Abstract
Adenosine 3′,5′-cyclic monophosphate (cAMP) modulates proximal tubule sodium and bicarbonate absorption by decreasing the rate of apical Na+-H+ exchange and basolateral sodium bicarbonate efflux, through activation of protein kinase A (PKA). The electrogenic sodium bicarbonate cotransporter kNBC1 mediates basolateral sodium and bicarbonate efflux in the proximal tubule by coupling the transport of 1 Na+ cation to that of 3 HCO3− anions. In this work we studied the effects of cAMP on the function of kNBC1 expressed heterologously in a proximal tubule cell line.
A mouse renal proximal tubule cell line, deficient in electrogenic sodium bicarbonate cotransport function, was transfected with kNBC1. Cells were grown on a permeable support to confluence, mounted in an Ussing chamber and permeabilized apically with amphotericin B. Current through the cotransporter was isolated as the difference current due to the reversible inhibitor dinitrostilbene disulfonate. The HCO3−:Na+ stoichiometry of kNBC1 was calculated from its reversal potential by measuring the current-voltage relationships of the cotransporter at different Na+ concentration gradients.
Addition of the potent cAMP agonsit 8-Br-cAMP caused the stoichiometry of kNBC1 to shift from 3 HCO3−: 1 Na+ to 2 HCO3−:1 Na+. Pretreatment of the cells with the PKA inhibitor H-89 abolished the effect of the agonist on the stoichiometry change. Replacing Ser982 at the C-terminus consensus PKA phosphorylation site with alanine resulted in a failure of PKA to phosphorylate the transporter and induce a stoichiometry shift.
Our data indicate that cAMP modulates the stoichiometry of kNBC1 through activation of PKA. The change in stoichiometry from 3:1 to 2:1 is predicted to cause a shift in the direction of basolateral membrane sodium bicarbonate transport from efflux to influx. Ser982 in the C-terminus of kNBC1 is a target for PKA phosphorylation. This is the first example of modulation of the stoichiometry of a membrane transporter by phosphorylation.
The renal proximal convoluted tubule in mammals is responsible for the reabsorption of most of the filtered load of bicarbonate (HCO3−) by secreting protons into the lumen and an equal number of base equivalents across the basolateral membrane into the peritubular space (Alpern & Rector, 1996). The sodium bicarbonate cotransporter (kNBC1) has been identified as the main pathway for bicarbonate efflux across the basolateral membrane in the proximal tubule (Romero et al. 1997; Abuladze et al. 1998b; Schmitt et al. 1999). Loss of function mutations in the NBC1 gene result in a severe ocular and renal phenotype characterized by blindness, cataracts, glaucoma and proximal renal tubular acidosis (Igarashi et al. 1999; Bok et al. 2001).
kNBC1 is transcribed from the NBC1 gene (Abuladze et al. 2000). Another variant transcribed from the same gene, pNBC1, contributes to pancreatic ductal bicarbonate secretion where it mediates basolateral sodium bicarbonate influx (Abuladze et al. 1998a; Marino et al. 1999; Gross et al. 2001a). An important functional property of these electrogenic transporters is their HCO3−:Na+ coupling ratio, which sets the transporter reversal potential and determines the direction of sodium bicarbonate flux (Gross et al. 2001b). Thermodynamic considerations indicate that for kNBC1 to mediate sodium bicarbonate efflux, its reversal potential must be more positive than the membrane potential. This constraint requires that at least 3 HCO3− anions be cotransported with 1 Na+ cation. However when kNBC1 was expressed in various heterologous systems, the HCO3−:Na+ stoichiometry was 2:1 in Xenopus oocytes (Heyer et al. 1999) and mouse collecting duct (mCD) cells (Gross et al. 2001b), and 3:1 in mouse proximal convoluted tubule (mPCT) cells (Gross et al. 2001b). Moreover, in studies using isolated proximal tubules, the stoichiometry of basolateral sodium bicarbonate cotransport was 2:1 or 3:1 depending on the experimental conditions utilized (Muller-Berger et al. 1997; Kunimi et al. 2000). However, neither the molecular mechanism nor the structural basis of these changes was identified. We have recently demonstrated that the stoichiometries of both kNBC1 and pNBC1 are not fixed, and that they are 3:1 or 2:1 depending on the cell type in which each transporter is expressed (Gross et al. 2001b). These findings were the first definitive evidence that the stoichiometry, and hence the reversal potential, of these electrogenic transporters could be altered in a cell type-specific fashion.
