Abstract
The CXC chemokine CXCL12 and its cognate receptor CXCR4 play an important role in inflammation, immunodeficiency virus (HIV) infection and cancer metastasis. The signal transduction and intracellular trafficking of CXCR4 are involved in these functions, but the underlying mechanisms remain incompletely understood. In the present study, we demonstrated that the CXCR4 formed a complex with the cytolinker protein plectin in a ligand-dependent manner in HEK293 cells stably expressing CXCR4. The glutathione-S-transferase (GST)-CXCR4 C-terminal fusion proteins co-precipitated with the full-length and the N-terminal fragments of plectin isoform 1 but not with the N-terminal deletion mutants of plectin isoform 1, thereby suggesting an interaction between the N-terminus of plectin and the C-terminus of CXCR4. This interaction was confirmed by confocal microscopic reconstructions showing co-distribution of these two proteins in the internal vesicles after ligand-induced internalization of CXCR4 in HEK293 cells stably expressing CXCR4. Knockdown of plectin with RNA interference (RNAi) significantly inhibited ligand-dependent CXCR4 internalization, attenuated CXCR4-mediated intracellular calcium mobilization and activation of extracellular signal regulated kinase 1/2 (ERK1/2). CXCL12-induced chemotaxis of HEK293 cells stably expressing CXCR4 and of Jurkat T cells was inhibited by the plectin RNAi. Moreover, CXCR4 tropic HIV-1 infection in MAGI (HeLa-CD4-LTR-Gal) cells was inhibited by the RNAi of plectin. Thus, plectin appears to interact with CXCR4 and play an important role in CXCR4 signaling and trafficking and HIV-1 infection.
Introduction
Chemokines are a group of small molecular mass proteins primarily found to play a role in migration of leukocytes to the inflammatory sites or to the second lymphoid organs. Chemokines exert their functions through binding to the cell surface G protein-coupled receptors, namely chemokine receptors, which are designated as CXCR1 through CXCR6, CCR1 through CCR11, XCR1, and CX3CR1 according to their specific preference for certain chemokines [1]. Among these chemokine receptors, CXCR4 has received extensive studies because this receptor is involved in multiple physiological and pathological processes, including type 1 human immunodeficiency virus (HIV-1) infection [2], hematopoiesis [3], embryonic development [4-8], tumorigenesis and metastasis [9]. Its ligand, CXCL12, which also binds to RDC1 that has been proposed to be renamed as CXCR7 [10], plays an important role in the migration of peripheral blood lymphocytes [11], CD34+ progenitor cells [12], and pre- and pro-B cell lines [13]. Ligand stimulation of CXCR4 induces activation of a number of signaling pathways [14], followed by the receptor endocytosis, a process involving phosphorylation of the receptors by G protein-coupled receptor kinases and formation of clathrin-coated pits [15]. Endocytosis of CXCR4 is a major component of the mechanism of chemokine inhibition of viral infection [16], and may be necessary to activate several pathways and functions such as chemotaxis [17]. However, mechanisms that regulate CXCR4 signaling and intracellular trafficking remain not fully understood.
