Abstract
In order to further characterize the role of lamina I as the source of central ascending neural pathways for thermoreception and thermoregulation, experiments were performed on anaesthetized cats to determine the quantitative response characteristics of warming-specific lumbosacral spinothalamic lamina I neurones.
We identified 10 neurones out of 474 that were selectively excited by cutaneous warming (Warm cells). Their thresholds were all in the range 35-37 °C at a baseline of 34.5 °C, and their discharge linearly encoded the temperature of graded, innocuous warming stimuli with a sensitivity of 2.1 Hz °C−1.
The stimulus-response function of the Warm cells plateaued at temperatures that were in the noxious heat range.
The Warm cells were distinguished from other classes of spinothalamic lamina I neurones by their peripheral inputs, central conduction velocities and level of ongoing activity.
The discharge of Warm cells compares well with the known human psychophysics of warm sensibility, and these neurones are likely to be crucial to discriminative thermoreception. Additionally, a role in thermoregulation, a defining feature of mammalian homeostasis, is suggested.
Cutaneous nerve fibres specifically excited by innocuous thermal stimuli (Warm and Cool fibres) with appropriate characteristics for thermoreceptors have been identified in almost every mammalian species studied to date, including humans (for review see Hensel, 1981). In contrast, much less is known about central mechanisms of thermoreception. The crossed trigemino/spinothalamic tract is critical for pain and temperature sensations (Craig, 2000; Villaneuva & Nathan, 2000), and cooling-specific neurones have been identified in the most superficial layer (lamina I) of the spinal and medullary dorsal horns (Christensen & Perl, 1970; Dostrovsky & Hellon, 1978; Craig & Kniffki, 1985; Dostrovsky & Craig, 1996). These Cool neurones have appropriate response properties to account for discriminative cooling sensibility (Craig et al. 2000, 2001). However, neurones specifically excited by innocuous warming are rare in comparison: Dostrovsky & Hellon (1978) identified 133 cooling-specific cells but only 15 warming-specific cells in lamina I of the cat's subnucleus caudalis. At spinal levels, Warm cells have been reported very infrequently, their projections are unknown and their responses to quantitative stimulation have not been tested. Combined human psychophysics and recordings from monkey Cool fibres showed that warmth discrimination cannot be solely accounted for by the inhibition of cooling (Darian-Smith & Dykes, 1971), but central Cool neurones are inhibited in a graded manner by warm stimuli (Craig et al. 2001), indicating that the inhibition of Cool cell activity could contribute to warmth perception. Nonetheless, warm has a different sensory quality from cool, and warming produces a pattern of cortical activation that differs from that produced by cooling in human imaging studies (Craig et al. 1996). In the current experiments we have investigated the presence and encoding properties of spinothalamic tract (STT) lamina I Warm cells. Our results support the hypothesis that Warm and Cool lamina I STT neurones provide discrete ascending sensory channels for temperature sensations.
METHODS
Animal preparation
All experimental protocols were approved by the local Institutional Animal Care and Use Committee and they complied with the guiding principles issued by the American Physiological Society and the National Institutes of Health. The data reported here were obtained during the course of three different series of experiments (Andrew & Craig, 2001a, b; Craig et al. 2001) that were performed on anaesthetized cats (2.0-6.5 kg) over several years. The animals were anaesthetized throughout the experiments with either sodium pentobarbital (Nembutal; Abbott, Chicago, IL, USA; 40 mg kg−1i.p. then 5-10 mg kg−1 h−1i.v.) or ketamine (25 mg kg−1i.m.) and α-chloralose (80 mg kg−1i.v.). They were injected with the neuromuscular blocker pancuronium (400 μg h−1; ESI, Cherry Hill NJ, USA) and monitored to ensure an adequate depth of anaesthesia (stable blood pressure, constricted pupils and no withdrawal reflexes when neuromuscular blockade wore off; Craig et al. 2001). At the end of the experiment the animals were killed with an overdose of barbiturates. Room temperature was maintained at 27 °C.
