Abstract
The role of phosphorylcreatine (PCr) and creatine (Cr) in the regulation of mitochondrial respiration was investigated in permeabilised fibre bundles prepared from human vastus lateralis muscle.
Fibre respiration was measured in the absence of ADP (V̇0) and after sequential additions of submaximal ADP (0.1 mm ADP, V̇submax), PCr (or Cr) and saturating [ADP] (V̇max).
V̇submax increased by 55% after addition of saturating creatine (P < 0.01; n = 8) and half the maximal effect was obtained at 5 mm[Cr]. In contrast, V̇submax decreased by 54% after addition of saturating phosphorylcreatine (P < 0.01; n = 8) and half the maximal effect was obtained at 1 mm[PCr]. V̇max was not affected by Cr or PCr.
V̇submax was similar when PCr and Cr were added simultaneously at concentrations similar to those in muscle at rest (PCr/Cr = 2) and at low-intensity exercise (PCr/Cr = 0.5). At conditions mimicking high-intensity exercise (PCr/Cr = 0.1), V̇submax increased to 60% of V̇max (P < 0.01 vs. rest and low-intensity exercise).
Eight of the subjects participated in a 16 day Cr supplementation programme. Following Cr supplementation, V̇0 decreased by 17% (P < 0.01 vs. prior to Cr supplementation), whereas ADP-stimulated respiration (with and without Cr or PCr) was unchanged.
For the first time evidence is given that PCr is an important regulator of mitochondrial ADP-stimulated respiration. Phosphorylcreatine decreases the sensitivity of mitochondrial respiration to ADP whereas Cr has the opposite effect. During transition from rest to high-intensity exercise, decreases in the PCr/Cr ratio will effectively increase the sensitivity of mitochondrial respiration to ADP. The decrease in V̇0 after Cr supplementation indicates that intrinsic changes in membrane proton conductance occur.
The control of mitochondrial respiration is a cardinal issue in the field of muscle energetics. Early work on isolated mitochondria identified ADP as an important stimulator of mitochondrial respiration (Lardy & Wellman, 1952; Chance & Williams, 1955). Later, the [ADP]/[ATP] ratio or the inverse phosphorylation potential [ADP][Pi]/[ATP] as well as the redox state of the electron transport chain were recognised as important regulators of respiration (for a review see Balaban, 1990). Although isolated mitochondria have proved to be an ideal tool for measurements of certain parameters of mitochondrial control and function (e.g. coupling efficiency), some of the sophisticated respiratory control mechanisms present in intact skeletal muscle are lost during the isolation procedure. For example the sensitivity of respiration to ADP and the effect of creatine (Cr) appear to be altered during the isolation procedure (Saks et al. 1995). An additional experimental limitation is that only a fraction (10-25 %) of the mitochondria are harvested from the muscle.
An alternative method for studying muscle oxidative function is the use of chemically permeabilised (skinned) muscle fibres. Unlike isolated mitochondria, skinned fibres allow virtually the entire mitochondrial population of the muscle sample to be studied in their natural structural environment (i.e. the connections between the mitochondria and the cytoskeleton remain undisturbed) (Kay et al. 1997; Saks et al. 1998). All the soluble cytosolic enzymes and metabolites are removed during the preparation, enabling experimental manipulations of the environment surrounding the functionally intact mitochondria (Kay et al. 1997; Saks et al. 1998).
In contrast to isolated mitochondria, the connections between mitochondria and cytoskeleton are preserved in skinned fibres and this is considered to be crucial for the maintenance of a high Km for ADP and for the ability of Cr to stimulate respiration (Saks et al. 1991, 1994, 1995, 1998; Kay et al. 2000). Creatine kinase (CK) is located in the cytosol and in the mitochondrial intermembrane space (CKmit). The location of CK in the muscle cell, together with the low permeability of the outer mitochondrial membrane for adenine nucleotides, is the basis for the Cr shuttle model of muscle energetics (Saks et al. 1976; Bessman, 1985). The model states that Cr is transported from the ATP utilising sites (e.g. myofibrils) to mitochondria, while PCr is transported in the reverse direction. Due to the presence of CKmit at the inner mitochondrial membrane, Cr will react with ATP formed by oxidative phosphorylation. This will increase local [ADP] and stimulate respiration. There is evidence that Cr-stimulated respiration is an important feature of oxidative muscles, including cardiac tissue, but is absent in fast-twitch muscles (Kuznetsov et al. 1996). Recent data demonstrate that mitochondria are incorporated into functional complexes with the ADP-producing systems (Seppet et al. 2001). Since the CK reaction is reversible, increased [PCr] is expected to decrease [ADP] and thus have the opposite effect to creatine on mitochondrial respiration. Therefore, we hypothesise that the presence of PCr may reduce respiration at rest and that decreases in the PCr/Cr ratio during exercise may be an important activator of respiration in vivo. If both PCr and Cr modulate mitochondrial respiration, it is of physiological importance to investigate the sensitivity of respiration to changes in [Cr] and [PCr] and combinations of PCr and Cr that mimic in vivo conditions.