Cyclic AMP serves as a second messenger for a variety of hormones in various cell types (Soderling et al. 1973). In the renal proximal tubule cAMP is known to regulate bicarbonate absorption by decreasing the rate of apical Na+-H+ exchange and basolateral sodium bicarbonate efflux (McKinney & Myers, 1980; Ruiz & Arruda, 1992; Kurashima et al. 1997). Given the potent effect of cAMP in altering basolateral sodium bicarbonate cotransport in the proximal tubule, we studied its role in potentially modulating the stoichiometry of kNBC1 in a mammalian transient expression system. By regulating the HCO3−:Na+ stoichiometry of kNBC1, proximal tubule cells could alter the direction of sodium bicarbonate flux through the cotransporter without a change in the gradient of these ions across the basolateral membrane. Our data indicate that phosphorylation of Ser982 in the C-terminus of kNBC1, by cAMP-dependent PKA, shifts the HCO3−:Na+ stoichiometry of kNBC1 from 3:1 to 2:1.
METHODS
Cell culture
Experiments were carried out with the mouse cell line mPCT 1296 (d) derived from the proximal tubule. The cell line was generated by microdissecting and culturing a single S1 proximal tubule segment from a mouse carrying at least one copy of the H-2Kb-tsA58 transgene (Immortomouse, Charles River Laboratories, Wilmington, MA, USA), as previously described (Gross et al. 2001b). Mice were killed by an increasing concentration of CO2 and all animal protocols were approved by the Institutional Review Board. The H-2Kb-tsA58 transgene codes for a thermolabile mutant of the SV40 large T antigen under the control of an interferon-γ promoter (Jat et al. 1991). Cells were used at passages between 15 and 25.
Transfection
mPCT cells were transiently transfected with kNBC1 or with the indicated kNBC1 construct as previously described (Gross et al. 2001b). Briefly, cells were grown in mouse renal tubular epithelium (mRTE) medium containing a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F12 medium and the following additives: 10 ng ml−1 epidermal growth factor (EGF), 5 μg ml−1 insulin, 5 μg ml−1 transferrin, 4 μg ml−1 dexamethasone, 10 units ml−1 interferon-γ, 2 mm glutamine and 5 % fetal bovine serum, on filters to form a high-resistance confluent monolayer (Rt≥ 1000 Ω cm2). Cells were transfected with the corresponding plasmid using Effectene (Qiagen, Valencia, CA, USA) as per the manufacturer's protocol. Mock transfected mPCT cell lines were generated by transfecting the corresponding cell line with the vector only. All plasmids were purified with the Endofree plasmid purification kit (Qiagen) prior to their use.
Mutagenesis
The coding region of human kNBC1 was cloned into the EcoRI and EcoRV sites in the pcDNA3.1 vector (Clontech, Palo Alto, CA, USA). An N-terminal enhanced green fluorescent protein (EGFP) fusion protein was produced by inserting kNBC1 into the EcoRI and ApaI sites in the EGFP-C3 vector (Clontech). To generate the S982A mutant, a site directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used with the following pair of primers:
The mutations were verified by DNA sequencing.
Fluorescence microscopy
To confirm that EGFP-WT-kNBC1 was targeted to the plasma membrane, the cells were transfected with the corresponding plasmid using Effectene. The cells were washed in phosphate-buffered saline three times, and mounted in Cytoseal 60 (Stephens Scientific, Riverdale, NJ, USA). Confocal images were captured with a Leica TCS SP inverted confocal microscope (Leica, Germany).
Phosphorylation assays
EGFP-WT-kNBC1 or the EGFP-S982A-kNBC1 mutants were transiently expressed in mPCT cells. The mutants were immunoprecipitated using an anti-GFP antibody (Clontech) and protein A-Sepharose 4B (Amersham Pharmacia Biotech, Piscatawy, NJ, USA). The proteins were eluted from the beads with 50 μl of 30 mm glycine, pH 2.8, and neutralized with 6 μl of 200 mm Tris, pH 11. The immunoprecipitated proteins were phosphorylated using a PKA catalytic subunit (Promega, Madison, WI, USA) and [γ-32P]ATP as described (Zizak et al. 1999) and were separated on a 7.5 % sodium dodecyl sulphate polyacrylamide gel. The proteins from the gel were electrotransferred onto nitrocellulose membranes (Amersham Pharmacia Biotech) for Western blotting with an anti-kNBC1 antibody and for 32P incorporation measurements.