Full details of the preparation and maintenance of the animals and the recording procedures are given in our recent studies (Andrew & Craig, 2001a; Craig et al. 2001). Single spinal dorsal horn lamina I STT neurones with receptive fields on the left hindlimb were recorded extracellularly with glass-insulated tungsten microelectrodes. Units were confirmed as STT neurones if they followed a train of six antidromic stimuli delivered at 250 Hz from an array of electrodes in the contralateral thalamus, and if collision between antidromic and orthodromic action potentials was observed (Fig. 1A and B). The recording sites of neurones were marked with electrolytic lesions (+20 μA DC for 10 s), and the recording and stimulating sites were identified in 50 μm frozen sections that were stained with thionin (Fig. 1D and E).
Figure 1. Identification of STT lamina I Warm neurones.
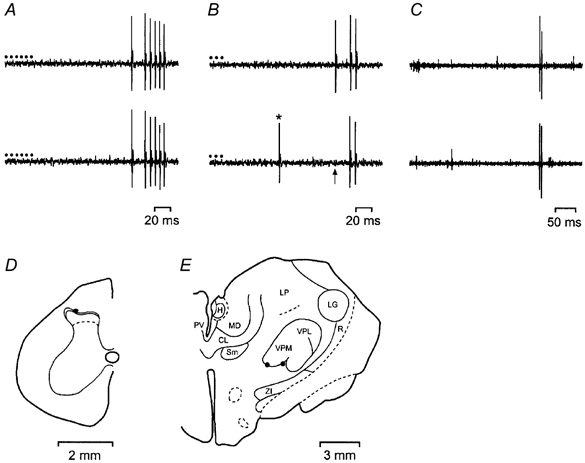
A, one-for-one following of a train of 6 antidromic pulses (•, 60 μA, 2 ms, 250 Hz) delivered from the electrode in the contralateral basal part of the ventromedial nucleus (vVMb). Conduction distance, 330 mm. B, collision of the first impulse in a train of 3 antidromic action potentials (150 Hz) due to an orthodromic impulse (*) occurring within the critical interval. The arrow indicates the point at which the first antidromic impulse would have occurred. C, a pair of responses from the same unit during electrical stimulation of the receptive field showing time-locked (monosynaptic) responses at C-fibre latency. The stimuli were applied at the beginning of each trace. Conduction distance, 360 mm. D, location of the recording site in lamina I of the spinal cord. E, contralateral thalamus showing effective sites for antidromic activation. CL, central lateral n.; H, habenula; LG, lateral geniculate n.; LP, lateral posterior n.; MD, medial dorsal n.; PV, paraventricular n.; R, reticular n.; Sm, submedial n.; VPL, ventral posterior lateral n.; VPM, ventral posterior medial n.; ZI, zona incerta.
Unit characterization
Each STT neurone was tested with cutaneous thermal stimuli (cooling with a beaker of wet ice for 30 s, radiant warming, heating to noxious levels for 5 s) and mechanical stimuli (brushing, blunt pressure, pinching with forceps) to determine its receptive properties. Warm cells were identified by their brisk response to innocuous warming with a radiant heat lamp, inhibition of their ongoing (background) activity by cooling and their insensitivity to innocuous and noxious mechanical stimulation. The three major classes of lamina I STT neurone (cooling specific, Cool; polymodal nociceptive, HPC; and nociceptive specific, NS) were recognized as previously described (Craig et al. 2001). The receptive fields of Warm cells were determined using radiant heat and shading. Warm neurones were tested quantitatively with both innocuous warm and noxious heat stimuli that were applied with a 40 mm × 40 mm thermoelectric (Peltier) element under feedback control. A graded warming sequence consisted of ramp-and-hold (ramp rate 10 °C s−1) pulses of 20 s duration from a baseline of 34.5 °C to final skin-thermode interface temperatures of 35.1, 35.9, 37.0, 38.0 and 38.7 °C. Individual pulses were separated by 60 s. The heat sequence was of identical duration and baseline to the warming sequence, except the skin-thermode interface temperatures tested were 38.5, 42.7, 45.5, 48.5 and 53.0 °C.
