Abstract
Episodic hypoxia (EH) is followed by increased ventilatory motor output in the recovery period indicative of long-term facilitation (LTF). We hypothesized that episodic hypoxia evokes LTF of genioglossus (GG) muscle activity in humans during non-rapid eye movement sleep (NREM) sleep. We studied 12 normal non-flow limited humans during stable NREM sleep. We induced 10 brief (3 minute) episodes of isocapnic hypoxia followed by 5 minutes of room air. Measurements were obtained during control, hypoxia, and at 5, 10, 20, 30 and 40 minutes of recovery, respectively, for minute ventilation (V̇I), supraglottic pressure (PSG), upper airway resistance (RUA) and phasic GG electromyogram (EMGGG). In addition, sham studies were conducted on room air. During hypoxia there was a significant increase in phasic EMGGG (202.7±24.1% of control, p<0.01) and in V̇I (123.0±3.3% of control, p<0.05); however, only phasic EMGGG demonstrated a significant persistent increase throughout recovery (198.9±30.9%, 203.6±29.9% and 205.4±26.4% of control, at 5, 10, and 20 minutes of recovery, respectively, p<0.01). In multivariate regression analysis, age and phasic EMGGG activity during hypoxia were significant predictors of EMGGG at recovery 20 minutes. No significant changes in any of the measured parameters were noted during sham studies. Conclusion: 1) EH elicits LTF of GG in normal non-flow limited humans during NREM sleep, without ventilatory or mechanical LTF. 2) GG activity during the recovery period correlates with the magnitude of GG activation during hypoxia, and inversely with age.
Keywords: Long-term facilitation, sleep, episodic hypoxia, genioglossus, minute ventilation
1. INTRODUCTION
Exposure to intermittent hypoxia elicits a sustained increase in ventilatory motor output, referred to as respiratory long-term facilitation (LTF). The occurrence and expression of LTF varies across species, CNS arousal state, experimental paradigms and medullary motor nuclei. Episodic hypoxia elicits LTF in many (Maltais et al. 1991; Cao et al. 1992; Hayashi et al. 1993; Mateika and Fregosi, 1997; Turner and Mitchell, 1997; Fregosi and Mitchell, 2000), but not all animal models (Janssen and Fregosi, 2000). Likewise, episodic hypoxia did not elicit LTF during wakefulness in humans (McEvoy et al. 1996, Jordan et al. 2002, Morris and Gozal. 2004). In contrast, studies from our laboratory have demonstrated LTF in a subset of sleeping humans, specifically, in individuals who snore and who demonstrate inspiratory airflow limitation during sleep, (Babcock and Badr, 1998, Shkoukani et al. 2002, Babcock et al. 2003), as well as in patients with obstructive sleep apnea (OSA) (Aboubakr et al. 2001). Experimental conditions also influence the development of LTF. An example is the occurrence of LTF following episodic hypoxia in awake human subjects, only in the presence of concomitant hypercapnia (Harris et al. 2006). Finally, LTF may manifest differently across different motor nuclei, manifesting in the phrenic motorneuron (Fuller et al. 2000) or hypoglossal motorneuron (Bach and Mitchell, 1996).
Respiratory LTF in sleeping humans is associated with decreased upper airway resistance and no change in the activity of thoracic pump muscles (Shkoukani et al. 2002) suggesting that episodic hypoxia elicits ventilatory LTF by preferentially activating upper airway dilating muscles. Findings from animal studies support this conclusion. However, it is unknown whether episodic hypoxia evokes LTF of the genioglossus muscle in sleeping humans. Thus, the purpose of this study was to test the hypothesis that episodic hypoxia elicits LTF of the genioglossus muscle activity during NREM sleep in normal humans, manifesting as increased genioglossus muscle activity after termination of the hypoxic exposure. Given the potential confounding effects of varying negative pressure with changing upper airway resistance (Malhotra et al. 2002), we chose to investigate this phenomenon in healthy subjects, free of snoring and flow limitation.
2. METHODS
2.1 Subjects
All the experimental protocols described were approved by the Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center, and conformed to the standards set by the Declaration of Helsinki. Subjects were recruited via newspaper advertisements and local postings. Informed written consent was obtained from healthy participants free of daytime sleepiness, sleep-disordered breathing or other medical disorders. Twelve individuals successfully completed the experimental protocol. All participants were non-flow limited by definition. The absence of sleep-disordered breathing was confirmed by sham studies in seven of these participants.
2.2 Breathing Circuit
Each participant was connected to the breathing circuit with an airtight silicone rubber mask, strapped and glued to the face to prevent leaks. The mask was connected to a Plateau Exhalation Valve® (Respironics, Inc, Pittsburgh, PA), via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected on the inspiratory line. Three cylinders containing the following gases: 100% nitrogen (N2), 8% oxygen (FiO2 0.08%, balanced with N2), or 100% O2 were connected to the inspiratory line. To maintain isocapnia, supplemental carbon dioxide (CO2) (FiCO2 0.07%, balanced with N2), was added to the inspiratory line from an external source to maintain end-tidal PCO2 (Petco2) at or near control levels.
2.3 Measurements
Electroencephalograms (EEG), electrooculograms (EOG), and chin electromyograms (EMG) were recorded using the International 10–20 system of electrode placement (EEG: C3-A2 and C4-A1; EOG F7-A2, F8-A2, O-A2). Inspiratory airflow was measured by a heated pneumotachometer (Model 3700A, Hans Rudolph, Kansas City, MO) that was attached to a pressure transducer (Validyne, Northridge, CA). The tidal volume (VT) was obtained from the electronic integration of the flow signal (model FV156 Integrator, Validyne, Northridge, CA). Supraglottic airway pressure was measured using a pressure transducer tipped catheter (Model TC-500XG, Millar Instruments, Houston, TX), with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip for 2 cm after it disappeared behind the tongue. Petco2 was measured using air sampled continuously from the nasal mask by an infrared analyzer (Model CD-3A, AEI Technologies, Pittsburgh, PA). Arterial O2 saturation (SaO2) was measured by a pulse oximeter (Biox 3700, Ohmeda). The signals were displayed on a polygraph recorder (model 7-D, Grass, West Warwick, RI) and recorded using Biobench data acquisition software (National Instruments, Austin, TX) for further analysis. The above technique has been used previously (Aboubakr et al. 2001).
Surface inspiratory muscle EMG (EMGdia) was recorded using two surface electrodes (3M Red Dot, 3M Company, St. Paul, MN) placed 2–4 cm above the right costal margin in the anterior axillary line. One pair was positioned at the percussed dullness at total lung capacity and another pair was positioned at the point of percussed dullness at functional residual capacity. The electrode pair with the best signal-to-noise ratio was selected for analysis. The EMGGG was measured with a pair of unipolar intramuscular electrodes referenced to a single ground, thus producing a bipolar recording. Two stainless steel Teflon-coated 36-gauge wire electrodes were inserted 15–20 mm into the body of the genioglossus muscle 3 mm lateral to the frenulum of the tongue on each side, using a 25-gauge needle, which was quickly removed, leaving the wires in place. This technique has been described previously (Vitti et al. 1975).
