. Regardless of their thermosensitivity, these ‘EPSP-driven’ neurones were treated as a separate population because their predominant activity was not intrinsic but, rather, was synaptically derived from other neurones.
Figure 1 illustrates a procedure that was used to further distinguish EPSP-driven neurones from inherently firing neurones whose action potentials were primarily determined by intrinsic depolarizing prepotentials. As shown halfway through the records in Fig. 1A and B, brief depolarizing current injections (2 ms, 200 pA) were applied to spontaneously firing neurones in order to shift or reset the neurone's spike activity. If a neurone's action potentials were due solely to synaptic input from another neurone, the tick marks at the top of the record show the predicted times of these action potentials, based on the firing rate pattern before the current injection. In Fig. 1B, the neurone's spiking activity appeared to be dependent on this synaptic activity, since the current injection did not alter the times at which action potentials occurred. Moreover, at the first tick mark (dotted line) after the current injection, no action potential occurred, but instead, a prominent EPSP was evident. In contrast, the activity of the neurone in Fig. 1A was intrinsically generated, and even though the neurone had postsynaptic potentials, it was the depolarizing prepotentials that primarily determined the generation of action potentials. As illustrated by the dotted lines at the top of Fig. 1A, when a depolarizing pulse produced a premature action potential in this neurone, all subsequent action potentials were shifted in time and no longer occurred at times predicted by the firing rate pattern before the pulse.
Recent tissue slice studies of supraoptic neurones identified miniature or spontaneous postsynaptic potentials/currents that are resistant to tetrodotoxin (TTX) and presumably not dependent on action potentials in presynaptic axons (Inenaga et al. 1998; Kombian et al. 2000; Stern et al. 2000). In the present hypothalamic tissue slice study, a separate set of TTX experiments was performed to determine the relative incidence of action potential-dependent EPSPs and IPSPs in preoptic neurones. Figure 2A shows an EPSP-driven neurone displaying IPSP and subthreshold EPSP activity during the interval between EPSP-generated action potentials. Injection of small hyperpolarizing current suppressed the action potentials (Fig. 2B). Figure 2C shows the same neurone (without hyperpolarizing current) during the application of 0.5 μm TTX, which eliminated most postsynaptic potentials and action potentials. In these separate TTX experiments, EPSPs and IPSPs were recorded in ten preoptic neurones (i.e. two warm-sensitive, five temperature-insensitive, and three silent neurones) before and after 0.5 μm TTX application. TTX reduced the average EPSP activity by 68 % (i.e. control: 9.6 EPSPs s−1; TTX: 3.1 EPSPs s−1). Similarly, TTX reduced the average IPSP activity by 67 % (i.e. control: 5.8 IPSPs s−1; TTX: 1.9 IPSPs s−1). Since 0.5 μm TTX did not completely eliminate all recorded action potentials, it might be suggested that more than 68 % of the postsynaptic activity reported in the Results is due to action potentials in presynaptic axons. Therefore, most of the recorded synaptic activity reflects the firing activity and thermosensitivity of nearby neurones.
Histological preparation
The microelectrode's large (2 μm) tip diameter permitted the fluorescent dye lucifer yellow and the intracellular stain biocytin to passively diffuse into a neurone during recording. At the end of the recording, each tissue slice was removed from the chamber and fixed in a 4 % paraformaldehyde solution for at least 2 h (Viana et al. 1990). When only lucifer yellow was added to the electrode solution, the tissue slice was cleared in dimethyl sulfoxide (DMSO) for 20 min, mounted in DMSO on a microscope slide and coverslipped. Each neurone's morphology and location were examined with an epifluorescence microscope. At different magnifications, serial photographs were taken of each neurone in different focal planes. These photographs were used to produce two-dimensional reconstruction drawings, which indicated each neurone's location, soma diameter and dendritic orientation. Location was determined by the neurone's distance from the caudal edge of the third ventricle and the distance from the fornix and anterior commissure.
When biocytin was added to the electrode solution, tissue slices were placed in a 30 % sucrose solution for at least 2 h, after fixation in 4 % paraformaldehyde. The slices were then resectioned at a thickness of 50 μm. Sections were stained for biocytin using a Vector ABC kit and intensified through a silver-gold procedure described by Breder et al. (1993). The tissue was then counter-stained with a giemsa stain, rehydrated, cleared in xylene and coverslipped. Camera lucida drawings were made of identified neurones to record somal and dendritic morphology and location.
Determination of neuronal morphological characteristics
For each neurone, average soma diameter was determined by the mean of two independent measurements. Proximal dendrites were counted and the radial orientation (1-360 deg) of each dendrite was measured. To determine the direction of dendritic projections, orientation measurements were made by a line drawn from the somal centre to a point 50 μm from the observed dendritic ending. To be considered a proximal dendrite, a process had to originate either from the soma or within 50 μm of the soma. Orientation measurements were made only for dendrites that exceeded 200 μm in length. If a dendrite branched within 100 μm of the soma, orientation measurements were made for each separate branch, as long as each branch extended beyond 200 μm from the cell body. If a dendrite branched beyond 100 μm from the soma (and the branches extended 200 μm from the cell body), only one orientation measurement was made, and this was determined by a line drawn between the somal centre and the branch point.
For each cell type, dendritic orientations were grouped to determine if these processes were primarily parallel or perpendicular to the midline third ventricle. In horizontal slices, for example, an orientation of 360 deg is rostral and parallel to the ventricle, while an orientation of 90 deg is lateral and perpendicular to the ventricle. In the horizontal plane, dendrites were grouped into four quadrants or ranges of orientation: rostral (315-45 deg), lateral (45-135 deg), caudal (135-225 deg) and medial (225-315 deg). In the coronal plane, dendrites were similarly grouped into four quadrants: dorsal (315-45 deg), lateral (45-135 deg), ventral (135-225 deg) and medial (225-315 deg). Thus, for the neurones in horizontal slices, dendrites that were more parallel to the third ventricle would lie in the rostral and caudal quadrants, and dendrites that were more perpendicular to the third ventricle would lie in the lateral and medial quadrants. Similarly, for neurones in coronal slices, dendrites that were more parallel to the third ventricle would lie in the dorsal and ventral quadrants, and dendrites that were more perpendicular to the third ventricle would lie in the lateral and medial quadrants.