RESULTS
General properties
Recordings were made from 474 lamina I STT neurones. Ten of these units were Warm cells, and quantitative data were obtained from eight. The other 464 cells comprised 169 Cool cells, 177 HPC cells and 118 NS cells. Innocuous warming with a radiant heat lamp briskly excited these 10 Warm cells. They were not excited by cool or cold stimuli (one unit was tested with graded cooling to demonstrate inhibition of its ongoing activity) or by innocuous or noxious mechanical stimuli. Their central conduction velocities were in the range 1.5-3.0 m s−1 (mean 2.3 m s−1, s.d. 0.5 m s−1), indicating that their central axons were unmyelinated. These conduction velocities were significantly slower than those of Cool and HPC neurones (P < 0.001, ANOVA; P < 0.0002, Tukey's test post hoc;Andrew & Craig, 2001a; Craig et al. 2001), but no different from those of NS neurones (P > 0.7). All of the Warm cells were antidromically activated from the ventral periphery of the ventrobasal complex and one was also activated from nucleus submedius. A broad range of antidromic thresholds was observed (60 μA to 1.2 mA, 2 ms duration, bipolar stimulation), but the thresholds were dependent on the position of the array, which was not always optimal. Warm cells had low levels of background (ongoing) activity: their mean firing rate over a 2 min recording period at room temperature, prior to quantitative characterization, was 0.22 impulses s−1 (range 0.0-0.61 impulses s−1, s.d. 0.19 impulses s−1). This level of activity is significantly lower than that of Cool cells (P < 10−7, ANOVA; P < 0.0002, Tukey's test post hoc) and HPC cells (P < 0.03) but no different from that of NS neurones (P > 0.9). Warm cells had moderately sized receptive fields. The unit with the smallest receptive field was activated over all of the glabrous pads of the hindlimb, whereas the cell with the largest field responded over all the glabrous and hairy skin of the plantar hindlimb. The conduction velocities of the peripheral inputs to three Warm neurones were determined by intracutaneous electrical stimulation with needle electrodes after quantitative study. All three units received time-locked (monosynaptic) inputs from unmyelinated (C-) fibres only, and their conduction velocities were in the range 0.8-1.2 m s−1 (Fig. 1C).
Quantitative characterization
The quantitative responses of all eight Warm cells to graded thermal stimulation of the glabrous hindpaw are shown in Fig. 2. A variety of responses in terms of threshold, temperature encoding, overall discharge rate and adaptation were observed. Most neurones showed a brief, phasic (‘dynamic’) response at threshold temperatures. Latencies from stimulus onset varied between 480 and 1440 ms (mean 977 ms, s.d. 311 ms), although this delay includes the conduction time (300-450 ms estimated from conduction velocity measurements) and synaptic transmission in the dorsal horn. At supra-threshold temperatures, the Warm neurones exhibited sustained (‘static’) firing. Adaptation was prominent in six units during supra-threshold stimulation, two neurones had mixed adapting and maintained responses, and one showed an augmenting discharge. After-discharges were observed in one unit, and they were preceded by a brief silent period when the thermode temperature returned to baseline, suggestive of inhibition. The thresholds of the Warm cells were between 35.1 and 37.0 °C (mean 36.1 °C, s.d. 0.8 °C), i.e. they were all activated within the first three temperature pulses. The discharge of Warm neurones increased with a linear trend as warming intensity increased (P < 10−5, repeated-measures ANOVA). The individual stimulus-response functions and the population mean are shown in Fig. 3.
Figure 2. Quantitative response characteristics of Warm neurones.