Dietary supplementation with Cr has been shown to increase intramuscular levels of PCr + Cr (TCr) (Harris et al. 1992) and has been shown to enhance performance during high-intensity exercise (Balsom et al. 1993; Greenhaff et al. 1993). It is well known that the CK reaction is the most rapid process for ATP generation and increased PCr will therefore effectively increase the energetic power and may, at least partly, explain the improvement in supramaximal performance after Cr supplementation.
The influence of Cr supplementation on oxidative energy supply has not been studied in detail. Since endurance during moderate intensity exercise is not improved by Cr supplementation (Balsom et al. 1993; Stroud et al. 1994; Vandebuerie et al. 1998), it may be argued that oxidative function during the steady state is not affected by increased [Cr]. However, it is possible that, at least in some of these studies, the increased body weight associated with Cr supplementation has confounded the results (Balsom et al. 1993; Stroud et al. 1994). There is some evidence to support the hypothesis that creatine supplementation enhances oxidative phosphorylation. First, it has been shown that Cr supplementation can increase the rate of PCr resynthesis during recovery after intense exercise (Greenhaff et al. 1994). Second, during low-intensity exercise performed intermittently, utilisation of PCr was reduced after Cr supplementation (Rico-Sanz, 2000). These findings may be due to an effect of increased [Cr] as such but may also be due to intrinsic changes in mitochondrial function. The effect(s) (if any) of Cr supplementation on oxidative function remains elusive and measurements with the skinned fibre technique may provide a mechanistic approach to the problem.
The purpose of this study is (i) to investigate the physiological role of PCr in oxidative metabolism, (ii) to assess the sensitivity of respiration to Cr and PCr and the effects of physiological combinations of [PCr] and [Cr] on respiration, and (iii) to investigate whether Cr supplementation alters mitochondrial respiratory control.
METHODS
Subject data
Thirteen healthy male subjects participated in the study. The subjects’ mean age, weight and height (range) were: 24.8 (19-28) years, 81.3 (73.5-96.0) kg and 179.9 (173.0-189.5) cm, respectively. None of the subjects who participated in the study had used Cr as a dietary supplement but no measurements or tests were conducted to verify this statement. All subjects were fully informed of the possible risks and discomforts involved in the experiment before giving their written voluntary consent. The experimental design of the study was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. All experiments conformed with the Declaration of Helsinki.
Experimental procedure
Maximal oxygen uptake during cycling (V̇02,peak) was determined on a Monark 829e cycle. Subjects performed a discontinuous incremental cycle ergometer test (80 r.p.m.) until exhaustion. Expired air was collected in Douglas bags and analysed for O2 and CO2 (Beckman Instruments, Fullerton, CA, USA).
Two to three days following the V̇02,peak test, muscle biopsies were taken from the vastus lateralis. After local anaesthesia (1-2 ml carbocain; 20 mg ml−1, Astra), an incision was made through the skin and fascia at one-third of the distance from the upper margin of patella to the anterior superior iliac spine. The biopsies were performed using the technique of Bergström with suction, and were randomised between legs.
Each biopsy was divided with a surgical blade into at least two portions. One portion was frozen and stored at –80 °C until analysis of fibre type composition. A second portion was further separated into several small sections and placed in an ice-cold medium consisting of (mmol l−1): EGTA-CaEGTA buffer, 10 (free Ca2+ concentration 100 nm); imidazole, 20; KH2PO4, 3; dithiothreitol, 0.5; ATP, 5.3; PCr, 15; MgCl2, 9.5; 2-[N-morpholino]ethanesulphonic acid (Mes), 53.5; pH 7.00. The fibre bundles were separated with sharp-ended needles, leaving only small areas of contact, and incubated in 1.5 ml of the above medium (4 °C) containing 50 μg ml−1 saponin for 30 min with mild stirring. In order to completely remove saponin and metabolites, the fibres were washed three times with mild stirring for 5 min in 1.2 ml of cooled (4 °C) washing and oxygraph medium consisting of (mmol l−1): EGTA-CaEGTA buffer, 10 (free Ca2+ concentration 100 nm); imidazole, 20; KH2PO4, 3; pyruvate, 5; malate, 2; MgCl2, 4; Mes, 100; dithiothreitol, 0.5; taurine 20; and BSA, 2 mg ml−1; pH 7.00. After washing, the fibres were stored on ice until determination of respiratory activity. The maximal time between fibre preparation and measurement of respiratory activity was 2.5 h. Previous studies in our laboratory have shown that fibre respiration remains unchanged during this period (Tonkonogi et al. 1998). In eight subjects, a third portion was immediately quenched in liquid nitrogen and stored at –80 °C for metabolite determination.