Chi square tests were used to determine if the dendritic orientations were randomly distributed or confined to specific quadrants. In addition, analysis of variance (ANOVA) and Fisher's exact probabilty test were used to compare the frequencies of synaptic potentials and morphological characteristics of neurones in each of the thermosensitive classes. Differences were considered significant at P < 0.05. Each value reported in this paper is the mean ± standard error of the mean.
RESULTS
Synaptic characteristics
Postsynaptic potentials (PSPs) were analysed in 53 hypothalamic neurones, including 40 preoptic and anterior hypothalamic neurones. Other recorded locations included dorsal medial hypothalamus (n = 5), reuniens nucleus (n = 3), septum (n = 2), paraventricular nucleus (n = 1), bed nucleus of the solitary tract (n = 1) and posterior hypothalamus (n = 1). Of the 53 neurones, 15 were warm sensitive, 19 were temperature insensitive, 10 were silent and 9 were EPSP driven. At normothermic temperatures, these neurones had the following resting membrane potentials and input resistances: warm-sensitive neurones, -53 ± 2 mV, 398 ± 35 MΩ; temperature-insensitive neurones, -52 ± 1 mV, 301 ± 38 MΩ; silent neurones, -60 ± 1 mV, 244 ± 20 MΩ; and EPSP-driven neurones, -54 ± 1 mV, 389 ± 46 MΩ. Both excitatory and inhibitory postsynaptic potentials were recorded in each of these neuronal types; however, in some cases, only EPSPs or IPSPs were recorded in a given neurone.
For individual warm-sensitive neurones, Fig. 3A shows the IPSP and EPSP frequencies recorded in three different temperature ranges. While IPSPs were observed in all of these warm-sensitive neurones (15/15), EPSPs were observed in only one-third of the neurones (5/15). Based on PSP frequencies, there appeared to be two distinct groups of warm-sensitive neurones; i.e. one group having IPSP and EPSP frequencies greater than 5 PSP s−1 and the other group having frequencies less than 3 PSP s−1. With few exceptions, temperature had little or no effect on IPSP and EPSP frequencies. This suggests that the predominant local synaptic input to warm-sensitive neurones comes from nearby temperature-insensitive neurones. Table 1 shows the effect of temperature on PSP frequencies for each neuronal population. Like the individual neurones in Fig. 3A, Table 1 indicates that for the population of warm-sensitive neurones, IPSP and EPSP frequencies remained constant at cool, neutral and warm temperatures, again suggesting that synaptic input is primarily derived from temperature-insensitive neurones.
Figure 3. Effect of temperature on the frequency of IPSPs and EPSPs.
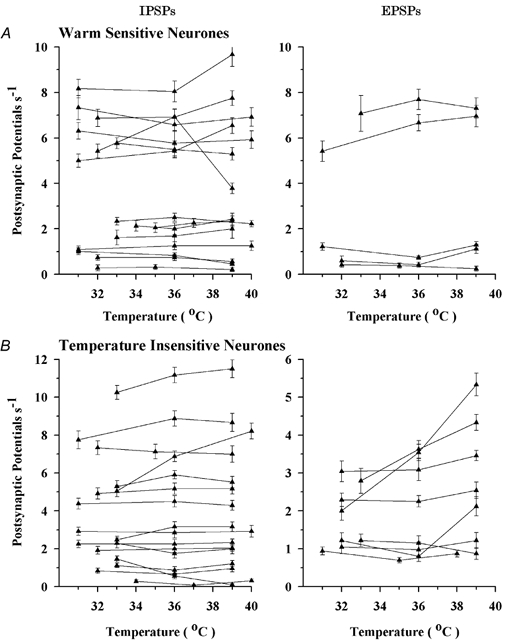
A, individual warm-sensitive neurones; B, temperature-insensitive neurones. Vertical bars indicate standard errors of the mean.
Table 1.
Frequency (postsynaptic potentials s−1) of inhibitory postsynaptic potentials (IPSPs) and excitatory postsynaptic potentials (EPSPs) recorded from hypothalamic neurones at different temperature ranges
| IPSPs | EPSPs | |||||||
|---|---|---|---|---|---|---|---|---|
| Neurone type | n | (31–33 °C) | (34–37 °C) | (38–40 °C) | n | (31–33 °C) | (34–37 °C) | (38–40 °C) |
| Silent | 7 | 3.3 ± 1.5 | 3.7 ± 1.6 | 4.0 ± 1.7 | 9 | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 |
| Temperature insensitive | 16 | 3.8 ± 0.7 | 4.0 ± 0.8 | 4.1 ± 0.8 | 8 | 1.8 ± 0.3 | 2.0 ± 0.4 | 2.6 ± 0.6 |
| Warm sensitive | 15 | 3.8 ± 0.7 | 3.8 ± 0.7 | 3.9 ± 0.8 | 5 | 3.0 ± 1.4 | 3.2 ± 1.6 | 3.4 ± 1.6 |
| EPSP driven | 9 | 4.9 ± 1.8 | 4.6 ± 1.5 | 4.5 ± 1.4 | 9 | 2.8 ± 0.6 | 3.7 ± 0.8 | 5.0 ± 1.0 * |
EPSP-driven neurones showed a significant increase in the frequency of EPSPs at 38–40 °C compared to 31–33 °C (P < 0.05).
Figure 3B shows the effect of temperature on PSP frequencies in temperature-insensitive neurones. IPSP frequencies and most of the EPSP frequencies were not affected by temperature. In a few neurones, however, EPSP frequency increased with increasing temperatures, suggesting that some temperature-insensitive neurones were synaptically excited by nearby thermosensitive neurones. These trends are reflected in Table 1; however, for the population of recorded temperature-insensitive neurones, there were no significant differences in IPSP and EPSP frequencies over the three ranges of temperature.
Figure 4 presents similar analyses for the silent and EPSP-driven neurones. Figure 4A shows that, like the other neuronal types, silent neurones received both excitatory and inhibitory synaptic inputs. Again, most of this input appeared to come from temperature-insensitive neurones since, with few exceptions, EPSP and IPSP frequencies remained relatively stable over the temperature range tested. This is substantiated in Table 1, which indicates that temperature had no effect on IPSP and EPSP frequencies for the population of silent neurones.
Figure 4. Effect of temperature on the frequency of IPSPs and EPSPs.