Plectin is a versatile, high molecular weight (>500,000) protein originally identified as an abundant intermediate filament-associated protein in C6 glioma cells. Multiple isoforms of plectin generated by alternative splicing have been identified and different N-termini of the isoforms profoundly affect their subcellular localization [18, 19]. Plectin belongs to the spectrin superfamily of actin-binding proteins that share a conserved ABD [20, 21]. Its N-terminus contains the ABD that associates with filamentous actin [22], and its C-terminal part contains six tandem repeat domains (R1–R6) that bind to several types of intermediate filament proteins [23, 24]. In addition, plectin interacts with microtubules [25, 26], thereby suggesting that plectin interlinks all three major protein cytoskeletal systems. The important role of plectin in cytoskeleton organization has been evidenced by the result that plectin deficient mice exhibit severe defects in skin, skeletal muscle and heart and die shortly after birth [27]. Apart from acting as a cytolinker protein, plectin plays an important role as a scaffolding platform of proteins in cellular signaling [28]. More interestingly, recent studies have shown that CXCL12 stimulation of the peripheral blood T lymphocytes induces plectin redistribution [29], and that plectin isoform 1 deficient T lymphocytes exhibit reduced migration towards CXCL12 gradients [30], thereby suggesting the involvement of plectin in the functions of CXCR4. In the present study, we demonstrate that plectin formed a complex with CXCR4 in HEK293 cells stably expressing the receptor most likely through the plectin N-terminal domain associating with the CXCR4 C-terminus. Knockdown of plectin by RNA interference (RNAi) resulted in the inhibition of CXCR4 endocytosis and attenuation of CXCR4-mediated calcium mobilization and extracellular signal regulated kinase 1/2 (ERK1/2) activation in HEK293 cells stably expressing CXCR4, and resulted in inhibition of chemotaxis of the CXCR4-expressing HEK293 cells or Jurkat T cells. Moreover, knockdown of plectin reduced CXCR4-trpoic HIV-1 infection in MAGI (HeLa-CD4-LTR-Gal) cells. These findings indicate novel roles of plectin in CXCR4 signaling and trafficking as well as CXCR4-mediated HIV-1 infection.
Materials and Methods
Plasmids
Plasmids encoding CXCR4, Myc-CXCR4 and glutathione S-transferase (GST)-conjugated CXCR4 C-terminus were obtained from Dr. Gang Pei (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences). These epitope tagged CXCR4 have been tested by radioligand binding assay and cyclic AMP assay, and were confirmed to function similarly as the non-tagged receptors. HA-tagged plectin isoform 1 and its truncation mutants (plectin 1−339, 1−606, 1−1154, 284−1154, 284−2532) were generously provided by Dr. Arnoud Sonnenberg (The Netherlands Cancer Institute, Division of Cell Biology) [31]. A control siRNA consisting of a scramble sequence that does not lead to the specific degradation of any cellular message and two plectin specific siRNAs, siRNA-1 targeting at the 21 nucleotides starting from 1085 and siRNA-2 targeting the 21 nucleotide starting from 2030, were purchased from GeneChem Co., Ltd.
Cell culture and transfection
Human embryonic kidney (HEK293) cells and MAGI (HeLa-CD4-LTR-Gal) cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin in 5% CO2-95% air at 37°C. Cells were cultured in P-100 dishes or on 22- by 22-mm glass coverslips for transfections and immunofluorescence microscopy, respectively. Transfection was performed with LipofectAMINE 2000 reagent (Invitrogen). Cells stably expressing Myc-CXCR4 were selected with 560 μg/ml Geneticin (G418). Jurkat T cells were grown in RPMI medium containing 10% fetal bovine serum and a 1:100 dilution of penicillin/streptomycin, at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Superfect transfection reagent (Qiagen) was used to transfect Jurkat cells, according to the manufacturer's directions. The cells were treated (with EtOH or DEX) 24 h after transfection and were collected for analysis after an additional 24 h of incubation.
Confocal microscopy
For the co-localization between endogenous plectin and CXCR4, HEK293 cells stably expressing CXCR4 were grown on coverslips for 1 or 2 days. Cells were treated with or without CXCL12 (10nM) (10 nM) for 60 min before being fixed with methanol. Cells were washed with phosphate-buffered saline (PBS) and incubated with an antibody mixture containing a goat polyclonal plectin antibody (Santa Cruz Biotechnology Inc.) and a mouse monoclonal CXCR4 antibody (Santa Cruz Biotechnology Inc.) for 30 min. Cells were washed and incubated with a mixture of secondary antibodies containing a CY3-conjugated anti-goat antibody (Molecular Probes, OR) and a FITC-conjugated anti-mouse antibody (Molecular Probes, OR) for 30 min. Confocal microscopy was performed on an LSM-510 laser scanning microscope (Carl Zeiss) with a 63 × 1.4 numerical aperture oil immersion lens using dual excitation (488 nm for FITC, 543 nm for Cy3) and emission (505−530 nm band pass for FITC, 560 nm long pass for Cy3) filter sets. Final images were processed using Adobe Photoshop software.
