Abstract
The purpose of this investigation was to determine if activation of the exercise pressor reflex in the decerebrate rat induced circulatory responses comparable to those reported in large mammalian species.
To activate both mechanically and metabolically sensitive afferent fibres, static hindlimb contractions were induced by stimulating the cut ends of L4 and L5 spinal ventral roots in Sprague-Dawley rats (300–400 g). To selectively stimulate mechanically sensitive receptors, hindlimb muscles were passively stretched.
In intact halothane-anaesthetized animals (n = 10), static contraction and passive stretch induced a decrease in mean arterial pressure (ΔMAP =−17 ± 3 and −8 ± 1 mmHg for contraction and stretch, respectively) and heart rate (HR). In contrast, MAP increased 23 ± 2 mmHg during contraction and 19 ± 3 mmHg during stretch in decerebrate rats (n = 10). These pressor responses were accompanied by a significant tachycardia. In decerebrate animals, the reintroduction of halothane attenuated the increase in MAP and HR caused by both contraction and stretch.
In both anaesthetized and decerebrate rats, sectioning the spinal dorsal roots innervating the activated skeletal muscle eliminated responses to contraction and stretch. This finding indicated that an intramuscular neural reflex mediated the response to each stimulus.
The results demonstrate that a decerebrate preparation in the rat is a reliable model for the study of the exercise pressor reflex. Development of the model would enable the study of this reflex in a variety of pathological conditions and allow investigation of the mechanisms controlling cardiovascular responses to exercise in health and disease.
Afferent signals from contracting skeletal muscle are an important source of neural input to the brain stem during exercise (Alam & Smirk, 1937; Coote et al. 1971; McCloskey & Mitchell, 1972; Kaufman & Forster, 1996). These exercise-induced signals are generated by activation of group III (predominantly mechanically sensitive A-δ fibres) and IV (predominantly metabolically sensitive C fibres) skeletal muscle afferents which reflexively increase arterial blood pressure (ABP) and heart rate (McCloskey & Mitchell, 1972; Kaufman et al. 1984). Haemodynamic regulation by this reflex loop, termed the exercise pressor reflex, is primarily mediated by increased efferent sympathetic nerve activity (Mitchell et al. 1983).
The rodent is an attractive candidate for the study of the exercise pressor reflex as disease models (e.g. heart failure, hypertension and diabetes) are readily available or easily produced. This is important as previous studies have suggested the exaggerated increases in ABP, sympathetic nerve activity and vascular resistance to exercise in patients with cardiovascular disease are due, in part, to an overactive exercise pressor reflex (Pickering, 1987; Magnusson et al. 1997; Piepoli et al. 1999). Furthermore, more genomic information is currently known about rodents than about larger mammals, presenting the opportunity to study this reflex at the level of cellular and molecular physiology.
Unfortunately, induction of static muscle contraction in the rat has been reported to elicit either no change (Vissing et al. 1991), an increase (Freda et al. 1999) or a decrease in arterial blood pressure (Overton & Stremel, 1992; Toney & Mifflin, 1996). The most obvious difference between these investigations was the method of anaesthesia. It is known that anaesthetic agents influence central haemodynamics as well as the cardiovascular responses to stresses such as haemorrhage and exercise (Vatner, 1978; Longnecker et al. 1982). However, experiments comparing the haemodynamic effects of pharmacological anaesthesia and decerebration in rats have shown that the circulatory responses to haemorrhage in the decerebrate animal closely resemble those elicited in the conscious state (Seyde et al. 1985). Therefore, decerebration, which renders the animal insentient and so obviates the need for anaesthesia, may provide a superior model for the study of cardiovascular control in rodents.
The purpose of the present investigation was to compare the effects of static muscle contraction and passive stretch on cardiovascular control in anaesthetized and decerebrate rats. Intramuscular afferent fibres were stimulated by (i) involuntary muscle contractions induced by either electrical stimulation of sectioned spinal ventral roots or direct electrical stimulation of the sciatic nerve and (ii) passive muscle stretch. We hypothesized that the use of a decerebrate model would elicit circulatory responses to exercise similar to those previously observed in humans and large animals (McCloskey & Mitchell, 1972; Fisher & Nutter, 1974; Rowell et al. 1981). A preliminary report of these findings has been published (Smith et al. 2000).
METHODS
All procedures outlined in this investigation complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care Committee of the University of Texas Southwestern Medical Center. Experiments were performed on 37 Sprague-Dawley male rats (Harlan, body weight 300-400 g). Animals were housed in standard rodent cages and regulated on a 12 h light-dark schedule. Food and water were made available ad libitum.
Surgical preparation
General procedures
Anaesthesia was induced with halothane (2-3 %) in pure oxygen. The trachea was exposed and an endotracheal tube inserted into the airway. Catheters (polyethylene tubing, PE-50) were inserted into the external jugular vein for the administration of drugs and into the common carotid artery for the measurement of ABP. Levels of inhalant gas were increased as indicated by a withdrawal reflex to pinching of the hindpaw, presence of a corneal reflex, and/or spontaneous increases in HR. A continuous infusion (2 ml 1 m NaHCO3 and 10 ml 5 % dextrose in 38 ml Ringer solution) was established via the jugular vein (3-5 ml h−1 kg−1) to stabilize fluid balance and maintain basal ABP (Quintin et al. 1989). In addition, dexamethasone (0.2 mg) was given intramuscularly to minimize oedema (Tian & Duffin, 1996). Animals were artificially ventilated using a mechanical respirator (Model 680, Harvard Apparatus) throughout the experiment. Arterial blood gases and pH were assessed periodically by an automated blood gas analyser (Model ABL 5, Radiometer) and maintained within normal ranges (arterial PO2 > 80 mmHg, arterial PCO2 35-45 mmHg, pH 7.3-7.4). Body temperature was monitored using a rectal thermometer (YSI series 400) and maintained between 36.5 and 38.0 °C by a temperature-controlled water-perfused heating pad. All animals were held in a stereotaxic head unit (Kopf Instruments) and customized spinal frame. At the conclusion of the experiment, animals were humanely killed by intravenous administration of sodium pentobarbital (120 mg kg−1).