Measurements of mitochondrial respiration
Mitochondrial oxygen consumption was measured polargraphically with a Clark-type electrode (Hansatech DW1, King's Lynn, Norfolk, UK) in a water-jacketed glass chamber maintained at 25 °C. Measurements were carried out in 0.3 ml of the above-described washing and oxygraph solution. In all fibres, respiration was measured in the absence of ADP (V̇0) and following the addition of 0.1 mm ADP (V̇submax). Following measurement of V̇0 and V̇submax, respiration in skinned fibres from eight subjects was measured after sequential additions of Cr (final [Cr] 5 mm and 20 mm) and ADP (V̇max, final [ADP] 5 mm). In another fibre bundle (from the same muscle biopsy) respiration was measured after sequential additions of PCr (final [PCr] 1 mm and 20 mm) and ADP (V̇max, final [ADP] 5 and 10 mm). Due to the inhibition of respiration with PCr a higher concentration of ADP was required to reach V̇max.
The effect of different combinations of PCr + Cr was investigated in muscle samples from five subjects. Following measurement of respiration at V̇submax, PCr and Cr were added at concentrations (mm) similar to those in muscle at rest (24 PCr + 12 Cr; PCr/Cr = 2), and during low-intensity work (12 PCr + 24 Cr; PCr/Cr = 0.5) or high-intensity work (3 PCr + 33 Cr; PCr/Cr = 0.1). Mitochondrial respiration was maximally stimulated by the addition of ADP to a final concentration of 10 mm (V̇max).
Immediately following respiratory measurements, the fibre bundles were removed, quick frozen in liquid nitrogen, freeze-dried and weighed. Wet weight was used as a reference base for respiration and was obtained from the dry weight assuming 77 % water content (Bergström, 1962).
Creatine supplementation
After the first biopsy, the eight subjects in whom the sensitivity of mitochondrial respiration to PCr or Cr was studied began a 16 day creatine supplementation programme. During the first 5 days of supplementation 20 g creatine + 180 g glucose were ingested daily (divided into two equal portions taken in the morning and evening). During days 6-16 of the supplementation period subjects ingested 2 g creatine + 18 g glucose (one portion per day). On day 17, muscle biopsies were taken, and fibre respiration was measured with an identical procedure to the first muscle biopsy.
Fibre type determination
Part of each biopsy was used for fibre type determination. Muscle samples were mounted in an embedding medium (Tissue-Tek O.C.T. Compound 4583, Histolab Products AB, Frolunda, Sweden), frozen in liquid nitrogen-cooled isopentane and stored at –80 °C. Transverse sections (6 μm) were cut with a cryostat (Leica Jung Frigocut 2800E) and the slides were stored at –80 °C. The fibre sections were incubated in 2 % formaldehyde solution for 20 min, rinsed in distilled water, and washed in Earle's balanced salt solution (EBSS) (14050-041; Life Technologies, Paisley, UK) (including Hepes 1 % and saponin 0.1 %) for 3 × 3 min. The fibres were prepared for the addition of the primary antibody through incubation in a solution containing EBSS + saponin + 1 % H2O2+ 2 % NaN3 in darkness for 1 h. The fibres were then rinsed for 3 × 3 min in EBSS and the primary antibody (monoclonal anti-myosin (skeletal, slow) M8421, Sigma) was added. Incubation with the primary antibody lasted 18 h. After overnight storage, the fibres were washed in EBSS for 3 × 3 min and exposed to Histostain Plus reagents A, B and C (commercial kit available from Zymed Laboratories Inc., San Francisco, USA). Following treatment with Histostain Plus, the slides were developed in an AEC (3-amino-9-ethyl-carbazole, A-6926, Sigma) solution (30 mg AEC + 12 ml DMSO + 0.002 % H2O2/ 100 ml EBSS buffer) for 10 min in darkness. The slides were washed for 3 × 3 min and the background was coloured by exposing the slides to Mayer's haematoxylin solution (Apoteket, Sweden) for 30-40 s.
In six of the eight subjects participating in the Cr supplementation programme it was possible to determine fibre type composition in both pre- and post-supplementation samples. There was no effect of Cr supplementation on the relative numbers of type I fibres or fibre type area following the supplementation period (n = 6; data not shown).