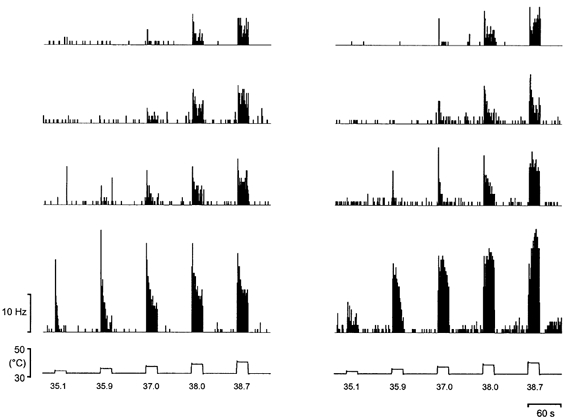
Original histogram records (1 s bins) from all 8 Warm neurones that were tested quantitatively. The lower pair of records are specimen temperature traces obtained from a thermocouple fixed to the surface of the thermode.
Figure 3. Innocuous temperature encoding by Warm neurones.
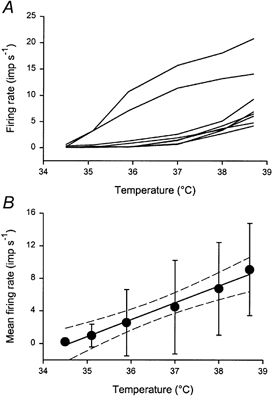
A, individual stimulus-response curves for all 8 Warm neurones that were quantitatively characterized. B, population means (± 1 s.d.). The continuous line is the linear regression (r = 0.60), and the dashed lines are the 95 % confidence limits. Firing rates are given in impulses (imp) per second.
As can be seen from both the unitary histograms (Fig. 2) and the individual curves (Fig. 3A), two subsets of Warm cells are suggested. These two groups differ in terms of threshold, absolute firing rate and rate of rise, although the sample size prevents statistical differentiation. The population stimulus-response function could be fitted with a linear regression of the form y = mx + c (r = 0.60), with a slope of 2.1 Hz °C−1 (Fig. 3B). The 95 % confidence limits span a width of 1.3 °C on the abscissa, meaning this is the smallest temperature difference that this population of neurones can reliably discriminate (P < 0.05). The population response could also be fitted with a power function of the form y = y0+ axb (r = 0.59) with an exponent of 1.1. Expressing the discharge of the Warm neurones as a percentage of the response at 38.7 °C (100 %) reduced the effect of differences in absolute discharge rates (r = 0.87); the slope was 20.5 % °C−1 and the width of the confidence limits was 0.6 °C. The correlation obtained for a power function fit using normalized data was 0.88, and the exponent was 1.72.
All eight Warm cells were also tested with graded heat stimuli that included temperatures in the noxious range. An example of the response of a Warm neurone to an innocuous and a noxious heat stimulus is shown in Fig. 4A. Individual and population responses are shown in Fig. 4B and C. All of the neurones continued to respond as temperature increased, and none suddenly stopped firing during stimulation as has been reported for Warm fibres (LaMotte & Campbell, 1978; Darian-Smith et al. 1979). However, the evoked response peaked at 42.7 °C, and plateaued at noxious temperatures (45.5-53.0 °C). There was no relationship between firing rate and temperature over the range 42.7-53.0 °C (P < 0.2, ANOVA).
Figure 4. Warm neurones do not encode noxious heat stimuli.
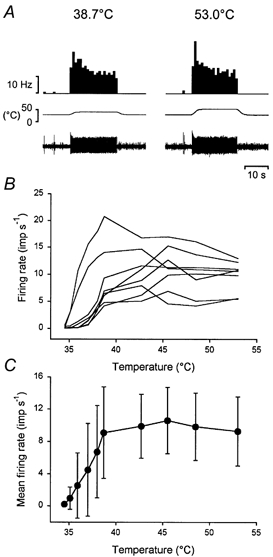
A, pair of records from the same Warm neurone illustrating its response to an innocuous (38.7 °C) and a noxious (53.0 °C) heat stimulus. The traces are, from the top downward, firing rate of the unit in 1 s bins, temperature and the single-unit recording. B, individual stimulus-response curves for all 8 Warm neurones to innocuous and noxious heat stimuli. C, population stimulus-response curve (means ± 1 s.d.).
