Abstract
Objective
To evaluate diurnal variation in Optical Coherence Tomography (OCT) measured retinal thickness in patients with center-involved diabetic macular edema (DME).
Methods
Serial OCT3 measurements were performed in 156 eyes of 96 subjects with clinically-diagnosed DME and OCT central subfield retinal thickness ≥225 microns at 8 a.m. Central subfield thickness was measured from OCT3 retinal thickness maps at 6 time points over a single day between 8 a.m. and 4 p.m. A change in central subfield thickening (observed thickness minus mean normal thickness) of at least 25% and of at least 50 microns at two consecutive time points or between 8 a.m. and 4 p.m. was considered to have met the composite outcome threshold.
Results
At 8 a.m., the mean central subfield thickness was 368 microns and the mean visual acuity was 66 letters (approximately 20/50). The mean change in relative central subfield retinal thickening between 8 a.m. and 4 p.m. was a decrease of 6% (95% CI -9% to -3%) and the mean absolute change was a decrease of 13 microns (95% CI -17 to -8). The absolute change was significantly greater in retinas that were thicker at 8 a.m. (P <0.001) but the relative change was not (P=0.14). The composite threshold of reduction in central subfield thickening (as defined above) was observed in 5 eyes of 4 subjects (3% of eyes, 95% CI, 1% to 8%) while 2 eyes of 2 subjects (1%, 95% CI, 0% to 5%) had an increase in central subfield thickening of this same magnitude. The maximum decrease was observed at 4 p.m. in all 5 eyes.
Conclusions
Although on average there are slight decreases in retinal thickening during the day, most eyes with DME have little meaningful change in OCT central subfield thickening between 8 a.m. and 4 p.m.
Introduction
Optical Coherence Tomography (OCT) is a noninvasive method for measuring the thickness of the central retina. It has become a standard tool in the management of patients with diabetic macular edema (DME). This imaging technique uses low-coherence interferometry to produce cross-sectional tomograms of the posterior segment eye structures. An 850 nm diode laser is used to measure the delay in light backscattering from different layers in the retina, from which the retinal thickness is calculated. Retinal thickness is determined from multiple individual axial scans arrayed in a radial pattern along each of six linear segments all intersecting at the center. A computer algorithm is used to determine the inner and outer retinal boundaries for each scan.
Several small studies have reported that retinal thickening may vary during the day in some eyes with DME, generally being thickest in the morning and thinner later in the day.1–3 Since OCT is a very sensitive method used to measure retinal thickening and judge response to DME treatment in the clinic and in clinical trials, it is important to have reliable information on the diurnal variation in retinal thickening. These findings will be essential for appropriate interpretation of OCT results and/or the design of studies using this technique. We designed the current study to evaluate retinal thickening measured by OCT throughout the day in eyes with DME.
Methods
The study was conducted at 25 sites participating in the Diabetic Retinopathy Clinical Research Network (DRCR.net). The study protocol was approved by the institutional review boards and each subject gave written informed consent for participation in the study.
To be eligible, a subject was required to have at least one eye with (1) definite retinal thickening due to DME based on clinical exam involving the center of the macula, (2) OCT central subfield ≥225 microns, (3) pupil dilation of 5 mm or larger, and (4) no treatment for DME within the prior 3 months. Subjects were excluded if there was a history of chronic renal failure requiring dialysis or kidney transplant, congestive heart failure currently under treatment, or blood pressure >180/110.
Study Procedures
Retinal thickness measurements were made with the OCT3 system (Carl Zeiss Meditec, Dublin, California) at 8 a.m., 9 a.m., 10 a.m., 12 noon, 2 p.m. and 4 p.m. (within ±30 minute time windows). In addition at 8 a.m., 12 noon, and 4 p.m., the following were measured: visual acuity (following a refraction) using the electronic ETDRS method,4 blood glucose using a home glucose meter, and blood pressure. On the day of testing, or within one month of the visit, fundus photographs were obtained. HbA1c was measured if not obtained during the prior 3 months. Additional health and past ocular history data were captured on electronic case report forms (Table 1).
Table 1.
