Abstract
In eccentric exercise the contracting muscle is forcibly lengthened; in concentric exercise it shortens. While concentric contractions initiate movements, eccentric contractions slow or stop them. A unique feature of eccentric exercise is that untrained subjects become stiff and sore the day afterwards because of damage to muscle fibres. This review considers two possible initial events as responsible for the subsequent damage, damage to the excitation-contraction coupling system and disruption at the level of the sarcomeres. Other changes seen after eccentric exercise, a fall in active tension, shift in optimum length for active tension, and rise in passive tension, are seen, on balance, to favour sarcomere disruption as the starting point for the damage. As well as damage to muscle fibres there is evidence of disturbance of muscle sense organs and of proprioception. A second period of exercise, a week after the first, produces much less damage. This is the result of an adaptation process. One proposed mechanism for the adaptation is an increase in sarcomere number in muscle fibres. This leads to a secondary shift in the muscle's optimum length for active tension. The ability of muscle to rapidly adapt following the damage from eccentric exercise raises the possibility of clinical applications of mild eccentric exercise, such as for protecting a muscle against more major injuries.
All forms of exercise, if carried out vigorously enough, can become painful. But only one form of exercise, eccentric exercise, if we are unaccustomed to it, leaves us stiff and sore the next day. During eccentric exercise the contracting muscle is forcibly lengthened. One commonly encountered example of eccentric exercise is downhill walking. As we step down the slope, the contracting quadriceps muscle controls the rate of knee flexion against the force of gravity and in the process the muscle undergoes an eccentric contraction with each step. Immediately after the exercise there is no pain. This sets in several hours later and peaks at about 48 h. It is thought to result from muscle damage produced by the eccentric exercise.
An interesting and important feature is the adaptation process. A second bout of eccentric exercise, a week after the first, leaves us much less stiff and sore. The ability of muscle to rapidly adapt to the damage from eccentric exercise, to prevent further damage, provides the opportunity for a number of clinical applications.
The subject of eccentric exercise and its mechanism has been discussed previously in a number of reviews (Armstrong et al. 1991; McHugh et al. 1999; Morgan & Allen, 1999; Warren et al. 2001; Allen, 2001). Brief perusal of the literature indicates that the subject is of growing interest. The aim in this review is to focus attention particularly on some of the indicators of the damage from unaccustomed eccentric exercise and their possible mechanisms. The discussion will consider how such indicators might be used to assess the degree of protection available to an individual in the event of exposure to further eccentric exercise and how this kind of knowledge might be useful in the clinic.
Initial event
It is generally agreed that there are two prominent signs of damage in a muscle immediately after it has been subjected to a series of eccentric contractions. There are the presence of disrupted sarcomeres in myofibrils and damage to the excitation-contraction (E-C) coupling system. It remains a point of controversy which of these two represents the primary event. The view taken here (see also Morgan & Allen, 1999) is that the damage process begins with overstretch of sarcomeres (Fig. 1). The alternative view is that the starting point is damage to components of the excitation-contraction (E-C) coupling process. In a recent review, Warren et al. (2001) summarised their position by declaring that 75 % or more of the decline in tension after eccentric exercise was attributable to a failure of the E-C coupling process. The remaining damage seen during the first few days after the exercise was attributed by the authors to physical disruption of the tension-bearing elements within the muscle. So the suggestion is that most of the primary damage arises in the E-C coupling system and only a small component occurs at the level of the sarcomeres. Supporting evidence comes from the observation that in mouse muscle the post-exercise deficit in tension can be recovered with caffeine (Warren et al. 1993; Balnave & Allen, 1995). In the first of these studies, tension was recovered with 50 mm caffeine, which releases Ca2+ from the sarcoplasmic reticulum and leads to development of a contracture in the muscle. In the second, 10 mm caffeine was used to potentiate tension in single fibres in response to direct electrical stimulation. It was concluded that in mouse fibres changes in E-C coupling may be a major contributor to the observed fall in tension after eccentric contractions (Allen, 2001).
Figure 1. Postulated series of events leading to muscle damage from eccentric exercise.
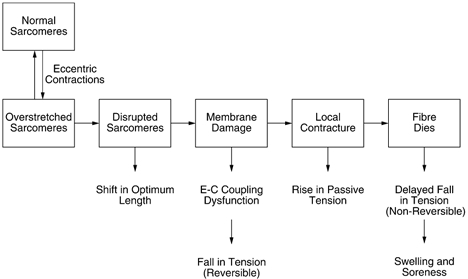
During an active lengthening, longer, weaker sarcomeres are stretched onto the descending limb of their length-tension relation where they lengthen rapidly, uncontrollably, until they are beyond myofilament overlap and tension in passive structures has halted further lengthening. Repeated overextension of sarcomeres leads to their disruption. Muscle fibres with disrupted sarcomeres in series with still-functioning sarcomeres show a shift in optimum length for tension in the direction of longer muscle lengths. When the region of disruption is large enough it leads to membrane damage. This could be envisaged as a two-stage process, beginning with tearing of t-tubules. Any fall in tension at this point would be reversible with caffeine (see text). It would be followed by damage to the sarcoplasmic reticulum, uncontrolled Ca2+ release from its stores and triggering of a local injury contracture. That, in turn, would raise muscle passive tension. If the damage was extensive enough, parts of the fibre, or the whole fibre, would die. This fall in tension would not be recoverable with caffeine. Breakdown products of dead and dying cells would lead to a local inflammatory response associated with tissue oedema and soreness.
However all of this still leaves open the question of what comes first, E-C coupling failure or sarcomere disruption. With respect to the effects of potentiators, there does seem to be a species difference. In single frog fibres (Morgan et al. 1996) and in toad muscle (Talbot, 1997; Allen, 2001) the fall in tension could not be recovered by potentiating Ca2+ release.
There is a specific hypothesis for the process of sarcomere disruption (Fig. 1). It has been known for some time that the descending limb of the sarcomere length-tension curve is a region where sarcomere inhomogeneities develop (Gordon et al. 1966). It has been proposed by Morgan (1990) that during active stretch of a muscle, most of the length change will be taken up by the weakest sarcomeres in myofibrils or, more strictly speaking, the weakest half-sarcomeres. On the descending limb of the length-tension curve, these sarcomeres will become progressively weaker and when they reach their yield point, they will lengthen rapidly, uncontrollably, to a point of no myofilament overlap, where tension in passive structures balances the active tension in adjacent sarcomeres that still have myofilament overlap. This process is repeated iteratively, with the next-weakest sarcomere stretching, and so on. It is postulated that overstretched sarcomeres are distributed at random along muscle fibres. At the end of the stretch, when the muscle relaxes, myofilaments in the majority of overstretched sarcomeres re-interdigitate so that they are able to resume their normal function. A few may fail to do so and become disrupted (Talbot & Morgan, 1996). During repeated eccentric contractions it is postulated that the number of disrupted sarcomeres grows, until a point is reached where membrane damage occurs. It is at this point that damage to elements of the E-C coupling machinery becomes apparent. Subsequently the fibre may die (Fig. 1).
