Abstract
In McArdle's disease, muscle glycogenolysis is blocked, which results in absent lactate and enhanced ammonia production in working muscle. Using McArdle patients as an experimental model, we studied whether lactate and ammonia could be mediators of the exercise pressor reflex.
Changes in muscle interstitial ammonia and lactate were compared with changes in blood pressure and muscle sympathetic nerve activity (MSNA) during static arm flexor exercise at 30 % of maximal contraction force. Muscle interstitial changes in lactate and ammonia were assessed by microdialysis of the biceps muscle, and MSNA by peroneal nerve microneurography, in six McArdle patients and 11 healthy, matched controls. One McArdle patient also had myoadenylate deaminase deficiency, a condition associated with abolished ammonia production in exercise.
Exercise-induced increases were higher in McArdle patients vs. controls for MSNA (change of 164 ± 71 vs. 59 ± 19 %) and blood pressure (change of 47 ± 7 vs. 38 ± 4 mmHg). Interstitial lactate increased in controls (peak change 1.3 ± 0.2 mmol l−1) and decreased in McArdle patients (peak change -0.5 ± 0.1 mmol l−1) during and after exercise. Interstitial ammonia did not change during exercise in either group, but was higher post-exercise in McArdle patients, except in the patient with myoadenylate deaminase deficiency who had a flat ammonia response. This patient had an increase in MSNA and blood pressure comparable to other patients. MSNA and blood pressure responses were maintained during post-exercise ischaemia in both groups, indicating that sympathetic activation was caused, at least partly, by a metaboreflex.
In conclusion, changes in muscle interstitial lactate and ammonia concentrations during and after exercise are temporally dissociated from changes in MSNA and blood pressure in both patients with McArdle's disease and healthy control subjects. This suggests that muscle acidification and changes in interstitial ammonia concentration are not mediators of sympathetic activation during exercise.
It is well recognized that sympathoactivation of cardiovascular and neuroendocrine responses in exercise is regulated by neural feedback from chemosensitive nerve endings in the interstitium of working muscle (Kaufman & Forster, 1996). It is unknown, however, which chemical substances elicit this reflex. A drop in pH, related to lactate production in exercise, has repeatedly been suggested to be a potent stimulator of the muscle reflex (Victor et al. 1988; Pryor et al. 1990; Ettinger et al. 1991; Sinoway et al. 1992). This has been inferred from both animal and human studies where a temporal correlation between sympathetic activation and the development of muscle acidosis in exercise has been observed (Thimm et al. 1984; Victor et al. 1988; Rotto et al. 1989; Stebbins & Longhurst, 1989). Furthermore, conditions that lower lactate production, such as glycogen depletion, myophosphorylase deficiency and dichloroacetate infusion, have been associated with an attenuated sympathetic activation during exercise (Pryor et al. 1990; Ettinger et al. 1991; Sinoway et al. 1992).
Another potential mediator of the exercise reflex is ammonia, which in intense exercise is produced primarily from the deamination of AMP by myoadenylate deaminase (Hellsten et al. 1999). Plasma ammonia concentration has been found to correlate closely with heart rate and fatigue during exercise in both healthy subjects (Roeykens et al. 1998; Harris et al. 1999) and patients with exaggerated ammonia production due to blocked muscle glycogenolysis (Coakley et al. 1992). These observations indicate that ammonia could be a mediator of the exercise reflex.
The study of potential chemical mediators in muscle that may activate reflex sympathetic responses in exercise has been limited by the inability to study metabolic events occurring in the interstitium of contracting muscle. Metabolic changes in muscle assessed by 31P-magnetic resonance spectroscopy preferentially reflect intracellular and not interstitial changes. Furthermore, the use of metabolite concentration in effluent venous blood from exercising muscle as an index of interstitial metabolite concentration relies on assumptions of steady state for metabolism and transport. Quantitative measurement of interstitial metabolites by microdialysis can more directly illustrate changes in the interstitial milieu of muscle. The use of this technique on resting skeletal muscle was first reported by Maggs et al. in 1995, and has subsequently been applied to contracting muscle (MacLean et al. 1999).
In the present study, microdialysis of exercising muscle was performed simultaneously with direct microneurographic recordings of muscle sympathetic nerve activity (MSNA). Specifically, we aimed to test whether interstitial accumulation of lactate and/or ammonia could be mediators of the exercise metaboreflex, leading to increased sympathetic traffic and blood pressure. To accomplish this we studied patients with myophosphorylase deficiency (McArdle's disease) and the rare combination of myophosphorylase and myoadenylate deaminase deficiencies. A lack of production or accumulation of lactate and an increased ammonia production during exercise characterize patients with McArdle's disease (McArdle, 1951; Rumpf et al. 1981; Haller & Bertocci, 1994; Vissing & Haller 2001). In contrast, myoadenylate deaminase deficiency is characterized by a lack of ammonia production during exercise.