Baseline Demographis and Clinical Characteristics
| Subject Characteristics | N=96 |
|---|---|
| Gender: Women - N (%) | 44 (46%) |
| Age (years) - Mean ± SD | 59 ± 11 |
| Race - N (%) | |
| White | 65 (68%) |
| African-American | 14 (15%) |
| Hispanic | 15 (16%) |
| Other | 2 (2%) |
| Diabetes Type - N (%) | |
| Type 1 | 14 (15%) |
| Type 2 | 79 (82%) |
| Uncertain | 3 (3%) |
| Duration of Diabetes (years) - Mean ± SD | 17 ± 9 |
| Currently Medically Treated for Hypertension - N (%) | 73 (76%) |
| History of Sleep Apnea - N (%) | 20 (21%) |
| History of Chronic Obstructive Pulmonary Disease - N (%) | 0 (0%) |
| Time Subject in Bed Prior Night (hours) - Mean ± SD | 6.7 ± 1.4 |
| HbA1c (%)* - Mean ± SD | 7.8 ± 1.6 |
| Blood Glucose mg/dl (8 a.m.) † - Mean ± SD | 178 ± 65 |
| Body Mass Index (kg/m2) | 34 ± 6 |
|
| |
| Baseline (8 a.m.) Eye Characteristics | N=156 |
| Central subfield Thickness (8 a.m.) – N (%) | |
| 225–300 microns | 71 (46%) |
| 301–450 microns | 53 (34%) |
| >450 microns | 32 (21%) |
| Mean ± SD – microns | 368 ± 132 |
| Cysts on OCT‡ - N (%) | |
| None/Questionable | 42 (27%) |
| Definite | 111 (73%) |
| Visual Acuity (8 a.m.) - N(%) | |
| ≥84 letters (20/20 or better) | 15 (10%) |
| 83–68 letters (20/25 to 20/40) | 73 (47%) |
| 67–51 letters (20/50 to 20/100) | 42 (27%) |
| 50–36 letters (20/125 to 20/200) | 19 (12%) |
| ≤35 letters (worse than 20/200) | 7 (4%) |
| Mean ± SD - Snellen Equivalent | 20/50 ± 3.4 lines |
| Prior Treatment for DME - N (%) | 124 (79%) |
| Laser Photocoagulation - N (%) | 117 (75%) |
| Intravitreal Triamcinolone Injection - N (%) | 27 (17%) |
| Peribulbar Triamcinolone Injection - N (%) | 8 (5%) |
| Vitrectomy - N (%) | 13 (8%) |
| Prior Scatter Photocoagulation - N (%) | 34 (22%) |
| Prior Cataract Extraction - N (%) | 42 (26%) |
Four subjects with missing HbA1c lab values.
1 subject had missing blood glucose value.
Cystoid space ungradable on 3 scans.
OCT Procedures
OCT images were obtained on each eye following pupil dilation by a certified operator using the OCT3 system. The same operator obtained the OCT images once at each time point. A second measurement at each time point was made by the same operator or a different operator. Scans of inadequate quality (e.g., standard deviation >10% or signal strength score <6) were to be repeated until they met these specified quality metrics. Scans were sent to the DRCR.net Reading Center at the University of Wisconsin-Madison for grading. For scans with standard deviations ≥10.5% of the center point thickness, inaccurately drawn automated boundary lines, or decentration,5 center point thickness was measured manually with calipers from the retina thickness map scans (13% of images). For such scans, central subfield thickness was imputed from the manually-determined center point thickness using a regression equation (since the correlation of the two measurements was 0.98 for 897 automated measurements).
Statistical Considerations
The sample size was estimated to be 120 eyes such that the half-width of a 95% confidence interval on a 0.20 point estimate of the proportion of eyes experiencing meaningful diurnal variation would be 0.07 and on a 0.10 point estimate would be 0.05.
Change in central subfield thickening was the primary outcome variable. Only eyes with both 8 a.m. and 4 p.m. OCT scans in which the 8 a.m. central subfield thickness was ≥225 microns and the initial OCT scan was obtained within ±45 minutes of 8 a.m. (analysis window 30 minutes larger than the protocol specified time window) were included in the analysis. Eleven subjects did not have either eye meeting these criteria and were excluded from analyses. OCT measurements obtained twice at the same time point were averaged for analysis.