Co-immunoprecipitation and Western blot analysis
HEK293 cells stably expressing Myc-CXCR4 were treated with CXCL12 (10 nM) for various time intervals, washed three times with ice-cold PBS, and lysed in 1 ml of RIPA buffer containing PBS (pH.7.0), 0.1% sodium deoxylcholate, 0.01% sodium dodecyl sulphate (SDS), and 1% NP-40. The cell debris was removed by centrifugation (15,000 × g, 15 min). The supernatant was pre-cleared by incubation with 40 μl of protein A/G agarose (Pierce Chemical, Rockford, IL) for 1 h at 4°C to reduce nonspecific binding. After removing the protein A/G agarose by centrifugation (15,000 × g, 1 min), the cleared supernatant was collected and 10 μl of mouse monoclonal anti-Myc antibody (Santa Cruz Biotechnology Inc.) was added for overnight precipitation at 4°C. Protein A/G (40 μl) was then added and incubation was continued at 4°C for 2 h. The protein A/G–antibody–antigen complex was then collected by centrifugation while washing three times with ice-cold immunoprecipitation buffer. The final pellets were resuspended in 40 μl of SDS sample buffer containing 5% β-mercaptoethanol and heated to 50°C for 10 min. Forty microliters of this preparation was separated by 6% SDS-polyacrylamide gel electrophoresis (PAGE) and the proteins on the gel were transferred to nitrocellulose membranes (Bio-Rad). The co-immunoprecipitated plectin was detected by Western blotting using a goat polyclonal anti-plectin antibody (Santa Cruz Biotechnology Inc.).
Purification of GST-CXCR4 tail fusion protein and in vitro protein binding assay
For the purification of the GST or GST-CXCR4 C-terminus fusion proteins, bacteria strain DH5α transformed with plasmids encoding GST or GST-CXCR4 C-terminus fusion proteins were cultured overnight at 37 °C, then isopropyl-D-thiogalactopyranoside was added and incubation was continued for another 3 h to induce protein expression. The bacteria were lysed in RIPA buffer and then sonicated on ice for 10 seconds. The supernatant of the bacterial lysate was incubated with glutathione-Sepharose at 4 °C for 30 min. After centrifugation and washing three times with RIPA buffer, the purified GST- or GST-CXCR4 C-terminus fusion protein-bound beads were resuspended in RIPA buffer. For the GST-pull down assay, aliquots of the purified GST or GST fusion proteins were incubated with the lysates of HEK293 cells with or without transient expression of HA-tagged full-length or truncation mutants of plectin isoform 1 at 4 °C for 2 h with rotation. Beads were collected by centrifugation (15,000 × g, 2 min) and washed four times with RIPA buffer. Bound plectin proteins were released by boiling in SDS-PAGE sample buffer containing 5% β-mercaptoethanol for 5 min and detected by SDS-PAGE and immunoblotting using an antibody against plectin or the HA-tag.
Mitogen activated protein kinase assay
HeLa cells, which endogenously express CXCR4 [32], were transfected with scramble siRNA or plectin siRNAs. Cells were treated with CXCL12 (10 nM) for different time intervals before being lysed in RIPA buffer. Lysates containing equal amount of proteins were subjected to 10% SDS-PAGE. Phosphorylated ERK1/2 was detected by Western blot analysis using a phosphor-specific ERK1/2 (Santa Cruz Biotechnology Inc.). The blots were stripped and reprobed with ERK2 antibody to confirm equal loading. For the densitometry analysis, the relative amount of the Western blot bands was measured by densitometry analysis using NIH Image software (http://rsb.info.nih.gov/nih-image/). The relative density of the protein bands was calculated in the area encompassing the immunoreactive protein band and subtracting the background of an adjacent nonreactive area in the same lane of the protein of interest.