Decerebration procedures
Decerebration was carried out using a method similar to that described by Sapru & Krieger (1978). This technique was used in 15 animals in which the ventral roots were exposed and in all animals in which the sciatic nerve was isolated (n = 12). To minimize cerebral haemorrhage, the remaining intact common carotid artery was isolated and ligated. A bilateral craniotomy was performed by drilling burr holes into the parietal skull. Subsequently, the portion of bone superior to the central sagittal sinus was removed. The dura mater was breached and reflected. The cerebral cortex was gently aspirated to visualize the superior and inferior colliculi. Using a blunt instrument, the brain was sectioned pre-collicularly and the transected forebrain aspirated. Small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the exposed surfaces of the brain and cotton balls were used to pack the cranial cavity. A minimum recovery period of 1.25 h was employed post-decerebration before data collection began. This allowed sufficient time for the effects of halothane anaesthesia to be eliminated from the preparation (Kohn, 1997).
Procedures for exposure of the sciatic nerve and lumbar ventral roots
In order to activate both mechanically and metabolically sensitive skeletal muscle afferent fibres, static hindlimb contractions were induced using two distinct methods. In one group of animals (n = 25), a laminectomy exposing the lower lumbar portions of the spinal cord (L2-L6) was performed. The dura of the cord was cut and reflected allowing visual identification of the L4-L6 spinal roots. The dorsal and ventral roots of L4 and L5 were carefully separated. The ventral roots were sectioned and the cut peripheral ends were positioned on insulated bipolar platinum electrodes. In a second set of rats (n = 12), the sciatic nerve was exposed using a dorsal approach. Connective tissue was carefully removed and the nerve placed on an insulated bipolar platinum electrode. In each method of preparation, the exposed neural tissue was covered in a pool of warm mineral oil (37 °C). The animals were secured within the spinal frame by clamps placed on rostral lumbar vertebrae. Further, the pelvis was stabilized with steel posts within the frame and the exercising limb was fixed in one position using clamps attached to the tibial bone. The calcaneal bone was sectioned and the Achilles’ tendon connected to a force transducer (Grass Instruments, FT10) for the measurement of muscle tension. Electrical stimulations were performed using a Grass Instruments S88 stimulator. In animals in which ventral root stimulation was performed, preferential activation of mechanically sensitive intramuscular afferents was achieved by passively stretching the triceps surae muscles using a calibrated 9.5 mm rack and pinion system (Harvard Apparatus, Inc.). Care was taken to match the peak tension developed in response to electrical stimulation during the passive stretch experiments.
Data acquisition
ABP was recorded by connecting the carotid artery catheter to a pressure transducer (Model DTX plus-DT-NN12, Ohmeda). MAP was obtained by integrating the arterial signal with a time constant of 1-4 s. Heart rate was derived from the ABP pulse using a biotachometer (Gould Instruments). All data for these experiments were recorded directly by an eight channel thermal recorder (Astro-Med, Inc.) and subjected to A/D conversion (CED micro 1401, Cambridge Electronic Design Ltd) using commercially available data acquisition software (Spike 2, version 3, Cambridge Electronic Design Ltd) on a personal computer (Pentium III, 550 MHz, Dell Computer Corp.). Computer-acquired data were used in post hoc analyses.
Experimental protocols
Halothane-anaesthetized animals
Rats were divided into two groups based upon their method of surgical preparation. (1) Ventral root stimulation: electrically induced static muscle contraction of the triceps surae was performed by stimulating the L4 and L5 ventral roots for 30 s. Constant-current stimulation was used at 3 times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. These stimulation parameters have been shown to elicit consistent force generation during electrically induced contraction (McCloskey & Mitchell, 1972). Subsequently, passive stretch of the muscle was performed while attempting to generate the same magnitude and pattern of muscle tension developed during electrical stimulation. (2) Sciatic nerve stimulation: static contraction of hindlimb muscle was generated by depolarizing the sciatic nerve for 30 s at twice motor threshold (0.025 ms duration at 40 Hz). These parameters have been reported to minimize direct activation of skeletal muscle afferent fibres within the sciatic nerve (Rybicki & Kaufman, 1985). In all testing protocols each contraction or stretch was separated by a minimum of 10-15 min. In addition, the triceps surae muscles were pre-loaded with 70-100 g of tension prior to any manipulation.
Decerebrate animals
In decerebrate rats the same techniques and protocols as described for halothane-anaesthetized animals were used. Additionally, in a subset of rats in which the ventral roots had been exposed (n = 5), a wide range of stimulus intensities (1-5 times motor threshold) were executed randomly to assess the relationship between tension development and cardiovascular responsiveness. Further, four different magnitudes of tension (250-1000 g) were used to assess the stimulus-response relationship to passive stretch. In a subgroup of rats in which the sciatic nerve was exposed (n = 5), variable intensities (1-3 times motor threshold) were developed randomly to assess the stimulus-response relationship to static contraction.