Retinal thickening, i.e. excess retinal thickness, was defined as the observed thickness minus the mean normal central subfield thickness of 202 microns (based on unpublished data provided by Carl Zeiss Meditec from a study of 260 nondiabetic eyes with a normal macula). Therefore, relative change in retinal thickening was calculated as (4 p.m. thickness minus 8 a.m. thickness)/(8 a.m. thickness minus mean normal thickness). The mean change in absolute and relative retinal thickening in the central subfield between 8 a.m. and 4 p.m. was computed and a 95% confidence interval constructed, accounting for the inter-eye correlation in subjects with two study eyes using repeated measures mixed models. The proportions of study eyes with change in central subfield thickening meeting the composite threshold endpoint of at least 25% and at least 50 microns at consecutive time points or between 8 a.m. and 4 p.m. were calculated. The proportion of eyes with a change in central subfield thickening of at least 50 microns was also calculated. The threshold of 25% was arbitrarily chosen as a relative change that was likely to be clinically meaningful. The threshold of 50 microns was likewise considered to be clinically meaningful and exceeded the 95% half-width confidence interval for detection of change of 40 microns (based on reproducibility data from 1147 eyes). The composite endpoint was chosen to utilize the relative change measurement while assuring that the magnitude of change exceeded this 95% confidence interval and was clinically meaningful. Results were evaluated in three subgroups based on the baseline central subfield retinal thickness: 225–300 microns, 301–450 microns, and >450 microns. The effect of baseline thickness on the change in thickening from 8 a.m. to 4 p.m. was assessed using a repeated measures model adjusting for inter-eye correlation in subjects with two study eyes. The effects of additional clinical and ocular characteristics on the mean change in central subfield thickening were evaluated by ANCOVA also adjusting for 8 a.m. central subfield thickness and accounting for inter-eye correlation. Correlations were used to define the relationship between change in central subfield thickening and the change in visual acuity or blood glucose. They were calculated in repeated measures models (to account for the correlation within subjects with two study eyes) based on the likelihood ratio as defined by Magee.6 To satisfy parametric assumptions change in visual acuity was truncated at ±3 standard deviations from the mean, truncation did not meaningfully change the results. Statistical analyses were performed using SAS software version 9.1.
Results
The study included 96 subjects, of whom 36 (38%) had one study eye and 60 (63%) two study eyes, for a total of 156 study eyes. The baseline characteristics of the subjects and study eyes are shown in Table 1. Among the 156 study eyes, the mean central subfield retinal thickness at baseline (8 a.m.) was 368 ± 132 microns. There were 71 (46%) study eyes with baseline thickness of 225–300 microns, 53 (34%) with 301–450 microns, and 32 (21%) with >450 microns. Of the 96 subjects included in the analyses all with 8 a.m. and 4 p.m. measurements, one subject missed both 9 a.m. measurements and 2 subjects missed the second 10 a.m. measurement. All 96 subjects completed both measurements at 12 p.m. and 2 p.m.
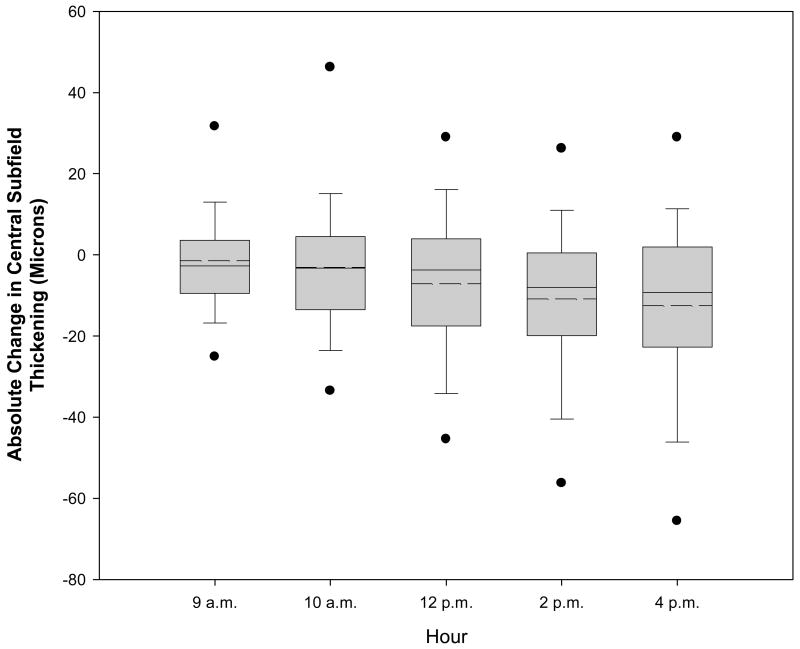
The mean change in relative central subfield retinal thickening from 8 a.m. to 4 p.m. was a decrease of 6% (95% confidence interval -9% to -3%) and the mean absolute change was a decrease of 13 microns (95% confidence interval -17 to -8, Figure 1 and Table 2). Five eyes (3%, 95% confidence interval 1% to 8%) of 4 subjects reached the predefined composite outcome of ≥25% decrease in central retinal thickening and ≥50 micron decrease in central retinal thickening between 8 a.m. and 4 p.m. (Table 3). There were no additional eyes that met these criteria at two consecutive earlier time points but not between 8 a.m. and 4 p.m. Among the 5 eyes reaching the composite decrease in central subfield thickening, this decrease was first achieved at 2 p.m. in 2 eyes, and 4 p.m. in 3 eyes. The maximum relative decrease was observed at 4 p.m. in all 5 eyes (Figure 2). Two eyes (1%, 95% confidence interval 0% to 5%) of 2 subjects reached the predefined composite outcome for an increase in central subfield thickening ≥25% and ≥50 microns. Tables 2 and 3 detail these changes subgrouped by 8 a.m. central subfield thickness. On avera2ge, central subfield thickening decreased throughout the day by approximately 9.2 microns (95% confidence interval 6.1 to 12.2) for every 100 microns of increased central subfield thickness at 8 a.m., but there was considerable variation between individuals (Figure 3). The absolute change was significantly greater in retinas that were thicker at 8 a.m. (P<0.001) but the relative change was not (P=0.14) (Table 2). No additional factors were found to be significantly associated with the degree of diurnal change (P≥0.01, Table 4).