2.4 Protocol
Experimental study
Participants assumed the supine position for the entire experimental protocol that was conducted during stable NREM sleep. All trials were conducted while subjects were in stable stage 2 or stage 3 sleep (Night 1). Twelve subjects without flow limitation (non-flow limited, NIFL) participated in this protocol. Ten cycles of episodic hypoxia were induced in each subject (Fig. 1). The subjects breathed room air for 5 minutes (control period), followed by hypoxia that was rapidly induced by having the subject breathe 1 or 2 breaths of 100% N2, followed by continuous 8% O2 for 3 minutes to maintain hypoxia (SaO2:80–85%). Supplemental CO2 was titrated to maintain isocapnia during hypoxia guided by Petco2 on a breath-by-breath basis. After 3 minutes, hypoxia was abruptly terminated with one to two breaths of 100% O2. This was followed by 5 minutes of room air. The breathing pattern was monitored for 40 minutes of recovery period following the tenth cycle of hypoxia.
Figure 1.
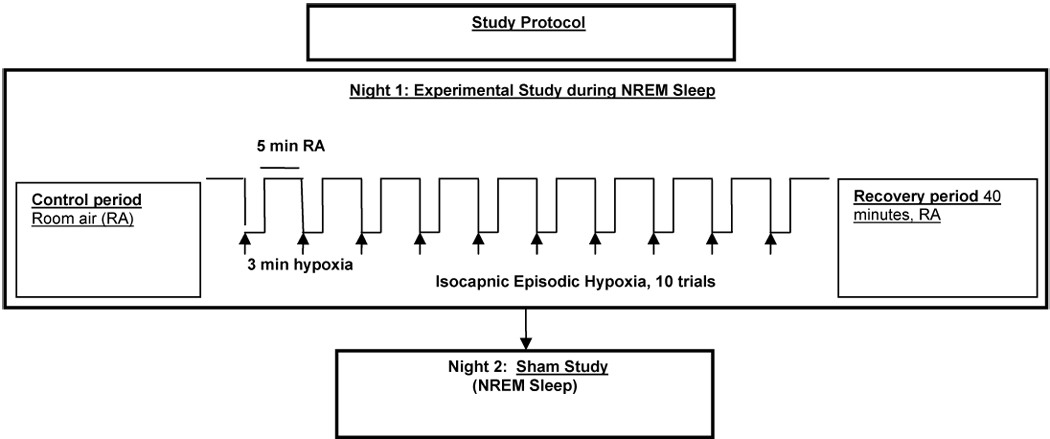
Algorithm of the study protocol.
Sham study
Sham studies (Night 2) were conducted to ascertain the presence of time dependent changes in ventilation and EMGGG, independent of the experimental protocol. For this purpose, seven of the twelve subjects were restudied during stable NREM sleep. Of the remaining five subjects, who had participated in the experimental study, three could not be contacted to return for the sham study and two did not agree to undergo a repeat study. The subjects who underwent sham studies were connected to the same breathing circuit with identical instrumentation except that they breathed ambient air for a study time that was equivalent to the study time of the experimental night.
2.5 Data Analysis
All analyses were done during stable NREM sleep at different time points: baseline room air, during each of the ten hypoxic periods and during recovery, at five (R5), ten (R10), twenty (R20), thirty (R30) and forty (R40) minutes after the tenth hypoxic episode (H10). Wakefulness and sleep stages were scored according to standard criteria (Rechtschaffen and Kales, 1968) and transient arousals were scored using the ASDA criteria (Task Force. 1992). The subjects were required to be in stable stage 2 or stage 3 sleep during control period, hypoxic exposures and recovery period data collection. Trials were eliminated from analysis if there was any arousal or change in sleep stage to a lighter stage.
Inspired tidal volume (VT), inspiration time (TI), total time for a breath (TTOT), breathing frequency (fR), minute ventilation (V̇I), Petco2, and arterial O2 saturation were calculated breath by breath during stable sleep during the first normoxic period (‘control’ period), during each of the hypoxic (H) periods and during recovery at R5, R10, R20, R30 and R40. To ensure that changes in ventilation were not due to subtle changes in sleep state, an independent observer confirmed the stability of sleep state. For each variable, a mean value was computed from ten consecutive breaths during each of the hypoxia trials and at R5, R10, R20, R30, and R40, respectively. For the control period two sets of ten consecutive breaths were analyzed. Shkoukani et al. (2002) have previously described this methodology. For graphical comparisons, V̇I data was normalized to the control period data and reported as a percentage of baseline activity.
To ascertain changes in upper airway mechanics, a pressure-flow loop was plotted for every breath for 20 breaths of the control period, for 10 breaths at each of hypoxic periods and for 10 breaths each at R5, R10, R20, R30, and R40. Upper airway resistance (RUA) at a maximum linear flow (peakflow, V̇peakflow) was computed from each loop as a numeric representation of the slope of the linear part of the pressure-flow loop. To ascertain the presence or absence of inspiratory flow limitation in each subject, the pressure-flow loop of each breath was analyzed. Flow limitation was defined as a plateau in flow despite ≥1 cmH2O decrease in the supraglottic pressure. Inspiratory flow limitation (IFL) has been previously described in detail by our laboratory (Rowley et al. 2001; Aboubakr et al. 2001; Shkoukani et al. 2002, Mansour et al. 2002).
To analyze GG, the raw EMG signal was amplified, filtered with a band pass filter of 50–10,000 Hz (Grass model 7-D Polygraph) and full wave rectified. EKG artifacts were “blanked” with an EKG blanker (CWE model SB-1). The processed signal was integrated with a moving-time averager with a time constant of 100 milliseconds. (CWE model MA-821 RES.) Phasic EMG activity was determined from the moving time average. Diaphragm EMG was analyzed similarly (Aboubakr et al. 2001). The GG EMG activity was quantified as a percent of maximal activation. To define maximal GG EMG activity, subjects performed three maneuvers: each subject maximally inspired against an occluded inspiratory line, maximally protruded the tongue against the back of the teeth, and swallowed (Mezzanotte et al. 1992). Each of these maneuvers was performed several times, with the maximal value recorded (from any maneuver) being called 100%. Electrical zero was then determined, with subsequent muscle activity being quantified as a percentage of maximal activation for that individual. Peak EMGGG was determined from the peak integrated activity of the moving time average and expressed in arbitrary units (au) (Mezzanotte et al. 1992). Phasic EMGGG was calculated as the difference between peak and tonic EMGGG. Again measurements were made for every breath for 20 breaths of the baseline control period (prior to trial 1), for 10 breaths of the control period (room air) prior to the onset of each of hypoxia trials (trials 2 to 10), for 10 breaths at each of hypoxic periods, and for 10 breaths each at R5, R10, R20, R30, and R40, respectively. To ensure that changes in EMGGG were not due to changes in sleep state, the segments chosen for analysis were those during stable sleep. For comparison, EMGGG data were normalized to the control period data and reported as a percentage of baseline activity.
For the sham study, data were sampled on ambient air at periods corresponding to the individual’s control period and a hundred and twenty minutes of study time. The latter period corresponds to the total duration of the episodic hypoxia trials and the recovery period during the experimental protocol. Accordingly, sham ‘recovery’ periods were analyzed at 85 and 100 minutes from the beginning of the control period and represent R5 and R20, respectively. As noted earlier, conducted sham studies were conducted on seven of twelve subjects.