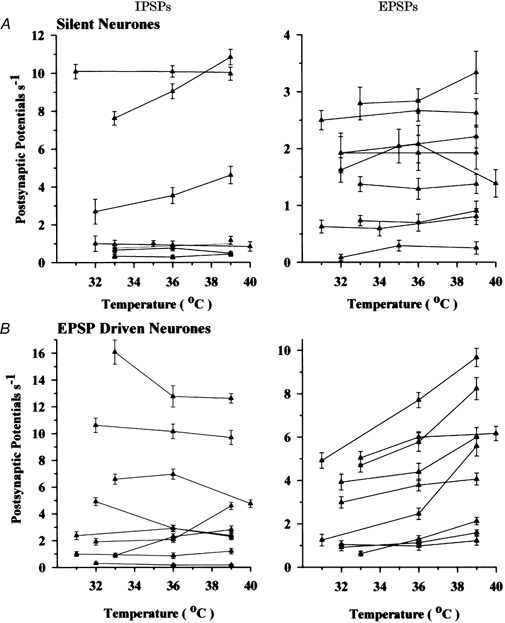
A, individual silent neurones having no spontaneous firing rate; B, EPSP-driven neurones. Vertical bars indicate standard errors of the mean.
Figure 4B shows that temperature did not affect IPSP frequencies for most of the EPSP-driven neurones; however, approximately half of these neurones showed increases in EPSP frequencies with increases in temperature. Many EPSP-driven neurones, therefore, appeared to be synaptically excited by nearby neurones having some degree of thermosensitivity. This is also reflected in the population of EPSP-driven neurones in Table 1; i.e. compared to cool temperatures, warm temperatures nearly doubled the frequency of EPSPs. This increase from 2.8 EPSP s−1 at 31-33 °C to 5.0 EPSP s−1 at 38-40 °C was the only statistically significant change in PSP frequencies observed in all neuronal populations.
Neuronal morphology
Morphological characteristics and locations were determined for 96 neurones which were classified according to their temperature sensitivity. These included the 53 neurones mentioned in the previous section, as well as 43 additional neurones in which postsynaptic potentials (meeting criteria described in Methods) either were not observed or were not analysed. Of the total of 96 neurones, 29 (30 %) were warm sensitive, 37 (39 %) were temperature insensitive, 21 (22 %) were silent and 9 (9 %) were EPSP driven. Most of these neurones (n = 68) were recorded in preoptic and anterior hypothalamic regions. Other recorded locations included dorsal medial hypothalamus (n = 8), reuniens nucleus (n = 5), paraventricular nucleus (n = 4), perifornical area (n = 4), septum (n = 3), bed nucleus of the solitary tract (n = 2), organum vasculosum lamina terminalis (n = 1) and posterior hypothalamus (n = 1). There was no distinct region in which one type of neurone was concentrated. Of the 96 neurones, 51 were labelled with lucifer yellow (resting membrane potential: -54 ± 1 mV), and 45 were labelled with biocytin (resting membrane potential: -57 mV ± 1 mV). There were no significant differences between lucifer yellow and biocytin neurones in terms of their resting membrane potentials or the proportions of different neurones recorded.
Figure 5 shows an example of a biocytin-filled, warm-sensitive neurone recorded in a coronal tissue slice. One of the neurone's dendrites projected laterally into the fibres of the fornix, and another dendrite projected medially toward the third ventricle. As shown in Fig. 5C, this neurone increased its firing rate (imp s−1) with an increase in tissue temperature, and the regression coefficient (m) of 1.1 imp s−1°C−1 met the criterion for a warm-sensitive neurone (see Methods). In Fig. 5D, the superimposed average action potentials indicate that, compared to 34 °C, warming to 38 °C increased the rate of the prepotential depolarization to threshold.
Figure 5. Morphological and physiological characteristics of a warm-sensitive neurone recorded in the perifornical area of a coronal tissue slice.

A, camera lucida drawing of the biocytin-filled neurone. B, photograph of the biocytin-filled neurone. Dorsal is at the top. 3V, third ventricle; PVN, hypothalamic paraventricular nucleus; fx, fornix. C, effect of temperature on the neurone's firing rate (impulses per second or imp s−1). The regression coefficient, m, defines the neurone's thermosensitivity, which was 1.10 imp s−1°C−1. Each point represents a sample taken every 10 s. D, superimposed averages of 15 action potentials recorded at two different temperatures, 34 and 38 °C. Warming to 38 °C increased the rate of rise in the depolarizing prepotential.
Figure 6 is an example of a temperature-insensitive neurone recorded in a coronal slice near the medial preoptic nucleus (MPN). The neurone had very little dendritic branching. In addition to a ventral dendrite, there were two dorsal dendrites, one projecting to the MPN and the other projecting toward the third ventricle. As indicated in Fig. 6C, the neuron's firing rate was relatively unaffected by changes in tissue temperature, and the regression coefficient (m) of 0.20 imp s−1°C−1 met the criterion for a temperature-insensitive neurone. Unlike the warm-sensitive neurone in Fig. 5, the averaged action potentials in Fig. 6D indicate that temperature had no effect on the depolarizing prepotential for this temperature-insensitive neurone.
Figure 6. Morphological and physiological characteristics of a temperature-insensitive preoptic neurone recorded in a coronal tissue slice.
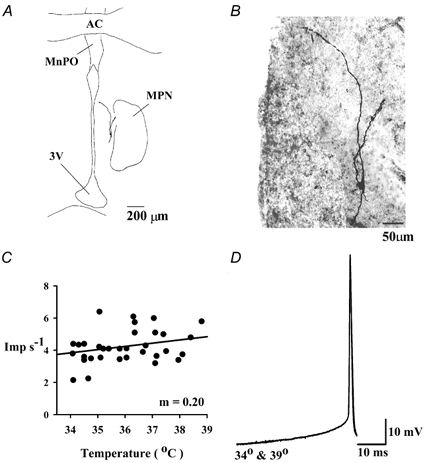
A, camera lucida drawing of the biocytin-filled neurone. B, photograph of the biocytin-filled neurone. Dorsal is at the top. 3V, third ventricle; MPN, medial preoptic nucleus; MnPO, median preoptic nucleus; AC, anterior commissure. The left side of the photograph (B) is the edge of the third ventricle. C, effect of temperature on the neurone's firing rate (imp s−1). The regression coefficient, m, defines the neurone's thermosensitivity, which was 0.20 imp s−1°C−1. Each point represents a sample taken every 10 s. D, superimposed averages of 15 action potentials recorded at two different temperatures, 34 and 39 °C. Warming to 39 °C had no effect on the depolarizing prepotential.