METHODS
The study was approved by the ethical committee of Copenhagen and Frederiksberg and the institutional review board of the University of Texas, Southwestern Medical Center and Presbyterian Hospital, Dallas. The study conformed to the standards set by the Declaration of Helsinki. The subjects were informed of the nature and risks of the study, and gave written consent to participate.
Subjects
Three women and three men, aged 25-50 years (mean 36 years), with McArdle's disease and six healthy subjects individually matched for age (22-50 years, mean 37 years), sex, weight and approximate arm strength were used for the experiments. Another five healthy subjects, also matched for age, sex, weight and approximate arm strength, were used for a series of additional experiments aimed at disclosing the effect of post-exercise ischaemia on sympathetic responses.
In muscle biopsies of each McArdle patient, myophosphorylase activity had been documented to be absent by biochemical analyses. In addition to myophosphorylase deficiency, one McArdle patient had myoadenylate deaminase deficiency (MADD) as demonstrated by histochemical staining, a previous ischaemic forearm exercise test showing no increase in venous effluent ammonia and genetic analysis demonstrating the common homozygous C→T point mutation at nucleotide 34 in the second exon of the MAD gene on chromosome 1. All patients had lifelong exercise intolerance and had experienced myoglobinuria after strenuous exercise. All subjects had normal 12-lead ECGs and neurological examination. No subject took any medication.
Experimental set-up
All subjects were studied supine. The exercising arm was in a flexed position and supported by a cast to avoid changes in force transduction. At the beginning of each protocol, the individual maximal voluntary contraction force (MVC) was determined.
Microdialysis technique
Probes were made from artificial kidney dialysis tubes (GFE 18) with a molecular mass cut-off at 3000 Da. Each end of the probe was inserted into a nylon tube (inner diameter = 0.50 mm, outer diameter = 0.63 mm) and glued. The free probe length between the two nylon tubes was approximately 3 cm with an inner diameter of 0.20 mm and an outer diameter of 0.22 mm. The sites on the skin where probes entered and exited were anaesthetized with xylocain containing adrenaline. Care was taken not to anaesthetize the underlying muscle. Three microdialysis catheters were placed in the biceps muscle of the dominant arm by use of a 16 g curved cannula inserted parallel to the muscle fibres. The distance between the probe entry and exit points on the skin was 6-7 cm and the distance between probes in the muscle was 2-3 cm. One patient had only two probes inserted because her biceps muscle was small. All probes were perfused at a rate of 7.5 μl min−1 with the same Ringer-acetate solution, containing 3.0 mm of glucose and 0.5 mm of lactate, in order to minimize the risk of draining the interstitial space (Lonnroth et al. 1987), and approximately 0.2 μCi ml−1 of l-[U-14C]lactate, which acted as an internal reference for determination of the recovery rate of lactate as previously described (Scheller & Kolb, 1991). The probes were perfused for at least 60 min before data collection was started, to allow the tissue to recover from possible changes in the interstitial environment induced by the insertion. The distal exteriorized tip of the dialysate catheters was placed in a microtube for dialysate collection at rest, and during exercise and recovery. The timing of dialysate collections was delayed according to the transition time of the perfusate in the probes. To determine the actual probe perfusion rate, each dialysate tube was weighed before and after each collection. Dialysate samples were collected 1 h after the exercise bout to ensure that the interstitial concentration of lactate and ammonia returned to baseline values following exercise. Dialysates from different probes were not pooled.
Due to the limited volume of dialysate that could be collected during exercise, not all probes could be evaluated for lactate and ammonia at all time points. For this reason, results are presented both as data from all probes with sufficient volume of dialysate to permit analysis (Table 2) and as paired data derived from probes that could be evaluated at all time points (Fig. 1). The number of probes from which probe recovery and concentrations of lactate and ammonia were obtained is provided in Fig. 1 and Tables 1 and 2.
Table 2.
Interstitial and dialysate concentrations of lactate and ammonia in biceps muscle at rest, during exercise and recovery, and at rest 1 h after exercise
| Post-exercise | ||||
|---|---|---|---|---|
| Rest | Exercise | Recovery | Rest | |
| Lactate (mM) | ||||
| McArdle | 2.3 ± 0.2 (16) | 1.8 ± 0.3 *† (5) | 2.0 ± 0.2 † (9) | 2.9 ± 0.2 (13) |
| Controls | 2.2 ± 0.2 (16) | 2.5 ± 0.2 * (3) | 3.5 ± 0.5 * (6) | 2.5 ± 0.2 (17) |
| Ammonia (μM) | ||||
| McArdle | 23 ± 3 (15) | 28 ± 8 (7) | 50 ± 7 *† (12) | 24 ± 4 (13) |
| Controls | 16 ± 2 (16) | 12 ± 3 (7) | 24 ± 4 (13) | 19 ± 2 (15) |
| McArdle + MADD | 22 (1) | 13 (1) | 27 (1) | 17 (1) |
Values are means ±s.e.m. The exercise protocol was static arm flexor exercise at 30% of MVC in six patients with McArdle's disease and six healthy control subjects.