Statistical Analysis
For normally distributed data, comparisons between time-points were made using one-way ANOVA with repeated measures, followed by post-hoc analysis for all pairwise comparisons using the Holm-Sidak method. If the normality test failed, then ANOVA on Ranks was performed followed by pair-wise comparisons test using the Tukey method. To ascertain the time course of increased EMGGG, we plotted the phasic EMGGG during successive room air and hypoxia periods, respectively, for each individual subject. We then performed linear regression analyses to determine the slope of EMGGG, for each subject during the trials, and used paired t-tests to determine if the slopes of the regressions were significantly different than zero. In addition, Pearson’s correlation analyses evaluated correlations between the EMGGG activity at R20 and multiple demographic and physiologic variables (see below). We used multivariate linear regression analyses to determine which independent variables predicted EMGGG during the recovery period. Several independent variables were considered for inclusion in the linear regression models, on the basis of an a priori reasoning that they might predict EMGGG activity at R20 (dependent variable). The following independent variables were tested: 1) age, 2) gender, 3) BMI, 3) V̇I and RUA (as a % of control) during hypoxia, 5) percent change in oxygen saturation during the hypoxia trials, and 6) phasic EMGGG during hypoxia. The level of statistical significance was set at p<0.05. All results are presented as mean±standard error of mean (S.E.M).
3. RESULTS
The demographic data of the subjects are given in Table 1. Twelve subjects participated in the study with satisfactory ventilation data; satisfactory GG signal during stable sleep was available during the first 20 minutes of recovery in 10 subjects. All selected subjects were non-snorers and had no evidence of inspiratory flow limitation by an a priori definition as described above. Thus, in these 12 individuals, only 8±3% of breaths were flow limited during the control period.
Table 1.
Subject demographics (n=12).
| Variable | Results |
|---|---|
| Age (years) | 25.8±2.0 |
| Male/Female (n) | 7/5 |
| Neck circumference (cm) | 35.7±1.1 |
| BMI (kg/m2) | 22.4±1.3 |
Values are mean±SEM, BMI body mass index
3.1 Hypoxia Periods
During the episodic hypoxia trials, oxyhemoglobin saturation decreased to 87.1±0.4%. The Petco2 during hypoxia was 38.9±1.0 mmHg compared to room air control values of 40.3±0.9 mmHg (Table 2). Trials were eliminated from analyses if there was any arousal or change in sleep stage to a lighter stage; a mean of 8±0.6 trials were averaged for each individual. Overall, there was a significant increase in V̇I, VT and V̇peakflow during the episodic hypoxia trials, p<0.05, but no significant change in RUA. Likewise, phasic EMGGG increased significantly to 202.7±24.1% of control period (p<0.01) (Table 2, Fig. 3). The increase in phasic EMGGG was related to a corresponding increase in peak EMG with no significant change in tonic EMGGG, therefore only phasic activity is reported. Satisfactory EMGdia signals were available in 6 subjects; EMGdia increased during hypoxia (131.5±19.1% of control). While this was a numerically increased value, it failed to reach statistical significance owing to small number of subjects.
Table 2.
Group data for ventilation, upper airway mechanics and EMG during control, hypoxia and recovery periods.
| Variables | Control | Hypoxia | R5 | R10 | R20 |
|---|---|---|---|---|---|
| TI, sec | 1.7±0.1 | 1.6±0.1 | 1.7±0.1 | 1.6±0.1 | 1.6±0.1 |
| TE, sec | 2.1±0.1 | 2.1±0.1 | 2.2±0.1 | 2.1±0.1 | 2.2±0.1 |
| fr, breath/min | 16.4±0.9 | 16.6±0.8 | 16.1±0.8 | 16.4±0.9 | 16.5±0.6 |
| Petco2 (mmHg) | 40.3±0.9 | 38.9±1.0 | 40.7±1.0 | 40.8±1.2 | 40.1±1.0 |
| V̇I, L/min | 6.8±0.6 | 8.5±0.7** | 6.7±0.5 | 6.9±0.6 | 7.0±0.6 |
| VT, L | 0.42±0.31 | 0.51±0.32** | 0.44±0.33 | 0.47±0.45 | 0.45±0.37 |
| RUA, cmH2O/L/sec | 5.8±1.0 | 4.9±0.7 | 5.9±0.8 | 5.5±1.1 | 6.3±0.9 |
| PSG cmH2O | −2.6±0.3 | −3.0±0.4 | −2.5±0.4 | −2.6±0.4 | −2.8±0.4 |
| V̇peakflow, L/sec | 0.32±0.03 | 0.40±0.03** | 0.32±0.02 | 0.31±0.03 | 0.33±0.03 |
| EMGGG, % control | - | 202.7±24.1* | 198.9±30.9* | 203.6±29.9* | 205.4±26.4* |
Values are mean±SEM
p<0.01 recovery, hypoxia vs control period.
p<0.05 hypoxia vs control, TI:inspiratory time, TE:expiratory time, fr:respiratory rate, Petco2:end-tidal CO2 level, V̇I:minute ventilation, VT:tidal volume, RUA:upper airway resistance, PSG:nadir supraglottic pressure, V̇peakflow:peak flow, EMGGG:phasic EMGGG; R5 R10 R20 recovery at 5, 10 and 20 minutes after hypoxia, respectively. EMGGG is expressed as percent of the control values.
Figure 3.
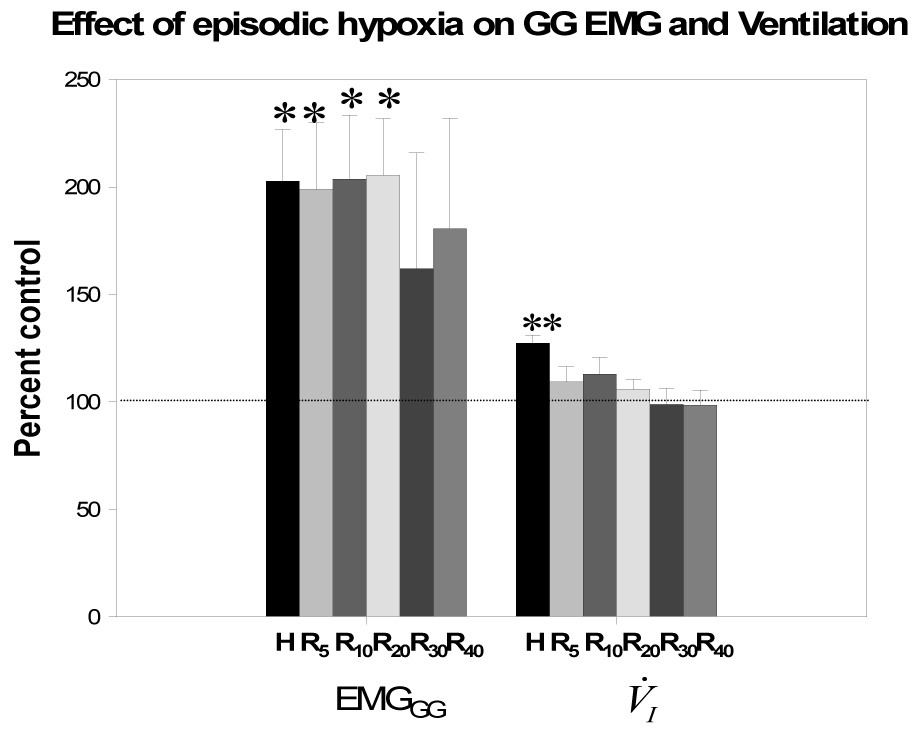
Group data (all subjects) for phasic EMGGG and V̇I during hypoxia and recovery periods normalized to baseline as a percent of control. H represents averaged data of the hypoxia trials while R5, R10, R20, R30, R40 are averaged data as a percent of room air control during recovery 5, 10, 20, 30 and 40 minutes, respectively. Both V̇I and phasic EMGGG activity increased significantly during hypoxia vs room air control. However, only EMGGG phasic activity was significantly increased during recovery periods R5, R10, and R20, as a percent of room air control; **p<0.05, hypoxia vs control period; *p<0.01, hypoxia, R5, R10, R20 vs control period. While phasic EMGGG remained elevated in R30 and R40, the values did not reach statistical significance due to the small number of subjects with stable sleep during this period, see text for details.