Fluorescence-activated cell sorting analysis
HEK293 cells stably expressing CXCR4 and transiently transfected with a scramble siRNA or a plectin specific siRNA were incubated in HEPES (20 mM)-buffered DMEM at 37°C for 30 min in the presence or absence of CXCL12 (10 nM) before being fixed in 2% formaldehyde in PBS. Cells were incubated with a PE-conjugated monoclonal anti-CXCR4 antibody at 4°C for 60 min. As a control, the parental HEK293 cells without expressing CXCR4 were incubated with the PE-conjugated CXCR4 antibody. The cell surface CXCR4 receptors were analyzed by flow cytometry equipped with CellQuest software (BD Biosciences).
Intracellular calcium mobilization assay
HEK293 cells stably expressing CXCR4 were transfected with a scramble siRNA or a plectin specific siRNA. Cells were plated on collagen IV-coated glass bottom microwell dishes (MatTek) for 30 min at 37°C. Cells were loaded with Fura 2-AM (5 μM) (Invitrogen) for 15 min at 37°C and then washed in Hank's buffered salt solution. For some experiments, Fura 2-AM-loaded cells were suspended initially in Ca2+-free Hank's buffered salt solution (Cambrex) containing 2 mM EGTA (Sigma-Aldrich) that was supplemented with 2 mM CaCl2 (Sigma-Aldrich) after 5 min. The dishes were mounted on an inverted Nikon TE2000 microscope at room temperature. Images were collected using a 40×, 1.3 numerical aperture, oil immersion Plan Fluor objective lens and a side-mounted CoolSNAPHQ2 camera. Fluorescence was monitored using dual excitation wavelengths (340/380 nm) and a single emission wavelength (510 nm). Fluorescence at 340 nm indicates dye bound to Ca2+, whereas that at 380 nm corresponds to free dye. Basal readings were taken for 60 s before stimulation. Nikon Elements Advanced Research imaging software (Nikon Instruments) was used for automated collection of images at approximately 1 s intervals over a 5−10 min period. During this time, cells maintained healthy morphology. Average fluorescence measurements were determined using Nikon Elements software. Data are expressed as the ratio of bound/free Fura 2-AM fluorescence intensities after background subtraction.
Cell adhesion assay
A 96-well plate was coated with recombinant human intercellular adhesion molecule-1 (ICAM-1) (R&D Systems, Minneapolis, MN, USA) prepared at 1.0 g/ml in bicarbonate buffer (pH 9.0) and incubated overnight at 4°C. Wells were then washed with bicarbonate buffer and coated with recombinant human SDF-1 (Santa Cruz Biotechnology, CA) prepared at 1.0 g/ml in bicarbonate buffer for 30 min at room temperature or with the buffer alone. Wells were subsequently washed and blocked with 20% FBS in PBS for 1 h at 37°C, followed by washing with Hank's balanced salt solution (HBSS) supplemented with HEPES. HeLa cells transiently transfected with plasmids encoding full-length plectin or its truncation mutants were resuspended in of adhesion medium (HBSS buffered with HEPES and supplemented with 0.5% BSA), and were added in triplicate to the wells and allowed to settle for 30 min at 37°C, followed by four washes with adhesion medium to remove nonadhered cells. The number of adhered cells was determined using CyQUANT Cell Proliferation Kit (Molecular Probes, Eugene, OR, USA).
Preparation of HIV virus stock
Infectious proviral clones expressing the luciferase gene in place of nef, pNL-Luc-HXB (CXCR4-tropic), were generated as described previously [33]. HIV-1 virus stocks were prepared in 293T cells as described previously [34-36]. Briefly, 293T cells were transfected with 2 μg of the proviral expression plasmids carrying a luciferase reporter gene, pNL-Luc-HXB, by using FuGENE 6. The culture medium was replaced 16 h later, and the culture supernatants were harvested 40 h after transfection and filtered through 0.45-μm-pore-size filters, and virus yield was measured by enzyme-linked immunosorbent assay. Virus stocks were stored at 80 °C until needed.