Significantly different change from Rest in all McArdle patients compared to controls.
Significant change from rest in all subjects. Ammonia results in the combined myophosphorylase-and myoadenylate deaminase-deficient patient (McArdle + MADD) are shown separately. Numbers of probes from which data are derived are shown in parentheses.
Figure 1.
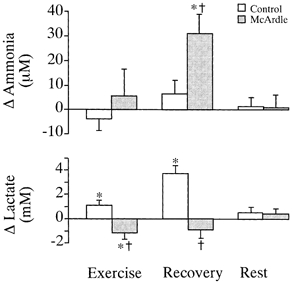
Changes during and after exercise in interstitial lactate and dialysate ammonia concentrations in biceps muscle of six patients with McArdle's disease and six healthy matched controls. Changes are from pre-exercise resting levels to: (1) exercise, (2) the first 4 min of recovery from exercise and (3) at rest 1 h following static arm flexor exercise, in probes from which data could be obtained at all time points. The number of probes evaluated at all time points was (McArdle/control) 6/7 for ammonia and 4/3 for lactate. Ammonia results do not include data from the patient with combined myophosphorylase and myoadenylate deaminase deficiency. Ammonia data from this patient are shown separately in Table 2. Values are means ±s.e.m.† Significantly different change from pre-exercise rest in all patients vs. controls. * Significantly different change from pre-exercise rest in all subjects.
Table 1.
Probe recoveries of lactate at rest, during static arm flexor exercise at 30 % of MVC and during recovery, and at rest 1 h after exercise
| Post-exercise | ||||
|---|---|---|---|---|
| Rest | Exercise | Recovery | Rest | |
| McArdle | 12.8 ± 1.8 (15) | 21.8 ± 3.8 * (7) | 18.4 ± 2.0 * (9) | 13.5 ± 1.8 (13) |
| Controls | 12.7 ± 1.7 (17) | 21.2 ± 6.5 * (7) | 17.3 ± 2.0 (12) | 14.4 ± 2.0 (16) |
Values (%) are means ±s.e.m.
Significant change (P < 0.05) from Rest. Numbers of probes from which data are derived are shown in parentheses.
Microneurographic technique
Multiunit recordings of post-ganglionic muscle sympathetic nerve activity (MSNA) were obtained with tungsten microelectrodes inserted into muscle nerve fascicles of the peroneal nerve, using the microneurographic technique of Vallbo et al. (1979). The neural signals were amplified, filtered (bandwidth of 700-2000 Hz), rectified and integrated to obtain a mean voltage display of MSNA. Requirements for an acceptable recording have been described in detail previously (Vissing, 1997). Nerve traffic was expressed both as bursts per minute (an index of the frequency of sympathetic discharge), and as bursts per minute multiplied by the mean burst amplitude, which was expressed as a percentage of the control value (an estimate of relative changes in integrated activity). All subjects undergoing microdialysis had microneurography performed simultaneously.
The response of MSNA to baroreceptor unloading during a Valsalva manoeuvre was used as a standard non-exercise stimulus to evaluate the validity of MSNA responses obtained during exercise (Vallbo et al. 1979).
Specific protocols
Biceps muscle interstitial lactate and ammonia and reflex sympathetic activation during fatiguing isometric arm flexion
The aim of this protocol was to test whether sympathoexcitation during exercise is influenced by changes in interstitial lactate and ammonia in contracting muscle. In five patients with McArdle's disease, one with combined McArdle's disease and MADD, and six age- and sex-matched healthy subjects, MSNA, blood pressure (Finapres BP monitor, Ohmeda, Englewood, CO, USA), heart rate (from ECG) and force of muscle contraction were recorded and microdialysate was collected simultaneously before, during and after static arm flexor exercise to exhaustion at 30 % of MVC. The exercise was performed by pulling a hand-held lever upwards against a force transducer. In this way, the subjects recruited both upper arm and forearm flexor muscles during exercise. One dialysate sample from each probe was collected at rest and one during recovery. The sampling time was the 4 min immediately preceding and following exercise. One sample from each probe was also collected during exercise, but since the average duration of exercise was only a little more than 2 min, dialysate sample volume was correspondingly lower than rest and recovery samples.
Venous effluent blood lactate and ammonia during fatiguing isometric handgrip
. It was not possible to sample venous effluent blood from the exercised biceps muscle. However, to investigate the relationship between interstitial and effluent venous changes in lactate and ammonia concentrations, handgrip exercise at 30 % MVC was performed in the same subjects with the arm contralateral to the microdialysis site. Plasma levels of lactate and ammonia were then measured in effluent blood from a cubital vein at the times depicted in Fig. 4. The duration of the handgrip exercise was the same as that of the exercise performed during the arm flexor protocol.
Figure 4.