An early Golgi study (Millhouse, 1979) indicated that rostral hypothalamic neurones have 15-25 μm soma diameters and spherical, fusiform or triangular shapes with two or three primary dendrites. Similar characteristics were observed in the present study. Table 2 shows the average soma diameters and number of proximal dendrites for neurones in each population. The cell bodies of warm-sensitive and temperature-insensitive neurones had similar sizes, and these neurones were significantly smaller than the silent and EPSP-driven neurones. There was no correlation between the neuronal types and the shapes of the cell bodies. Of the 96 neurones characterized, somas were spherical in 68, fusiform in 19 and triangular in 9. For the entire population, the average number of primary dendrites was 3.1 ± 0.1. As indicated in Table 2, the silent neurones had significantly more dendrites than the other neuronal types. The EPSP-driven neurones had the fewest dendrites, and these were significantly fewer than the warm-sensitive neurones and silent neurones.
Table 2.
Characterization of soma diameter and number of primary dendrites from hypothalamic neurons
| Neurone type | n | Soma diameter (μm) | No. of primary dendrites |
|---|---|---|---|
| Temperature insensitive | 37 | 15.8 ± 0.8 | 2.7 ± 0.2 |
| Warm sensitive | 29 | 15.6 ± 1.0 | 2.8 ± 0.1 |
| Silent | 21 | 21.1 ± 1.0 * | 4.3 ± 0.2 † |
| EPSP driven | 9 | 19.9 ± 2.3 * | 2.3 ± 0.2 ‡ |
Significantly different from temperature-insensitive and warm-sensitive neurones (P < 0.05).
Significantly different from all other classes (P < 0.05).
Significantly different from warm-sensitive neurones (P < 0.05).
The camera lucida drawings in Fig. 7 show the projected locations and morphologies of several temperature-insensitive neurones (left side) and warm-sensitive neurones (right side) recorded in coronal tissue slices. For both types of neurones the dendrites displayed little branching. The dendrites of many temperature-insensitive neurones were oriented dorsally or ventrally, and they were relatively parallel to the third ventricle. In contrast, most dendrites of the warm-sensitive neurones projected laterally or medially, and they were more perpendicular to the third ventricle.
Figure 7. Camera lucida drawings showing projected locations and morphologies of temperature-insensitive neurones (left side) and warm-sensitive neurones (right side) recorded in coronal tissue slices.
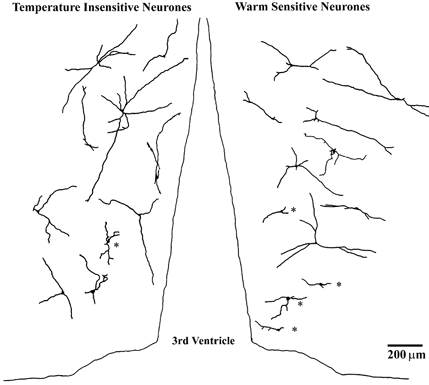
Neurones identified at different rostral-caudal locations are projected onto a single coronal plane. Dorsal is at the top. Cells marked with asterisks had dendrites shorter than 200 μm and were not included in the averages shown in Fig. 9 (see Methods).
Figure 8 shows similar camera lucida drawings of temperature-insensitive neurones (left side) and warm-sensitive neurones (right side) recorded in horizontal tissue slices. Most dendrites of the temperature-insensitive neurones projected either rostrally or caudally and, again, were aligned parallel to the third ventricle. The warm-sensitive neurones, however, had dendrites projecting laterally and medially, perpendicular to the third ventricle.
Figure 8. Camera lucida drawings showing projected locations and morphologies of temperature-insensitive neurones (left side) and warm-sensitive neurones (right side) recorded in horizontal tissue slices.
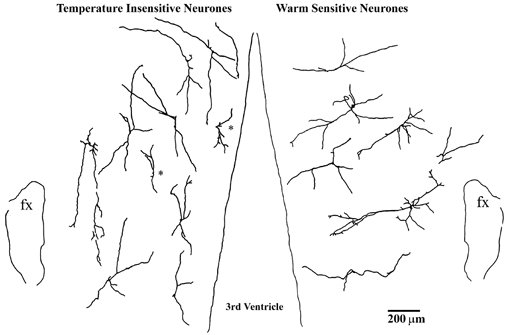
Neurones identified at different dorsal-ventral locations are projected onto a single horizontal plane. Rostral is at the top; fx, fornix. Cells marked with asterisks had dendrites shorter than 200 μm and were not included in the averages shown in Fig. 9 (see Methods).
Figure 9 shows the average dendritic orientations for the temperature-insensitive neurones (I) and warm-sensitive neurones (W) recorded in either horizontal or coronal tissue slices. As described in Methods, these average orientations were derived only from neurones having dendrites that exceeded 200 μm in length. Neurones in Fig. 7 and Fig. 8 (marked with asterisks) having shorter dendrites were not included in the averages in Fig. 9; however, it should be noted that even these neurones had dendritic orientations that were similar to the other neurones in their population. Orientation averages were determined only if there were at least four dendritic projections in a quadrant. For the horizontal plane in Fig. 9, temperature-insensitive neurones had two primary dendritic orientations, rostral 11.2 ± 7.3 deg (n = 6) and caudal 189.1 ± 5.5 deg (n = 11); and warm-sensitive neurones also had two primary orientations, lateral 88.3 ± 7.6 deg (n = 7) and medial 264.3 ± 5.5 deg (n = 6). For the coronal plane in Fig. 9, temperature-insensitive neurones had two orientations, i.e. dorsal 354.0 ± 9.4 deg (n = 9) and ventral 185.5 ± 15.9 deg (n = 4); and warm-sensitive neurones had three dendritic orientations, lateral 112.9 ± 6.2 deg (n = 7), medial 275.0 ± 9.0 deg (n = 5) and dorsal 333.5 ± 11.3 deg (n = 4).
Figure 9. Average dendritic orientations of temperature-insensitive neurones (I) and warm-sensitive neurones (W).
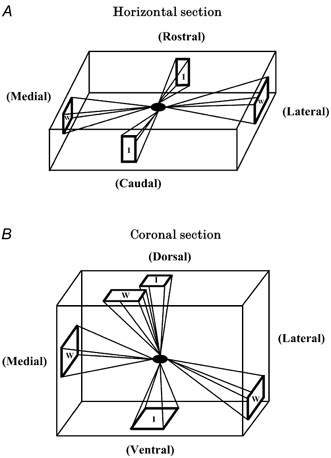
A, in horizontal tissue slices, dendrites of temperature-insensitive neurones projected rostrally and caudally, while warm-sensitive neurones projected medially and laterally. B, in coronal tissue slices, dendrites of temperature-insensitive neurones projected in dorsal and ventral directions, while warm-sensitive neurones projected in medial, lateral and dorsal directions.