DISCUSSION
The current findings are the first quantitative recordings of warming-specific STT neurones. Below we compare their response characteristics to those of peripheral Warm fibres and central neurones, and also to human sensation.
Response properties of Warm fibres
Warming-specific primary afferents have unmyelinated (C-fibre) axons and they are spontaneously active at normal skin temperatures. They are selectively excited by small increases in temperature and are inhibited by cooling (LaMotte & Campbell, 1978; Darian-Smith et al. 1979; Duclaux & Kenshalo, 1980). All of these properties were observed in the current study of Warm neurones. Warm fibre activity depends on basal skin temperature and the rate of temperature rise (Darian-Smith et al. 1979; Duclaux & Kenshalo, 1980). At 35 °C, Warm fibres have thresholds within a degree of skin temperature (LaMotte & Campbell, 1978), and the thresholds of the most sensitive Warm neurones compare well to these data. Temperatures in the innocuous warming range are encoded remarkably linearly by the discharge of Warm fibres. In monkey glabrous skin, Warm fibres have a sensitivity of 2.3 Hz °C−1 for temperature steps in the range 2-8 °C above a baseline of 34 °C (Darian-Smith et al. 1979), which is very close to the responsiveness of lamina I STT Warm cells of 2.1 Hz °C−1 reported here. The corresponding figure for human Warm fibres is 2.0 Hz °C−1 for temperature steps of 1-5 °C above a baseline of 37 °C (Konietzy & Hensel, 1977).
A notable difference between the responses of Warm fibres and Warm STT neurones was the continued firing of Warm neurones at noxious temperatures, whereas Warm fibres can become silent after an abrupt burst of impulses (LaMotte & Campbell, 1978; Darian-Smith et al. 1979). Thus the stimulus-response functions of Warm fibres are bell shaped with maxima at 41-47 °C (Hensel, 1981), although testing Warm fibres with noxious heat has not been performed extensively. In contrast, the stimulus-response function of the Warm cell population was piecewise linear, plateauing at approximately 43 °C. However, examples of Warm fibres that continue to respond to noxious temperatures have been noted previously (see Fig. 8 in Darian-Smith et al. 1979, and Fig. 3E and G in Duclaux & Kenshalo, 1980). The maintained responses of lamina I STT Warm neurones to noxious heat could be explained if they received inputs from different classes of Warm fibres with different stimulus-response functions.
Response properties of Warm neurones
Warming-specific central neurones have been reported quite rarely. They seem to be more common in the trigeminal region, where they are present at a ratio of about one Warm cell to nine Cool cells (Dostrovsky & Hellon, 1978). At spinal levels, Warm neurones have been noted very infrequently (Christensen & Perl (1970) mention a ‘few’ neurones, one was documented by Kumazawa et al. (1975) and one was intracellularly stained by Light (1992)). In the present study the Warm:Cool ratio was about 1:17, which underscores their scarcity. This scarcity does not seem to be due to any systematic bias in the recordings, as we routinely record from nociceptive and chemoselective neurones with slow central conduction velocities and no ongoing activity (see Andrew & Craig, 2001a). The low Warm:Cool spinal neurone ratio could explain why cats cannot discriminate warm temperatures as well as cool temperatures with their paws (Norrsell, 1983).