3.2 Recovery Period
Figure 2 shows a representative polygraph segment from a subject during NREM sleep. Results for the group during recovery (R5, R10 and R20) are shown in Table 2. Table 2 shows that there was no significant change in V̇I, VT, V̇peakflow or RUA during recovery compared to the control period. The aforementioned increase in the phasic EMGGG activity persisted into the recovery period. Phasic genioglossus EMG at R20 was 205.4±26.4% of RA control. In contrast, during recovery V̇I returned to RA control levels; V̇I was 7.0±0.6 L/min at R20 vs. 6.8±0.6 L/min during control, p=ns, where the statistical power of the test was >0.90. Likewise, EMGdia (n=6 subjects) returned to baseline RA control values in the recovery period (Fig. 4). There was no change in RUA during the hypoxia or the recovery periods.
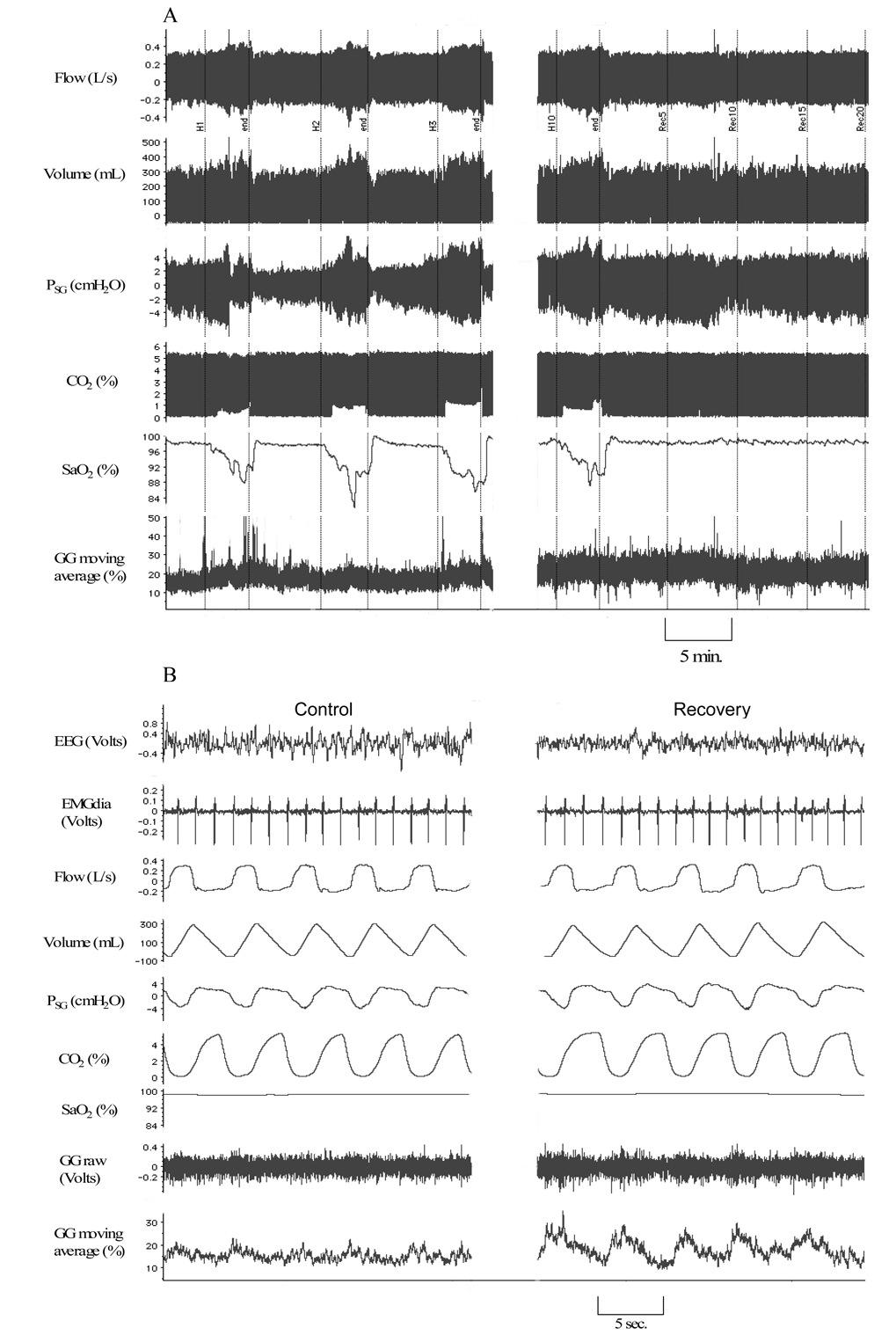
Figure 2.
Representative polygraph segments from a subject during stable NREM sleep at different time points: A. Compressed, high-speed polygraph segments during room air control condition, hypoxia periods (H1, H2, H3, H10) and recovery (R5, R10, R15 and R20). Note elevated phasic EMGGG amplitude during isocapnic hypoxia that persists during recovery relative to control; VT is increased during hypoxia alone. B. Polygraph at usual speed, for better visualization of EEG, EMGGG moving time average and EMGdia, clearly demonstrates increase in phasic EMGGG in during recovery period relative to control.
Figure 4.
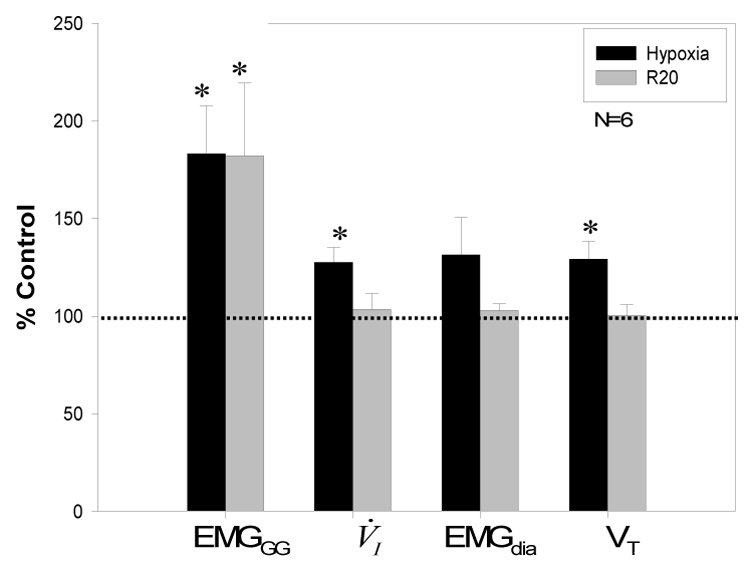
Group data for 6 subjects with satisfactory EMGdia signals are presented. Data for phasic EMGGG, V̇I, EMGdia and VT during hypoxia and recovery period (R20) were normalized to baseline as a percent of control. Phasic EMGGG, V̇I, and Vt, all increased significantly during hypoxia vs room air control. However, only phasic EMGGG activity was significantly increased during R20; *p<0.05.