Experimental controls
Following the contraction and stretch protocols, several control experiments were completed. Firstly, the neuromuscular blocking agent pancuronium bromide (200 μg kg−1) was administered i.v. Electrical activation of either the ventral roots or sciatic nerve was repeated using the stimulus parameters described previously. This manoeuvre was instituted to eliminate the possibility that cardiovascular responses were mediated by direct activation of sensory afferent fibres during stimulation protocols. In halothane-anaesthetized and decerebrate animals HR and ABP were stable between pressure-altering manoeuvres, providing evidence of the efficacy of each anaesthetic method during this period. Secondly, the L4, L5 and L6 dorsal roots were sectioned. These dorsal roots innervate the hindlimb and are carried via the sciatic nerve (Greene, 1963). Following dorsal rhizotomy, contraction and stretch protocols were repeated. A lack of responsiveness to manipulation after dorsal rhizotomy would confirm that contraction or stretch-induced haemodynamic alterations were of neural reflex origin. Thirdly, halothane anaesthesia was reintroduced to decerebrate animals for a minimum of 1 h. The level of halothane anaesthesia implemented (2 % in pure oxygen) was identical to that used during the testing of brain-intact animals. Subsequently, the contraction and stretch protocols were repeated. These trials were completed to assess the effect of decerebration on the cardiovascular responses elicited by static muscle contraction and stretch. Finally, the viability of the preparation after neuromuscular blockade or dorsal root transection was confirmed by obtaining a response to a brief (30 s) hypoxic stimulus after discontinuing the use of the mechanical respirator.
Statistical analysis
Baseline values were determined by analysing 30 s of data immediately prior to a given manoeuvre. The peak response of each variable was defined as the greatest change from baseline elicited during the 30 s execution of the contraction or stretch. Analyses conducted within and between halothane-anaesthetized or decerebrate animal populations were made using one- or two-way ANOVA, as appropriate. When significance was indicated, a Student-Newman-Keuls post hoc test was executed. Linear regression analyses were used to characterize the stimulus-response relationships between the changes in MAP and tension produced in response to contraction or stretch. The α level was set at P < 0.05. Results are presented as means ±s.e.m.
RESULTS
Cardiovascular responses elicited by ventral root stimulation and passive stretch
Muscle contraction induced by ventral root stimulation and passive muscle stretch resulted in significant alterations in MAP and HR from baseline as illustrated in Fig. 1. Both static contraction and stretch produced depressor and bradycardic responses in the halothane-anaesthetized animal (Fig. 1A). In contrast, increases in ABP and HR were elicited during both manoeuvres in the decerebrate rat under conditions of equal tension development (Fig. 1B). Figure 2 is a summary of the population studies illustrated in the representative tracings shown in Fig. 1. Static muscle contraction significantly depressed MAP (-17 ± 3 mmHg) and HR (-7 ± 2 beats min−1) in animals anaesthetized by halothane (Fig. 2A) while both variables increased (23 ± 2 mmHg and 12 ± 2 beats min−1, respectively) in decerebrate rats (Fig. 2B). Likewise, in halothane-anaesthetized rats, passive muscle stretch significantly decreased MAP and HR by -8 ± 1 mmHg and -7 ± 1 beats min−1, respectively, whereas in decerebrate animals MAP (19 ± 3 mmHg) and HR (12 ± 2 beats min−1) were elevated in response to this manoeuvre. The re-administration of halothane in decerebrate animals (n = 5) attenuated the increase in MAP and HR by 48 ± 11 % and 57 ± 8 %, respectively, during static contraction and 67 ± 2 % and 69 ± 6 %, respectively, during passive stretch (Fig. 2B). In all cases, cardiovascular responses to either contraction or stretch were eliminated by sectioning the dorsal roots innervating the activated skeletal muscle (Fig. 2). Further, stimulation of the ventral roots after neuromuscular blockade did not elicit cardiovascular responses in either halothane-anaesthetized or decerebrate rats (Fig. 2). Basal and peak HR, MAP and tension, expressed as raw values for each study population, are presented in Table 1. Baseline and peak values for HR and MAP were significantly augmented in decerebrate animals compared to those of their halothane-anaesthetized counterparts.
Figure 1. Cardiovascular responses elicited by spinal ventral root stimulation (contraction) and passive muscle stretch in halothane-anaesthetized (A) and decerebrate (B) rats.
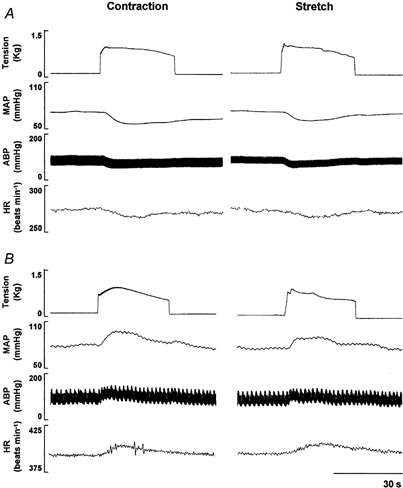
The contraction- and stretch-induced decreases in mean arterial pressure (MAP), arterial blood pressure (ABP) and heart rate (HR) in the pharmacologically anaesthetized animal were in direct contrast to the marked pressor and tachycardic responses elicited in the decerebrate model. The tracings depicted in A and B are from two different animals. Stimulation parameters: 3 × motor threshold, 40 Hz, 0.1 ms duration.