For each neuronal type, chi square tests determined if dendritic orientations were random or confined to specific quadrants in combined horizontal and coronal planes. Temperature-insensitive neurones showed a greater number of dendrites oriented parallel to the third ventricle, rostrally and caudally in the horizontal plane and dorsally and ventrally in the coronal plane. A chi square test showed that this distribution of dendrites was not random (P < 0.05) and that temperature-insensitive neurones showed a greater tendency to have dorsal/ rostral or ventral/caudal dendrites. The following are the probabilities that a temperature-insensitive neurone had a dendrite in a specific quadrant: dorsal/rostral, 0.83 ± 0.14 and ventral/caudal, 0.83 ± 0.14, compared to lateral, 0.28 ± 0.11 and medial, 0.33 ± 0.11. In contrast, warm-sensitive neurones showed a greater number of dendrites oriented laterally or medially in the combined coronal and horizontal planes. A chi square test showed that the distribution of these dendrites was not random (P < 0.05) and that warm-sensitive neurones showed a greater probability of having dendrites which were lateral (0.93 ± 0.15) and medial (0.73 ± 0.15), rather than dorsal/ rostral (0.4 ± 0.13) and ventral/caudal (0.06 ± 0.06).
It is important to note that the above statistical differences and probabilities apply only if the criterion for neuronal warm sensitivity is > 0.8 imp s−1°C−1. Some studies have used lower criteria for warm sensitivity; however, if lower criteria had been applied in the present study, there would not be significant distinctions between the dendritic orientations in warm-sensitive and temperature-insensitive neurones. As an example, if the criterion for warm sensitivity was lowered to > 0.6 imp s−1°C−1, the probabilities for dendrites in specific quadrants would be: lateral, 0.71 ± 0.14; medial, 0.62 ± 0.13; dorsal/rostral, 0.48 ± 0.11; and ventral/ caudal, 0.33 ± 0.13; and the chi square test would indicate that the distribution is random (P > 0.1).
Figure 10 shows camera lucida drawings of several silent neurones. Depending on the tissue slice, these silent neurones are projected on either a coronal plane (Fig. 10 left side) or a horizontal plane (Fig. 10 right side). Regardless of the plane of the tissue slice, compared to the other neuronal types, the silent neurones displayed more branching and (as indicated in Table 2) had more proximal dendrites. Unlike the warm-sensitive and temperature-insensitive neurones, the dendrites of the silent neurones did not show a predominant direction of orientation.
Figure 10. Camera lucida drawings showing projected locations and morphologies of silent neurones.

These are recorded in coronal tissue slices (left side: dorsal, top) and horizontal tissue slices (right side: rostral, top; fx, fornix). Neurones identified at different rostral-caudal locations are projected onto a single coronal plane (left), and neurones identified at different dorsal-ventral locations are projected onto a single horizontal plane (right).
Figure 11 shows camera lucida drawings of EPSP-driven neurones projected on either a coronal plane (Fig. 11 left side) or a horizontal plane (Fig. 11 right side). Compared to the other neuronal types, EPSP-driven neurones had fewer dendrites, which were relatively short and displayed the least amount of branching. The most prominent feature of EPSP-driven neurones was that their dendrites tended to be unidirectional, with orientations that were either medial or lateral. This suggests that EPSP-driven neurones receive more selective synaptic input from one particular direction.
Figure 11. Camera lucida drawings showing projected locations and morphologies of EPSP-driven neurones.
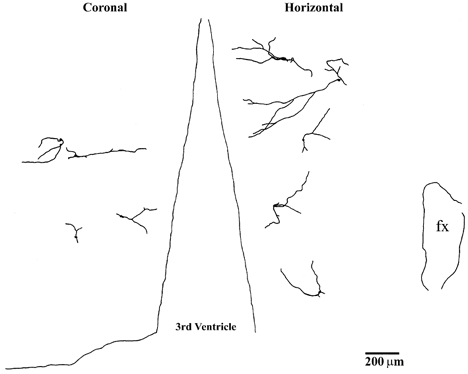
These are recorded in coronal tissue slices (left side: dorsal, top) and horizontal tissue slices (right side: rostral, top; fx, fornix). Neurones identified at different rostral-caudal locations are projected onto a single coronal plane (left), and neurones identified at different dorsal-ventral locations are projected onto a single horizontal plane (right).
DISCUSSION
Our results show that in the rostral hypothalamus, different types of physiologically identified neurones have different dendritic organizations. Warm-sensitive neurones have medial-lateral dendritic fields, whereas temperature-insensitive neurones tend to have dorsal- ventral and rostral-caudal dendritic fields. Silent neurones have more extensive and less polarized dendritic trees, but neurones driven by local EPSPs have shorter dendrites that project either in a medial direction or in a lateral direction. These highly differentiated dendritic trees may be related to the sources of inputs that these neurones receive which, in turn, may shape their physiological roles.
Warm-sensitive neurones
Warm-sensitive neurones in the rostral hypothalamus integrate both central and peripheral thermal information. Whole-animal studies found that about 60 % of warm-sensitive PO/AH neurones received afferent input from spinal and skin thermoreceptors (Boulant & Hardy, 1974). The present study indicates that these warm-sensitive neurones orient their dendrites to receive this afferent information ascending over both medial and lateral pathways. Although ascending thermoreceptive pathways are not well understood, a major source may be the spinohypothalamic tract. Burstein et al. (1991) estimated that about 43 % of spinohypothalamic neurones in the lumbar enlargement responded to innocuous heating or cooling, and Dado et al. (1994) found that 29 % of spinohypothalamic neurones in the cervical spinal cord responded to innocuous cold. Malick et al. (2000) reported that nearly all low-threshold trigeminohypothalamic neurones responded to innocuous heat and cold.