It is conceptually more difficult to envisage damage to the E-C coupling process as the primary event. Observations by Takekura et al. (2001) of abnormal t-tubular arrangements after eccentric exercise could provide the basis for such a hypothesis. Here the first step in the damage process would be t-tubule rupture. Torn tubule ends would then seal off leading to inactivation of some sarcomeres. If such sarcomeres were concentrated in particular myofibrils it could lead to a fall in tension. This tension would be recoverable by a caffeine contracture but not by potentiators. If inactivated sarcomeres were scattered at random amongst myofibrils, the situation would be very similar to that where the primary event was sarcomere disruption. That is, ultrastructurally, a non-uniform sarcomere length distribution would be observed and mechanically, a shift in optimum length for active tension would occur, in the direction of longer muscle lengths. The main difficulty with such a hypothesis is trying to account for why t-tubules should be the primary site for damage and why this only occurs at lengths beyond the optimum. The reverse sequence, beginning with sarcomere disruptions and leading to t-tubule damage, could, of course, account equally readily for the observations of Takekura et al. (2001).
Structural signs
It is no longer a matter of controversy that eccentric exercise leads to structural signs of muscle damage (Friden et al. 1981; Newham et al. 1983). Most of the evidence comes from electron microscopic examinations and these show sarcomeres out of register with one another, Z-line streaming, regions of overextended sarcomeres or half-sarcomeres, regional disorganisation of the myofilaments and t-tubule damage; for a review see Morgan & Allen (1999).
The precise details of the sarcomere disruption process following eccentric contractions remain the subject of speculation. They may involve the elastic filament titin, which anchors thick filaments to Z discs, or the structural protein desmin, which links adjacent Z discs (Allen, 2001). It is conceivable that because of small alignment errors, thick and thin filaments of overstretched sarcomeres may butt up against one another. Inactivation of some sarcomeres from damage to t-tubules may also play a part. Whatever the precise details, there is evidence of overextended sarcomeres and half-sarcomeres in muscle which has undergone eccentric contractions (see, for example, Brown & Hill, 1991; Lieber et al. 1991; Wood et al. 1993; Talbot & Morgan, 1996). It has recently been shown with permeabilised segments of single fibres of rat muscles that regions of long sarcomere lengths before an active stretch contained the majority of disrupted sarcomeres after the stretch. These disrupted sarcomeres were longer than the rest (Macpherson et al. 1996).
A diagnostic structural feature of disruption after eccentric exercise is the presence of overstretched half-sarcomeres, with the adjacent half-sarcomere contracted down to a short length (for examples, see Brown & Hill, 1991; Talbot & Morgan, 1996; Macpherson et al. 1997). This consistent feature prompted us to speculate about the arrangement of elastic filaments within sarcomeres. Titin is believed to anchor the ends of the thick filaments to the Z-line (Horowits, 1999). In a simple mechanical model of a sarcomere, if the titin was attached just to the ends of the thick filaments, the two half-sarcomeres would behave independently of one another and overstretch of one half would not be expected to lead to shortening of the other half (Fig. 2). If, however, a second elastic element is included, one that spans the full length of the sarcomere, the structural changes observed in electronmicrographs are achieved. That is, a shortened half-sarcomere facing an overstretched half-sarcomere (Fig. 2).
Figure 2. Postulated distribution of elastic filaments in sarcomeres.
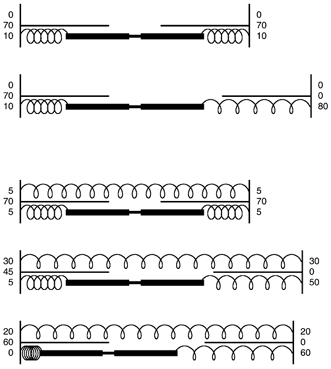
The top two diagrams consider a sarcomere with elastic filaments only linking the ends of the thick filaments to the Z-lines. The numbers indicate the distribution of tension. Total tension is set at 80 % of maximum to indicate that the sarcomere is on the descending limb of its length-tension relation. When one half-sarcomere becomes over-stretched, as a result of an eccentric contraction, tension borne by its myofilaments drops to zero and the full tension is borne by the elastic filament. The other half-sarcomere is unaffected since its isometric tension capability remains the same. When a second elastic element is included in the model, one which spans the full length of the sarcomere (lower 3 diagrams), overstretch of one half-sarcomere leads 50 % of the tension to be distributed to the elastic element in series with the thick filaments while, because of its proportionately smaller extension, that spanning the sarcomere bears less (30 %). It will also contribute 30 % of the tension to the other half-sarcomere, the remaining 50 % being distributed between the series elastic element (5%) and the cross-bridges (45 %). Since this half-sarcomere's isometric tension capability remains at 70 %, it shortens until tension in the series element has fallen to zero and myofilament overlap is somewhere on the ascending limb, generating 60 % of the tension, leaving 20 % in the elastic element spanning the sarcomere. This is what is observed under the electron microscope, one half-sarcomere over-stretched to beyond overlap, the other half very short. This kind of model indicates that passive tension in the whole sarcomere becomes significant when one half becomes over-stretched.
There is less information available about structural damage to the E-C coupling system. In a recent study of rats which had been exercised by downhill running, forelimb muscles showed a number of ultrastructural abnormalities including more longitudinal t-tubule segments, changes in disposition of triads, caveolar clusters and apposition of multiple t-tubule segments with terminal cisternae elements (Takekura et al. 2001). The disordered membrane systems were seen widely distributed throughout muscle fibres. These findings were interpreted as consistent with a hypothesis starting with focal sarcomere damage, sliding of myofibrils past one another, and eventual damage to t-tubules.
Shift in optimum length
Is there any evidence from the mechanical properties of muscle to support the existence of overstretched sarcomeres in damaged muscle? It has been proposed that the presence of overstretched sarcomeres increases series compliance, leading to a shift in the muscle's active length-tension relation in the direction of longer muscle lengths (Morgan, 1990). Such a shift was first described by Katz (1939) and since then has been shown for single frog fibres (Morgan et al. 1996), whole amphibian muscle (Wood et al. 1993; Talbot & Morgan, 1996) and human muscle (Jones et al. 1997; Brockett et al. 2001). A modelled example is shown in Fig. 3A.
Figure 3. Changes in mechanical properties of muscle following a series of eccentric contractions.