Figure 1.
Box-whisker plot demonstrating mean (dashed horizontal line), median (solid horizontal line), 25–75th percentiles (extremes of the box), 10–90th percentiles (whiskers), and 5–95th percentiles (solid circles) for change in central subfield thickening from baseline at each time point.
Table 2.
Change in Central Subfield Thickening from 8 a.m. to 4 p.m. According to 8 a.m. Thickness (156 eyes from 96 subjects)
| Change in Central Subfield Thickening Mean (95% Confidence Interval)* | |||
|---|---|---|---|
| Total N | Relative Change in Thickening | Absolute Change (Microns) | |
| Overall | 156 | −6% (−9%, −3%) | −13 (−17, −8) |
|
| |||
| Central Subfield Thickness (8 a.m.) | |||
| 225–300 microns | 71 | −4%(−10%, +2%) | −4 (−8, 0) |
| 301–450 microns | 53 | −8% (−11%, −4%) | −14 (−20, −8) |
| >450 microns | 32 | −8% (−13%, −2%) | −30 (−48, −11) |
| P-value† for Effect of 8 a.m. Thickness on Change | 0.14 | <0.001 | |
Accounting for the inter-eye correlation within subjects with two study eyes.
P-value calculated from repeated measures mixed model regressing change in thickness from 8 a.m. to 4 p.m. on 8 a.m. thickness.
Note: Negative change implies decrease in thickening.
Table 3.
Proportion of Eyes Meeting Change in Central Subfield Thickening Thresholds from 8 a.m. to 4 p.m.
| Reduction in Central Subfield Thickening | Increase in Central Subfield Thickening | ||||
|---|---|---|---|---|---|
| Total N | ≥25% and ≥50 Microns | ≥50 Microns | ≥25% and ≥50 Microns | ≥50 Microns | |
| Overall - N (%) [95% C.I.*] | 156 | 5 (3%) [1%, 8%] | 13 (8%) [4%, 14%] | 2 (1%) [0%, 5%] | 2 (1%) [0%, 5%] |
| Central Subfield Thickness (8 a.m.) - N (%) | |||||
| 225–300 microns | 71 | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) |
| 301–450 microns | 53 | 2 (4%) | 2 (4%) | 0 (0%) | 0 (0%) |
| >450 microns | 32 | 3 (9%) | 11 (34%) | 1 (3%) | 1 (3%) |
Accounting for the inter-eye correlation within subjects with two study eyes.
Figure 2.
Central Subfield Thickness throughout the Day for Eyes with Relative Decrease ≥25% and Absolute Decrease ≥50 Microns
Figure 3.
Absolute Change in Central Subfield Thickening from 8 a.m. to 4 p.m. by 8 a.m. The solid line represents the regression line and the dotted lines represent the 95% confidence interval for the mean.
Table 4.