Spinohypothalamic tract axons ascend through the lateral hypothalamus. A number of these fibres cross the midline in the ventral supraoptic commissure such that the tract distributes bilaterally to medial hypothalamic nuclei (Cliffer et al. 1991; Newman et al. 1996). Their terminal branches approach the medial nuclei from both the lateral direction and along the wall of the ventricle, from the medial direction. Other possible sources of thermosensitive inputs to the hypothalamus include relays in the periaqueductal grey matter, which approach the medial hypothalamus primarily from the medial side through the periventricular fibre system (Floyd et al. 1996; Thompson & Swanson, 1998), and the nucleus of the solitary tract, parabrachial nucleus, or ventrolateral medulla. These latter relays receive spinal sensory afferents (Cechetto et al. 1985; Menetrey & Basbaum, 1987; Thompson and Swanson, 1998) and project to hypothalamic nuclei both medially via the periventricular route, and laterally via the medial forebrain bundle (Ricardo & Koh, 1978; Saper & Loewy, 1980). Thus, the medial-lateral dendritic orientation of the warm-sensitive cells may reflect the predominant orientation of afferent inputs to these neurones.
Some thermosensitive PO/AH neurones are affected by endogenous factors in cerebrospinal fluid (CSF) and blood (via circumventricular organs). Endogenous factors influencing thermoregulation include fever-producing cytokines, hormones, metabolites and osmolality (Boulant, 1996). As indicated in Fig. 7 and Fig. 8, several warm-sensitive neurones had medial dendrites close to the third ventricle. This raises the possibility that these neurones sense factors in the CSF, in addition to receiving synaptic information from ascending afferent pathways.
An important component of our analysis is the classification of neuronal thermosensitivity using a (firing rate/temperature) regression coefficient of 0.8 imp s−1°C−1 as the minimal criterion for warm sensitivity (Boulant & Dean, 1986; Boulant, 1996). This criterion is based on a study in rabbits in which PO/AH neurones were recorded during changes in central and peripheral temperature (Boulant & Hardy, 1974). In that study, of the neurones classified as insensitive to hypothalamic temperature, only 3 % responded to skin or spinal cord temperature. In contrast, 60 % of the warm-sensitive neurones responded to these peripheral temperatures. Therefore, PO/AH thermosensitivity of 0.8 imp s−1°C−1 is the demarcation between neurones that either do or do not respond to peripheral temperatures. Neurones having slightly lesser PO/AH thermosensitivities do not respond to peripheral temperature. For this reason, a PO/AH thermosensitivity of at least 0.8 imp s−1°C−1 appears to identify thermal integrative neurones that receive afferent information.
The present study supports this criterion for defining warm-sensitive neurones. In the hypothalamus, afferent signals ascend medially in the periventricular system and laterally in the medial forebrain bundle (Palkovits & Zaborszky, 1979). As summarized in Fig. 9, warm-sensitive neurones send their primary dendrites medially and laterally, presumably to receive afferent information from these two ascending pathways. In contrast, neurones having thermosensitivities less than 0.8 imp s−1°C−1 are defined as temperature insensitive, and these neurones orient their dendrites in the opposite direction, parallel to the third ventricle.
When the demarcation criterion of 0.8 imp s−1°C−1 is used, chi square tests show that warm-sensitive and temperature-insensitive neurones have dendritic orientations that are specific and not random. On the other hand, this is not the case if a lesser criterion is used. As an example, if the criterion for warm sensitivity is lowered to 0.6 imp s−1°C−1, it is not possible to show significant differences between the dendritic morphologies of warm-sensitive and temperature-insensitive neurones. This is an important point because in several electrophysiological studies the minimum criterion for warm sensitivity has been either 0.6 imp s−1°C−1 (Nakashima et al. 1987) or 0.5 imp s−1°C−1 (Hori et al. 1980; Morimoto et al. 1988; Kobayashi & Takahashi, 1993). Based on the present study showing morphological distinctions and the previous study (Boulant & Hardy, 1974) showing afferent input distinctions, there is strong evidence that the minimum criterion for neuronal warm sensitivity should be 0.8 imp s−1°C−1.
In addition to synaptic input from ascending afferent pathways, PO/AH warm-sensitive neurones also receive inhibitory and excitatory synaptic input from nearby hypothalamic neurones. As indicated in Fig. 3A, local inhibitory input to warm-sensitive neurones is more prevalent than excitatory input. In addition, both types of inputs appear to be derived from nearby temperature-insensitive neurones, since temperature has little or no effect on IPSP and EPSP frequencies. Recent studies have shown that hypothalamic temperature-insensitive neurones exert considerable synaptic inhibition on nearby warm-sensitive neurones in the PO/AH (Curras et al. 1991) and suprachiasmatic nucleus (Burgoon & Boulant, 1998). These studies indicate that synaptic inhibition can increase the thermosensitivity of the postsynaptic neurone since IPSP amplitudes and durations are affected by temperature. Hypothalamic cooling, for example, increases IPSP durations, thereby slowing the firing rate of the postsynaptic neurone. In this way, inhibitory synaptic input from temperature-insensitive neurones may partially contribute to the thermosensitivity of warm-sensitive neurones.
The present current-clamp study relied on postsynaptic potentials to characterize the types of nearby neurones that make synaptic contact on the recorded neurones. Voltage-clamp techniques would permit better identification of single synaptic events, recorded as either excitatory or inhibitory postsynaptic currents. Indeed, a recent study used both current- and voltage-clamp techniques to compare IPSPs and IPSCs recorded in the same population of neurones in the suprachiasmatic nucleus (SCN; Burgoon & Boulant, 1998). While this SCN study reported greater IPSC frequencies compared to IPSP frequencies, the identification of presynaptic thermosensitivities remained the same with both techniques. Moreover, like the present study, the SCN study concluded that temperature-insensitive neurones are responsible for most of the inhibitory synapses on both temperature-sensitive and -insensitive SCN neurones.
The present study found that, with a few exceptions (i.e. Fig. 4B), warm-sensitive neurones do not appear to play a major role in local synaptic networks. This suggests that warm-sensitive neurones send axonal projections out of the local network to influence networks at more remote locations. Previous studies support this hypothesis. Recordings made in horizontal tissue slices found that PO/AH warm-sensitive neurones synaptically inhibited neurones in more caudal locations, especially in perifornical areas (Dean et al. 1992). Whole-animal studies (using central stimulation and lesions) concluded that it is primarily the warm-sensitive neurones in the PO/AH that influence more caudal brainstem areas controlling heat loss and heat production responses (Zhang et al. 1995; Kanosue et al. 1998).