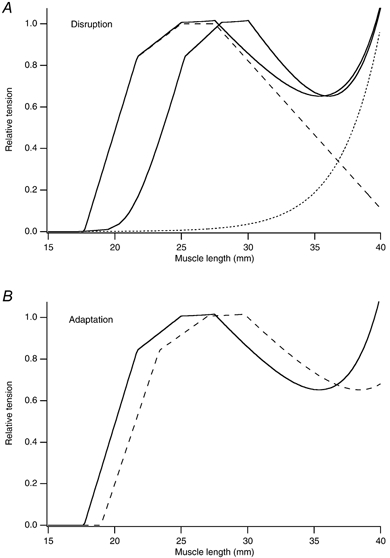
A, disruption of sarcomeres. Computer simulated sarcomere length-tension relations. The dashed line is the active length-tension relation taken from Gordon et al. (1966). The dotted line is an exponential curve representing passive tension; the continuous line is the total tension. Tension is normalised relative to the maximum active tension. Length is given as that of a postulated muscle fibre comprising 10 000 sarcomeres with a sarcomere length of 2.5 μm at optimum length. The control curve is the continuous curve on the left. After a series of eccentric contractions 10 % of the sarcomeres have their active force set to zero to simulate becoming disrupted, leading to a shift in optimum length of the total tension curve by 3 mm (continuous curve to the right). B, adaptation of the muscle fibre following injury from eccentric exercise. The continuous curve is the control total tension curve as in the upper panel, the dashed curve, that after the number of sarcomeres in series has been increased by 10 %, without changing the length of the tendon. It has led to an increase in optimum length by 2 mm.
If it were argued that the primary cause for the tension deficit after eccentric contractions was E-C coupling failure (Warren et al. 1993), leading to a reduced but uniform Ca2+ release, a shift in the length-tension relation could be interpreted as indicating nothing more than a reduced level of activation, so that the muscle had to be stretched further to achieve maximum activation (Endo, 1973). There is evidence that such an explanation does not always hold. For single frog fibres (Morgan et al. 1996) and whole rat muscle (Fig. 4), examples can be found where the length-tension curves from before and after the eccentric contractions cross at long lengths (Katz, 1939; see also Brockett et al. 2001a, b). At these long lengths, the post-exercise tensions are higher than before the exercise so that here incomplete activation cannot be used to explain the change in the curve (Fig. 4).
Figure 4. Activation and the length-tension relation.
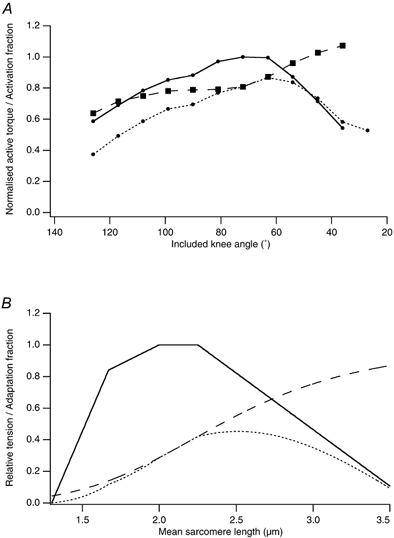
A, torque-angle relationship for the vastus intermedius muscle of the anaesthetised rat. Torque-angle curves were measured before (continuous line) and after (dotted line) a series of eccentric contractions of the muscle. Here the muscle was stretched through 27 deg in 33 ms while being stimulated at 90 pulses per second. Stretches were arranged to start 5 deg short of the optimum angle and finished at 22 deg beyond optimum. Included knee angle is the angle subtended between knee and thigh. At each length, the ratio of torque before and after the contractions has been calculated (dashed line) giving an estimate of the activation fraction (modified from Allen, 1999). B, computer simulation of partial activation. The continuous curve is the fully activated sarcomere length-tension curve (Gordon et al. 1966). The dashed curve is a Hill plot (Hill, 1913), as a reasonable estimate of length dependence of the activation fraction. The dotted curve is the resulting partial activation curve. The partial activation simulation gives a realistic shift in optimum length for tension but is unable to simulate active tension seen at long lengths after a series of eccentric contractions. That is, at long lengths tension lies well below the control curve. Note also the greater fall in tension at short lengths.
The sarcomere non-uniformity hypothesis predicts that damage will only occur if sarcomeres are actively stretched to beyond optimum length. If sarcomere disruption and damage are especially prevalent on the descending limb of the muscle's length-tension curve, indicators of damage should show a length dependence. That is indeed the case. For both rat and toad muscle the shift in optimum length and the fall in active tension post-contraction depended on the length range over which stretches were given (Lynn & Morgan, 1998; Talbot & Morgan, 1998).
A shift in the optimum length for active tension, in the direction of longer muscle lengths, is a rather indirect indicator of increased muscle compliance, post-exercise. Are there any other mechanical changes in the muscle, consistent with an increase in compliance? In his original account, Katz (1939) commented on the 2-3 times slower rise of the isometric tetanic tension and fall in twitch:tetanus ratio consistent with a ‘partial transformation of active contractile into passive elastic tissue’. In a recent series of experiments on the medial gastrocnemius muscle of the anaesthetised cat, it was found that after a series of eccentric contractions the tension rise in response to a stretch of the passive muscle was delayed, compared with the response before the contractions (Whitehead et al. 2001). Our interpretation is that the eccentric contractions led to overextension and disruption of some sarcomeres. When the muscle relaxed, some overextended sarcomeres did not re-interdigitate (Talbot & Morgan, 1996) and this meant that neighbouring sarcomeres returned to a shorter length than before the contractions. The muscle would therefore have to be stretched further before passive tension in these shorter sarcomeres rose to measurable levels.
Decline in tension
An important indicator of damage after a period of eccentric exercise is the drop in active tension. This may be as much as 60 %, but here it must be remembered that the tension decline will include a component of metabolic fatigue that is common to all forms of exercise, not just eccentric exercise. It has been suggested that any estimate of the decline in tension from eccentric exercise should use as a control a comparable period of concentric exercise, but which took into account the difference in energetic cost of concentric and eccentric contractions (Allen, 2001).
The disruption of sarcomeres lying in series with still functioning sarcomeres, by itself, would not be expected to produce a fall in tension, provided fibre length was adjusted to the new, longer optimum length. But as the damage spread across the myofibril, and to other myofibrils, membranous structures would become involved, leading to damage and impairment of the E-C coupling process. Any tension decline at this point would be recoverable with a caffeine contracture. Subsequently a point would be reached where part of the fibre, or the whole fibre died. Consistent with such a two-stage process is the report of a secondary, delayed fall in tension (Jones et al. 1989; Faulkner et al. 1993; MacIntyre et al. 1995). In human subjects it was observed that the initial fall in tension after eccentric exercise was followed by a slow rise over 2-4 h, presumably recovery from metabolic exhaustion. Then 24 h later there was a second fall in tension (MacIntyre et al. 1995). It may be that here some fibres die, so that this component of a fall in tension would not be expected to be recoverable with caffeine (Fig. 1).