Evaluation of Diurnal Change According to Clinical Characteristics (N=156 Eyes of 96 Patients)
| Change in Central Subfield Thickening from 8 a.m. to 4 p.m. Stratified by Baseline Characteristics | N | Absolute Mean Change in Central Subfield Thickening ± SD (8 a.m. to 4 p.m.) | P-value* |
|---|---|---|---|
| Patient Characteristics | |||
| Gender | 0.79 | ||
| Women | 64 | −13 ± 34 | |
| Men | 92 | −12 ± 23 | |
| Race | 0.07 | ||
| White | 111 | −8 ± 26 | |
| African American | 19 | −29 ± 32 | |
| Hispanic | 24 | −19 ± 27 | |
| Diabetes Type† | 0.47 | ||
| Type I | 21 | −17 ± 31 | |
| Type II | 129 | −12 ± 27 | |
| HbA1c | 0.67 | ||
| <8% | 99 | −13 ± 25 | |
| ≥8% | 52 | −13 ± 33 | |
| Sleep Apnea | 0.01 | ||
| Yes | 32 | −25 ± 30 | |
| No | 124 | −9 ± 26 | |
| BMI (kg/m2) | 0.03 | ||
| ≤25 | 14 | +2 ± 42 | |
| >25 | 142 | −14 ± 26 | |
| Ocular Characteristics | |||
| Prior Treatment for DME | 0.65 | ||
| Yes | 124 | −14 ± 28 | |
| No | 32 | −6 ± 26 | |
| Prior Laser Photocoagulation | 0.80 | ||
| Yes | 117 | −13 ± 27 | |
| No | 39 | −10 ± 29 | |
| Prior Scatter Photocoagulation | 0.75 | ||
| Yes | 34 | −13 ± 39 | |
| No | 122 | −12 ± 24 | |
| Prior Cataract Extraction | 0.76 | ||
| Yes | 42 | −9 ± 29 | |
| No | 114 | −14 ± 27 | |
| Prior Vitrectomy | 0.13 | ||
| Yes | 13 | −23 ± 36 | |
| No | 143 | −12 ± 27 | |
| Visual Acuity (8 a.m.) - N (%) | 0.72 | ||
| 20/40 or better | 82 | −7 ± 21 | |
| 20/50–20/100 | 52 | −19 ± 28 | |
| Worse than 20/125 | 22 | −17 ± 42 | |
| Cystoid Space on OCT‡ | 0.42 | ||
| None/Questionable | 42 | −2 ± 13 | |
| Definite | 111 | −16 ± 31 | |
P-value obtained from ANCOVA, adjusted for 8 a.m. central subfield thickness and accounting for correlation within subjects with two study eyes.
N=6 eyes of 3 subjects that were uncertain of their diabetes type were not included.
have ungradeable cystoid spaces.
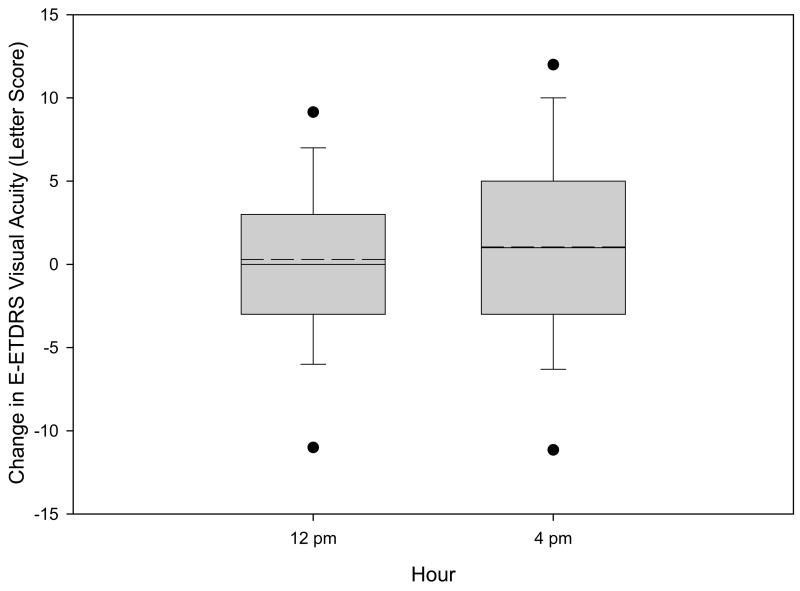
From 8 a.m. to 4 p.m. best corrected E-ETDRS visual acuity letter score increased on average by 1.0 letters (95% confidence interval −0.05 to +2.1 letters, Figure 4), improving by ≥10 letters in 16 eyes (10%) and worsening by ≥10 letters in 9 eyes (6%). Of the 5 eyes with the composite outcome for a reduction in retinal thickening, visual acuity improved ≥5 letters in 1 eye, worsened ≥5 letters in 2 eyes, and changed <5 letters in 2 eyes. Change in retinal thickening from 8 a.m. to 4 p.m. correlated poorly with change in visual acuity (r=−0.05, Figure 4), reflecting the large proportion of eyes with little change in thickening and the variability inherent in the measurements of both acuity and retinal thickening.
Figure 4.
Box-whisker plot demonstrating mean (dashed horizontal line), median (solid horizontal line), 25–75th percentiles (extremes of the box), 10–90th percentiles (whiskers), and 5–95th percentiles (solid circles) for change in visual acuity from baseline at each time point.