Temperature-insensitive neurones
Temperature-insensitive neurones are located in medial areas of the hypothalamus, and their dendrites project parallel to the third ventricle (as shown in Fig. 7 and Fig. 8). In a Golgi study by Millhouse (1979), neurones with similar dendritic orientations were characterized in the medial hypothalamus, especially in close proximity to the third ventricle. Given the orientation of their dendrites, it is less likely that temperature-insensitive neurones receive peripheral thermoreceptive inputs ascending over lateral and medial afferent pathways. This is supported by a whole-animal study indicating that only 3 % of the temperature-insensitive neurones receive afferent input from spinal and skin thermoreceptors (Boulant & Hardy, 1974). The paucity of peripheral thermal afferent input, however, does not necessarily mean that temperature-insensitive neurones do not participate in thermoregulation. On the contrary, temperature-insensitive neurones constitute the largest neuronal population in the rostral hypothalamus. Moreover, as shown in Fig. 3 and Fig. 4, these neurones form most of the synaptic connections in this area, including synapses on other temperature-insensitive neurones.
One might speculate that the predominant rostral-caudal and dorsal-ventral dendrites of the temperature-insensitive neurones primarily receive synaptic input from nearby temperature-insensitive neurones. On the other hand, the medial-lateral dendrites of the warm-sensitive neurones appear to receive afferent input from peripheral thermoreceptors. If this is the case, Fig. 9 shows that, in the coronal plane, some warm-sensitive neurones also have dorsally projecting dendrites, similar to the temperature-insensitive neurones. Accordingly, warm-sensitive neurones may receive their peripheral afferent information via the medial-lateral dendrites, but their local synaptic information (from nearby temperature-insensitive neurones) may come via their dorsal dendrites.
Silent neurones
Silent neurones, which do not fire spontaneously in the slice preparation, represent 22 % of all neurones recorded in our sample. This proportion is similar to previous studies reporting that silent neurones have lower input resistances and more negative resting membrane potentials (Curras et al. 1991; Griffin & Boulant, 1995). In addition, voltage-clamp recordings show that silent neurones display greater transient K+ currents (IA) and delayed rectifier K+ currents (IK(V)) (Griffin et al. 1996). It is likely, therefore, that silent neurones have greater outward K+ conductances, leading to hyperpolarization and a suppression of action potentials.
While the role of silent neurones is not clear, it is possible that these neurones are recruited in hypothalamic regulatory networks, depending on changes in the body's external or internal environment. Despite their lack of spontaneous activity, silent neurones appear to be most dependent on synaptic input. Figure 4 shows that silent neurones receive substantial amounts of excitatory and inhibitory inputs from nearby temperature-insensitive neurones. Moreover, of the different neuronal types, silent neurones have the most extensive and branched dendritic trees, projecting in all directions. Therefore, the lack of action potentials in these neurones may be due to the loss of synaptic input or the lack of endogenous factors that occurs in tissue slices. In the intact animal, however, these same neurones may be quite active, when afferent input and endogenous factors are high. In addition, a previous study suggests that neuronal temperature sensitivity is dependent on transient K+ currents (IA) that determine interspike intervals between action potentials (Griffin et al. 1996). Since transient K+ currents are prominent in silent neurones, these same cells may become thermosensitive when action potentials are elicited.
The dendrites of silent neurones project in many different directions. As indicated in Fig. 10, most silent neurones have some dendrites that project in both medial and lateral directions. Therefore, like the warm-sensitive neurones, silent neurones in the intact animal may serve to integrate central and peripheral thermal information by sensing changes in their own local temperature and receiving synaptic input from ascending medial and lateral pathways relaying skin and spinal thermal afferent signals.
EPSP-driven neurones
EPSP-driven neurones also are highly dependent on excitatory synaptic input. In this case, the input is local and comes from nearby neurones. Many of these nearby neurones appear to be thermosensitive since (as indicated in Table 1) EPSP-driven neurones were the only population to show a significant increase in EPSP frequency during an increase in temperature. The relatively short dendrites of the EPSP-driven neurones would be consistent with receiving predominantly local input.
In 1965, H. T. Hammel proposed a neuronal model for set-point temperature regulation. In this model, PO/AH warm-sensitive and temperature-insensitive neurones synaptically control effector neurones that evoke various thermoregulatory responses, such as evaporative heat loss and cutaneous blood flow. Hammel's model proposed that heat loss effector neurones are synaptically excited by warm-sensitive neurones and inhibited by temperature-insensitive neurones. The EPSP-driven neurones in the present study are similar to the heat loss effector neurones proposed by Hammel. Figure 4B indicates that in many of these neurones, EPSPs are derived from neurones that are either warm-sensitive or moderately thermosensitive, while IPSPs come primarily from temperature-insensitive neurones.
In addition to electrophysiological differences, the morphology of EPSP-driven neurones is different from other rostral hypothalamic neurones. As indicated in Fig. 11, EPSP-driven neurones have fewer and shorter dendrites that show very little branching. Most importantly, their dendrites project primarily in only one direction, usually either medially or laterally. This would suggest that EPSP-driven neurones are more specialized to receive input from a single source rather than more integrative inputs from two or three different sources.
The present study shows similarities between EPSP-driven neurones and heat loss effector neurones predicted in Hammel's model. EPSP-driven neurones and warm-sensitive neurones may be the source for descending pathways from the preoptic area to specific thermoregulatory effector areas in the midbrain, pons and medulla (Kanosue et al. 1998). This suggests that further work is needed to determine the physiological significance of these PO/AH neurones and their efferent projections in the brainstem.
Finally, it should be noted that in addition to thermoregulation, the rostral hypothalamus plays an important role in other regulatory systems (e.g. controlling reproductive responses, body water and metabolites) (Boulant & Silva, 1989). It is possible that each of these regulatory networks is composed of the various neurones identified in the present study. The proportions of temperature-sensitive and -insensitive neurones are relatively constant throughout much of the hypothalamus (Dean & Boulant, 1989). Temperature affects most regulatory systems, and alternatively, non-thermal factors (e.g. osmolality, glucose, reproductive hormones) affect body temperature (Boulant & Silva, 1989). In the rostral hypothalamus, these same non-thermal factors affect similar proportions of both temperature-sensitive and insensitve neurones (Boulant & Silva, 1988). Therefore, it is likely that different hypothalamic regions use both temperature-sensitive and -insensitive neurones to form neuronal networks that influence both thermoregulatory and non-thermoregulatory systems.