Furthermore, we determined the duration of GG LTF by analyzing all the stable sleep epochs in the recovery period. Stable NREM sleep was maintained during R30 and R40 in five individuals from the group. GG LTF was apparent during R30 and R40, as phasic EMGGG remained elevated at 207±56.4% of control, Fig. 3 (p=ns due to small number of subjects during R30 and R40); there was no evidence of ventilatory LTF as V̇I was only 98.7±7.4% of control during this period. In addition, exposure to episodic hypoxia resulted in a gradual increase of phasic EMGGG that occurred between the hypoxic episodes on room air as well as during the hypoxic periods, Fig. 5A and 5B. The phasic EMGGG data were plotted for each individual subject, then least square regression lines were calculated for each individual (see Statistical Analysis). The slopes for the regression lines were significantly different than zero (p<0.05).
Figure 5.
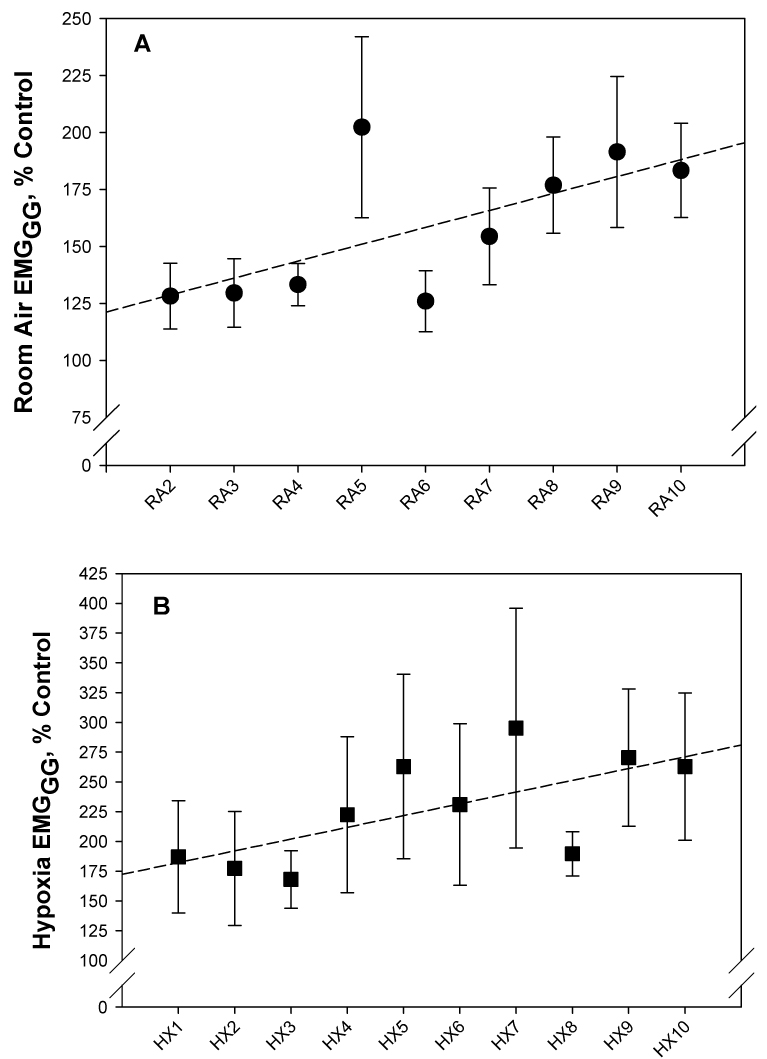
Least square regression analysis: A. There was a gradual increase in the phasic EMGGG during the course of the room air control periods. The slope of the regression line was significantly different than zero (p<0.05) and illustrates the augmentation of LTF in the normoxic intervals between hypoxic episodes (RA2 to RA10). B. There was also a progressive augmentation of the EMGGG during successive hypoxia episodes (HX1 to HX10); slope of the regression line was significantly different than zero (p<0.05).
To ascertain the determinants of phasic EMGGG during the recovery period, we used regression analysis to test several variables that were deemed as potential determinants of genioglossus activity (see Data analysis). Two determinants were identified; the level of phasic EMGGG during hypoxia (r=0.88, p<0.001, Fig. 6) correlated with the genioglossus activity during the recovery period. In contrast, there was a negative correlation between the age of the participant and EMGGG at R20 (r=−0.64, p=0.04, Fig. 7). There was no correlation between the EMGGG at R20 and gender, percent change in oxygen saturation during hypoxia, or change in V̇I or RUA during either hypoxia or recovery (Table 3). In a multivariate regression model, both age and EMGGG during hypoxia significantly predicted phasic EMGGG (model r2= 0.93, p=0.001). In addition, in Fig. 6, after excluding the far right data point, the coefficient was 0.6, p=0.06; but in the multiple linear regression model, the coefficient for phasic EMGGG during hypoxia was significant, (coefficient: 0.7, p<0.01), i.e., after adjusting for age the EMGGG during hypoxia is a significant independent predictor of EMGGG at R20. Similarly in Fig. 7, even after excluding the highest left point there remained a significant negative significant correlation between recovery EMGGG and age, r=−0.74, p=0.04; age remained a significant independent predictor in the multiple regression model, p<0.01. While there was no significant correlation between recovery genioglossus activity and the change in saturation during hypoxia, the phasic EMGGG activity during hypoxia tended to be positively correlated with the degree of desaturation achieved during the hypoxia trials (r=0.5, p=0.07).
Figure 6.
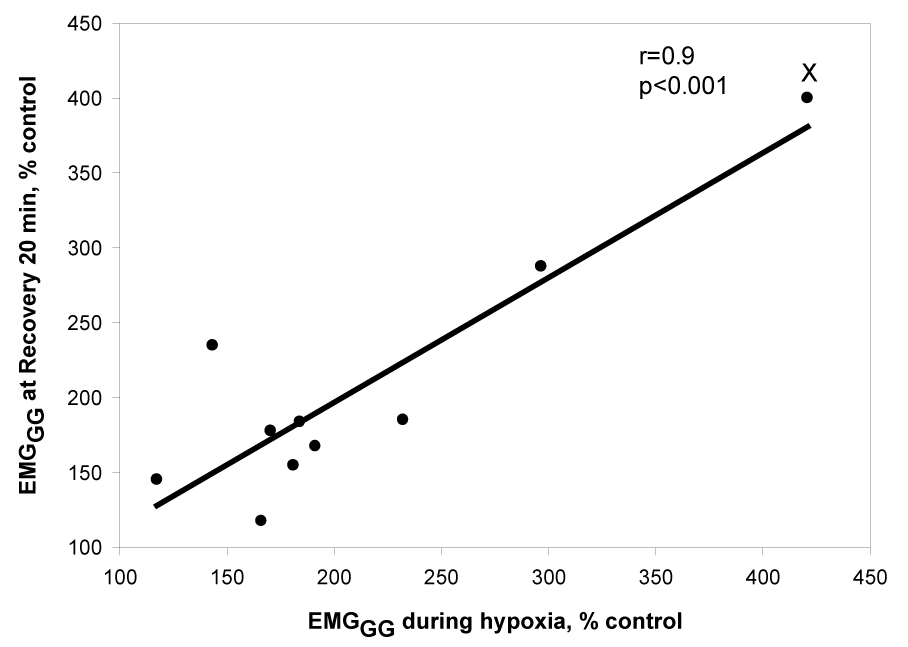
Demonstrates a significant positive relationship between phasic EMGGG (% control) during recovery at 20 minutes and phasic EMGGG (% control) during hypoxia. This correlation declined to r=0.6, p=0.06 after eliminating the highest right point (marked with X); but when adjusted for age in the multiple regression model, the coefficient of EMGGG during hypoxia was significant, see text for explanation.