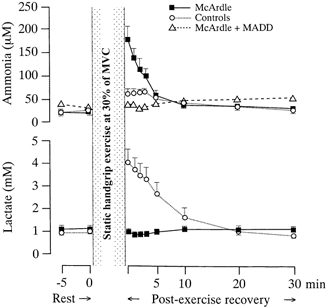
Plasma concentrations of lactate and ammonia in cubital venous blood at rest and after exhaustive static handgrip exercise at 30 % of MVC in six patients with McArdle's disease (□) and six matched healthy controls (○). Values are means ±s.e.m. Ammonia data from the patient with combined myophosphorylase and myoadenylate deaminase deficiency (▵) are shown separately from those of the other five McArdle patients in the top panel. All other data points are based on findings in six subjects in each group.
Reflex sympathetic activation and blood pressure during isometric biceps flexion and post-exercise ischaemia
To investigate whether exercise-induced sympathetic activation was caused by mechanisms other than a metaboreflex (i.e. baroreceptor resetting, central command or muscle mechanoreflexes), we restudied three of the McArdle patients and five new healthy subjects during post-exercise ischaemia. All eight subjects were studied with microneurography at rest, during static elbow-flexing exercise at 30 % MVC until near exhaustion, during 1 min of post-exercise ischaemia induced by inflating a narrow blood pressure cuff to above 280 mmHg as proximal as possible on the upper arm, and during recovery. Ischaemic forearm exercise produces painful cramps in McArdle's disease. Therefore, post-exercise ischaemia was limited to 1 min, the subjects were not pushed quite to exhaustion and exercise was performed with isolated elbow flexion by having the subjects pull a strap around the wrist instead of pulling a handle with their hands as in the main experiments.
Data analysis and calculations
Blood was spun at 4000 r.p.m. for 4 min in a cooled centrifuge and the plasma was stored with dialysate samples at -80 °C until analysis for lactate (using a YSI 2300 STAT glucose/lactate analyser) and ammonia (Kun & Kearney, 1974). The volume of dialysate needed for analysis of ammonia and lactate was 5 and 12 μl, respectively. Before the dialysates were frozen, 5 μl of the dialysate from each probe was added to 3 ml of scintillation fluid and counted in a scintillation counter to determine the activity of l-[U-14C]lactate, so that recovery of lactate could be calculated for individual probes. Recovery of lactate was calculated according to the internal reference method (Scheller & Kolb, 1991), as:
where Pdpm and Ddpm represent disintegrations per minute in the perfusate and dialysate, respectively, for labelled lactate. The interstitial concentration of lactate was then calculated as:
where Dc and Pc represent dialysate and perfusate concentrations of lactate, respectively.
Statistical analysis was performed with Page's test for ordered alternatives followed by multiple comparisons with a control, or by Wilcoxon's ranked sum test. Results are expressed as means ±s.e.m.P < 0.05 was considered significant.
RESULTS
Rest and Valsalva manoeuvre data
At rest, plasma and interstitial concentrations and probe recovery of lactate (Fig. 4, Tables 1 and 2), plasma and dialysate concentrations of ammonia (Fig. 4, Table 2), MSNA, heart rate and blood pressure (Table 3) did not differ between McArdle patients and controls. Interstitial lactate and dialysate ammonia concentrations returned to pre-exercise values 1 h after static arm flexor exercise in all subjects (Fig. 1, Table 2). The Valsalva manoeuvre evoked comparable increases in total MSNA in McArdle patients (change of 230 ± 131 %) and healthy subjects (change of 314 ± 118 %).
Table 3.
MSNA, heart rate and blood pressure (BP) responses to static arm flexor exercise at 30% MVC
| Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 0.5 min | 1.0 min | 1.5 min | 2.0 min | Exhaustion or 3 min | Recovery | ΔMax | |
| MSNA (bursts min-1) | ||||||||
| McArdle | 25 ± 5 | 28 ± 5 | 33 ± 6 | 44 ± 7 † | 44 ± 8 † | 47 ± 7 † | 31 ± 6 | 22 ± 6 † |
| Controls | 20 ± 3 | 20 ± 4 | 24 ± 4 | 25 ± 5 | 23 ± 4 | 30 ± 4 | 19 ± 4 | 10 ± 2 |
| McArdle + MADD | 22 | 34 | 34 | 41 | 46 | 41 | 25 | 24 |
| MSNA (total activity) | ||||||||
| McArdle | — | 25 ± 27 | 48 ± 22 | 130 ± 64 | 128 ± 77 † | 164 ± 71 † | 29 ± 15 † | 164 ± 71 † |
| Controls | — | 0 ± 21 | 14 ± 16 | 22 ± 23 | 28 ± 25 | 59 ± 19 | 4 ± 22 | 59 ± 19 |
| McArdle + MADD | — | 72 | 74 | 92 | 159 | 92 | 14 | 159 |
| Heart rate (beats min-1) | ||||||||
| McArdle | 70 ± 3 | 78 ± 5 | 81 ± 5 | 85 ± 5 | 89 ± 7 | 90 ± 6 | 75 ± 4 | 20 ± 3 |
| Controls | 66 ± 2 | 84 ± 3 | 84 ± 2 | 86 ± 2 | 89 ± 2 | 92 ± 2 | 67 ± 4 | 25 ± 2 |
| McArdle + MADD | 66 | 69 | 73 | 90 | 93 | 93 | 79 | 27 |
| Mean BP (mmHg) | ||||||||
| McArdle | 108 ± 12 | 130 ± 16 | 135 ± 17 | 142 ± 14 | 143 ± 14 | 160 ± 13 † | 132 ± 13 | 47 ± 7 † |
| Controls | 122 ± 11 | 134 ± 12 | 142 ± 15 | 147 ± 14 | 155 ± 14 | 160 ± 13 | 125 ± 17 | 38 ± 4 |
| McArdle + MADD | 117 | 134 | 138 | 152 | 160 | 160 | 138 | 43 |
Data are from five patients with myophosphorylase deficiency (McArdle), six healthy sedentary subjects (Controls) and one patient with combined myophosphorylase and myoadenylate deaminase deficiency (McArdle + MADD). MSNA is expressed both as bursts per min and total activity (% change from rest). ΔMax, maximal increase from Rest to Exercise. The separately shown results in the myophosphorylase- and myoadenylate-deficient patient are also integrated in the grouped data for all McArdle patients. Values are means ±s.e.m.