Stoichiometry
The stoichiometry of the cotransporter was determined from its reversal potential (Erev) and eqn (1), as described previously (Gross et al. 2001b):
| (1) |
where n is the number of bicarbonate anions cotransported with each sodium cation, and the subscripts i and o represent intra- and extracellular concentrations of the indicated ion. R, T and F have their usual meanings. For a symmetrical HCO3− concentration, the ratio [HCO3−]in/[HCO3−]on = 1, and the reversal potential depends logarithmically only on the magnitude and direction of the Na+ concentration gradient. We thus measured Erev of the cotransporter for several different sodium concentration gradients while keeping bicarbonate concentrations symmetrical across the basolateral membrane. For experiments, confluent cells on filters were mounted vertically in a thermostatically controlled Ussing chamber equipped with gas inlets for CO2 bubbling. Cells were then permeabilized apically with 10 μm amphotericin B to remove the electrical resistance of the apical membrane. A 5-fold sodium concentration gradient was applied across the monolayer, by perfusing either the apical or the basolateral compartment with solution 50Na containing (mm): 50 sodium gluconate, 50 N-methyl-d-glucamine (NMDG), 2.5 calcium gluconate, 1.1 magnesium gluconate, 70 Hepes, 25 d-glucose, 22 tetramethylammonium (TMA)-HCO3 (pH 7.4); and the contralateral compartment with solution 10Na containing (mm): 10 sodium gluconate, 90 NMDG, 2.5 calcium gluconate, 1.1 magnesium gluconate, 70 Hepes, 25 d-glucose, 22 TMA-HCO3 (pH 7.4). Solutions were Cl− free. To measure Erev of the cotransporter, current-voltage relationships were collected with an epithelial voltage-clamp amplifier (EC825, Warner Instument Corp., Hamden, CT, USA). The data were digitized at 100 kHz and recorded through an A/D converter (PowerLab/400, ADInstruments, Castle Hill, Australia) on a Pentium PC for further analysis. Data were filtered at 0.5 Hz. Current-voltage relationships were obtained by stepping the voltage command from -60 mV to +60 mV with a 10 mV step, using the stimulator utility of the Chart program (ADInstruments). The current through the cotransporter is defined as the difference in current measured in the absence of the cotransporter inhibitor dinitrostilbene disulfonate (DNDS), and that measured 10 min after the addition of 2 mm basolateral DNDS. In a separate experiment DNDS was found to inhibit the current through kNBC1 in transformed mPCT cells with a Ki value of 0.10 mm. This value is compatible with the value of 0.11 mm found previously for the cotransporter in rat proximal tubule cells (Gross & Hopfer, 1999). Only transfected cell monolayers for which the DNDS-sensitive current was at least 10-fold larger then that of the corresponding mock-transfected cells were included in this study. About 30 % of all transfected cell monolayers met the inclusion criteria.
Materials
Amphotericin B, Hepes, d-glucose, NMDG, gluconic acid, 8-Br-cAMP and all salts were purchased from Sigma Chemical Co. (St Louis, MO, USA). DNDS was obtained from Pfaltz & Bauer, Inc. (Waterbury, CT, USA). H-89 was obtained from Calbiochem (San Diego, CA, USA) and H-85 from Seikagaku (Fulmouth, MA, USA). Filters were purchased from Millipore (Bedford, MA, USA).
Statistics
Experiments were performed at least 4 times. The results for the reversal potential and stoichiometry are presented as means ±s.e.m. Student's unpaired t test and linear regression analysis were used as required.
RESULTS
To study the effect of cAMP agonists on the stoichiometry of kNBC1 clones, we expressed the cotransporter in a previously characterized mouse proximal convoluted tubule (mPCT) cell line that is deficient in electrogenic sodium bicarbonate cotransporter activity (Fig. 1A–D, circles) (Gross et al. 2001b).
Figure 1. Current-voltage relationships of kNBC1 constructs.