When making measurements of the decline in tension after eccentric exercise, it is not only important to consider any effects of metabolic fatigue but the consequences of a shift in optimum length for active tension. The optimum length before the exercise will be short of the optimum length after the exercise because of the shift and so risks leading to an underestimate of the remaining tension. It is also likely to contribute to the observed change in the force-frequency relationship (Newham et al. 1983).
Different fibre types
Many studies have considered the susceptibility of different muscles and parts of muscles to damage from eccentric contractions. A question that has been raised repeatedly concerns the vulnerability of different types of motor units in muscles of mixed, slow-fast fibre composition. Claims have been made for a predisposition to damage in slow units during locomotion (Armstrong et al. 1983; Mair et al. 1992). The reasons put forward include the low recruitment threshold and important postural roles of these motor units. When muscles of mixed, slow-fast fibre composition were subjected to maximal active lengthenings, the large, fast-fatiguable motor units were more vulnerable, it was suggested, because of their lack of oxidative capacity (Friden & Lieber, 1998), or the higher tensions generated by them (Appell et al. 1992). In another recent study demonstrating preferential fast-oxidative-glycolytic fibre damage, it was suggested that fibre phenotype or lower contractile workload might be responsible (Vijayan et al. 2001). Others have suggested a combination of factors involving both active and passive properties of muscle fibres (Macpherson et al. 1996). In a recent review, Lieber & Friden (1999) proposed that the larger amount of fibre injury in fast-glycolytic fibres after eccentric exercise was a result of the ‘increased strain and injury due to their short fibre length’.
An important feature of the sarcomere non-uniformity hypothesis is the dependence of damage on the length range over which eccentric contractions are carried out. It raises the possibility that optimum lengths for different fibre types may not be the same so that in a muscle of mixed composition, stretch of the whole muscle leads some fibres to be stretched further down the descending limb of their length-tension curve than others. In a recent study from our laboratory (J. Talbot, M. Homewood & D. L. Morgan, unpublished observation), the predominantly fast-twitch, tibialis anterior muscles and slow-twitch soleus muscles of rats were subjected to a series of eccentric contractions. The applied active stretches were carefully arranged to cover the same portion of each muscle's length-tension relation. Shifts in optimum length for active tension were observed in both muscles, indicating that the contractions had produced damage in both fibre types. The size of the shift was not significantly different between the two muscles. The result suggested that fibre-type composition, as such, was not a determinant of the amount of damage from eccentric contractions, provided the contractions covered an equivalent range of muscle lengths.
However, it still remained to explain the reports of others concerning susceptibility to damage of fibres within the same muscle, of mixed fibre composition. In a second experiment (Brockett et al. 2001b), slow-twitch and fast-twitch motor units were studied in the medial gastrocnemius muscle of the cat. It was found that the majority of fast-twitch units had an optimum length for tension that was shorter than the whole muscle optimum length. Conversely, slow-twitch units had an optimum length, on average, longer than the whole muscle optimum. When motor units were subjected to a series of eccentric contractions, using stretches that began at the whole-muscle optimum, all units showed a shift in their length-tension relation, indicative of damage. However, the slow-twitch units showed a smaller shift than fast-twitch units. Statistical analysis showed that a motor unit's optimum length for tension, relative to the whole-muscle optimum, was a better indicator than motor unit type for the susceptibility of a unit to damage from eccentric exercise. The difference in optimum length of the two motor unit types was thought to be due to differences in numbers of sarcomeres in series in muscle fibres. This, in turn, was thought to relate to the role of the different motor unit types in gastrocnemius during standing and walking in the cat (Brockett et al. 2001b). The result now needs to be confirmed for other muscles of mixed fibre composition.
Rise in passive tension
It has been known for some time that after a period of eccentric exercise there is a rise in passive tension in the muscle. For human elbow flexors this is indicated by the relaxed arm adopting a slightly flexed posture (Jones et al. 1987). When muscle stiffness was measured, this more than doubled after the exercise and remained elevated for the next 4 days (Howell et al. 1993). The immediate rise in stiffness post-exercise was postulated to result from stretch-activated Ca2+ release (Howell et al. 1993). Other explanations have been based on shortening of parallel, non-contractile elements in the muscle (Howell et al. 1985; Jones et al. 1987). The scheme shown in Fig. 1 has passive tension rising from development of a local contracture in fibre segments, following a rise in myoplasmic Ca2+, as a result of membrane damage.
Several studies have demonstrated increases in resting Ca2+ levels in muscle fibres damaged by eccentric contractions (Balnave & Allen, 1995; Balnave et al. 1997; Ingalls et al. 1998). However, within the limits of resolution of the method, measurements showed that the rises in Ca2+ levels were uniformly distributed along muscle fibres (Balnave et al. 1997). Perhaps the rise in Ca2+ is sufficient to trigger a low level of activation to increase passive tension, although there is no direct evidence for this (Whitehead et al. 2001, p. 602).
When measured across the full physiological range, the rise in passive tension after a series of eccentric contractions peaks at a length close to the optimum for active tension (Whitehead et al. 2001). Measurements of work absorption by the passive muscle in response to large, slow lengthening and shortening movements showed a significant rise after the eccentric contractions. It was suggested that the increase in work absorption was a result of actively cycling cross-bridges in the damaged segments of muscle fibres (Whitehead et al. 2001). There is some structural evidence for contracted fibre segments in muscle damaged by eccentric exercise. Thus, it was found that after downhill running, fibres of soleus muscles of rats showed Z-line dissolution, A-band disruption and fibre clotting (Ogilvie et al. 1988). Similarly Friden & Lieber (1998) found that in rabbit muscle after eccentric contractions there was cytoskeletal disruption and the presence in fibres of hypercontracted regions.
Muscle swelling and soreness
Eccentric exercise is followed by sensations of stiffness and soreness the next day (Hough, 1902). The current view of the mechanism is that damage at the level of sarcomeres leads, during repeated contractions, to more extensive damage and, ultimately, to death of some muscle fibres. The injury triggers a local inflammatory response that is accompanied by some oedema. The breakdown products of injured tissues sensitise nociceptors (Smith, 1991; MacIntyre et al. 1995).