Metabolite analysis
PCr and Cr were analysed in the biopsy samples from the subjects who participated in the Cr supplementation programme. The samples were freeze-dried and dissected free of solid non-muscle constituents, powdered, and extracted with perchloric acid (0.5 mol l−1) and neutralised with KHCO3 (2.2 mol l−1). Analyses were performed by NAD(P)H-coupled specific enzymatic reaction adapted for spectrophotometric assays of NAD(P)H (Harris et al. 1974).
Data analysis
All values are presented as means ±s.e.m. Differences between means were tested for statistical significance with Student's paired t test. Significance was set at P < 0.05.
RESULTS
The subjects’ maximal oxygen uptake reached during the cycle test (V̇02,peak) was 4.2 ± 0.1 l min−1 or 52.2 ± 1.9 ml O2 (kg body weight)−1 and the area of type I fibres in the vastus lateris was 53.1 ± 3.9 % (n = 13).
Representative oxygraphic traces of mitochondrial respiration in permeabilised muscle fibres after sequential additions of submaximal ADP, PCr (or Cr), and saturating ADP concentrations are shown in Fig. 1. Muscle fibre respiration in the presence of 0.1 mm ADP (V̇submax) averaged 42 % of maximal ADP-stimulated respiration (V̇max, n = 13). Addition of 20 mm PCr (sufficient to elicit maximal effect; data not shown) decreased respiration by 54 % (P < 0.01, n = 8, Fig. 2A). About half the maximal effect of PCr was achieved at 1 mm. In contrast, the addition of 20 mm Cr (sufficient to elicit maximal effect; data not shown) to skinned muscle fibres respiring at 0.1 mm ADP increased respiration by 55 % (P < 0.01, n = 8, Fig. 2B). The addition of 5 mm Cr was sufficient to reach half the maximal Cr-stimulated increase in respiration. Although the presence of PCr or Cr decreased or increased the respiration in the presence of 0.1 mm ADP, V̇max was identical regardless of the presence of PCr or Cr (1.87 ± 0.12 vs. 1.85 ± 0.12 mmol O2 min−1 (kg wet wt)−1, respectively). Therefore, additions of PCr or Cr altered the sensitivity of respiration to ADP ((V̇submax – V̇0)/(V̇max – V̇0)). In the presence of 20 mm PCr the ADP sensitivity decreased by 84 ± 4 %, while 20 mm Cr increased the ADP sensitivity by 83 ± 10 %.
Figure 1. Representative oxygraphic traces of mitochondrial respiration in permeabilised fibre bundles from human muscle.
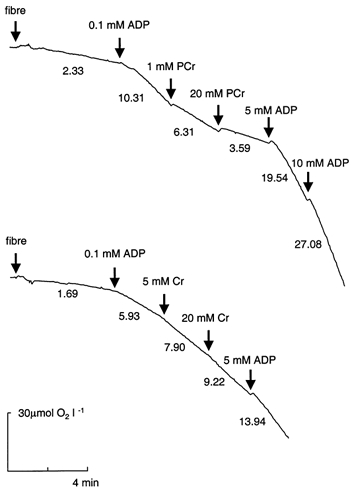
The initial oxygen concentration was 237.5 μm. Pyruvate (5 mm) + malate (2 mm) were used as respiratory substrates. The numbers indicate rates of oxygen consumption as μmol O2 min−1 l−1. The weight of the fibre bundles was 0.99 mg dry wt (upper trace) and 0.55 mg dry wt (lower trace).
Figure 2. The effect of maximal and submaximal additions of PCr or Cr on submaximally ADP-stimulated respiration in skinned muscle fibres.
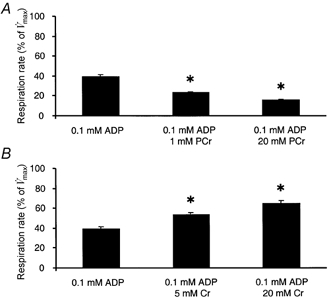
Respiration was measured in skinned muscle fibres following the sequential addition of 0.1 mm ADP, 1 mm PCr and 20 mm PCr (A) or 0.1 mm ADP, 5 mm Cr and 20 mm Cr (B). Values are expressed relative to maximal ADP-stimulated respiration (V̇max) and are means ±s.e.m., n = 8. *P < 0.05vs. respiration at 0.1 mm ADP.
The effect of three combinations of PCr + Cr on fibre respiration at a constant [ADP] (0.1 mm) was studied. The combinations of Cr + PCr were chosen to mimic in vivo concentrations (mm) at rest (24 PCr/12 Cr; PCr/Cr = 2), during low-intensity exercise (LI: 12 PCr/24 Cr; PCr/Cr = 0.5) and during high-intensity exercise (HI: 3 PCr/33 Cr, PCr/Cr = 0.1). Respiration at LI conditions was similar to that at rest, whereas under HI conditions it was about twofold higher (P < 0.01vs. both rest and low-intensity work conditions; Fig. 3).