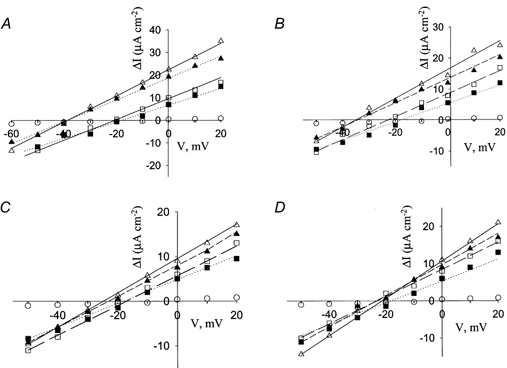
A, current-voltage relationships of WT kNBC1 (open symbols) and EGFP-WT-kNBC1 (filled symbols) in the absence (squares) and presence (triangles) of 8-Br-cAMP. B, effect of 8-Br-cAMP on I–V relationships of WT kNBC1 (open symbols) and EGFP-WT-kNBC1 (filled symbols) in cells pre-incubated with H-85 (triangles) or H-89 (squares). C, I–V relationships of S982A-kNBC1 (open symbols) and EGFP-S982A-kNBC1 (filled symbols) in the absence (squares) and presence (triangles) of 8-Br-cAMP. D, effect of 8-Br-cAMP on I–V relationships of S982A-kNBC1 (open symbols) and EGFP-WT-kNBC1 (filled symbols) in cells pre-incubated with H-85 (triangles) or H-89 (squares). I–V relationships of mock-transfected cells are shown with circles in A–D. All I–V relationships were measured at 5-fold Na+ concentration gradient (apical/basolateral = 10 mm/50 mm) in transformed mPCT cells.
The stoichiometry, n, of the cotransporter in these cells was calculated from the reversal potential of the cotransporter using eqn (1). Figure 1 shows the current-voltage relationships of the different kNBC1 clones in mPCT cells for a 5-fold Na+ concentration gradient (basolateral/apical = 50 mm/10 mm). The reversal potential is the voltage at which the current-voltage graph crosses the X-axis. As can be seen, in the absence of any treatment Erev of WT kNBC1 was -20 mV (Fig. 1A).
To study the effect of cAMP agonists on WT kNBC1, transformed mPCT cells were treated with the cAMP analogue 8-Br-cAMP for 15 min. As a result of this treatment, a shift in the cotransporter's reversal potential from -20 to -38 mV was observed (Fig. 1A). The change in Erev represents a corresponding shift in the HCO3−:Na+ stoichiometry from 3:1 to 2:1 (Table 1).
Table 1.
Effects of PKA modulators on reversal potential (Erev) and stoichiometry (n) of recombinant kNBC1 clones in mPCT cells
| WT kNBC1 | EGFP-WT-kNBC1 | S982A-kNBC1 | EGFP-S982A-kNBC1 | |||||
|---|---|---|---|---|---|---|---|---|
| [Na+]AP/[Na+]BL | Erev | n | Erev | n | Erev | n | Erev | n |
| No treatment | ||||||||
| 10/50 | −20.1 ± 2(6) | 3.1 ± 0.3 | −19.4 ± 2(4) | 3.2 ± 0.3 | −18.6 ± 2(7) | 3.2 ± 0.3 | −19.4 ± 2(6) | 3.2 ± 0.3 |
| 50/10 | 23.2 ± 2(5) | 2.8 ± 0.3 | 21.5 ± 2(4) | 2.9 ± 0.3 | 19.1 ± 2(8) | 3.2 ± 0.3 | 20.3 ± 2(4) | 3.1 ± 0.3 |
| 8-Br-cAMP | ||||||||
| 10/50 | −38.2 ± 4(7)* | 2.1 ± 0.2* | −37.6 ± 4(6)* | 2.1 ± 0.2* | −22.4 ± 2(6) | 2.9 ± 0.3 | −21.3 ± 2(4) | 3.0 ± 0.3 |
| 50/10 | 37.5 ± 4(8)* | 2.1 ± 0.2* | 44.2 ± 4(5)* | 1.9 ± 0.2* | 18.6 ± 2(7) | 3.2 ± 0.3 | 23.1 ± 2(5) | 2.8 ± 0.3 |
| H-89 + 8-Br-cAMP | ||||||||
| 10/50 | −23.7 ± 2(8) | 2.8 ± 0.3 | −22.6 ± 2(4) | 2.8 ± 0.3 | −18.8 ± 2(7) | 3.2 ± 0.3 | −20.4 ± 2(6) | 3.1 ± 0.3 |
| 50/10 | 22.4 ± 2(5) | 2.9 ± 0.3 | 24.1 ± 2(6) | 2.7 ± 0.3 | 22.9 ± 2(6) | 2.8 ± 0.3 | 23.5 ± 2(4) | 2.8 ± 0.3 |
| H-85 + 8-Br-cAMP | ||||||||
| 10/50 | −35.2 ± 3(4)* | 2.2 ± 0.2* | −36.6 ± 2(4)* | 2.1 ± 0.2* | −24.1 ± 2(4) | 2.7 ± 0.3 | −21.6 ± 2(4) | 2.9 ± 0.3 |
| 50/10 | 37.4 ± 3(4)* | 2.1 ± 0.3* | 34.3 ± 2(4)* | 2.2 ± 0.2* | 23.2 ± 2(4) | 2.8 ± 0.3 | 22.5 ± 2(4) | 2.8 ± 0.3 |
[Na+]AP/[Na+]BL, apical/basolateral sodium gradient. Number of experiments is shown in parentheses.