Figure 2. Effect of dorsal rhizotomy, neuromuscular blockade and halothane readministration (decerebrate animal only) on the cardiovascular responses evoked by ventral root stimulation and passive muscle stretch.
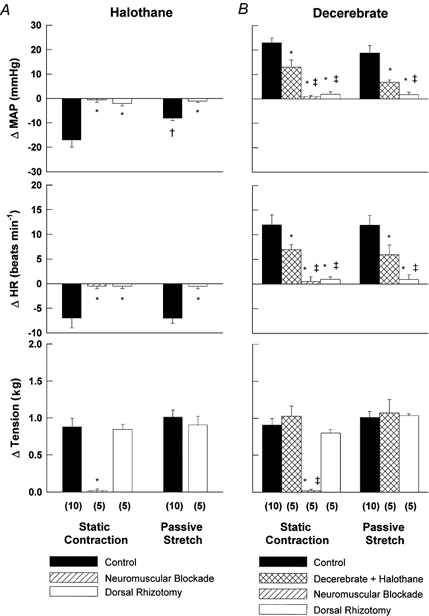
The contraction- and stretch-induced decreases in mean arterial pressure (MAP) and heart rate (HR) in the halothane-anaesthetized animal (A) were reversed in the decerebrate rat (B). Denervation and/or neuromuscular blockade eliminated the responses to static muscle contraction and passive stretch in both animal preparations. B, the reintroduction of gas anaesthesia in decerebrate rats attenuated the increase in MAP and HR caused by both manoeuvres. (n), the number of rats tested in each protocol. Stimulation parameters, 3 × motor threshold, 40 Hz, 0.1 ms duration. * Significantly different from control perturbation. † Significantly different from control contraction. ‡ Significantly different from decerebrate + halothane condition. P < 0.01.
Table 1.
Cardiovascular responses to contraction via ventral root stimulation and passive stretch in halothane-anaesthetized and decerebrate rats
| Halothane | Decerebrate | |||
|---|---|---|---|---|
| Contraction | Stretch | Contraction | Stretch | |
| MAP (mmHg) | ||||
| Baseline | 79 ± 4 | 75 ± 6 | 91 ± 6† | 92 ± 7† |
| Peak response | 62 ± 2* | 67 ± 5 | 114 ± 7*† | 111 ± 8*† |
| HR (beats min−1) | ||||
| Baseline | 270 ± 17 | 271 ± 14 | 398 ± 14 † | 404 ± 13 † |
| Peak response | 263 ± 15 | 264 ± 14 | 410 ± 13 † | 416 ± 12 † |
| Tension (kg) | ||||
| Baseline | 0.074 ± 0.010 | 0.081 ± 0.010 | 0.081 ± 0.003 | 0.088 ± 0.005 |
| Peak response | 0.953 ± 0.112* | 1.095 ± 0.091* | 0.990 ± 0.087* | 1.101 ± 0.085* |
| n | 10 | 10 | 10 | 10 |
Data are means ±s.e.m. MAP, mean arterial pressure; HR, heart rate. Stimulation parameters: 3 times motor threshold, 40 Hz, 0.1 ms.
Significantly different from baseline.
Significantly different from animals anaesthetized by halothane. P < 0.05.
In a subset of decerebrate animals, graded contraction and stretch elicited pressor responses that were linearly related (r = 0.844 and 0.836, respectively) to tension development (Fig. 3A and C). A significant main effect for each variable in response to graded electrical stimulation and passive stretch was calculated (MAP and tension, P < 0.001; Fig. 3B and D). In order to control for inter-subject variability in tension development, regression analyses were conducted by normalizing this variable to the maximal value obtained for each animal during contraction (1.001 ± 0.160 kg) and stretch (1.021 ± 0.027 kg). Cardiovascular responses were then matched to the corresponding percentage of maximal tension developed.
Figure 3. Changes in mean arterial pressure (MAP) and tension in response to graded electrical stimulation of spinal ventral roots (A and B, n = 5) and passive muscle stretch (C and D, n = 5).
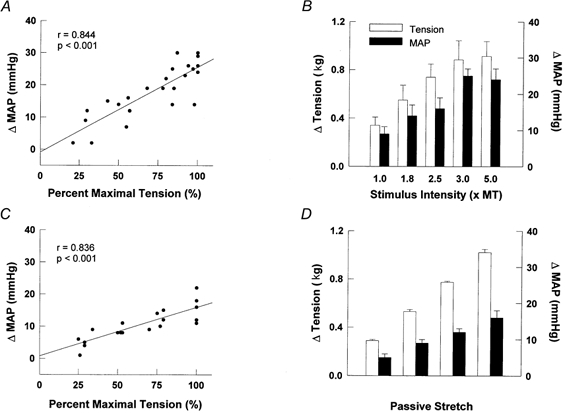
Regression analyses were used to describe the line of best-fit depicted for both testing protocols. To control for inter-subject variability, tension was expressed as a percentage of maximum for each animal in A and C. MT, motor threshold. Stimulation parameters: 40 Hz, 0.1 ms duration.