Figure 7.
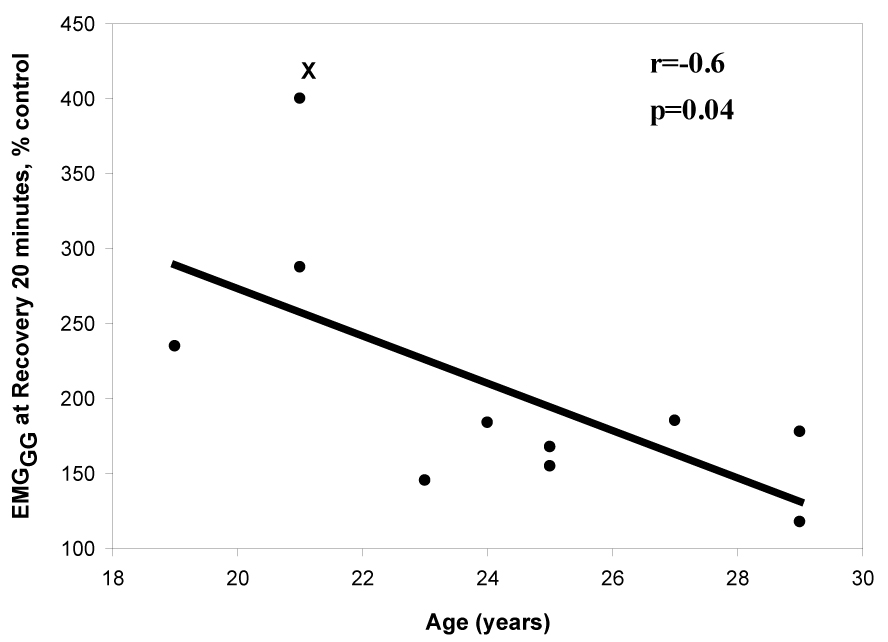
Demonstrates a significant negative relationship between the phasic EMGGG (% control) during recovery at 20 minutes and age (years). This correlation remained significant, r=−0.74, p=0.044 even after deleting the highest left point (marked with X).
Table 3.
Results of correlation analyses to determine relationship between recovery EMGGG activity during NREM sleep and demographic and physiologic variables.
| Variables | Correlation coefficient | p-value |
|---|---|---|
| EMGGG during hypoxia (% control) | 0.88 | <0.001 |
| Age (years) | −0.64 | 0.04 |
| RUA during hypoxia (% control) | −0.14 | 0.7 |
| Gender | 0.04 | 0.9 |
| BMI (kg/m2) | 0.09 | 0.8 |
| Percent change in SaO2 | 0.46 | 0.1 |
| V̇I during hypoxia (%control) | 0.36 | 0.3 |
See text for explanation.
3.3 Sham study
Seven subjects underwent the sham protocol and met the criteria for stable NREM sleep. Fig. 8 is a polygraph segment from a representative sham study. Averaged results for the sham studies are tabulated in Table 4. There were no significant changes in V̇I, VT, TI, respiratory frequency, peak flow or PSG during the designated ‘recovery’ period at R20. In addition, there was no significant change in EMGGG phasic activity during recovery.
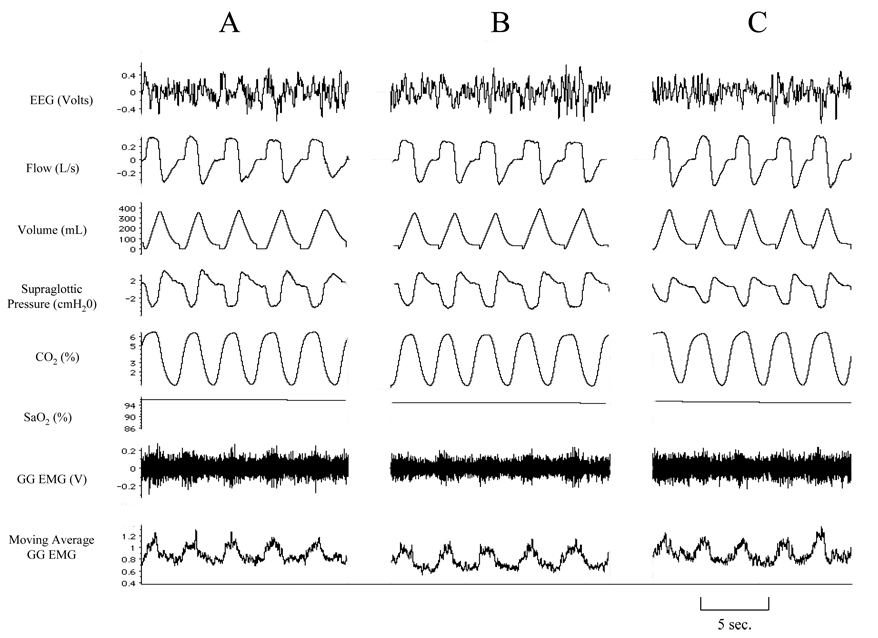
Figure 8.
Representative polygraph segment from a sham protocol during stable NREM sleep. A represents room air control condition, while B represents 85 minutes and C represents 100 minutes, corresponding to the recovery periods at R5 and R20, respectively, of the subject’s experimental study. Note unchanged VT, PSG and EMGGG during B and C compared to segment A of the study.
Table 4.
Ventilation, upper airway mechanics and EMGGG during the control and recovery (R20) periods of the sham protocols, n=7.
| Variables | Control | Sham R20 |
|---|---|---|
| TI, sec | 1.7±0.1 | 1.8±0.1 |
| TE, sec | 2.1±0.2 | 2.0±0.1 |
| fr, breath/min | 16.3±0.7 | 16.2±0.6 |
| Petco2 (mmHg) | 38.5±1.7 | 38.6±1.6 |
| V̇I, L/min | 6.6±0.7 | 6.5±0.6 |
| VT, L | 401.3±32.8 | 387.6±33.1 |
| RUA, cmH2O/L/sec | 6.7±1.0 | 7.1±1.3 |
| PSG, cmH2O | −2.9±−0.4 | −2.8±−0.5 |
| V̇peakflow, L/sec | 0.32±0.02 | 0.33±0.02 |
| EMGGG, % of control | -- | 111.4±4.2 |
Data are expressed as mean±SEM; no significant change in measured variables during sham R20 compared to control period. See Table 2 for abbreviations.