Significantly greater increase from rest in McArdle patients compared to controls (P <0.05).
Exercise duration and force of contraction
In the main protocol, MVC did not differ significantly between McArdle patients (23 ± 5 kg) and healthy controls (27 ± 6 kg). Thus, the average force development during arm flexor exercise was similar in the two groups (McArdle patients 7 ± 2 kg; controls 8 ± 2 kg). The duration of exercise in the intended 3 min protocol was similar in the McArdle patients (155 ± 11 s) and control subjects (144 ± 16 s).
In the isolated biceps exercise protocol with post-exercise ischaemia, the MVC was lower in McArdle patients (11 ± 3 kg) than in healthy subjects (16 ± 2 kg). The duration of exercise was similar in the two groups (McArdle patients 160 ± 26 s; healthy subjects 168 ± 7 s).
Muscle sympathetic discharge and cardiovascular responses to exercise
MSNA, heart rate and blood pressure all increased significantly with exercise in both groups. After 1.5 min of exercise, MSNA was higher in McArdle patients than in corresponding control subjects, and at exhaustion both MSNA and increases in blood pressure were higher in every McArdle patient vs. the corresponding control (Table 3, Fig. 2). MSNA and pressor responses in the patient with combined McArdle's disease and myoadenylate deaminase deficiency were comparable to those in other McArdle patients (Table 3). Heart rate responses to exercise were comparable in McArdle patients and controls (Table 3).
Figure 2.
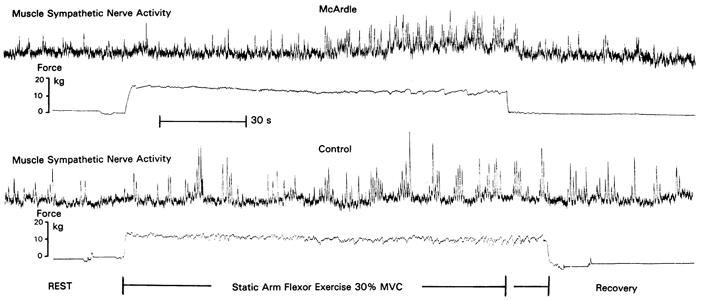
Integrated neurograms of muscle sympathetic nerve activity and force development during static arm flexor exercise at 30 % of MVC in a patient with McArdle's disease and a corresponding, healthy, control subject.
Where responses to post-exercise ischaemia were evaluated, the same pattern of sustained MSNA and pressor responses during post-exercise ischaemia was observed in the two groups (Fig. 3). MSNA and blood pressure returned to basal levels during recovery in both groups. The magnitude of exercise-induced increases in MSNA and blood pressure was numerically higher in healthy controls due to very high responses in two of the five healthy subjects, but changes did not differ statistically between groups.
Figure 3.
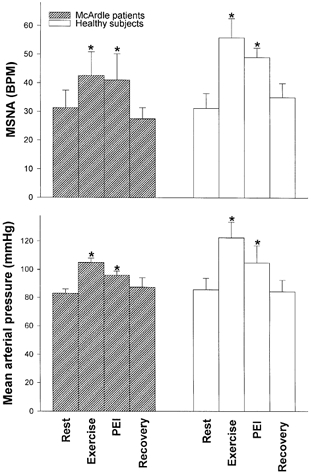
MSNA expressed as bursts min−1 (BPM) and mean arterial blood pressure in three patients with McArdle's disease and five healthy matched subjects at rest, at the end of 2-3 min of static elbow flexing exercise at 30 % of MVC (Exercise), during post-exercise ischaemia (PEI) and during recovery. Values are means ±s.e.m.* Significantly different from Rest values in all subjects. All data points are based on observation in three McArdle patients and five healthy subjects, except blood pressure data, which are missing for one healthy subject, and recovery data, which are missing for one of the McArdle patients.