Cardiovascular responses elicited by sciatic nerve stimulation
The population data obtained in halothane-anaesthetized and decerebrate rats in response to static muscle contraction induced via electrical stimulation of the sciatic nerve are presented in Fig. 4. In halothane-anaesthetized rats, static muscle contraction increased MAP (ΔMAP = 10 ± 3 mmHg). This change in blood pressure was significantly less than the pressor response elicited by contraction in decerebrate animals (MAP = 49 ± 7 mmHg). There was no significant difference in the HR response to static contraction between halothane-anaesthetized or decerebrate animals (13 ± 3 and 15 ± 2 beats min−1, respectively). After neuromuscular blockade in decerebrate rats, sciatic nerve stimulation induced increases in HR (3 ± 1 beats min−1) and MAP (28 ± 3 mmHg) that were significantly attenuated from those elicited in the non-paralysed state. The increases in both HR and MAP post-paralysis were completely abolished by dorsal rhizotomy (n = 2).
Figure 4. Cardiovascular responses elicited by sciatic nerve stimulation in halothane-anaesthetized and decerebrate rats (n = 10).
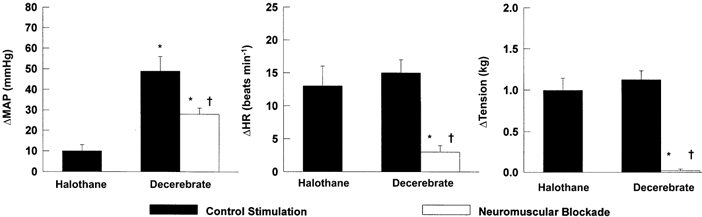
The large pressor response elicited by electrical stimulation in the decerebrate animal could not be abolished by neuromuscular blockade, indicating direct activation of somatosensory afferent fibres. This finding may also explain the increases in mean arterial pressure (MAP) and heart rate (HR) produced in the halothane-anaesthetized rat. Stimulation parameters: 2 × motor threshold, 40 Hz, 0.025 ms duration. * Significantly different from control stimulation in halothane-anaesthetized rats. † Significantly different from control stimulation in decerebrate animals. P < 0.01.
Graded blood pressure responses were produced by stimulating the sciatic nerve over a range of one to three times motor threshold in a subgroup of decerebrate animals (Fig. 5A). The data indicate that the changes in arterial pressure were dependent upon contraction intensity, as we observed a significant main effect for both MAP and tension (P < 0.001). However, electrical stimulation of the sciatic nerve produced graded pressor responses that persisted after neuromuscular blockade. This persistent pressor response was evident at all levels of stimulation tested despite the lack of tension development (Fig. 5B).
Figure 5. Changes in mean arterial pressure (MAP) and tension in response to graded electrical stimulation of the sciatic nerve before (A) and after neuromuscular blockade (B).
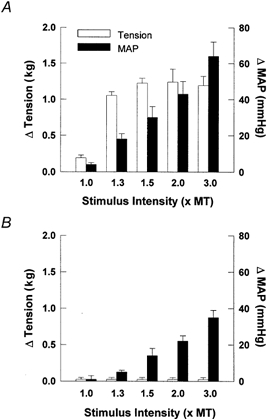
The increases in MAP persisted after induction of neuromuscular blockade in the absence of tension development. This finding suggests that electrical activation of muscle afferents must have occurred. MT, motor threshold. Stimulation parameters: 40 Hz, 0.025 ms duration. n = 5.
Preparation viability
At any given stimulus intensity, both static contraction and passive stretch elicited reproducible cardiovascular responses during multiple trials (i.e. two to three manipulations). In addition, viability testing of animals 4-5 h post-decerebration confirmed that all preparations were able to elicit pronounced circulatory responses after neuromuscular blockade or following dorsal rhizotomy. Induction of hypoxic/hypercapnic stimuli produced increases in MAP of 40 ± 3 mmHg (n = 20). This finding suggests that the lack of responsiveness to electrical stimulation or stretch reported after administration of pancuronium bromide or deafferentation was not due to deterioration of the physiological preparation.
DISCUSSION
The results of this investigation provide evidence that static muscle contraction induced by ventral root stimulation and passive muscle stretch were able to elicit increases in HR and MAP in the decerebrate rat. Furthermore, these changes were mediated by a neural reflex, since transection of the dorsal roots innervating the activated skeletal muscle abolished the response. Moreover, neuromuscular blockade eliminated the increase in HR and MAP caused by ventral root stimulation, indicating that the response elicited was due exclusively to muscle contraction. In addition, graded activation of intramuscular afferent fibres by both contraction and stretch elicited increases in MAP that were linearly related to the intensity of the stimulus in a manner similar to that reported in other mammalian models (Perez-Gonzalez, 1981; Mitchell et al. 1983). Given that models of cardiovascular pathology are readily accessible and easily produced in the rat, this investigation describes a novel preparation that can be used to study the exercise pressor reflex in both health and disease.