It has been suggested that a component of the stiffness after eccentric exercise is due to the swelling accompanying the damage. Thus Howell et al. (1985) suggested that delayed increases in stiffness in exercised elbow flexor muscles were the result of volume changes exerting strain on perimysial and epimysial connective tissue elements. A quantitative biomechanical model supported this view (Purslow, 1989). However, passive tension and stiffness change immediately post-exercise (Howell et al. 1993; Chleboun et al. 1998; Whitehead et al. 2001), when there is not yet any evidence of swelling. In our experiments, there was a rise in passive tension in ankle extensor muscles which reached close to its peak value immediately after the exercise. At 24 h after the exercise, when swelling peaked, there was no significant further increase in passive tension. So under the conditions of our experiments there did not appear to be a close link between passive tension rises and muscle swelling. The swelling began to subside by 4 days post-exercise.
Soreness sets in at about 6-8 h after the exercise and peaks at about 48 h (MacIntyre et al. 1995; Jones et al. 1997). The current view of the mechanism is that the tissue breakdown products sensitise nociceptors so that these respond to stimuli that are normally non-noxious. So the muscle is tender to local palpation, stretch and contraction. It has recently been proposed that a component of the delayed soreness from eccentric exercise involves large-fibre mechanoreceptors (Barlas et al. 2000; Weerakkody et al. 2001). It has been postulated that input from mechanoreceptors, including afferents of muscle spindles, is able to access the pain pathway at the level of the spinal cord.
Adaptation
We have all had the experience that the stiffness and soreness following a period of exercise become very much less when the exercise is repeated a week later, the result of adaptation by the muscle. This has been known for some time (Hough, 1902; Friden et al. 1983; Schwane & Armstrong, 1983; Clarkson & Tremblay, 1988). However, the underlying mechanism remains controversial (for a review see McHugh et al. 1999).
In his original proposal for a mechanism for the damage from eccentric exercise, Morgan (1990) suggested that the subsequent adaptation process involved an increase in the number of sarcomeres in series in muscle fibres. As a consequence, at a set muscle length, average sarcomere length would be shorter (Fig. 3B). Therefore less of the muscle's working range would include the region of potential instability, the descending limb of the length-tension curve. Supporting evidence comes from experiments in which rats were exercised on an inclined or declined treadmill (Lynn & Morgan, 1994; see also Lynn et al. 1998). Fibres of a muscle known to undergo eccentric contractions during downhill running, vastus intermedius, were fixed, digested and sarcomere numbers counted after a week of exercise. It was found that the mean number of sarcomeres was, on average, 11 % greater in the muscles of animals which had run downhill, compared with those which had run uphill.
This proposal has been challenged (Koh & Herzog, 1998; see also Koh & Brooks, 2001). However, the contrary findings are difficult to evaluate since in that study of rabbit muscle no indication was given of the length range, with respect to the optimum length, over which the muscles were stretched, nor was it stated whether the eccentric contraction protocol used was followed by any detectable signs of disruption or damage.
Scepticism over an adaptation mechanism involving the addition of extra sarcomeres to muscle fibres centres around the time course (McHugh et al. 1999). If muscle fibres are going to be re-modelled after injury from eccentric exercise, this process must be rapid enough to be substantially complete by the end of a week following the injury. Is muscle able to undergo such a rapid adaptation process at the level of the sarcomeres? It was shown back in 1973 that immobilising a muscle in the lengthened position with a plaster cast led to an increase in sarcomere number in muscle fibres within 5 days (Williams & Goldspink, 1973). This increase was rapidly reversible. While we do not yet understand the precise details, at the cellular level, of the events that lead to the adaptation of sarcomere numbers (Goldspink, 1998; see also Wretman et al. 2001), the speed of the process is obviously sufficient to account for the adaptation observed after eccentric exercise.
If the muscle adapts to the injury from eccentric exercise with an increase in sarcomere numbers in muscle fibres, what implications does this have for muscle-tendon relations? We have tried to model this situation (Fig. 5). Consider a muscle composed of muscle fibres containing 10 000 sarcomeres and 20 mm of tendon. In this model muscle tension would begin to rise when the muscle was stretched to 35 mm length, representing a sarcomere length of 1.5 μm. Tension would peak at 45 mm or 2.5 μm sarcomere length. If the aim was to shift the muscle's length-tension curve in the direction of longer lengths, to provide protection against injury from eccentric exercise, a simple adaptation would be to increase the length of the tendon by 5 mm. That would shift the optimum length by 5 mm. However, tension would be less well maintained at short muscle lengths, falling to zero at 40 mm rather than at 35 mm. If the number of sarcomeres in the fibre was increased by 20 %, without changing the length of the tendon, the requisite shift in optimum length would still be obtained but the working range of the muscle would be reduced by 3 mm. Only if sarcomere number was increased further and the tendon shortened could both a shift in optimum length and maintenance of the original working range of the muscle be achieved (Fig. 5). In practice, it is unlikely that the tendon is able to be remodelled within a week, although this remains an option for adaptation in the longer term.
Figure 5. Sarcomere length-muscle length relation.
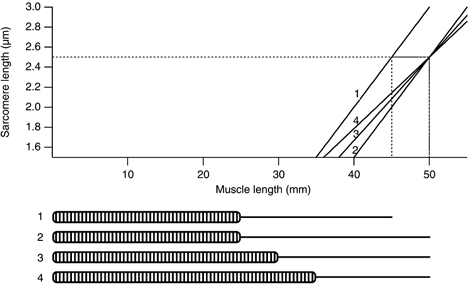
Relation between sarcomere length and muscle length for theoretical muscle fibres comprising different numbers of sarcomeres and different lengths of tendon. It is assumed that optimum sarcomere length is 2.5 μm. For a muscle fibre with 10 000 sarcomeres and 20 mm of tendon (fibre no. 1) tension begins to rise at 35 mm and the optimum is reached at 45 mm (dashed line). A shift in optimum length for active tension by 5 mm in the direction of longer lengths (dashed line) can be achieved by increasing the length of tendon to 25 mm (fibre no. 2). The drawback is that active tension is not developed until the muscle is stretched to 40 mm, that is, the working range of muscle lengths has been reduced. Increasing the number of sarcomeres from 10 000 to 12 000, and leaving tendon length at 20 mm (fibre no. 3) produces the required increase in optimum length and leads to a smaller reduction in the muscle's working range, where tension begins to rise at 39 mm. Increasing sarcomere number further to 14 000, while at the same time reducing the length of tendon to 15 mm (fibre no. 4) produces the most satisfactory result, the required shift in optimum length by 5 mm and reduction of the working range by only 1 mm.