Interstitial lactate and dialysate ammonia concentrations in biceps muscle during and after exercise
As evident from Table 1, probe recoveries for lactate increased similarly in McArdle patients and control subjects with exercise. During exercise, interstitial lactate decreased in all McArdle patients and increased in all healthy controls (Table 2). During recovery, interstitial lactate increased further in controls. Dialysate concentrations of ammonia did not change significantly with exercise in either of the two groups but, during recovery from exercise, dialysate ammonia increased significantly in McArdle patients above that observed in controls (Table 2). In the patient with combined McArdle's disease and myoadenylate deaminase deficiency, lactate responses were similar to findings in other McArdle patients, and therefore lactate data from this patient were pooled with those from other McArdle patients. In contrast, the patient showed no increase in dialysate ammonia compared to other McArdle patients (Table 2). Muscle interstitial lactate and ammonia concentrations assessed by pooling data from all probes that could be evaluated (Table 2) were similar to the concentrations obtained from probes that could be evaluated at all time points (Fig. 1).
Plasma lactate and ammonia
Systemic plasma lactate and ammonia levels were similar to those reported at rest in Fig. 4, and did not change at any time during the microdialysis protocol. In line with the changes in dialysate ammonia and interstitial lactate, McArdle patients had higher post-exercise ammonia and lower lactate levels in venous effluent plasma from the exercised arm than controls (Fig. 4). Thus, plasma changes mirrored interstitial conditions for these substances.
DISCUSSION
Using inborn errors of muscle lactate and ammonia metabolism as models, this study evaluated whether exercise-induced sympathetic activation is affected by changes in muscle interstitial lactate and ammonia concentrations in contracting muscle. The principal findings were twofold. First, muscle acidification related to exercise-induced lactate production is not needed to produce a reflex sympathoexcitation during exercise. Thus, MSNA and cardiovascular pressor responses to static exercise were preserved in patients in whom lactate production was abolished, and peak interstitial lactate levels coincided with a drop in MSNA to basal values in the immediate recovery period after exercise in healthy controls. Second, ammonia does not seem to be a mediator of the exercise reflex either. This is suggested by normal increases in blood pressure and MSNA in a patient in whom ammonia production was abolished, and the finding in other patients with enhanced ammonia production of high interstitial ammonia concentrations only in the recovery period after exercise when pressor and MSNA responses had returned to basal values. In addition, sustained exercise-induced pressor and MSNA responses to post-exercise ischaemia in both McArdle patients and healthy control subjects, indicate that the exercise-induced sympathetic activation was at least partly caused by a metaboreflex arising in the muscle.
Other studies have suggested a pivotal role of muscle acidosis/lactate for reflex sympathoactivation in exercise. This proposal has been based on a temporal correlation between changes in muscle pH and sympathetic activation during exercise (Thimm et al. 1984; Victor et al. 1988; Rotto et al. 1989; Sinoway et al. 1989; Stebbins & Longhurst 1989) and an attenuation of sympathetic responses in conditions associated with low lactate levels (Pryor et al. 1990; Ettinger et al. 1991; Sinoway et al. 1992). These studies did not assess muscle interstitial lactate or pH. They also did not address the contradiction to the pH theory represented by the fact that a fall in sympathetic activity to basal levels immediately post-exercise coincides with a further decline in muscle pH (Arnold et al. 1984), and with an increase in muscle interstitial lactate levels, as shown by the present study. Lactate is an important surrogate marker for mitochondrial energy metabolism, but our results indicate that lactate accumulation is merely an epiphenomenon with respect to sympathetic reflex activation during exercise.
We have previously investigated the role of muscle pH/lactate for reflex sympathoactivation in McArdle patients (Vissing et al. 1998), in a study where forearm muscles, and not combined forearm and upper arm flexor muscles, as in the present study, were exercised. In the previous study, we correlated sympathetic responses to 31P-magnetic resonance spectroscopy-assessed intracellular and not interstitial metabolic changes. Since the metaboreflex is elicited by chemical stimulation of nerve endings in the muscle interstitium, the present microdialysis study provides the opportunity to better correlate metabolic changes to sympathoexcitation. Furthermore, by using inborn errors of ammonia metabolism, the present study provides new information on the role of ammonia for sympathetic activation during exercise.
The higher sympathetic activation in McArdle patients during exercise in the present study compared to findings in the previous study (Vissing et al. 1998) could relate to the recruitment of a larger muscle mass in the present study. In line with this, McArdle patients also have an enhanced pressor and neurohormonal response when larger muscle masses are recruited during cycling (Andersen et al. 1969; Vissing et al. 1992). Similar exercise-induced increases in MSNA and blood pressure in patients and controls in the post-exercise ischaemia protocol, where subjects were not pushed quite to exhaustion, may also indicate that part of the exercise MSNA and pressor responses to exhaustive exercise in the main protocol could be caused by increased central command in the patients.