The use of the rat as a model for the exercise pressor reflex is controversial. In some studies, hindlimb muscle contractions have been shown to produce large depressor responses (Overton & Stremel, 1992; Toney & Mifflin, 1996). In this investigation, a marked decrease in MAP was produced in response to muscle contraction via ventral root stimulation in halothane-anaesthetized rats. In contrast, others have been able to elicit robust pressor responses to muscle contraction induced by electrical stimulation of the tibial nerve in chloral hydrate-anaesthetized rats (Caringi et al. 1997; Freda et al. 1999). In the current study, static contraction via sciatic nerve stimulation produced an increase in MAP and HR in both anaesthetized and decerebrate animals. However, this response was not abolished by induction of neuromuscular blockade in the decerebrate rat. Therefore, it is likely that the pressor responses induced by sciatic nerve stimulation in both study populations were due, in part, to direct electrical activation of afferent fibres, a conclusion supported by the complete elimination of the response after dorsal rhizotomy. This finding was surprising as the stimulation parameters used (i.e. twice motor threshold, 0.025 ms duration, 40 Hz) have been shown not to activate group III and IV fibres within the sciatic nerve of larger mammals (Rybicki & Kaufman, 1985). Based on these results, there is a need to define the operating characteristics (e.g. the activation threshold) of afferent fibres within whole nerves innervating the hindlimb of the rat. Given these findings, the differences in the responses produced in this and previous studies (Overton & Stremel, 1992; Toney & Mifflin, 1996; Caringi et al. 1997; Freda et al. 1999) are probably due to the technique employed to induce muscle contraction and/or to the regimen used for anaesthesia.
Evidence exists which suggests that the neural circuitry of the rat possesses the ability to drive sympathoexcitatory responses mediated by intramuscular afferent input. For example, significant increases in adrenal sympathetic nerve activity have been described in anaesthetized rats in response to static hindlimb contraction (Vissing et al. 1991). Toney & Mifflin (2000) demonstrated that neurons in the rat nucleus tractus solitarii (NTS), a medullary autonomic nucleus intimately involved in cardiovascular regulatory control, receive excitatory input from skeletal muscle receptors in response to both muscle contraction and stretch. Although the effects of activating NTS neurons remains undetermined (i.e. sympatho-inhibition vs. excitation) in the rat, these findings do provide electrophysiological evidence that input from peripheral muscle afferents is projected to the NTS in this species. Furthermore, previous experimentation in decerebrate rats has elicited increases in blood pressure and HR in response to stimulation of the mesencephalic locomotor region (MLR) that were similar to those in dynamically exercising conscious animals (Bedford et al. 1992). More recently, Potts et al. (2000) have reported that activation of forelimb intramuscular receptors via electrically induced contraction produced a vasoconstrictor-mediated increase in pressure in an in situ decerebrate rat preparation. The data presented in this investigation extend the latter finding to the larger muscle groups of the hindlimb in an in vivo model. In addition, this study demonstrates that the responses can be driven by various somatic sensory stimuli (i.e. mechanical and metabolic).
It is unclear why decerebration was necessary to reverse the depressor response to contraction and stretch elicited in anaesthetized animals. Examining locations within the central nervous system where halothane is known to alter neuronal function may provide a viable explanation. Using expression of the immediate-early proto-oncogene c-fos as a marker, several investigators have identified the medulla, pons, midbrain, hypothalamus, thalamus and cerebrum as sites of action for halothane in the rat (Takayama et al. 1994; Clement et al. 1996). More importantly, halothane-induced c-fos protein expression in these areas is essentially equal (Takayama et al. 1994). It is possible, therefore, that the depressor response elicited in this study could have been driven by halothane-induced changes in neuronal activity in each of these brain structures. The re-administration of halothane after removal of the supra-mesencephalic brain elicited an attenuated increase in arterial pressure to contraction and stretch (as compared to decerebrate conditions), not a depressor response. Thus, it would appear that halothane, although still effective on the brain stem in the decerebrate preparation, cannot drive a depressor response unless all sites of its action remain intact.
Given the known depressive cardiovascular effects and varied sites of action of halothane within the central nervous system, we contend that the pressor response elicited within this investigation was attributable to the discontinuation of pharmacological anaesthesia rather than secondary to the decerebration procedure. In support of this finding, it has been shown in other models that a larger pressor reflex is generated in decerebrate animals (in the absence of anaesthesia) in response to exercise than in anaesthetized animals (Iwamoto et al. 1985). Even more compelling is that the increases in HR and MAP produced in the present study complement previous reports of pronounced pressor responses to both static (Tipton et al. 1988) and dynamic (Baum & Shropshire, 1975; Sturek et al. 1984) exercise in conscious rats. Therefore, the decerebrate model developed in this investigation mimics the physiological response to exercise in conscious animals.
An alternative explanation is that brain centres critical to the muscle reflex were removed by decerebration (Eldridge et al. 1981; Vertes & Crane, 1996). For example, a significant proportion (i.e. approximately 50 %) of the contraction-evoked depressor response described in anaesthetized rats has been attributed to the action of adrenal catecholamines on β-adrenoreceptors (Toney & Mifflin, 1996). Since adrenal catecholamine release, a sympathetically mediated response, can be elicited by stimulation of hypothalamic nuclei (Vissing et al. 1989), it is possible that the decerebration procedure used in the present study eliminated a potentially important component of the muscle reflex. However, neuroanatomical and electrophysiological studies have demonstrated that skeletal muscle afferent fibres project to several medullary nuclei known to control sympathetic nerve activity (Kalia et al. 1981; Iwamoto et al. 1989; Bauer et al. 1992). Since the medulla was fully intact in this preparation, it is unlikely that activation of this region of the brain stem was disrupted, preserving its ability to drive sympatho-excitatory responses. In support of this contention, it has been shown in other animal models that the cardiovascular response to muscle contraction is essentially complete at the level of the medulla, being only slightly diminished when supra-mesencephalic input is eliminated (Iwamoto et al. 1985).