Is there any evidence for a shift in the length-tension curve as a sign of a muscle's adaptation to the injury from eccentric contractions? First, it is important to distinguish between the two kinds of shift that may occur in association with eccentric exercise. Following a period of unaccustomed exercise there will be a shift in optimum length as a result of the increase in series compliance from disrupted sarcomeres (Fig. 3A). This is followed by a second, delayed shift representing the adaptation by increasing sarcomere numbers (Fig. 3B). In amphibian muscles, within about 6 h of the injury from eccentric contractions, the initial shift in optimum length has reversed and the optimum has returned near to its pre-exercise value (Jones et al. 1997). Here we assume that following the eccentric exercise, in some muscle fibres disrupted sarcomeres will have managed, over time, to re-establish their normal pattern of myofilament interdigitation and ability to generate tension (Talbot & Morgan, 1996). In other fibres, areas of disruption may have progressed to a more major lesion. Such damaged fibres will no longer contract and therefore cannot contribute to the shift in the length-tension curve. Both of these factors will lead to a reversal of the shift.
In a study of human triceps surae, it was possible to identify a damage-related shift in optimum angle for torque in the direction of longer muscle lengths after a period of eccentric exercise, but this had reversed back to control values by 2 days post-exercise (Jones et al. 1997). No subsequent shift due to adaptation could be detected. In a subsequent study of the hamstrings muscle group, a sustained shift in optimum angle was able to be demonstrated (Brockett et al. 2001a). Here, while a training effect was clearly evident, it was not possible to identify its point of onset, nor any reversal from a shift attributable to injury. Presumably in situations like this, the time course of recovery from the initial shift and the onset of the adaptation process may overlap.
In animal experiments it has been shown that fibres of vastus intermedius muscles of rats, trained to run downhill for a week, had more sarcomeres than fibres from an uphill trained group. At the end of training, in response to an acute series of eccentric contractions, beginning from the same knee angle, the downhill-trained group showed a smaller shift in optimum angle for torque than the uphill-trained group. The smaller shift was considered an indication of less damage (Lynn et al. 1998).
There is also limited evidence of an adaptation process in the opposite direction (Whitehead et al. 1998). A group of human subjects was required to carry out a period of concentric exercise with triceps surae of one leg, the other leg acting as a control. In response to a test period of eccentric exercise a week later, the concentrically trained muscles showed a larger shift in optimum, indicative of more damage, than the control muscles. It was suggested that during concentric exercise muscle fibres may lose sarcomeres, leading to a greater vulnerability to damage from eccentric exercise.
Muscle sense organs
While quite a lot is known about the effects of fatigue from exercise on local reflex action of muscle afferents (for a review, see Gandevia, 2001), the question of whether eccentric exercise leads to damage in muscle receptors remains an open one. Here the discussion will be restricted to the two prominent muscle receptors, the muscle spindle and tendon organ.
In an elbow position matching task, carried out after a period of eccentric exercise of elbow flexors of one arm, it was found that the exercised arm consistently adopted a more flexed position in matching the position of the unexercised arm (Saxton et al. 1995). Given that the main proprioceptors signalling limb position are the muscle spindles (Gandevia, 1996; Proske et al. 2000), the result suggests that the signal from muscle spindles had increased as a result of the exercise. That is, to get a set level of proprioceptive signal, the exercised muscle had to be stretched less than the control muscle. The current view is that the level of resting activity from muscle spindles signals the length of the muscle and, so, the position of the elbow. If as a result of the exercise some intrafusal fibres of spindles had become damaged and developed an injury contracture, this would be expected to raise the level of resting activity for a given muscle length and so explain the above results.
A second experiment by Brockett et al. (1997) employed a rather milder exercise regime and elbow flexors of one arm underwent eccentric contractions, while flexors of the other arm underwent concentric contractions, over the same period. Here there was only a small, transient drop in tension, post-exercise, suggesting minimal damage to the muscle, but over the next 48 h subjects matched the position of the concentrically exercised arm by placing the eccentrically exercised arm in a more extended position. This result was therefore the opposite of that of Saxton et al. (1995). The explanation probably relates to the difference in severity of the exercises. Mild eccentric exercise might be expected to produce some sarcomere disruption, leading to an increase in series compliance. That, in turn, would increase the length threshold for tension (Whitehead et al. 2001) and the muscle would have to be stretched further to achieve a set level of spindle discharge. If the spindles had actually become damaged the opposite result would have been obtained. These kinds of propositions should now be tested on single, identified receptors in animal experiments.
Some observations have also been made on tendon organs after a period of eccentric exercise (Gregory et al. 2001). In anaesthetised cats, the medial gastrocnemius muscle was subjected to a series of lengthening contractions and the responses of tendon organs measured to both passive and active tension changes before and after the exercise. It was found that in response to a slow stretch after the exercise, tendon organs commenced firing at a shorter muscle length. This was attributed to the rise in whole-muscle passive tension, post-exercise. In one animal, all of the sample of six tendon organs isolated signalled this increase, suggesting that the damage producing the rise in passive tension was widespread throughout the muscle. However tension threshold and tendon organ sensitivity had not changed suggesting that the exercise had not disturbed, in any way, the normal functioning of the receptors.
Clinical applications
Since eccentric exercise produces muscle damage, weakness and soreness, it raises the question of whether the mild symptoms we all experience on occasions may, at times, lead to more major injuries. A specific case in point is the hamstring tear (Brockett et al. 2001a). Clinical reports suggest that hamstring injuries occur most often as a result of eccentric contractions (Stanton & Purdham, 1989; Garrett, 1990; Kujala et al. 1997; Sallay et al. 1996). It is possible that in some elite sports such as track and field events, football and rugby, the micro-damage from mild eccentric exercise may, as a result of the demands placed on the muscle by the competitive event, progress to more major tears. If so, then a way to combat the problem would be to subject athletes to a mild eccentric exercise program to effect an adaptation that protected the muscles at risk against further damage. This proposition is currently being put to the test in our laboratory.
Another area where the adaptation effect from eccentric exercise may prove useful is with a condition known as idiopathic toe walking (equinus gait). It is a condition commonly found in children. They adopt a strongly plantar-flexed posture with their foot and walk on the heads of their metatarsals, rather than with a heel-to-toe action. Toe walking is sometimes associated with cerebral palsy but it also presents in the absence of neurological signs, in otherwise normal children. Current standard treatments designed to bring the heel down onto the ground include botulinum toxin injections into triceps surae to relax the muscle, plaster casts applied with the muscle in a dorsiflexed position, and insertion of stiff graphite plates in the shoes of afflicted children. We have recently explored the possibility of effecting an adaption in triceps surae by giving the children a specific programme of eccentric exercise of ankle extensor muscles, using a motorised foot plate. While the work is still ongoing, preliminary data suggest that, in the future, the more invasive treatments may be able to be replaced by exercise regimes (D. L. Morgan, C. Blackburn & P. Percival, unpublished observations).