Blood glucose concentration increased from 8 a.m. to 4 p.m. ≥50 mg/dl in 21 of the 95 subjects (8 a.m. blood glucose for one subject was missing) and decreased by ≥50 mg/dl in 23 subjects. Change in retinal thickening from 8 a.m. to 4 p.m. correlated weakly (r=−0.13) with change in blood glucose.
Results for other OCT measures such as center point thickness, average of central and inner subfield thickness, and macular volume were similar to the results for the central subfield (data not shown).
Discussion
This study demonstrates that although on average there are slight decreases in retinal thickening during the day, most eyes with DME have little meaningful change in OCT central subfield thickening between 8 a.m. and 4 p.m. Only 3% of eyes (5 of 156) met the composite threshold of both ≥25% and ≥50 micron decrease in retinal thickening at two consecutive time points or between 8 a.m. and 4 p.m. However, 2 eyes (1%) increased by these amounts over this period as well. While a change in retinal thickening of 50 microns may be a meaningful clinical change in an individual patient, with current OCT technology it is not possible to differentiate true change in retinal thickening from the variability of measurement when the difference is less than 40 microns, the measurement of variability.
A study by Frank, et al.1 suggested that OCT measured retinal thickening in DME varied according to time of day. In that study of 10 subjects, retinal thickness was measured at 8:00 a.m., 11:00 a.m., 2:00 p.m., and 5:00 p.m. Four of the 10 subjects had decreased thickening in one or more macular subfields in one or both eyes over the course of the day. The eyes with greater initial retinal thickening tended to show a greater absolute decline over the day than did the eyes with less initial thickening. The magnitude of the decrease in retinal thickening was relatively small (estimated at approximately a mean of 15 microns from the published figures), although statistically significant by paired t-test comparing 8 a.m. with later times. In the study by Frank, et al., almost all of the change in retinal thickening occurred by 11 a.m. In contrast, the 5 eyes in our study with a composite decrease in retinal thickening changed throughout the data collection period through the final 4 p.m. evaluation (Figure 2). An earlier study by Sternberg, et al.7 that employed psychovisual assessments of three patients with DME noted that visual function in patients with macular edema may vary with time of day, being generally worse in the morning. Such a finding supports the anatomic observations in the study by Frank, et al. Both authors suggested that the diurnal change may be related to nocturnal recumbency playing a role in retinal vascular homeostasis. In both studies, patients were admitted to the hospital so that the measurements could be obtained immediately on arising, a methodological difference from the current study. Larsen, et al.2 evaluated change in retinal thickening from evening to morning in 12 eyes of 12 patients with DME. There was a statistically significant mean increase in thickening overnight of 21 microns in the DME group, which was statistically significant compared with the results in 14 eyes of 7 nondiabetic control subjects. Reproducibility of measurements was not addressed in that study using the OCT1 machine. Patients with DME also demonstrated a mean loss of 5 ETDRS letters of acuity overnight. Recently, Polito, et al.,3 published data from 15 eyes of diabetic macular edema patients and 10 eyes of non-diabetic controls who had OCT3 fast macular thickness map scans at four time points between 9 a.m. and 6 p.m. on a single day. They did not detect any suggestion of diurnal variation in the control eyes or the macular edema eyes with center subfield thickness <300 microns. However, they found that among the 9 eyes with center subfield thickness ≥300 microns, 4 demonstrated a consistent decrease in thickness over the course of the day and this subgroup had a mean decrease of central subfield thickness of 9.4%. Two of these 4 eyes had a 5 letter increase in visual acuity.
Our data suggest that decreasing retinal thickening in eyes with DME is a real phenomenon that may occur over the course of a single day in some patients. This change is generally of small magnitude and only occasionally achieves levels that may be considered clinically relevant. Indeed, the mean change of approximately −15 microns measured in a small cohort of DME patients by Frank, et al.,1 and the 9.4% reduction measured among 9 subjects by Polito, et al.3 during daytime hours are similar to the mean change in the 301–450 micron group in our study.
There was also little correlation between change in retinal thickening and change in visual acuity. This is in agreement with the study of Larsen, et al.2 and not surprising given the small absolute changes in retinal thickening noted in this study. A separate paper authored by the DRCRnet details the relationship between central retinal thickening and visual acuity both before and after laser photocoagulation.8
In summary, although some patients with CSME appear to have progressive decreases in retinal thickening during the course of the day, the extent and frequency of these changes do not appear to be of a sufficient magnitude to substantially impact clinical trials (especially if there is a randomly assigned control group and no systematic difference between treatment groups in the time of day of measurement). Our study did not address whether the meaningful diurnal changes exhibited by the small number of patients would be consistently present on a daily basis. However, the clinical impact of these changes is likely to be small.