P < 0.05 compared with the corresponding WT kNBC1 construct in untreated cells.
Cyclic AMP is a potent activator of PKA. To determine whether PKA mediated the effect of cAMP on the stoichiometry of WT kNBC1, we pre-treated the cells with the PKA inhibitor H-89. In mPCT cells pre-incubated with H-89, 8-Br-cAMP failed to shift the stoichiometry of WT kNBC1 (Fig. 1B and Table 1). Furthermore, the inactive analogue H-85 failed to block the effect of 8-Br-cAMP (Fig. 1B and Table 1). These results suggested that the shift in stoichiometry is mediated by PKA.
PKA phosphorylates serine or threonine residues at specific consensus sites on target proteins. kNBC1 has a single PKA consensus phosphorylation site (Ser982) in its cytoplasmic C-terminus (970-KEDEKKKKKKKGS_LDSDNDDS-990). In order to determine whether PKA-mediated phosphorylation of this amino acid is involved in the shift of the stoichiometry of the cotransporter from 3:1 to 2:1, Ser982 was replaced with alanine. When the S982A mutant of kNBC1 was expressed in mPCT cells, it exhibited a 3:1 stoichiometry as did WT kNBC1 (Fig. 1C and Table 1). Treatment of mPCT cells expressing the S982A mutant with 8-Br-cAMP failed to alter the transporter stoichiometry (Fig. 1C and Table 1). This is in contrast to WT kNBC1, which shifts its stoichiometry in response to 8-Br-cAMP treatment from 3.0 ± 0.3 to 2.0 ± 0.2 (P < 0.05). The data suggest that phosphorylation of Ser982 in the C-terminus consensus PKA site of kNBC1 is necessary for the shift in stoichiometry. We have also examined the effect of cAMP in the presence of H-89 and H-85 on Erev and the stoichiometry of the S982A-kNBC1 mutant. None of these drugs affected Erev (Fig. 1D) or the stoichiometry of the mutant, which remained 3:1 (Table 1).
We next determined whether kNBC1 could be phosphorylated by the catalytic subunit of PKA. For this purpose we created EGFP-kNBC1 constructs by attaching an EGFP tag to the N-terminus of WT kNBC1 or of the S982A-kNBC1 mutant. To determine whether the tag might affect the stoichiometry of the corresponding cotransporter and its response to cAMP, we expressed the tagged constructs in mPCT cells and measured their stoichiometries under different experimental conditions. As can be seen from Fig. 1 and Table 1, the stoichiometries of the tagged constructs are not significantly different from that of their untagged counterparts. Furthermore, the EGFP tag did not seem to impair the targeting of the cotransporter to the plasma membrane (Fig. 2). The PKA-dependent incorporation of 32P from [γ-32P]ATP into immunoprecipitated EGFP-tagged constructs is shown in Fig. 3. PKA phosphorylated immunoprecipitated EGFP-WT-kNBC1 isolated from mPCT cells which had not been treated with 8-Br-cAMP. In contrast, prior treatment of mPCT cells with 8-Br-cAMP prevented the in vitro phosphorylation of the transporter. These results demonstrate that WT kNBC1 could be phosphorylated by the catalytic subunit of PKA. In order to confirm that PKA phosphorylates Ser982 of kNBC1, Ser982 was replaced with alanine in the EGFP-kNBC1 construct. As shown in Fig. 3, PKA failed to phosphorylate the mutant transporter isolated from mPCT cells which had not been treated with 8-Br-cAMP. Our data provide direct evidence that phosphorylation of Ser982 in the C-terminus of kNBC1 is necessary for the stoichiometry shift mediated by PKA.