Microdialysis probe recovery rates of lactate at rest and during exercise were virtually identical in patients and healthy subjects, suggesting that changes in interstitial lactate were directly comparable in the two groups. Furthermore, absolute concentrations of interstitial lactate were very similar to venous effluent lactate levels, which is in agreement with the rapid diffusion and exchange of lactate in working muscle (Juel & Halestrap, 1999).
Although an internal reference marker for ammonia was not used, the fivefold difference in magnitude of dialysate ammonia change between groups cannot be explained by differences in recovery. However, dialysate ammonia levels were lower than those observed in venous effluent blood from an exercised arm, indicating that dialysate ammonia concentration underestimates absolute interstitial ammonia levels, probably because of low probe recovery rates for ammonia. This indicates that absolute interstitial ammonia levels were higher during exercise and immediately post-exercise than reflected by dialysate levels. Still, the time course of ammonia changes, with higher levels post-exercise when sympathetic activity had returned to basal levels, and the normal sympathetic activation during exercise in the myoadenylate deaminase-deficient patient in whom ammonia production was abolished, indicate that ammonia does not reflexly stimulate sympathoexcitation in exercise. Like lactate, the association of ammonia with sympathoexcitation in exercise has previously been based exclusively on a temporal correlation between changes in the two parameters (Coakley et al. 1992; Roeykens et al. 1998; Harris et al. 1999).
The results of the present study call for the search for chemical compounds in muscle that can potentially act as mediators of the exercise reflex. Using the microdialysis technique while simultaneously monitoring sympathetic activity, as performed in the present study, will provide an opportunity to test temporal associations between changes in chemical compounds in the muscle interstitium and sympathetic exercise responses. Substances of interest to study have been reviewed recently (Vissing, 2000). Potassium may be particularly interesting to study because, unlike lactate and muscle pH, changes in potassium in effluent venous blood from an exercised limb, and therefore in all likelihood also muscle interstitial levels, appear to follow changes in sympathetic activity during and after exercise. Furthermore, changes in interstitial potassium have been implicated as a mediator of the exercise pressor reflex in healthy subjects (Wildenthal et al. 1968; Rybicki et al. 1985). In McArdle patients, potassium may also be an important mediator that can explain enhanced sympathetic activation in this condition during dynamic exercise (Vissing et al. 1992), where exercise consistently elicits an abnormally high increase in plasma potassium levels (McArdle, 1951; Kono et al. 1984; Paterson et al. 1990; Haller et al. 1998). A lower level of Na+-K+-ATPase in the sarcolemma of McArdle patients (Haller et al. 1998) may in part explain this increase.
In summary, the present study has provided evidence that lactate and ammonia are not important mediators that signal reflex sympathetic activation in working muscle. These findings suggest that the hypothesis of muscle acidification in exercise as a prerequisite for sympathoexcitation during exercise should be abandoned.
Acknowledgments
This study was supported by the Danish National Research Foundation (504-14). Additional financial support was provided by a NASA Center for Research and Training (NSCORT) grant, the Muscular Dystrophy Association and VA Merit Review. Excellent technical help was performed by Lynda Powell, Karin Juel and Carsten B. Nielsen.
References
- Andersen KL, Lund-Johansen P, Clausen G. Metabolic and circulatory responses to muscular exercise in a subject with glycogen storage disease (McArdle's disease) Scandinavian Journal of Clinical Laboratory Investigation. 1969;24:105–113. doi: 10.3109/00365516909080140. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magnetic Resonance Medicine. 1984;1:307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- Coakley JH, Wagenmakers AJ, Edwards RH. Relationship between ammonia, heart rate, and exertion in McArdle's disease. American Journal of Physiology. 1992;262:E167–172. doi: 10.1152/ajpendo.1992.262.2.E167. [DOI] [PubMed] [Google Scholar]
- Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. American Journal of Physiology. 1991;261:H1653–1658. doi: 10.1152/ajpheart.1991.261.5.H1653. [DOI] [PubMed] [Google Scholar]
- Haller RG, Bertocci LA. Exercise evaluation of metabolic myopathies. In: Engel AG, Franzini-Armstrong C, editors. Myology. 2. New York: McGraw-Hill; 1994. pp. 807–821. [Google Scholar]
- Haller RG, Clausen T, Vissing J. Reduced levels of skeletal muscle Na+-K+-ATPase in McArdle disease. Neurology. 1998;50:37–40. doi: 10.1212/wnl.50.1.37. [DOI] [PubMed] [Google Scholar]