Figure 3. The combined effect of PCr + Cr on submaximal respiration in skinned muscle fibres.
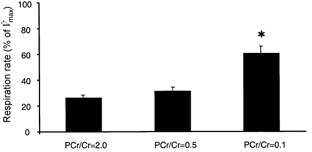
Following the measurement of respiration in the presence of 0.1 mm ADP, one of three combinations of PCr + Cr was added to the respiration medium, while keeping the sum of PCr and Cr constant (36 mm). The concentrations of PCr + Cr were chosen to mimic in vivo concentrations at rest (PCr/Cr = 2.0), and during low-intensity (LI; PCr/Cr = 0.5) and high-intensity (HI; PCr/Cr = 0.1) exercise. Values are expressed relative to maximal ADP-stimulated respiration (V̇max) and are means ±s.e.m., n = 8. Respiration in the presence of 0.1 mm ADP alone was 49 ± 3 % of V̇max. *P < 0.01vs. respiration at PCr/Cr = 2.0 and 0.5.
Eight subjects participated in a creatine supplementation programme for 16 days. TCr was higher in seven of the eight subjects after the supplementation period but due to a decrease in one subject the difference did not reach statistical significance (Table 1). This subject had already an exceptionally high TCr prior to the supplementation period (146.4 mmol (kg dry wt)−1). V̇0 decreased by 17 % (P < 0.01) following Cr supplementation; however, neither V̇submax, V̇max nor the rate of respiration in the presence of PCr or Cr were altered following the supplementation period (Table 1).
Table 1.
Effect of 16 days of creatine supplementation on muscle metabolites (mmol (kg dry wt)−1) and muscle fibre respiration (mmol O2 min−1 (kg wet wt)−1)
| Before Cr | After Cr | P | |
|---|---|---|---|
| ATP | 19.9 ± 1.3 | 20.1 ± 1.1 | 0.92 |
| PCr | 81.7 ± 3.1 | 78.6 ± 3.2 | 0.50 |
| Cr | 50.2 ± 3.5 | 61.3 ± 4.4 | 0.07 |
| PCr/ Cr | 1.68 ± 0.13 | 1.35 ± 0.14 | 0.18 |
| PCr + Cr | 131.9 ± 5.2 | 139.9 ± 2.8 | 0.10 |
| V̇0 | 0.24 ± 0.01 | 0.20 ± 0.01 | 0.005 |
| V̇submax | 0.70 ± 0.02 | 0.68 ± 0.03 | 0.43 |
| V̇max | 1.86 ± 0.11 | 1.79 ± 0.12 | 0.61 |
| V̇Cr/V̇submax | 1.55 ± 0.07 | 1.43 ± 0.11 | 0.36 |
| V̇PCr/V̇submax | 0.46 ± 0.02 | 0.42 ± 0.02 | 0.36 |
Results are presented as means ±s.e.m. (n = 8). Values of V̇0 (respiration in the absence of ADP), V̇submax (respiration at 0.1 mM ADP) and V̇max (maximal ADP stimulated respiration) are the means of experiments with Cr and PCr. V̇Cr is respiration in the presence of ADP (0.1 mM) and Cr (20 mM). V̇PCr is respiration in the presence of ADP (0.1 mM) and PCr (20 mM).
DISCUSSION
The primary finding of this study was that PCr reduces submaximal respiration but not maximal ADP-stimulated respiration. Previously, several investigators have reported that respiration increases when Cr is added to the respiration medium (Kuznetsov et al. 1996; Tonkonogi et al. 1998, 1999). The effect is believed to be due to the Cr shuttle system within skeletal muscle by which Cr will increase the local concentration of free ADP (see Introduction). Increases in [ADP] in the mitochondrial intermembrane space will increase respiration since ADP is a potent stimulator of respiration. The finding that PCr reduces respiration is fully compatible with the Cr shuttle hypothesis. The CK reaction is reversible and, therefore, the presence of PCr will reduce [ADP] and mitochondrial respiration. The maximal effect of PCr and Cr was reached at 20 mm and the magnitude (expressed either as change in ADP sensitivity or the relative change in V̇submax) was nearly identical. However, the concentration required to reach half the maximal effect was lower for PCr (1 mm) than for Cr (5 mm). Therefore, under the prevailing conditions, PCr appears to be a more potent regulator of respiration than Cr. It should also be noted that neither Cr nor PCr changed the maximal rates of ADP-stimulated respiration. This means that the presence of Cr and PCr influences the sensitivity of respiration to ADP, which is fully compatible with the creatine shuttle hypothesis.