Finally, Duchenne muscular dystrophy is a degenerative muscle disease related to the lack of a protein associated with the sarcolemma, dystrophin. It is know that dystrophin-deficient mice are particularly vulnerable to the damage from eccentric contractions (Head et al. 1992; Moens et al. 1993). It raises the possibility that degenerative changes in muscles of humans afflicted with the disease may be triggered by eccentric contractions. Strategies aimed at minimising such damage would be to avoid eccentric contractions altogether or alternatively embark on a programme of very mild, non-injurious eccentric exercise, in the hope of effecting an adaptation process in the affected muscles.
Concluding comments
This review has focused the discussion on the initial series of events leading to the damage to muscle from eccentric exercise (Fig. 1). In our view, the body of evidence in support of the sarcomere non-uniformity hypothesis has grown considerably in recent times and therefore a review of its current status is called for. Its appeal is that it is able to account for the length dependence of the amount of damage and for the observed differences in effects from concentric and eccentric exercise. It also helps to explain several other behaviours of skeletal muscle that have hitherto resisted satisfactory explanations (Noble, 1992; Morgan, 1994; Morgan et al. 2000). Another area of attention in the review has been the various signs of injury accompanying the damage. These may, in future, have practical applications. So to determine the extent of damage post-exercise, rather than measuring the tension deficit, which is complicated by metabolic factors, or a shift in the length-tension relation, which requires a series of measurements of active tension, a simple, non-invasive indicator is provided by the rise in passive tension. The training effect, produced by a period of unaccustomed exercise, is important because it represents a potential means of protecting athletes against muscle injuries. It may also be helpful for other clinical conditions. Its mechanism involves the addition of sarcomeres to regenerating muscle fibres, as shown by animal experiments. Such a mechanism provides further support for the proposal that the primary injury process is dependent on sarcomere length.
References
- Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiologica Scandanavica. 2001;171:311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- Allen TA. PhD Thesis. Clayton, Victoria, Australia: Monash University; 1999. Effect of eccentric contractions on the mechanical properties of skeletal muscle. [Google Scholar]
- Appell HJ, Soares JM, Duarte JA. Exercise, muscle damage and fatigue. Journal of Sports Medicine. 1992;13:108–115. doi: 10.2165/00007256-199213020-00006. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. Journal of Applied Physiology. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Journal of Sports Medicine. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. Journal of Physiology. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave CD, Davey DF, Allen DG. Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following stretch-induced injury. Journal of Physiology. 1997;502:649–659. doi: 10.1111/j.1469-7793.1997.649bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlas P, Walsh DM, Baxter GD, Allen JM. Delayed onset muscle soreness: effect of an ischaemic block upon mechanical allodynia in humans. Pain. 2000;87:221–225. doi: 10.1016/S0304-3959(00)00287-6. [DOI] [PubMed] [Google Scholar]
- Brockett C, Warren N, Gregory JE, Morgan DL, Proske U. A comparison of the effects of concentric versus eccentric exercise on force and position sense at the human elbow joint. Brain Research. 1997;771:251–258. doi: 10.1016/s0006-8993(97)00808-1. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Medicine and Science in Sports and Exercise. 2001a;33:783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Gregory JE, Proske U. Damage in different types of motor units following repeated active, lengthenings of the medial gastrocnemius muscle of the cat. Journal of Applied Physiology. 2001b doi: 10.1152/japplphysiol.00479.2001. in the Press. [DOI] [PubMed] [Google Scholar]
- Brown LM, Hill L. Some observations on variations in filament overlap in tetanized muscle fibres and fibres stretched during a tetanus, detected in the electron microscope after rapid fixation. Journal of Muscle Research and Cell Motility. 1991;12:171–182. doi: 10.1007/BF01774036. [DOI] [PubMed] [Google Scholar]
- Chleboun GS, Howell JN, Conatser RR, Giesey JJ. Relationship between muscle swelling and stiffness after eccentric exercise. Medicine and Science in Sports and Exercise. 1998;30:529–535. doi: 10.1097/00005768-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. Journal of Applied Physiology. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- Endo M. Length dependence of activation of skinned muscle fibers by calcium. Cold Spring Harbor Symposia on Quantitative Biology. 1973;37:505–510. [Google Scholar]
- Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Physical Therapy. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell and Tissue Research. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Friden J, Seger J, Sjostrom M, Ekblom B. Adaptive response in human skeletal muscle subjected to prolonged eccentric training. International Journal of Sports Medicine. 1983;4:177–183. doi: 10.1055/s-2008-1026031. [DOI] [PubMed] [Google Scholar]
- Friden J, Sjostrom M, Ekblom B. A morphological study of delayed muscle soreness. Experientia. 1981;37:506–507. doi: 10.1007/BF01986165. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinesthesia: Roles for afferent signals and motor commands. In: Rowell LB, Shepherd TJ, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological Reviews. in the Press. [DOI] [PubMed]
- Garrett WE., Jr Muscle strain injuries: clinical and basic aspects. Medicine and Science in Sports and Exercise. 1990;22:436–443. [PubMed] [Google Scholar]
- Goldspink G. Cellular and molecular aspects of muscle growth, adaptation and ageing. Gerodontology. 1998;15:35–43. doi: 10.1111/j.1741-2358.1998.00035.x. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. Journal of Physiology. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Brockett CL, Morgan DL, Whitehead NP, Proske U. Effect of eccentric muscle contractions on Golgi tendon organ responses to passive and active tension in the cat. Journal of Physiology. 2001 doi: 10.1113/jphysiol.2001.012785. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proceedings of the Royal Society B. 1992;248:163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Hill AV. The combinations of haemoglobin with oxygen and with carbon monoxide I. Journal of Biochemistry. 1913;7:471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. The physiological role of titin in striated muscle. Reviews of Physiology Biochemistry and Pharmacology. 1999;138:57–96. doi: 10.1007/BFb0119624. [DOI] [PubMed] [Google Scholar]
- Hough T. Ergographic studies in muscular soreness. American Journal of Physiology. 1902;7:76–92. [Google Scholar]
- Howell JN, Chila AG, Ford G, David D, Gates T. An electromyographic study of elbow motion during postexercise muscle soreness. Journal of Applied Physiology. 1985;58:1713–1718. doi: 10.1152/jappl.1985.58.5.1713. [DOI] [PubMed] [Google Scholar]