Prior quantitative characterization of a few trigeminal Warm neurones with unidentified projections has been reported by Dostrovsky & Hellon (1978; n = 5) and by Dickenson et al. (1979; n = 2). Like Warm fibres most of these units had bell-shaped stimulus-response functions (4/7); however, one neurone encoded temperature linearly up to 45 °C, the maximum intensity tested (Dostrovsky & Hellon, 1978). Thus, in general the properties of Warm neurones mirror those of Warm fibres. However, as noted above, the piecewise linear stimulus-response functions of warming-specific lamina I STT neurones could be explained by inputs from several classes of Warm fibre. Anterolateral quadrant axons responsive to warming or cooling of the spinal cord have been described previously (Simon & Iriki, 1971), and they have stimulus-response curves similar to those of Warm and Cool fibres. However, many of them also responded to whole-body stimulation (Simon, 1972), suggestive of inputs from both cutaneous and deep thermoreceptors. Additionally, the axons were localized in the ventrolateral funiculus, where lesions are ineffective at disrupting discriminative thermoreception (Norrsell, 1979, 1983). Thus, the thermoreceptive neurones studied by Simon and colleagues (Simon & Iriki, 1971; Simon, 1972) are probably different from those reported in the present study. The reversed Warm:Cool neurone ratio from an in vitro study of rat spinal cord (19:1; Pehl et al. 1997) supports this conclusion.
Warm STT neurones and human sensation
The responses of lamina I STT Warm cells compare favourably with the psychophysics of warmth sensation. The effects of the rate of temperature change and stimulus area on human warmth thresholds can be disregarded for rates > 0.1 °C s−1 and areas > 8 cm2 (Kenshalo, 1976). Thus human warmth thresholds of 0.3-0.5 °C (Kenshalo, 1976; Hensel, 1981) compare well to the thresholds of the most sensitive Warm neurones described here. The ability to discriminate warming increments as small as 0.05 °C has been reported using paired forced-choice protocols (Johnson et al. 1979) but these methods were not used in the current study. Magnitude estimation techniques have shown that warm sensation increases linearly across the innocuous range, and the discharge of Warm STT neurones is similarly linear for increases of 1-5 °C above a skin temperature of 34.5 °C. Power function fits of these sensory estimates have exponents close to 1.0 (Marks & Stevens, 1968; Molinari et al. 1977), and the exponent of the Warm cells using absolute firing rates was 1.1 in the present study. Temperatures > 45 °C produce pain (LaMotte & Campbell, 1978), and the appearance of this sensation coincides with activity in polymodal nociceptors and nociceptive STT neurones (LaMotte & Campbell, 1978; Craig et al. 2001). Thus the lamina I STT Warm neurones seem to be the only class of ascending neurone in the dorsal horn that encodes innocuous warming temperatures with a sensitivity comparable to human sensory judgements. Like ‘itch-specific’ cells (Andrew & Craig, 2001a), warming-specific STT neurones have long been predicted and here we describe their qualitative and quantitative characteristics. Both Warm and Cool neurones have the appropriate characteristics of ‘labelled lines’ for innocuous temperature sensations, but they also are likely to be of critical importance in thermoregulation, which is a defining feature of mammalian homeostasis.
Acknowledgments
We thank Maribeth Tatum and Sherri Jordan for excellent technical assistance. This work was supported by the NIH (NS 25616) and the Atkinson Pain Research fund administered by the Barrow Neurological Foundation.