In vivo, there is no condition in which PCr or Cr is absent intramuscularly. Rather, total creatine is maintained at a constant concentration and the ratio between PCr and Cr is altered in relation to energy requirements. Therefore, we investigated the combined effect of different relative concentrations of PCr and Cr, simulating conditions at rest as well as at LI and HI exercise. In the presence of 0.1 mm ADP, 20 mm Cr is sufficient to cause a maximal Cr effect on respiration. Interestingly, this study showed that respiration was not statistically different when [Cr] increased from 12 to 24 mm Cr in the presence of high [PCr] (transition from rest to LI). When PCr/Cr was 0.1 (simulating HI), respiration increased about twofold compared with that at a PCr/Cr at rest and LI. These results demonstrate how changes in PCr/Cr affect respiration when [ADP] is maintained constant at 0.1 mm. The quantitative effect of PCr/Cr will probably be different at other concentrations of ADP. In vivo respiration will, in addition to the effect of PCr/Cr, be modulated by changes in ADP (or phosphorylation potential) and redox drive. Previously, investigations have focused on the importance of the increased concentration of Cr during exercise in lowering the Km for ADP (Nemirovskaia et al. 1993; Walsh et al. 2001). However, the results from this study demonstrate that a reduction in [PCr] is equally or more important in achieving an increased sensitivity to ADP during exercise.
In isolated mitochondria, the Km for ADP has been determined to be 11-20 μm (Saks et al. 1995; Tonkonogi & Sahlin, 1997). Free ADP is generally calculated from the CK equilibrium and estimates of [free ADP] in skeletal muscle at rest (with both biochemical and 31P MRS techniques) range between 7 and 20 μm (Radda, 1986; Sahlin et al. 1997; Wackerhage et al. 1998). According to these estimates, respiration rates would be roughly 50 % of maximum at rest, which obviously cannot be correct. However, the Km for ADP is considerably lower in isolated mitochondria than in skinned fibres, and this has been explained as being due to damage to the outer mitochondrial membrane during the process of isolating mitochondria (Saks et al. 1995). Assuming Michaelis-Menten kinetics, it can be calculated from the present data that the Km for ADP in skinned fibres was 140 μm in the absence of PCr and Cr. In the presence of PCr and Cr at concentrations simulating those at rest (PCr/Cr = 2) the Km for ADP increased to 300 μm. This would correspond to a respiratory rate of 2-6 % of V̇max, which in vivo might be even lower due to a lower redox drive of the electron transport chain.
From studies in rat, it is known that the sensitivity of respiration to ADP is rather low (high Km) in fibre bundles from oxidative muscles (soleus and cardiac muscle) and that creatine can increase this sensitivity (Kuznetsov et al. 1996). In contrast, fast-twitch muscle (both red and white gastrocnemius) exhibits a high sensitivity to ADP and there is no effect of creatine in these skinned fibres. Human muscle is a mixture of fast- and slow-twitch fibres and the composition varies between subjects. We have previously reported that ADP sensitivity is negatively correlated with type I fibre area (Tonkonogi et al. 1998) and to oxidative potential (as indicated by the activity of citrate synthase) (Tonkonogi et al. 1999). We have also observed that the ADP sensitivity decreases after 6 weeks of endurance training (Walsh et al. 2001). These results are in accordance with the results from rat muscle discussed above. In the present study, there was no significant correlation between the proportion of type I fibre area and ADP sensitivity. The present study differs in experimental procedure from previous studies in our laboratory in that a large number of consecutive additions were made to the respiratory chamber. It is possible that this increased the experimental error to an extent that a significant correlation was obscured.
Oxidative muscles appear to have evolved a system where mitochondrial respiration has a low sensitivity to ADP but is activated by decreases in the PCr/Cr ratio. What could the physiological advantage be with this type of control? Firstly, modulation of respiration by the PCr/Cr system would reduce the extent of ADP fluctuation. This might be of advantage for cellular energetics since increases in ADP will influence other metabolic pathways (e.g. glycogenolysis, glycolysis and AMP deamination). Secondly, a low ADP sensitivity may indicate that the energetic system operates at a higher [free ADP]. Studies of quiescent cat muscle with 31P MRS have verified that [free ADP] is higher and phosphorylation potential lower in the slow-twitch soleus muscle than in the fast-twitch biceps brachi muscle (Meyer et al. 1985). A lower cytosolic phosphorylation potential implies that mitochondria can conduct oxidative phosphorylation with a lower driving force (i.e. a lower proton motive force; Δp) (Slater et al. 1973). The back leakage of protons into mitochondrial matrix increases at high Δp (Brand et al. 1994) and the conductance of the system at a lower Δp would therefore increase the efficiency (i.e. P/O ratio) of oxidative phosphorylation. Another advantage of operating at a reduced Δp would be that formation of reactive oxygen species is reduced (Vidal-Puig et al. 2000). To our knowledge, Δp has not been measured in the different fibre types of intact muscle but such experiments would provide a possible way to test this hypothesis.