- Howell JN, Chleboun G, Conatser R. Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. Journal of Physiology. 1993;464:183–196. doi: 10.1113/jphysiol.1993.sp019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. Journal of Applied Physiology. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Jones C, Allen T, Talbot J, Morgan DL, Proske U. Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. European Journal of Applied Physiology and Occupational Physiology. 1997;76:21–31. doi: 10.1007/s004210050208. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Clarkson PM. Skeletal muscle stiffness and pain following eccentric exercise of the elbow flexors. Pain. 1987;30:233–242. doi: 10.1016/0304-3959(87)91079-7. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Torgan C. Mechanical influences on long-lasting human muscle fatigue and delayed- onset pain. Journal of Physiology. 1989;412:415–427. doi: 10.1113/jphysiol.1989.sp017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. Journal of Physiology. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2001;281:R155–161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Herzog W. Eccentric training does not increase sarcomere number in rabbit dorsiflexor muscles. Journal of Biomechanics. 1998;31:499–501. doi: 10.1016/s0021-9290(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Orava S, Jarvinen M. Hamstring injuries. Current trends in treatment and prevention. Journal of Sports Medicine. 1997;23:397–404. doi: 10.2165/00007256-199723060-00005. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Mechanisms of muscle injury after eccentric contraction. Journal of Science and Medicine in Sport. 1999;2:253–265. doi: 10.1016/s1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25 % strain. Journal of Applied Physiology. 1991;70:2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- Lynn R, Morgan DL. Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. Journal of Applied Physiology. 1994;77:1439–1444. doi: 10.1152/jappl.1994.77.3.1439. [DOI] [PubMed] [Google Scholar]
- Lynn R, Talbot JA, Morgan DL. Differences in rat skeletal muscles after incline and decline running. Journal of Applied Physiology. 1998;85:98–104. doi: 10.1152/jappl.1998.85.1.98. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Journal of Sports Medicine. 1999;27:157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Journal of Sports Medicine. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- Macpherson PC, Dennis RG, Faulkner JA. Sarcomere dynamics and contraction-induced injury to maximally activated single muscle fibres from soleus muscles of rats. Journal of Physiology. 1997;500:523–533. doi: 10.1113/jphysiol.1997.sp022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson PC, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. American Journal of Physiology. 1996;271:C1438–1446. doi: 10.1152/ajpcell.1996.271.5.C1438. [DOI] [PubMed] [Google Scholar]
- Mair J, Koller A, Artner-Dworzak E, Haid C, Wicke K, Judmaier W, Puschendorf B. Effects of exercise on plasma myosin heavy chain fragments and MRI of skeletal muscle. Journal of Applied Physiology. 1992;72:656–663. doi: 10.1152/jappl.1992.72.2.656. [DOI] [PubMed] [Google Scholar]
- Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. Journal of Muscle Research and Cell Motility. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophysics Journal. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. An explanation for residual increased tension in striated muscle after stretch during contraction. Experimental Physiology. 1994;79:831–838. doi: 10.1113/expphysiol.1994.sp003811. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Allen DG. Early events in stretch-induced muscle damage. Journal of Applied Physiology. 1999;87:2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Claflin DR, Julian FJ. The effects of repeated active stretches on tension generation and myoplasmic calcium in frog single muscle fibres. Journal of Physiology. 1996;497:665–674. doi: 10.1113/jphysiol.1996.sp021798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. Journal of Physiology. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clinical Science. 1983;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Noble MI. Enhancement of mechanical performance of striated muscle by stretch during contraction. Experimental Physiology. 1992;77:539–552. doi: 10.1113/expphysiol.1992.sp003618. [DOI] [PubMed] [Google Scholar]
- Ogilvie RW, Armstrong RB, Baird KE, Bottoms CL. Lesions in the rat soleus muscle following eccentrically biased exercise. American Journal of Anatomy. 1988;182:335–346. doi: 10.1002/aja.1001820405. [DOI] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Progress in Neurobiology. 2000;60:85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. Journal of Biomechanics. 1989;22:21–31. doi: 10.1016/0021-9290(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Sallay PI, Friedman RL, Coogan PG, Garrett WE. Hamstring muscle injuries among water skiers. Functional outcome and prevention. American Journal of Sports Medicine. 1996;24:130–136. doi: 10.1177/036354659602400202. [DOI] [PubMed] [Google Scholar]
- Saxton JM, Clarkson PM, James R, Miles M, Westerfer M, Clark S, Donnelly AE. Neuromuscular dysfunction following eccentric exercise. Medicine and Science in Sports and Exercise. 1995;27:1185–1193. [PubMed] [Google Scholar]
- Schwane JA, Armstrong RB. Effect of training on skeletal muscle injury from downhill running in rats. Journal of Applied Physiology. 1983;55:969–975. doi: 10.1152/jappl.1983.55.3.969. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness. Medicine and Science in Sports and Exercise. 1991;23:542–551. [PubMed] [Google Scholar]
- Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation- contraction coupling in rat skeletal muscle. Journal of Physiology. 2001;533:571–583. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J. PhD thesis. Clayton, Victoria, Australia: Monash University; 1997. Muscle damage and recovery following eccentric contractions. [Google Scholar]
- Talbot JA, Morgan DL. Quantitative analysis of sarcomere non-uniformities in active muscle following a stretch. Journal of Muscle Research and Cell Motility. 1996;17:261–268. doi: 10.1007/BF00124247. [DOI] [PubMed] [Google Scholar]
- Talbot JA, Morgan DL. The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. Journal of Muscle Research and Cell Motility. 1998;19:237–245. doi: 10.1023/a:1005325032106. [DOI] [PubMed] [Google Scholar]
- Vijayan K, Thompson JL, Norenberg KM, Fitts RH, Riley DA. Fiber-type susceptibility to eccentric contraction-induced damage of hindlimb-unloaded rat AL muscles. Journal of Applied Physiology. 2001;90:770–776. doi: 10.1152/jappl.2001.90.3.770. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exercise and Sport Sciences Reviews. 2001;29:82–87. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. Journal of Physiology. 1993;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Whitehead NP, Canny BJ, Gregory JE, Proske U. Large-fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. Journal of Pain. 2001;2:209–219. doi: 10.1054/jpai.2001.22496. [DOI] [PubMed] [Google Scholar]
- Whitehead N, Weerakkody N, Gregory J, Morgan D, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. Journal of Physiology. 2001;533:593–604. doi: 10.1111/j.1469-7793.2001.0593a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Allen TJ, Morgan DL, Proske U. Damage to human muscle from eccentric exercise after training with concentric exercise. Journal of Physiology. 1998;512:615–620. doi: 10.1111/j.1469-7793.1998.615be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. Journal of Anatomy. 1973;116:45–55. [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. American Journal of Physiology. 1993;265:C792–800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]
- Wretman C, Lionikas A, Widegren U, Lannergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPKerk1/2 and MAPKp38 in isolated rat skeletal muscle. Journal of Physiology. 2001;535:155–164. doi: 10.1111/j.1469-7793.2001.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