References
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neuroscience. 2001a;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. The encoding of noxious mechanical stimuli by spinothalamic lamina I neurons. Society for Neuroscience Abstracts. 2001b;31 280.7. [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: Marginal zone of the dorsal horn. Journal of Neurophysiology. 1970;33:293–311. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Craig AD. Spinal location of ascending lamina I axons in the macaque monkey. Journal of Pain. 2000;1:33–45. [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craig AD, Kniffki K-D. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. Journal of Physiology. 1985;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. Journal of Neurophysiology. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EC, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Dykes RW. Peripheral neural mechanisms of thermal sensation. In: Dubner R, Kawamura Y, editors. Oral-Facial Sensory and Motor Mechanisms. New York: Appleton-Century Crofts Meredith Corp.; 1971. pp. 7–22. [Google Scholar]
- Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: Responses to thermal stimuli. Journal of Neurophysiology. 1979;42:1297–1315. doi: 10.1152/jn.1979.42.5.1297. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Hellon RF, Taylor DCM. Facial thermal input to the trigeminal spinal nucleus of rabbits and rats. Journal of Comparative Neurology. 1979;185:203–210. doi: 10.1002/cne.901850112. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Craig AD. Cooling-specific spinothalamic neurons in the monkey. Journal of Neurophysiology. 1996;76:3656–3665. doi: 10.1152/jn.1996.76.6.3656. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Hellon RF. The representation of facial temperature in the caudal trigeminal nucleus of the cat. Journal of Physiology. 1978;277:29–47. doi: 10.1113/jphysiol.1978.sp012258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclaux R, Kenshalo DR. Response characteristics of cutaneous warm receptors in the monkey. Journal of Neurophysiology. 1980;43:1–15. doi: 10.1152/jn.1980.43.1.1. [DOI] [PubMed] [Google Scholar]
- Hensel H. Thermoreception and Temperature Regulation. London: Academic Press; 1981. [PubMed] [Google Scholar]
- Johnson KO, Darian-Smith I, LaMotte C, Johnson B, Oldfield S. Coding of incremental changes in skin temperature by a population of warm fibers in the monkey: Correlation with intensity discrimination in man. Journal of Neurophysiology. 1979;42:1332–1353. doi: 10.1152/jn.1979.42.5.1332. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR. Correlations of temperature sensitivity in man and monkey, a first approximation. In: Zotterman Y, editor. Sensory Functions of the Skin. Oxford: Pergamon Press; 1976. pp. 305–330. [Google Scholar]
- Konietzny F, Hensel H. The dynamic response of warm units in human skin nerves. Pflügers Archiv. 1977;370:111–114. doi: 10.1007/BF00707956. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER, Burgess PR, Whitehorn D. Ascending projections from marginal zone (lamina I) neurons of the spinal dorsal horn. Journal of Comparative Neurology. 1975;162:1–10. [Google Scholar]
- LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. Journal of Neurophysiology. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Light AR. The Initial Processing of Pain and its Descending Control: Spinal and Trigeminal Systems. Basel: Karger; 1992. [Google Scholar]
- Marks LE, Stevens JC. Perceived warmth and skin temperature as functions of the duration and level of thermal irradiation. Perception and Psychophysics. 1968;4:220–228. [Google Scholar]
- Molinari HH, Greenspan JD, Kenshalo DR. The effects of rate of temperature change and adapting temperature on thermal sensitivity. Sensory Processes. 1977;1:354–362. [PubMed] [Google Scholar]
- Norrsell U. Thermosensory defects after cervical spinal cord lesions in the cat. Experimental Brain Research. 1979;35:479–494. doi: 10.1007/BF00236766. [DOI] [PubMed] [Google Scholar]
- Norrsell U. Unilateral behavioural thermosensitivity after transection of one lateral funiculus in the cervical spinal cord of the cat. Experimental Brain Research. 1983;53:71–80. doi: 10.1007/BF00239399. [DOI] [PubMed] [Google Scholar]
- Pehl U, Schmid HA, Simon E. Temperature sensitivity of neurones in slices of the rat spinal cord. Journal of Physiology. 1997;498:483–495. doi: 10.1113/jphysiol.1997.sp021874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. Temperature signals from skin and spinal cord converging on spinothalamic neurons. Pflügers Archiv. 1972;337:323–332. doi: 10.1007/BF00586649. [DOI] [PubMed] [Google Scholar]
- Simon E, Iriki M. Sensory transmission of spinal heat and cold sensitivity in ascending spinal neurons. Pflügers Archiv. 1971;328:103–120. doi: 10.1007/BF00592439. [DOI] [PubMed] [Google Scholar]
- Villaneuva L, Nathan PW. Multiple pain pathways. In Proceedings of the 9th World Congress on Pain. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Progress in Pain Research and Management. Vol. 16. Seattle: IASP Press; 2000. pp. 371–386. [Google Scholar]