Figure 5.
Absolute Change in Central Subfield Thickening on OCT versus Change in Visual Acuity from 8 a.m. to 4 p.m.
Acknowledgments
Writing Committee: Lead Authors: Ronald P. Danis, Adam R. Glassman. Additional Writing Committee Members: Lloyd Paul Aiello, Andrew N. Antoszyk, Roy W. Beck, David J. Browning, Antonio P. Ciardella, James L. Kinyoun, Timothy J. Murtha, Trexler M. Topping, Michel Shami, George S. Sharuk, John A. Wells, III
The Diabetic Retinopathy Clinical Research Network
Clinical Sites that Participated in this Protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Boston, MA - Joslin Diabetes Center (13) - George S. Sharuk (I); Paul G. Arrigg (I); Sabera T. Shah (I); Timothy J. Murtha (I); Deborah K. Schlossman (I); Ann Kopple (C); Margaret E. Stockman (C); Leila Bestourous (V); Jerry D. Cavallerano (V); Robert W. Cavicchi (P); James Strong (P); Tampa, FL - International Eye Center (12) - Don John Perez Ortiz (I); Madelyn Alvarez (C); Rita L. Johnson (C); Ross Jarrett (P); Lena M. Yagecic (P); Providence, RI - Retina Consultants (11) - Magdalena G. Krzystolik (I); Harold A. Woodcome (I); Paul B. Greenberg (I); Emiliya German (C); Sandra Henriques (V); Claudia Salinas (V); Erika Banalewicz (V); Mark Hamel (P); Dallas, TX - Texas Retina Associates (10) - Gary E. Fish (I); Rajiv Anand (I); Robert C. Wang (I); Jean Arnwine (C); Brenda Sanchez (V); Sally Arceneaux (V); Penny Ellenich (P); Diana Jaramillo (P); Hank Aguado (P); Seattle, WA - University of Washington Medical Center (7) - James L. Kinyoun (I); Susan A. Rath (C); Chuck Stephens (P); Slingerlands, NY - Retina Consultants, PLLC (6) - Paul M. Beer (I); Eugenia Olmeda (C); Robert Davis (P); Joe Fischer (P); Lubbock, TX - Texas Retina Associates (5) - Michel Shami (I); Phyllis Pusser (C); Linda Squires (V); Thom F. Wentlandt (P); Charlotte, NC - Charlotte Eye, Ear, Nose and Throat Assoc., PA (4) - David J. Browning (I); Andrew N. Antoszyk (I); Angela K. Price (C); Heather L. Murphy (V); Michael D. McOwen (P); Linda M. Davis (P); Loraine M. Clark (P); Columbia, SC - Palmetto Retina Center (4) - W. Lloyd Clark (I); John A. Wells (I); Mallie M. Taylor (C); Robbin Spivey (V); Amy B. Hickman (P); Paducah, KY - Paducah Retinal Center (4) - Carl W. Baker (I); Tracey M. Caldwell (C); Lynnette F. Lambert (V); Dawn D. Smith (P); Austin, TX - Retina Research Center (3) - Brian B. Berger (I); Margaret Rodriguez (C); Steve A. Jeffers (C); Diana S. Dorn (V); Bobbi Gallia (V); Ben Ostrander (P); Boston, MA - Ophthalmic Consultants of Boston (3) - Trexler M. Topping (I); Lori A. Tyler (C); Kathleen Marino (V); Margie Graham (P); Milwaukee, WI - Medical College of Wisconsin (3) - Judy E. Kim (I); William J. Wirostko (I); Christine Y. Lange (C); Troy S. Drescher (C); Kathy J. Selchert (P); Joseph R. Beringer (P); Sarasota, FL - Sarasota Retina Institute (3) - Keye L. Wong (I); John H. Niffenegger (I); Christine Holland (C); Mark Sneath (P); Syracuse, NY - Retina-Vitreous Surgeons of Central New York, PC (3) - G. Robert Hampton (I); Paul F. Torrisi (I); Bryan K. Rutledge (I); Cindy J. Grinnell (C); Lynn M. Kwasniewski (V); Peter B. Hay (P); Lynn A. Capone (P); Chicago, IL -Northwestern Medical Faculty Foundation (2) - Alice T. Lyon (I); Jeevan R. Mathura (I); Annmarie C. Munana (C); Lori A. Kaminski (C); Jonathan Shankle (P); Columbia, SC - Carolina Retina Center (2) - Jeffrey G. Gross (I); Barron C. Fishburne (I); Peggy W. Cummings (C); Regina A. Gabriel (V); Randall L. Price (P); Denver, CO - Denver Health Medical Center (2) - Antonio P. Ciardella (I); Dorothy L. Thomas (C); Colleen J. Smith (V); Debbie M. Brown (P); Lakeland, FL - Central Florida Retina Institute (2) - Scott M. Friedman (I); Steve Carlton (C); Damanda A. Fagan (V); McAllen, TX - Valley Retina Institute (2) - Victor H. Gonzalez (I); Jessica Herrera (C); Alma Herrera (V); Cassandra Garza (P); Rosie M. Corona (P); Daniel Cuellar (P); Nashville, TN - Vanderbilt University Medical Center (2) - Franco M. Recchia (I); Genise G. Mofield (V); Tony Adkins(P); Cynthia C. Recchia (P); Dublin, OH - Retinal Consultants, Inc. (1) - Frederick H. Davidorf (I); Robert B. Chambers (I); Jill D. Milliron (C); Jerilyn G. Perry (V); Scott J. Savage (P); Houston, TX - Vitreoretinal Consultants (1) - David M. Brown (I); Rebecca Delagarza (C); Karin A. Mutz (V); Eric N. Kegley (V); Lake Mary, FL - Central Florida Retina (1) - Preston P. Richmond (I); Douglas F. Lieb (I); Laverne Denise Davila (C); Ross Jarrett (P); Portland, OR -Retina Northwest, PC (1) - Mark A. Peters (I); Stephen Hobbs (C); Katie J. Reichenberger (C); Marcia Kopfer (V); Howard Daniel (P)
DRCR.net Coordinating Center – Tampa, FL: Roy W. Beck (Executive Director), Kimberly E. McLeod (DRCR.net Associate Director), Kelly A. Blackmer, Brian B. Dale, Adam R. Glassman, Nicola B. Hill, Paula A. Johnson, Craig Kollman, Alisha N. Lawson, Brenda L. Loggins, Ana C. Perez, Apryl C. Quillen, Cynthia R. Stockdale, Samara Strauber
DRCR.net Chairman’s Office – Boston, MA: Neil M. Bressler – Baltimore, MD (Network Chair), Lloyd P. Aiello – Boston, MA (Network Chair 2002 – 2005)
Fundus Photograph Reading Center – Madison, WI: Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Lambert (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator)
National Eye Institute: Päivi H. Miskala, Donald F. Everett (2002 – 2004)
DRCR.net Executive Committee: Lloyd P. Aiello (Chair 2002 – 2005), Roy W. Beck, Neil M. Bressler (Chair), David M. Brown, David J. Browning, Ronald P. Danis, Matthew D. Davis, Michael J. Elman, Frederick L. Ferris, Adam R. Glassman, Kimberly E. McLeod, Päivi H. Miskala
OCT Diurnal Variation Study Steering Committee: Lloyd P. Aiello, Roy W. Beck, Neil M. Bressler, Alexander J. Brucker, Steve Carlton, Emily Y. Chew, Ronald P. Danis (protocol chair), Frederick L. Ferris, Don S. Fong, Adam R. Glassman, Jeffrey G. Gross, Julia A. Haller, Helen K. Li, Kimberly McLeod, Päivi H. Miskala
Supported through a cooperative agreement from the National Eye Institute EY14231, EY14269, EY14229
Footnotes
There are no conflicts of interest.
An address for reprints will not be provided.
References
- 1.Frank RN, Schulz L, Abe K, Iezzi R. Temporal variation in diabetic macular edema measured by optical coherence tomography. Ophthalmology. 2004;111:211–17. doi: 10.1016/j.ophtha.2003.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Larsen M, Wang M, Sander B. Overnight thickness variation in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005;46:2313–16. doi: 10.1167/iovs.04-0893. [DOI] [PubMed] [Google Scholar]
- 3.Polito A, Del Borrello M, Polini G, Furlan F, Isola M, Bandello F. Diurnal variation in clinically significant diabetic macular edema measured by the stratus OCT. Retina. 2006;26:14–20. doi: 10.1097/00006982-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Beck RW, Moke PS, Turpin AH, Ferris FL, Sangiovanni JP, Johnson CA, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 5.Ray R, Stinnett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2005;139:18–29. doi: 10.1016/j.ajo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Amer Stat. 1990;44:250–3. [Google Scholar]
- 7.Sternberg P, Jr, Fitzke F, Finkelstein D. Cyclic macular edema. Am J Ophthalmol. 1982;94:664–9. doi: 10.1016/0002-9394(82)90012-5. [DOI] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. The relationship between OCT-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. doi: 10.1016/j.ophtha.2006.06.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]