Potential limitations in the design of this model are recognized. Firstly, baseline HR and MAP were significantly greater in decerebrate rats than in their halothane-anaesthetized counterparts. These increases were probably due to the discontinuation of pharmacological anaesthesia. Alternatively, it is possible that the decerebration procedure induced a hyperadrenergic condition. If the latter possibility occurred, the responses to static contraction and stretch may have been attenuated in decerebrate animals due to a diminished sympathetic reserve capacity. Finally, although baroreceptor signals from aortic and cardiopulmonary regions remained intact, carotid baroreflex function was compromised by the surgical preparation. As has recently been described, inputs from skeletal muscle and baroreceptor afferents undergo significant interaction within the central nervous system (Potts et al. 1998). Therefore, elimination of the carotid baroreflex may have affected the responses to contraction and stretch.
In this investigation, a novel exercise model has been developed in a species in which cardiovascular disease is readily available or easily induced. Alterations in skeletal muscle morphology and metabolism associated with the genesis of cardiovascular pathology have led to the hypothesis that the exercise pressor reflex may become hyperactive after development of disease (Piepoli et al. 1999). However, the contribution of this neurally mediated peripheral reflex to the evolution of abnormal circulatory control is poorly understood. The utility of this model lies in its ability to assess both peripheral and central autonomic control mechanisms using much of the physiological technology currently used in larger animal preparations. For example, the techniques of microdialysis and electrophysiological neuronal recordings can now be used at the level of the spinal cord and brain stem to assess synaptic events and cellular behaviour during disease. Further, much of the genome of the rat has been described allowing the use of cellular and molecular techniques previously unavailable in large mammalian populations. Equally important, the exercise-induced circulatory adjustments elicited in this preparation are similar to those reported in other mammalian models such as the mouse (Kramer et al. 2001), cat (Coote et al. 1971; McCloskey & Mitchell, 1972), dog (Fisher & Nutter, 1974) and human (Rowell et al. 1981; Hansen et al. 1994). Given these findings, in conjunction with the functional utility of the preparation, use of this model provides the potential to further our understanding of circulatory regulation during exercise in both health and disease.
Acknowledgments
The authors greatly appreciate the expert technical assistance provided by Margaret Robledo and Julius Lamar Jr. Further, we offer special thanks to Jeffrey Potts PhD and Geoffrey Kline PhD for their critical input into the scientific development of this project. This work was supported by National Heart, Lung, and Blood Institute Program Project Grant No. HL-06296 (J.H.M. and M.G.G.), the Lawson and Rogers Lacy Research Fund in Cardiovascular Diseases (J.H.M.) and National Institute of Health Individual National Research Service Award No. HL-10473 (S.A.S.).
References
- Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from voluntary muscles. Journal of Physiology. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RM, Waldrop TG, Iwamoto GA, Holzwarth MA. Properties of ventrolateral medullary neurons that respond to muscular contraction. Brain Research Bulletin. 1992;28:167–178. doi: 10.1016/0361-9230(92)90176-x. [DOI] [PubMed] [Google Scholar]
- Baum T, Shropshire AT. Responses to exercise in experimental hypertension. Cardiovascular Research. 1975;9:745–752. doi: 10.1093/cvr/9.6.745. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Loi PK, Crandall CG. A model of dynamic exercise: the decerebrate rat locomotor preparation. Journal of Applied Physiology. 1992;72:121–127. doi: 10.1152/jappl.1992.72.1.121. [DOI] [PubMed] [Google Scholar]
- Caringi D, Maher TJ, Chaiyakul P, Asmundsson G, Ishide T, Ally A. Extracellular glutamate increases in rostral ventrolateral medulla during static muscle contraction. Pflügers Archiv. 1997;435:465–471. doi: 10.1007/s004240050540. [DOI] [PubMed] [Google Scholar]
- Clement CI, Keay KA, Owler BK, Bandler R. Common patterns of increased and decreased fos expression in midbrain and pons evoked by noxious deep somatic and noxious visceral manipulations in the rat. Journal of Comparative Neurology. 1996;366:495–515. doi: 10.1002/(SICI)1096-9861(19960311)366:3<495::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. Journal of Physiology. 1971;214:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: Parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Fisher ML, Nutter DO. Cardiovascular reflex adjustments to static muscular contractions in the canine hindlimb. American Journal of Physiology. 1974;226:648–655. doi: 10.1152/ajplegacy.1974.226.3.648. [DOI] [PubMed] [Google Scholar]
- Freda BJ, Gaitonde RS, Lillaney R, Ally A. Cardiovascular responses to muscle contraction following microdialysis of nitric oxide precursor into ventrolateral medulla. Brain Research. 1999;828:60–67. doi: 10.1016/s0006-8993(99)01321-9. [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the Rat. New York: Hafner; 1963. [Google Scholar]
- Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human muscle. Amercian Journal of Physiology. 1994;266:H2508–2514. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Waldrop TG, Bauer RM, Mitchell JH. Pressor responses to muscular contraction in the cat: contributions by caudal and rostral ventrolateral medulla. In: Ciriello J, Caverson MM, Polosa C, editors. Progress in Brain Research. Vol. 81. Elsevier Science Publishers; 1989. pp. 253–263. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. Journal of Applied Physiology. 1985;59:459–467. doi: 10.1152/jappl.1985.59.2.459. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circulation Research. 1981;48:I48–62. [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. Reflexes controlling circulatory, ventilatory and airway responses to exercise; pp. 381–447. section 12. [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovascular Research. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Kohn DF. Anesthesia and Analgesia in Laboratory Animals. San, Diego, CA, USA: Academic Press; 1997. [Google Scholar]
- Kramer JM, Aragones A, Waldrop TG. Reflex cardiovascular responses originating in exercising muscles of mice. Journal of Applied Physiology. 2001;90:579–585. doi: 10.1152/jappl.2001.90.2.579. [DOI] [PubMed] [Google Scholar]
- Longnecker DE, Ross DC, Silver IA. Anesthetic influence on arteriolar diameter and tissue oxygen tension in hemorrhaged rats. Anesthesiology. 1982;57:177–182. doi: 10.1097/00000542-198209000-00006. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory response originating in exercising muscle. Journal of Physiology. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Slyven C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure only when a small muscle mass is exercised. Cardiovascular Research. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annual Review of Physiology. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Overton JM, Stremel RW. Hindlimb muscle contraction elicits depressor responses in anesthetized rats. The Physiologist. 1992;35:238. [Google Scholar]
- Perez-Gonzalez J. Factors determining the blood pressure responses to isometric exercise. Circulation Research. 1981;48:I87–92. [PubMed] [Google Scholar]
- Pickering TG. Pathophysiology of exercise hypertension. Herz. 1987;12:119–124. [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJS. A neural link to explain the ‘muscle hypothesis’ of exercise intolerance in chronic heart failure. American Heart Journal. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Potts JT, Hand GA, Li J, Mitchell JH. Central interaction between carotid baroreceptors and skeletal muscle receptors inhibits sympathoexcitation. Journal of Applied Physiology. 1998;84:1158–1165. doi: 10.1152/jappl.1998.84.4.1158. [DOI] [PubMed] [Google Scholar]
- Potts JT, Spyer KM, Paton JFR. Somatosympathetic reflex in a working heart-brainstem preparation of the rat. Brain Research Bulletin. 2000;53:59–67. doi: 10.1016/s0361-9230(00)00309-9. [DOI] [PubMed] [Google Scholar]
- Quintin L, Gillon JY, Saunier CF, Ghignone M. Continuous volume infusion improves circulatory stability in anesthetized rats. Journal of Neuroscience Methods. 1989;30:77–83. doi: 10.1016/0165-0270(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Freund PR, Hobbs SF. Cardiovascular responses to muscle ischemia in humans. Circulation Research. 1981;48:37–47. [PubMed] [Google Scholar]
- Rybicki KJ, Kaufman MP. Stimulation of group III and group IV afferents reflexly decreases total pulmonary resistance in dogs. Respiration Physiology. 1985;59:185–189. doi: 10.1016/0034-5687(85)90006-4. [DOI] [PubMed] [Google Scholar]
- Sapru HN, Krieger AJ. Procedure for the decerebration of the rat. Brain Research Bulletin. 1978;3:675–679. doi: 10.1016/0361-9230(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Seyde WC, McGowan L, Lund N, Duling B, Longnecker DE. Effects of anesthetics on regional hemodynamics in normovolemic and hemorrhaged rats. American Journal of Physiology. 1985;249:H164–173. doi: 10.1152/ajpheart.1985.249.1.H164. [DOI] [PubMed] [Google Scholar]
- Smith SA, Garry MG, Mitchell JH. Static muscle contraction elicits a pressor response in the decerebrate rat. The Physiologist. 2000;43:339. [Google Scholar]
- Sturek ML, Bedford TG, Tipton CM, Newcomer L. Acute cardiorespiratory responses of hypertensive rats to swimming and treadmill exercise. Journal of Applied Physiology. 1984;57:1328–1332. doi: 10.1152/jappl.1984.57.5.1328. [DOI] [PubMed] [Google Scholar]
- Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neuroscience Letters. 1994;176:59–62. doi: 10.1016/0304-3940(94)90871-0. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Experimental Brain Research. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Tipton CM, McMahon S, Youmans EM, Overton JM, Edwards JG, Pepin EB, Lauber C. Response of hypertensive rats to acute and chronic conditions of static exercise. Amercian Journal of Physiology. 1988;254:H592–598. doi: 10.1152/ajpheart.1988.254.3.H592. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Mediators of contraction-evoked skeletal muscle depressor response in anesthetized rats. Journal of Applied Physiology. 1996;81:578–585. doi: 10.1152/jappl.1996.81.2.578. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Sensory modalities conveyed in the hindlimb somatic afferent input to nucleus tractus solitarius. Journal of Applied Physiology. 2000;88:2062–2073. doi: 10.1152/jappl.2000.88.6.2062. [DOI] [PubMed] [Google Scholar]
- Vatner SF. Effects of anesthesia on cardiovascular control mechanisms. Environmental Health Perspectives. 1978;26:193–206. doi: 10.1289/ehp.7826193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Descending projections of the posterior nucleus of the hypothalamus: Phaseolus vulgaris leucoagglutinin analysis in the rat. Journal of Comparative Neurology. 1996;374:607–631. doi: 10.1002/(SICI)1096-9861(19961028)374:4<607::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Vissing J, Iwamoto GA, Rybicki KJ, Galbo H, Mitchell JH. Mobilization of glucoregulatory hormones and glucose by hypothalmic locomotor centers. Amercian Journal of Physiology. 1989;257:E722–728. doi: 10.1152/ajpendo.1989.257.5.E722. [DOI] [PubMed] [Google Scholar]
- Vissing J, Wilson B, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. American Journal of Physiology. 1991;261:R1307–1312. doi: 10.1152/ajpregu.1991.261.5.R1307. [DOI] [PubMed] [Google Scholar]