Figure 2. Membrane localization of EGFP-WT-kNBC1.
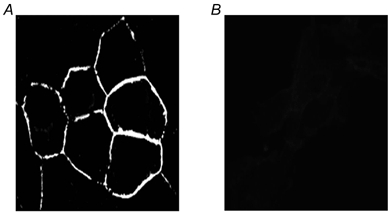
A, cells transfected with the EGFP-WT-kNBC1 construct showing plasma membrane localization of the fusion protein. B, mock-transfected cells.
Figure 3. Phosphorylation of kNBC1 by PKA.
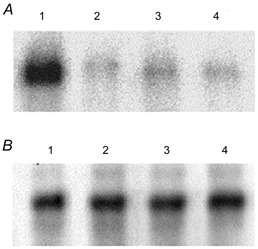
A, in vitro phosphorylation of WT kNBC1 (lanes 1 and 2) and the S982A-kNBC1 mutant (lanes 3 and 4) isolated from untreated mPCT cells (lanes 1 and 3) or cells treated with 100 μm 8-Br-cAMP (lanes 2 and 4) for 15 min. Immunoprecipitated EGFP-WT-kNBC1 and the EGFP-S982A-kNBC1 mutant were eluted from protein A-Sepharose beads and then phosphorylated in vitro using the PKA catalytic subunit and [γ-32P]ATP. A representative experiment is shown. B, the amount of the wild-type and mutant kNBC1 proteins used in these in vitro phosphorylation experiments was quantified using Western blotting with an affinity purified rabbit polyclonal kNBC1 antibody (Bok et al. 2001). Treatment of the cells with 8-Br-cAMP did not affect the size of EGFP-kNBC1 and the EGFP-S982A-kNBC1 mutant.
DISCUSSION
Cyclic AMP is a second messenger for several hormones that regulate bicarbonate transport in the proximal tubule segment of the nephron. An increase in intracellular cAMP by dopamine (Wiederkehr et al. 2001) or parathyroid hormone (PTH; Collazo et al. 2000) has been shown to decrease bicarbonate absorption in the proximal tubule in part by inhibition of apical NHE3. Conversely, a decrease in cAMP by angiotensin II stimulated bicarbonate absorption (Liu & Cogan, 1989) and sodium bicarbonate cotransport (Ruiz et al. 1995) in this segment.
In the present study we demonstrated, for the first time, that cAMP modulates the function of kNBC1 in mPCT cells derived from the proximal tubule. Addition of the cAMP agonist 8-Br-cAMP to the cells resulted in a shift in the HCO3− stoichiometry from 3:1 to 2:1. The change in stoichiometry was mediated by the PKA-dependent phosphorylation of Ser982 in the C-terminus of kNBC1. Phosphorylation of Ser982 could alter the stoichiometry of the transporter by several potential mechanisms: (i) association or dissociation of a second regulatory protein; or (ii) change in monomeric or oligomeric structure of the cotransporter. Regarding the latter possibility, when Ser982 is phosphorylated, the C-terminus of kNBC1 could act as a ‘plug’ by competing with bicarbonate for one of the three bicarbonate binding sites in a manner analogous to the ‘ball and chain’ model of the Shaker potassium channel or voltage-activated Na+ channels (Armstrong & Bezanilla, 1977; Hoshi et al. 1990). Further experiments are needed to address these possibilities.
The PKA-dependent phosphorylation of kNBC1 provides the proximal tubule with an efficient mechanism for modulating the direction of basolateral sodium bicarbonate flux through the cotransporter. The capacity of the proximal tubule cell to maintain intracellular Na+ and pH homeostasis despite large changes in trans-cellular Na+ and acid/base fluxes, in response to varying physiological conditions, depends on the coupling of apical Na+-H+ exchange mediated by NHE3, and basolateral sodium bicarbonate cotransport mediated by kNBC1. PKA decreases the activity of NHE3 by phosphorylating Ser605 in its C-terminus (Kurashima et al. 1997). On the basis of these data and our results demonstrating the PKA-meditated shift in the stoichiometry of kNBC1, it is likely that the coordinated phosphorylation of both transporters is an important mechanism for modulating the rates of transport across each membrane in concert.