4. DISCUSSION
4.1 Summary of findings
This study tested the effect of episodic hypoxic exposure on LTF of the genioglossus (GG) muscle in normal non-flow limited individuals during stable NREM sleep. Our major findings are: 1) Hypoxia results in increased activity of the genioglossus muscle, without a significant change in the magnitude of the supraglottic pressure. 2) GG LTF occurs following episodic hypoxia in normal sleeping humans; no such effect was noted in the sham studies. 3) The two independent predictors of GG LTF were the level of GG activity during hypoxia and the age of the participant. This is the first report concerning the direct assessment of respiratory LTF following episodic hypoxia in phasic activity of the genioglossus muscle in normal sleeping subjects. The data provide important confirmation that episodic hypoxia induces LTF in upper airway motor output during sleep in humans as it does in several anesthetized rodent or cat preparations. The findings suggest that respiratory LTF in upper airway motor output has the potential to be an important regulator of upper airway patency during sleep.
4.2 LTF of Genioglossus
We noted that episodic hypoxia, per se, elicited increased activity of the genioglossus muscle, independent of changes in upper airway mechanics. We also noted that the EMGGG was elevated during the recovery period relative to RA control; supporting our hypothesis that episodic hypoxia elicits LTF of GG during sleep. Our findings corroborate previous animal studies that have shown LTF of hypoglossal neural activity in male rats (Fuller 2005; Zabka et al. 2005); neonatal rats (McKay et al. 2004); anesthetized and vagotomized rats (Bach & Mitchell, 1996); and vagotomized cats (Mateika and Fregosi, 1997). We also observed an augmentation of phasic EMGGG in the normoxic intervals between hypoxic episodes as well as in the successive hypoxic episodes (Fig. 5). This progressive augmentation is characteristic of some studies (Turner and Mitchell, 1997; Millhorn et al., 1980), and not others (Bach et al., 1996). Interestingly, GG LTF occurred without accompanying changes in minute ventilation or upper airway resistance during the recovery period. Genioglossus LTF was directly correlated with the magnitude of the phasic genioglossus response during hypoxia, and inversely correlated with age through a limited range. The occurrence of GG LTF was not explained by the degree of ventilatory response during hypoxia or by gender. Notably, GG LTF significantly declined with increasing age, despite the narrow age range of our subjects, and even after adjusting for EMGGG during hypoxia. There is evidence that respiratory LTF is age dependent in male rats (McGuire and Ling, 2005; Zabka et al. 2005). The loss of LTF is potentially an important factor for the loss of upper airway patency noted with increasing age. This may be clinically relevant as a decline in LTF GG with increasing age might contribute to increased prevalence of sleep-disordered breathing in the elderly population (Bixler et al. 1998). However, in order to exactly determine the effect of age on LTF, a larger sample size with subjects in different age categories would be required. Our study was not designed to specifically investigate changes in LTF with aging, and while it raises an interesting correlation between age and LTF it does not establish this relationship. In addition, loss of LTF probably only partly explains the loss of upper airway patency with age. Other factors may include increased pharyngeal collapsibility (Eikerman et al. 2007) and changes in pharyngeal anatomy (Malhotra et al. 2006).
The occurrence of GG LTF in sleeping humans is in contrast to studies during wakefulness that have shown no evidence of GG LTF (McEvoy et al. 1996, Jordan et al. 2002), unless episodic hypoxia was accompanied by hypercapnia (Harris et al. 2006). The discrepancy between wakefulness and NREM sleep vis-à-vis GG LTF could be attributed to higher upper airway resistance during sleep, augmenting negative supraglottic pressure and the magnitude of GG activation. However, our subjects did not demonstrate high upper airway resistance during sleep and, by study design, did not have evidence of flow limitation. Moreover, they did not demonstrate a mechanical consequence for GG LTF (see below). Conversely, studies in unanaesthetized rats have demonstrated that LTF is most prominent during sleep. Unanaesthetized rats exhibit tidal volume LTF following episodic hypoxia while in documented NREM sleep, but not when awake (Mahamed and Mitchell, 2007). Thus, a possible explanation is that sleep may be more conducive to the development of LTF. Given that LTF of hypoglossal nerve activity recorded in rats has been noted to be dependent on serotonin pathways (Bach and Mitchell, 1996, Fuller et al. 2001), the occurrence of GG LTF in non-snoring, non-flow limited individuals suggests that sleep, per se, may be a determinant of LTF. This may relate to the fact that medullary serotonergic neuronal activity is decreased during NREM sleep, and discharges at higher, nearly maximal levels during wakefulness (Veasey et al. 1995). Differences in the basal serotonergic neuronal activity may explain why the peak magnitude and duration of changes in ventilation in the awake goat (+70%, 40 min, Turner et al. 1997) and dog (+40%; Cao et al. 1992) are somewhat lower than that recorded in anaesthetized rats (+70 to +100%, >60 min; Hayashi et al. 1993; Bach and Mitchell, 1996). Similar findings are noted during sleep where the average firing rate of raphe neurons in cats decreased during slow wave and rapid eye movement sleep compared to alert waking state and correlated with increases in ventilation (Veasey et al. 1995). Thus, decreased firing rate of the raphe neurons during NREM sleep relative to wakefulness may permit a greater dynamic range of increased caudal raphe neuron activity with episodic hypoxic stimulation.
4.3 Absence of ventilatory or mechanical LTF
Our study demonstrated no evidence of ventilatory LTF, corroborating other studies in neonatal rats (McKay et al. 2004), where LTF was expressed primarily as an increase in GG EMG burst amplitude, without a significant change in either ventilatory or timing parameters. Likewise, our findings corroborate previous work from our laboratory demonstrating no evidence of diaphragmatic LTF (Babcock and Badr, 1998) and the study by Mateika and Fregosi (1997) demonstrating, LTF of the genioglossus and the alae nasae without diaphragmatic LTF in vagotomized cats.
The aforementioned studies suggest that upper airway neuromuscular LTF would lead to ventilatory LTF only if the electrical activity of the genioglossus is translated into a mechanical response, dilating the upper airway and reducing resistance to flow (Brennick et al. 2007). Thus, the manifestations of LTF depend upon the mechanical properties of the upper airway. Accordingly, the mechanical and ventilatory manifestations of increased upper airway neuromuscular activity are more pronounced in individuals with inspiratory flow limitation (IFL) relative to those who do not display IFL. Episodic hypoxia in snoring subjects is followed by decreased RUA and an increased V̇I during the recovery period (Shkoukani et al. 2002). In addition, ventilatory LTF was abolished when IFL was eliminated using nasal CPAP (Babcock et al. 2003). Conversely, in the current study, low upper airway resistance and the absence of flow limitation would explain the dissociation between the occurrence of GG LTF and a ventilatory or mechanical consequence. Likewise, Schnall et al. (1995) have demonstrated that electrical stimulation of the genioglossus results in reduced upper airway resistance only if the upper airway is narrowed. Our methodology for measuring upper airway resistance is quite accurate, sensitive and was previously validated (Mansour et al. 2002, Shkoukani et al. 2002). Clark et al. (1998), also have used the relationship between pressure and flow to calculate resistance as a “gold-standard” to study upper airway resistance and flow limitation. Nevertheless, upper airway muscle activity and upper airway resistance are two distinct constructs that may be altered independently (Schnall et al. 1995). Our subjects were non-snorers and have a favorable upper airway anatomy, as evidenced by the absence of flow limitation. Activation of the GG muscle is unlikely to lead to upper airway dilatation or decreased resistance. Likewise, Fuller et al. (1999) showed that co-activation of the upper airway muscles, in a rodent model, decreased pharyngeal collapsibility but did not dilate the pharyngeal airway. Accordingly, we speculate that the role of GG may be to stiffen the airways and prevent collapse, rather than dilate, in sleeping normal non-flow limited individuals.