- Harris RC, Harris DB, Dunnett M, Harris PA, Fallowfield J, Naylor JR. Plasma ammonia and lactate responses using incremental and constant speed exercise tests. Equine Veterinary Journal. 1999;30(suppl.):546–551. doi: 10.1111/j.2042-3306.1999.tb05281.x. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Richter EA, Kiens B, Bangsbo J. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. Journal of Physiology. 1999;520:909–920. doi: 10.1111/j.1469-7793.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. Journal of Physiology. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Integration of Multiple Systems. New York: American Physiological Society, Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- Kono N, Mineo I, Sumi I, Shimizu T, Kang J, Nonaka K, Tarui S. Metabolic basis of improved exercise tolerance in muscle phosphorylase deficiency after glucagon administration. Neurology. 1984;34:1471–1476. doi: 10.1212/wnl.34.11.1471. [DOI] [PubMed] [Google Scholar]
- Kun E, Kearney EB. Ammonia. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1802–1805. [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. American Journal of Physiology. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- McArdle B. Myopathy due to a defect in muscle glycogen breakdown. Clinical Science. 1951;10:13–32. [PubMed] [Google Scholar]
- MacLean DA, Bangsbo J, Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. Journal of Applied Physiology. 1999;87:1483–1490. doi: 10.1152/jappl.1999.87.4.1483. [DOI] [PubMed] [Google Scholar]
- Maggs DG, Jacob R, Rife F, Lange R, Leone P, During MJ, Tamborlane WV, Sherwin RS. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadriceps and adipose tissue. Journal of Clinical Investigation. 1995;96:370–377. doi: 10.1172/JCI118043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DJ, Friedland JS, Bascom DA, Clement ID, Cunningham DA, Painter R, Robbins PA. Changes in arterial K+ and ventilation during exercise in normal subjects and subjects with McArdle's syndrome. Journal of Physiology. 1990;429:339–348. doi: 10.1113/jphysiol.1990.sp018260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's disease) Journal of Clinical Investigation. 1990;85:1444–1449. doi: 10.1172/JCI114589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeykens J, Magnus L, Rogers R, Meeusen R, De Meirleir K. Blood ammonia-heart rate relationship during graded exercise is not influenced by glycogen depletion. International Journal of Sports Medicine. 1998;19:26–31. doi: 10.1055/s-2007-971875. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. Journal of Applied Physiology. 1989;67:256–263. doi: 10.1152/jappl.1989.67.1.256. [DOI] [PubMed] [Google Scholar]
- Rumpf KW, Wagner H, Kaiser H, Meinck H-M, Goebel HH, Scheler F. Increased ammonia production during forearm ischemic work test in McArdle's disease. Klinische Wochenschrift. 1981;59:1319–1320. doi: 10.1007/BF01711182. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis muscle interstitial potassium concentrations stimulate group III and IV afferents. Journal of Applied Physiology. 1985;58:936–941. doi: 10.1152/jappl.1985.58.3.936. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methodology. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Phophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. Journal of Applied Physiology. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. American Journal of Physiology. 1992;263:H1499–1505. doi: 10.1152/ajpheart.1992.263.5.H1499. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Longhurst JC. Potentiation of the exercise pressor reflex by muscle ischemia. Journal of Applied Physiology. 1989;66:1046–1053. doi: 10.1152/jappl.1989.66.3.1046. [DOI] [PubMed] [Google Scholar]
- Thimm F, Carvalho M, Babka M, Meier Zul Verl E. Reflex increase in heart-rate induced by perfusing the hind leg of the rat with solutions containing lactic acid. Pflügers Archiv. 1984;400:286–293. doi: 10.1007/BF00581561. [DOI] [PubMed] [Google Scholar]
- Vallbo CB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. Journal of Clinical Investigation. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J. Muscle reflex and central motor control of neuroendocrine activity, glucose homeostasis and circulation during exercise. Acta Physiologica Scandinavica. 2000;170(suppl. 647):1–26. [PubMed] [Google Scholar]
- Vissing J, Haller RG. Metabolic myopathies. In: Pourmand R, editor. Neuromuscular Disease: Expert Clinician's Views. Boston: Butterworth-Heinemann; 2001. pp. 393–410. [Google Scholar]
- Vissing J, Lewis SF, Galbo H, Haller RG. Effect of deficient muscular glycogenolysis on extramuscular fuel production in exercise. Journal of Applied Physiology. 1992;72:1773–1779. doi: 10.1152/jappl.1992.72.5.1773. [DOI] [PubMed] [Google Scholar]
- Vissing J, Vissing SF, MacLean DA, Saltin B, Quistorff B, Haller RG. Sympathetic activation in exercise is not dependent on muscle acidosis: Direct evidence from studies in metabolic myopathies. Journal of Clinical Investigation. 1998;101:1654–1660. doi: 10.1172/JCI555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing SF. Differential activation of sympathetic discharge to skin and skeletal muscle in humans. Acta Physiologica Scandinavica. 1997;161(suppl.):1–32. [PubMed] [Google Scholar]
- Wildenthal K, Mierzwiak DS, Skinner NS, Mitchell JH. Potassium-induced cardiovascular and ventilatory reflexes from the dog hindlimb. American Journal of Physiology. 1968;215:542–548. doi: 10.1152/ajplegacy.1968.215.3.542. [DOI] [PubMed] [Google Scholar]