In most studies of dietary Cr supplementation, TCr has been shown to increase after the supplementation period (Harris et al. 1992; Hultman et al. 1996). In the present study, in seven of eight subjects TCr increased after Cr supplementation. The non-responding subject had an unusual high TCr content before the supplementation period (146 mmol (kg dry wt)−1). This is probably the explanation for the unique response, since the extent of Cr uptake is inversely related to the individual's initial muscle TCr content (Harris et al. 1992). Given the importance of both Cr and PCr in the regulation of oxidative function, it is possible that increases in intramuscular [Cr] and [PCr] will alter oxidative function in vivo. Several investigations have shown increases in Cr content with little or no change in PCr following Cr supplementation (Harris et al. 1992; Hultman et al. 1996). This would lead to a reduced PCr/Cr ratio, indicating increased [free ADP]. The combination of increases in [free ADP] and [Cr] at rest may indicate that respiration and oxidative phosphorylation are elevated after creatine supplementation. It may be speculated that the cellular energy requirement is augmented during creatine supplementation due to an anabolic effect of creatine (Kreider, 1999).
An alternative possibility is that the increased muscle TCr may cause intrinsic alterations in the control of oxidative function of the mitochondria (e.g. alterations independent of the immediate concentration of PCr/Cr and energy requirements). This hypothesis was investigated in the present study. Respiration in the absence of ADP decreased following Cr supplementation by 17 % (P < 0.01). It is noteworthy that the subject who had a paradoxical response in TCr was the only subject showing an increased V̇0 after the supplementation period. The decrease in V̇0 indicates that the back leakage of protons is reduced, possibly due to a reduced permeability of the inner mitochondrial membrane to protons or to a reduced Δp. The mechanism for this change and the physiological implications remain unclear. ADP-stimulated respiratory parameters remained unchanged following 16 days of Cr supplementation. These findings do not support the hypothesis that the control of oxidative phosphorylation is altered following supplementation with Cr, but the possibility that the supplementation period was too short for this adaptation cannot be ruled out.
In summary, it has been shown for the first time that PCr reduces mitochondrial respiration at submaximal concentrations of ADP. The decrease in respiration at saturating [PCr] was nearly identical to the increase in respiration at saturating [Cr] but at suboptimal concentrations respiration was more sensitive to PCr than to Cr. PCr and Cr at concentrations similar to that during high-intensity exercise increased respiration about twofold compared with that at rest or low-intensity exercise. The results demonstrate that PCr has an important role during high-intensity exercise, not only as a buffer of ATP but also as a modulator of the rate of mitochondrial ATP production. Finally, following 16 days of Cr supplementation, non-coupled respiration (i.e. in the absence of ADP) was reduced but ADP-stimulated respiration was unchanged. The results do not support the hypothesis that the control of mitochondrial ATP production is altered by Cr supplementation but indicate that intrinsic changes of membrane proton conductance occur.
Acknowledgments
The present study was supported by research grants from the Swedish National Centre for Research in Sport, the Swedish Medical Research Council (project 13020) and the Estonian Science Foundation grant 4928.