4.4 Implications
Long-term facilitation of the GG EMG following episodic hypoxia may contribute to the maintenance of upper airway patency during sleep in normal humans. Although we have not studied GG LTF in sleep apnea patients, it is likely that upper airway dilating muscle (GG) activity was responsible for the decreased upper airway resistance observed in the aftermath of episodic hypoxia (Aboubakr et al. 2001) indicating an improvement in upper airway mechanics. The occurrence of GG LTF and the ensuing improvement in upper airway mechanics may represent a protective mechanism mitigating upper airway narrowing and preserving alveolar ventilation following recurrent upper airway obstruction, and episodic hypoxia in sleep apnea. The LTF of upper airway dilating muscles in humans during sleep may restore upper airway patency following a series of apneas and hypopneas. Thus, we speculate that GG LTF may influence the expression of apneas during sleep.
The other implication of our findings is the observation that GG LTF inversely correlates with age, even within the narrow age range in our study sample. The decline in LTF with increasing age may render the aging individual more vulnerable to breathing instability during sleep explaining the increased prevalence of sleep-disordered breathing in the elderly (Bixler et al., 1998). Studies with larger sample size are required to confirm this relationship.
4.5 Methodological considerations
Several methodological factors have to be considered for proper interpretation of our findings. First, we excluded trials and epochs with unstable sleep. Thus, the data reported here were from periods with stable sleep state with no change in sleep state. Second, although we attempted to carefully maintain precise isocapnia during hypoxia, a small reduction in Petco2 did occur (Table 2). Mild hypocapnia might have dampened the magnitude of LTF. However, we were able to demonstrate GG LTF in these same individuals. Third, the degree of hypoxia may have been inadequate. However, a similar level of hypoxia has been attained in our prior studies with demonstration of LTF of V̇I, so the level of hypoxia alone does not explain the lack of rise in V̇I during recovery. Fourth, we measured the activity of the genioglossus muscle as representative of phasic upper airway dilators. However, other upper airway muscles, such as the tensor palatini, have a different pattern of activation and different innervations. In addition, whether LTF can be elicited in other upper airway muscles, including the tensor palatini and tongue retractors, cannot be determined from our data. Likewise, increased drive to the GG does not necessarily result in shortening of the muscle, i.e. electrical activation does not necessarily correlate with mechanical activity or with shortening of muscle fibers (Brennick et al. 2001, Fuller et al. 1998). Fifth, sham studies could not be done in all subjects as planned (explained in Methods). However, we do not think that a persistent rise in phasic EMGGG during the recovery period can be explained by time effect or by artifact alone. Sixth, we cannot be certain that we measured precisely EMGdia signals given the surface location of the electrodes. In addition, satisfactory EMGdia signals were available in only 6 subjects limiting meaningful statistical analysis for EMGdia. Finally, although full polysomnography was not done beforehand, to determine the absence or presence of obstructive sleep apnea, subjects were recruited based on the absence of symptoms or risk factors for obstructive sleep apnea. In addition, the seven sham studies did not reveal evidence of sleep apnea and all 12 subjects were non-flow limited subjects by definition.
In summary, our study results have demonstrated that episodic hypoxia during sleep in normal subjects elicits long term facilitation of the GG muscle manifested by increase EMGGG activity but without a change in ventilation or upper airway mechanics.
Acknowledgements
This work was supported by the Department of Veterans Affairs and the National Heart, Lung, and Blood Institute (NHLBI). We would like to thank Brenda Gillespie, PhD, University of Michigan, School of Public Health, for her valuable input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of Ventilation in Humans during NREM Sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol. 2003;94:53–59. doi: 10.1152/japplphysiol.00476.2002. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir. Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Parisi RA, England SJ. Genioglossal length and EMG responses to static upper airway pressures during hypercapnia in goats. Respir. Physiol. 2001;127:227–239. doi: 10.1016/s0034-5687(01)00253-5. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Gefter WB, Margulies SS. Mechanical effects of genioglossus muscle stimulation on the pharyngeal airway by MRI in cats. Respir Physiol Neurobiol. 2007;156:154–164. doi: 10.1016/j.resp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Cao KY, BerthonJones M, Zwillich CW, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dog. J.Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Clark SA, Wilson CR, Satoh M, Pegelow D, Dempsey JA. Assessment of inspiratory flow limitation invasively and noninvasively during sleep. Am J Respir Crit Care Med. 1998;158:713–722. doi: 10.1164/ajrccm.158.3.9708056. [DOI] [PubMed] [Google Scholar]
- Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477:469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller D. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol. 2005;98:1761–1767. doi: 10.1152/japplphysiol.01142.2004. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebrate rats. Am J Physiol Regulatory Integrative Comp Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- Maltais F, Dinh L, Cormier Y, Series F. Changes in upper airway resistance during progressive normocapnic hypoxia in normal men. J Appl Physiol. 1991;70:548–553. doi: 10.1152/jappl.1991.70.2.548. [DOI] [PubMed] [Google Scholar]
- Mansour KF, Rowley JA, Meshenish AA, Shkoukani MA, Badr MS. A mathematical model to detect inspiratory flow limitation during sleep. J Appl Physiol. 2002;93:1084–1092. doi: 10.1152/japplphysiol.01140.2001. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RH. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J.Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- McEvoy R, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol. 1996;81:866–875. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- McGuire M, Ling L. Ventilatory long-term facilitation is greater in 1- vs. 2-mo-old awake rats. J Appl Physiol. 2005;98:1195–1201. doi: 10.1152/japplphysiol.00996.2004. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldro TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir.Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol. 2004;140:1–8. doi: 10.1016/j.resp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. National Institute of Neurological Disease and Blindness. 1968 [Google Scholar]
- Rowley JA, Zhou ZS, Vergine I, Shkoukani M, Badr MS. The influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–2254. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- Schnall RP, Pillar G, Kelsen SG, Oliven A. Dilatory effects of upper airway muscle contraction induced by electrical stimulation in awake humans. J Appl Physiol. 1995;78:1950–1956. doi: 10.1152/jappl.1995.78.5.1950. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol. 2002;92:2565–2570. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- Task Force of the American Sleep Disorders Association. EEG arousals: Scoring and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–184. [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goat. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitti M, Basmajian JV, Ouellette PL, Mitchell DL, Eastmen WP, Seaborn RD. Electromyographic investigations of the tongue and circumoral muscular sling with fine-wire electrodes. J Dent Res. 1975;54:844–849. doi: 10.1177/00220345750540042401. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]