Luciferase Reporter Virus Assays
MAGI (HeLa-CD4-LTR-Gal) cells were maintained as monolayer in Dulbecco's modified Eagle's medium supplemented with 15% fetal bovine serum as described above. Cells (5 × 105) were transfected with a scramble siRNA or a plectin specific siRNA. Then the transfected cells were incubated with 20 ng of p24 antigen of a luciferase reporter virus, NL-Luc-HXB, for an additional 60 min. The cells were washed extensively with phosphate-buffered saline and cultured in fresh medium. After 48 h, the cells were lysed in 200 μl of lysis buffer, and luciferase activities were determined with a MicroLumatPlus LB96V microplate luminometer.
Results
Co-immunoprecipitation of plectin with CXCR4
In our preliminary proteomic studies, we identified plectin as a potential CXCR4 interacting protein. To replicate this interaction, we treated HEK293 cells stably expressing Myc-CXCR4 with CXCL12 (10 nM) for different time intervals, immunoprecipitated CXCR4 from the lysate, and detected plectin association by Western blot analysis using a specific anti-plectin antibody (Santa Cruz Biotechnology Inc.). In a parallel experiment, HEK293 cells transiently transfected with the Myc-containing vector was used as a control in the co-immunoprecipitation experiment, and our immunofluorescence experiments verified that approximately 90% of the cells were transfected with the Myc-containing vector (data not shown). As shown in Fig. 1, the immunoprecipitation revealed a dynamic interaction between plectin and CXCR4, which peaked between 30 to 60 min, and returned to near basal level after 120 min. The CXCR4-plectin interaction is apparently specific since the anti-Myc antibody failed to pull down any plectin from the cell lysate of HEK293 cells expressing the Myc alone.
Fig. 1.
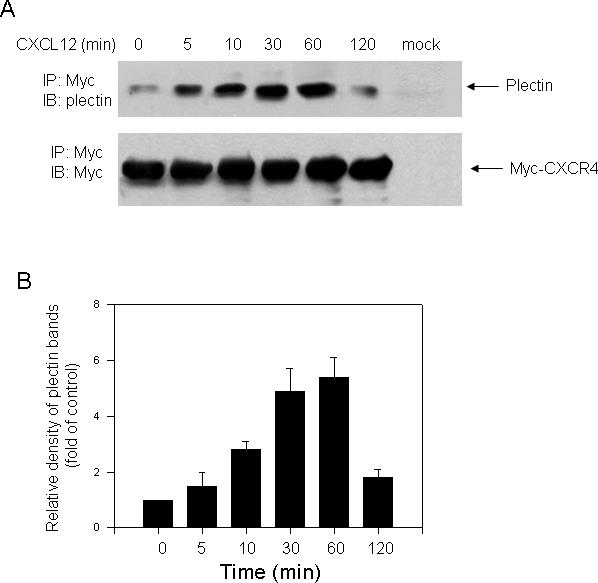
Plectin was co-immunoprecipitated with CXCR4. (A) HEK293 cells stably expressing Myc-CXCR4 were exposed to CXCL12 (10 nM) for the indicated time intervals and CXCR4 was immunoprecipitated from the cell lysate using a specific anti-Myc antibody. In a parallel experiment, parental HEK293 cells transiently transfected with vector containing Myc alone were treated with CXCL12 for 10 min, and immunoprecipitation was performed as described above (mock). Proteins were separated by 6% SDS-PAGE and transferred to a nitrocellulose membrane, and coprecipitated proteins were detected by Western blotting. The membrane was stripped and reblotted with a specific anti-Myc antibody to confirm equal loading of protein samples. (B) Quantification of the density of bands representing plectin, which was time-dependently associated with CXCR4, was determined by densitometric scanning. Data are the means ± S.E. from three independent experiments.