Acknowledgments
This work was supported by grants from the American Heart Association and Cystic Fibrosis Foundation to E.G.; NIH grants DK46976, DK54221, DK6976, the Iris and B. Gerald Cantor Foundation, the Max Factor Family Foundation, the Verna Harrah Foundation, the Richard and Hinda Rosenthal Foundation, and the Fredericka Taubitz Foundation to I.K.; NIH grant HL-41618 to U.H. N.A. is supported by a training grant from the National Kidney Foundation of Southern California J891002.
References
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. Journal of Biological Chemistry. 1998a;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I. Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. American Journal of Physiology. 1998b;274:F628–633. doi: 10.1152/ajprenal.1998.274.3.F628. [DOI] [PubMed] [Google Scholar]
- Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251:109–122. doi: 10.1016/s0378-1119(00)00204-3. [DOI] [PubMed] [Google Scholar]
- Alpern R, Rector RC., Jr . Renal acidification mechanisms. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia: W. B. Saunders; 1996. pp. 408–471. [Google Scholar]
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. Journal of General Physiology. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of the electrogenic sodium bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. American Journal of Physiology - Renal Physiology. 2001;281:F920–935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. Journal of Biological Chemistry. 2000;275:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3− :1 Na+ Journal of Physiology. 2001a;531:375–382. doi: 10.1111/j.1469-7793.2001.0375i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. Journal of Physiology. 2001b;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hopfer U. Effects of pH on kinetic parameters of the Na-HCO3 cotransporter in renal proximal tubule. Biophysical Journal. 1999;76:3066–3075. doi: 10.1016/S0006-3495(99)77459-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer M, Muller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na-HCO3-cotransporter expressed in Xenopus laevis oocytes. Pflügers Archiv. 1999;438:322–329. doi: 10.1007/s004240050916. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nature Genetics. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proceedings of the National Academy of Sciences of the USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimi M, Muller-Berger S, Hara C, Samarzija I, Seki G, Frömter E. Incubation in tissue culture media allows isolated rabbit proximal tubules to regain in-vivo-like transport function: response of HCO3− absorption to norepinephrine. Pflügers Archiv. 2000;440:908–917. doi: 10.1007/s004240000361. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Phosphorylation-dependent and -independent mechanisms. Journal of Biological Chemistry. 1997;272:28672–28679. doi: 10.1074/jbc.272.45.28672. [DOI] [PubMed] [Google Scholar]
- Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. Journal of Clinical Investigation. 1989;84:83–91. doi: 10.1172/JCI114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. American Journal of Physiology. 1999;277:G487–494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- McKinney TD, Myers P. Bicarbonate transport by proximal tubules: effect of parathyroid hormone and dibutyryl cyclic AMP. American Journal of Physiology. 1980;238:F166–174. doi: 10.1152/ajprenal.1980.238.3.F166. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Nesterov VV, Frömter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. II. Change of Na-HCO3 cotransport stoichiometry and of response to acetazolamide. Pflügers Archiv. 1997;434:383–391. doi: 10.1007/s004240050411. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na/HCO3 cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Ruiz OS, Arruda JAL. Regulation of the renal Na-HCO3 cotransporter by cAMP and Ca-dependent protein kinases. American Journal of Physiology. 1992;262:F560–565. doi: 10.1152/ajprenal.1992.262.4.F560. [DOI] [PubMed] [Google Scholar]
- Ruiz OS, Qiu YY, Wang LJ, Arruda JAL. Regulation of the renal Na-HCO3 cotransporter: IV. Mechanisms of the stimulatory effect of angiotensin II. Journal of the American Society of Nephrology. 1995;6:1202–1208. doi: 10.1681/ASN.V641202. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. American Journal of Physiology. 1999;276:F27–38. doi: 10.1152/ajprenal.1999.276.1.F27. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Corbin JD, Park CR. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. II. Hormonal regulation of the adipose tissue enzyme. Journal of Biological Chemistry. 1973;248:1822–1829. [PubMed] [Google Scholar]
- Wiederkehr MR, Di SF, Collazo R, Quinones H, Fan L, Murer H, Helmle-Kolb C, Moe OW. Characterization of acute inhibition of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney International. 2001;59:197–209. doi: 10.1046/j.1523-1755.2001.00480.x. [DOI] [PubMed] [Google Scholar]
- Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. cAMP-induced phosphorylation and inhibition of Na+/H+ exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. Journal of Biological Chemistry. 1999;274:24753–24758. doi: 10.1074/jbc.274.35.24753. [DOI] [PubMed] [Google Scholar]