References
- Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. American Journal of Physiology. 1990;258:C377–389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Balsom PD, Harridge SD, Soderlund K, Sjodin B, Ekblom B. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiologica Scandinavica. 1993;149:521–523. doi: 10.1111/j.1748-1716.1993.tb09649.x. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1962;68(suppl.):1–101. [Google Scholar]
- Bessman SP. The creatine-creatine phosphate energy shuttle. Annual Review of Biochemistry. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochimica et Biophysica Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilisation. Journal of Biological Chemistry. 1955;217:383–393. [PubMed] [Google Scholar]
- Greenhaff PL, Bodin K, Soderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. American Journal of Physiology. 1994;266:E725–730. doi: 10.1152/ajpendo.1994.266.5.E725. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Casey A, Short AH, Harris RC, Soderlund K, Hultman E. Influence of oral creatine supplementation on muscle torque during repeated bouts of maximal voluntary exercise in man. Clinical Science. 1993;84:565–571. doi: 10.1042/cs0840565. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo L-O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. Journal of Applied Physiology. 1966;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- Kay L, Li Z, Mericskay M, Olivares J, Tranqui L, Fontaine E, Tiivel T, Sikk P, Kaambre T, Samuel JL, Rappaport L, Usson Y, Leverve X, Paulin D, Saks VA. Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochimica et Biophysica Acta. 1997;1322:41–59. doi: 10.1016/s0005-2728(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Kay L, Nicolay K, Wieringa B, Saks V, Wallimann T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. Journal of Biological Chemistry. 2000;275:6937–6944. doi: 10.1074/jbc.275.10.6937. [DOI] [PubMed] [Google Scholar]
- Kreider RB. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Medicine. 1999;27:97–110. doi: 10.2165/00007256-199927020-00003. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. European Journal of Biochemistry. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Lardy HA, Wellman H. Oxidative phosphorylation: role of inorganic phosphate and acceptors systems in control of metabolic reactions. Journal of Biological Chemistry. 1952;1955:215–224. [PubMed] [Google Scholar]
- Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. American Journal of Physiology. 1985;248:C279–287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Nemirovskaia TL, Shenkman BS, Nekrasov AN, Kuznetsov AV, Saks VA. [Effect of training on the structural-metabolic indicators in athletes’ skeletal muscles] Biokhimiia. 1993;58:471–479. [PubMed] [Google Scholar]
- Radda GK. Control of bioenergetics: from cells to man by phosphorus nuclear-magnetic-resonance spectroscopy. Eighteenth Ciba medal lecture. Biochemical Society Transactions. 1986;14:517–525. doi: 10.1042/bst0140517. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J. Creatine reduces human muscle PCr and pH decrements and P(i) accumulation during low-intensity exercise. Journal of Applied Physiology. 2000;88:1181–1191. doi: 10.1152/jappl.2000.88.4.1181. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. American Journal of Physiology. 1997;273:C172–178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- Saks VA, Belikova YO, Kuznetsov AV, Khuchua ZA, Branishte TH, Semenovsky ML, Naumov VG. Phosphocreatine pathway for energy transport: ADP diffusion and cardiomyopathy. American Journal of Physiology. 1991;261:30–38. doi: 10.1152/ajplung.1991.261.4.L30. [DOI] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration. A synthesis. In: Saks VA, Ventura-Clapier R, editors. Cellular Bioenergetics. Role of Coupled Creatine Kinase. Dordrecht: Kluwer Academic Publishers; 1994. pp. 155–192. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. Journal of Molecular and Cellular Cardiology. 1995;27:625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- Saks V, Lipina N, Smirnov V, Casow E. Studies of energy transport in heart cells. The functional coupling between mitochondrial creatine phosphokinase and ATP-ADP translocase: kinetic evidence. Archives of Biochemistry and Biophysics. 1976;173:34–41. doi: 10.1016/0003-9861(76)90231-9. [DOI] [PubMed] [Google Scholar]
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Molecular and Cellular Biochemistry. 1998;184:81–100. [PubMed] [Google Scholar]
- Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M, Saks VA. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochimica et Biophysica Acta. 2001;1504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- Slater EC, Rosing J, Mol A. The phosphorylation potential generated by respiring mitochondria. Biochimica et Biophysica Acta. 1973;292:534–553. doi: 10.1016/0005-2728(73)90003-0. [DOI] [PubMed] [Google Scholar]
- Stroud MA, Holliman D, Bell D, Green AL, MacDonald IA, Greenhaff PL. Effect of oral creatine supplementation on respiratory gas exchange and blood lactate accumulation during steady-state incremental treadmill exercise and recovery in man. Clinical Science. 1994;87:707–710. doi: 10.1042/cs0870707. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: effects of prolonged exercise. Journal of Physiology. 1998;510:279–286. doi: 10.1111/j.1469-7793.1998.279bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiologica Scandinavica. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Walsh B, Tiivel T, Saks V, Sahlin K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflügers Archiv. 1999;437:562–568. doi: 10.1007/s004240050818. [DOI] [PubMed] [Google Scholar]
- Vandebuerie F, Vanden Eynde B, Vandenberghe K, Hespel P. Effect of creatine loading on endurance capacity and sprint power in cyclists. International Journal of Sports Medicine. 1998;19:490–495. doi: 10.1055/s-2007-971950. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. Journal of Biological Chemistry. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- Wackerhage H, Hoffmann U, Essfeld D, Leyk D, Mueller K, Zange J. Recovery of free ADP, Pi, and free energy of ATP hydrolysis in human skeletal muscle. Journal of Applied Physiology. 1998;85:2140–2145. doi: 10.1152/jappl.1998.85.6.2140. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilised muscle fibers. Pflügers Archiv. 2001;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]