In vitro association of CXCR4 C-terminus with plectin N-terminal domain
Like other GPCRs, CXCR4 receptors reside on the cell membrane with their N-terminal domains outside of the plasma membrane and their C-terminal domains inside. Based on the coimmunoprecipitation between plectin and CXCR4 even before ligand stimulation, we propose that the C-terminus of CXCR4 is involved in its association with plectin. To test this hypothesis, we chose plectin 1 to perform the in vitro binding assay because among all isoforms of plectin, plectin 1 contains the longest isoform-specific sequence (60 amino acid residues), increasing its potential to accommodate isoform-specific binding partners (19). We purified GST-CXCR4 C-terminal fusion proteins, incubated the purified GST-CXCR4 C-terminal fusion proteins with the cell lysate of HEK293 cells transiently overexpressing HA-tagged wild-type and several truncation mutants (1−339, 1−606, 1−1154, 284−1154, and 284−2532) of plectin 1, a full-length plectin isoform (Fig. 2A), and analyzed the co-precipitation of the plectin fragments by Western blot analysis using a mouse monoclonal anti-HA antibody (Santa Cruz Biotechnology Inc.). We observed that among these plectin forms overexpressed in HEK293 cells (Fig. 3B), only the full-length and the N-terminal fragments of plectin containing the ABD (1−339, 1−606, 1−1154) were co-precipitated with the GST-CXCR4 C-terminal fusion proteins, whereas N-terminal deletion mutants of plectin (284−1154, 284−2532) were either modestly (284−1154) or not (284−2532) co-precipitated (Fig. 2C), thereby suggesting that the plectin N-terminus are predominantly involved in the interaction with the CXCR4 C-terminus. It should be noted that these data do not necessarily indicate that the CXCR4 C-terminus directly binds to the N-terminus of plectin due to the use of cell lysate for the in vitro binding assay, rather, these data indicate that the C-terminus of CXCR4 and the N-terminus of plectin are involved in the complex formation.
Fig. 2.
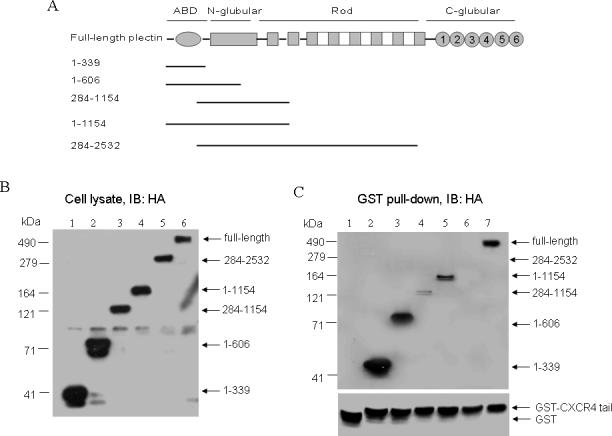
The N-terminal domain of plectin interacted with the C-terminal domain of CXCR4. (A) Diagrams of wild-type and truncation mutant forms of plectin, highlighting structurally distinct regions. (B) HEK293 cells transiently transfected with plasmids encoding HA-tagged full-length plectin or its truncation mutants (1−339, 1−606, 1−1154, 284−1154, and 284−2532) were lysed and proteins were separated by 10% SDS-PAGE. The expression of HA-tagged plectin forms was determined by Western blotting using a specific anti-HA antibody. (C) The GST-CXCR4 C-terminus fusion proteins were incubated with the cell lysate of HEK293 cells transiently expressing the above HA-tagged plectin forms (lanes 2−6). Equal amounts of GST proteins were incubated with the lysate of HEK293 cells overexpressing HA-tagged full-length plectin as control (lane 1). Co-precipitated plectin forms were analyzed by Western blotting using anti-HA antibody as described above.
Fig. 3.