Acknowledgments
This research was supported by NIH grants NS14644 from the National Institute for Neurological and Communicative Disorders and Stroke and NS07291 from the Neuroscience Predoctoral Training Program. J. A. Boulant is the Hitchcock Professor of Environmental Physiology. The authors express their gratitude to Dr P. W. Burgoon and M. L. Kaple for their assistance in this work.
References
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic neurons controlling body temperature. In: Blatteis CM, Fregly MJ, editors. Handbook of Physiology section 4, Environmental Physiology. New York: Oxford University Press; 1996. pp. 105–126. [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annual Review of Physiology. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. Journal of Physiology. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA, Silva NL. Neuronal sensitivities in preoptic tissue slices: interactions among homeostatic systems. Brain Research Bulletin. 1988;20:871–878. doi: 10.1016/0361-9230(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Silva NL. Multisensory homeostatic neurons may explain interactions among regulatory systems. News in Physiological Sciences. 1989;4:245–248. [Google Scholar]
- Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. The distribution and characterization of tumor necrosis factor alpha-like immunoreactivity in the murine central nervous system. Journal of Comparative Neurology. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Burgoon PW, Boulant JA. Synaptic inhibition: its role in suprachiasmatic nucleus neuronal thermosensitivity and temperature compensation in the rat. Journal of Physiology. 1998;512:793–807. doi: 10.1111/j.1469-7793.1998.793bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Cliffer KD, Giesler GJ., Jr Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. Journal of Neurophysiology. 1991;66:261–284. doi: 10.1152/jn.1991.66.1.261. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. Journal of Comparative Neurology. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ., Jr Distribution of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde tranpsort of PHA-L in rats. Journal of Neuroscience. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curras MC, Kelso SR, Boulant JA. Intracellular analysis of inherent and synaptic activity in hypothalamic thermosensitive neurones in the rat. Journal of Physiology. 1991;440:257–271. doi: 10.1113/jphysiol.1991.sp018707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado RJ, Katter JT, Giesler GJ., Jr Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. Journal of Neurophysiology. 1994;71:981–1002. doi: 10.1152/jn.1994.71.3.981. [DOI] [PubMed] [Google Scholar]
- Dean JB, Boulant JA. A diencephalic slice preparation and chamber for studying neuronal thermosensitivity. Journal of Neuroscience Methods. 1988;23:225–232. doi: 10.1016/0165-0270(88)90006-4. [DOI] [PubMed] [Google Scholar]
- Dean JB, Boulant JA. In vitro localization of thermosensitive neurons in the rat diencephalon. American Journal of Physiology. 1989;257:R57–64. doi: 10.1152/ajpregu.1989.257.1.R57. [DOI] [PubMed] [Google Scholar]
- Dean JB, Kaple ML, Boulant JA. Regional interactions between thermosensitive neurons in diencephalic slices. American Journal of Physiology. 1992;263:R670–678. doi: 10.1152/ajpregu.1992.263.3.R670. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Keay KA, Arias CM, Sawchenko PE, Bandler R. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neuroscience Letters. 1996;220:105–108. doi: 10.1016/s0304-3940(96)13240-7. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Boulant JA. Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. Journal of Physiology. 1995;488:407–418. doi: 10.1113/jphysiol.1995.sp020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Kaple ML, Chow AR, Boulant JA. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. Journal of Physiology. 1996;492:231–242. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT. Neurons and temperature regulation. In: Yamamoto WS, Brobeck JR, editors. Physiological Controls and Regulations. Philadelphia: Saunders; 1965. pp. 71–97. [Google Scholar]
- Hori T, Nakashima T, Kiyohara T, Shibata M, Hori N. Effect of calcium removal on thermosensitivity of preoptic neurons in hypothalamic slices. Neuroscience Letters. 1980;20:171–175. doi: 10.1016/0304-3940(80)90141-x. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Honda E, Hirakawa T, Nakamura S, Yamashita H. Glutamatergic synaptic inputs to mouse supraoptic neurons in calcium-free medium in vitro. Journal of Neuroendocrinology. 1998;10:1–7. doi: 10.1046/j.1365-2826.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Hosono T, Zhang Y-H, Chen X-M. Neuronal networks controlling thermoregulatory effectors. Progress in Brain Research. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Perlmutter MN, Boulant JA. Thermosensitive single-unit activity of in vitro hypothalamic slices. American Journal of Physiology. 1982;242:R77–84. doi: 10.1152/ajpregu.1982.242.1.R77. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Takahashi T. Whole-cell properties of temperature-sensitive neurons in rat hypothalamic slices. Proceedings of the Royal Society. 1993;251:89–94. doi: 10.1098/rspb.1993.0013. B. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Chen X, Pittman QJ. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. Journal of Neurophysiology. 2000;83:2542–2553. doi: 10.1152/jn.2000.83.5.2542. [DOI] [PubMed] [Google Scholar]
- Konnerth A. Patch-clamping in slices of mammalian CNS. Trends in Neurosciences. 1990;13:321–323. doi: 10.1016/0166-2236(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Malick A, Strassman AM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. Journal of Neurophysiology. 2000;84:2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. Journal of Comparative Neurology. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. A Golgi anatomy of the rodent hypothalamus. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus, vol. 1, Anatomy of the Hypothalamus. New York: Marcel Dekker, Inc.; 1979. pp. 221–265. [Google Scholar]
- Morimoto A, Murakami N, Watanabe T. Effect of prostaglandin E2 on thermoresponsive neurones in the preoptic and ventromedial hypothalamic regions of rats. Journal of Physiology. 1988;405:713–725. doi: 10.1113/jphysiol.1988.sp017357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Pierau F-K, Simon E, Hori T. Comparison between hypothalamic thermoresponsive neurons from duck and rat slices. Pflügers Archiv. 1987;409:236–243. doi: 10.1007/BF00583471. [DOI] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. Journal of Comparative Neurology. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Zaborszky L. Neural connections of the hypothalamus. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus, vol. 1, Anatomy of the Hypothalamus. Vol. 1. New York: Marcel Dekker, Inc.; 1979. pp. 379–510. [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. Journal of Physiology. 2000;526:109–114. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Research Reviews. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Viana F, Gibbs L, Berger AJ. Double- and triple-labeling of functionally characterized central neurons projecting to peripheral targets studied in vitro. Neuroscience. 1990;38:829–841. doi: 10.1016/0306-4522(90)90075-f. [DOI] [PubMed] [Google Scholar]
- Zhang Y-H, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? Journal of Physiology. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]