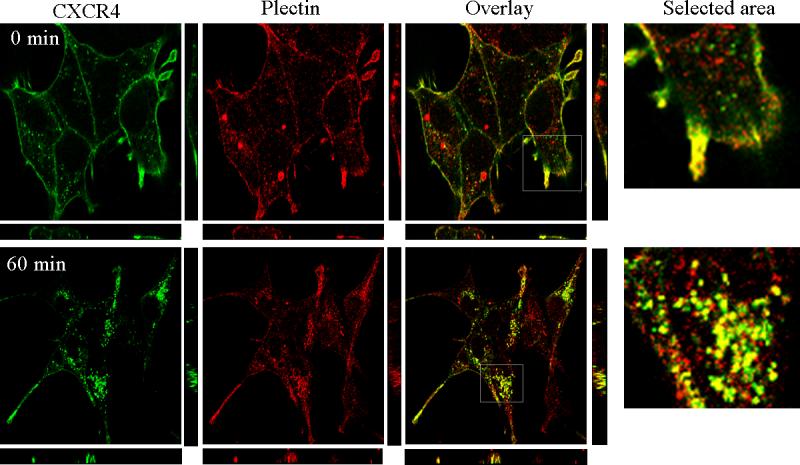
Plectin is co-distributed with CXCR4. HEK293 cells stably expressing CXCR4 were treated with or without CXCL12 (10 nM) for 60 min before being fixed in methanol. Cells were incubated with an antibody mixture of a goat polyclonal plectin antibody and a mouse monoclonal CXCR4 antibody for 30 min followed by incubation with a secondary antibody mixture containing a Cy3-conjugated anti-goat IgG and a FITC-conjugated anti-mouse IgG for 30 min. Images are look-through confocal microscopic reconstructions with Z axis projections below and right. Data are representatives of three independent experiments with similar results.
Co-distribution between plectin and CXCR4
The in vivo and in vitro interaction between plectin and CXCR4 suggest that CXCR4 is likely co-distributed with plectin. To test this hypothesis, we treated HEK293 cells stably expressing CXCR4 with or without CXCL12 (10 nM) for 60 min, immunostained CXCR4 and the endogenous plectin, and observed the subcellular localization of plectin and CXCR4 by confocal microscopy. As shown in Fig. 3, a proportion of plectin proteins were localized on the cell surface and partially colocalized with CXCR4 prior to CXCL12 stimulation. CXCL12 treatment for 60 min induced a robust internalization of CXCR4, and strikingly, the internalized CXCR4 receptors were co-localized with plectin in the internal vesicles (Fig. 3). The confocal microscopic reconstructions with Z axis projections confirmed the co-distribution between CXCR4 and plectin on the cell surface in quiescent cells and in the internal vesicles after ligand-induced CXCR4 internalization.
Plectin is involvement in CXCR4 signaling
To understand the functional significance of the interaction between plectin and CXCR4, we first examined the effect of plectin RNAi on CXCR4-mediated ERK1/2 activation. We transfected HEK293 cells stably expressing CXCR4 with a scramble siRNA or one of the two different plectin specific siRNAs (siRNA-1 and siRNA-2), treated these cells with CXCL12 (10 nM) for different time intervals, and determined ERK1/2 phosphorylation by Western blot analysis. As shown in Fig. 4, cells transfected with the scramble siRNA (control) exhibited time-dependent ERK1/2 phosphorylation in response to CXCL12 stimulation, which peaked at 5 min and returned to near basal level at 30 min. In contrast, cells transfected with the either of the two plectin siRNAs exhibited significantly reduced ERK1/2 phosphorylation in response to CXCL12 treatment and Western blots of plectin showed robust knockdown of the protein by the two individual siRNAs. These data indicate that plectin plays a role in CXCR4-mediated ERK1/2 activation.
Fig. 4.
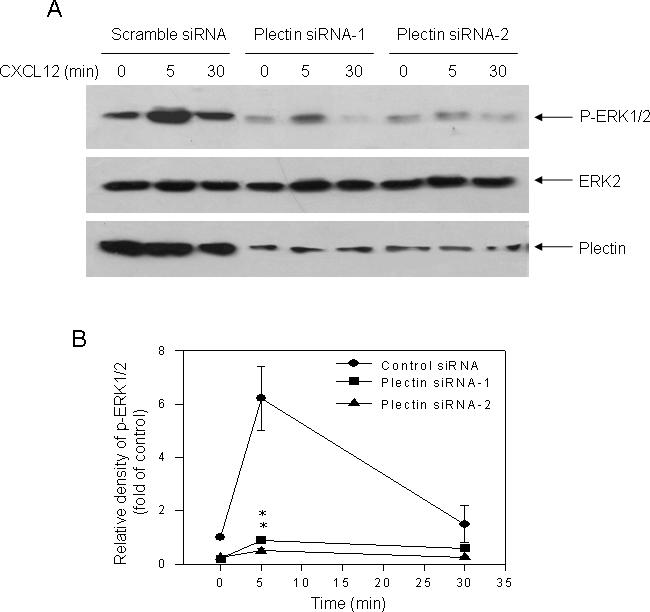
Effect of plectin RNAi on CXCR4-mediated ERK1/2 activation. (A) HEK293 cells stably expressing CXCR4 were transiently transfected with a scramble siRNA as a control or two plectin specific siRNAs (siRNA-1 and siRNA-2). Cells were treated with CXCL12 (10 nM) for indicated time intervals (0, 5, 30 min) and phosphorylation of ERK1/2 was assessed by Western blot analysis as described above. In a parallel experiment, equal amount of samples were subjected to 6% SDS-PAGE, and Western blot analysis was performed with an anti-plectin antibody to confirm the knockdown of plectin in the specific siRNAs transfected cells. (B) Quantification of the density of bands representing phosphorylated ERK1/2, which was normalized with the density of the total ERK2 bands, was performed using NIH Image software (http://rsb.info.nih.gov/nih-image). Data are the mean ± S.E. of three independent experiments. **P<0.01, compared to the control with the same treatment. (C) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells.
We then examined whether CXCR4-evoked calcium mobilization, an immediate signaling initiated by the receptor, is also affected by knockdown of plectin. HEK293 cells stably expressing CXCR4 were transfected with a scramble siRNA or one of the plectin siRNAs (siRNA-1), and CXCL12 (10 nM) induced calcium mobilization was assessed. As shown in Fig. 5, CXCL12 stimulation resulted in a time-dependent calcium mobilization in the cells transfected with the scramble siRNA (control). Interestingly, in the cells transfected with the plectin siRNA, CXCL12-evoked calcium mobilization was significantly reduced. These data indicate involvement of plectin in the early signaling of CXCR4.
Fig. 5.
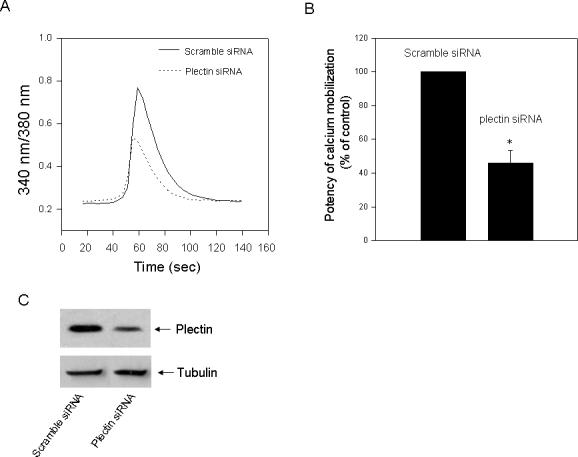
Effect of plectin RNAi on CXCL12–induced Ca2+ mobilization. (A) HEK293 cells stably expressing CXCR4 were transiently transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1). Cells were loaded with Fura-2, stimulated with CXCL12 (10 nM), and analyzed for ability to mobilize intracellular free Ca2+. The data shown are representative of at least three independent experiments with similar results. (B) The potency of Ca2+ mobilization was quantified from three independent experiments. *P<0.05, compared to the control. (C) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells.
Plectin is involved in CXCR4-mediated chemotaxis and cell adhesion
One of the major functions of CXCR4 is to mediate chemotaxis, a process involving multiple signal transduction pathways. A previous study has shown that plectin deficient T cells exhibit reduced chemotaxis towards CXCL12 gradients [30]. To examine whether knockdown of plectin affects CXCR4-mediated chemotaxis, we transfected HEK293 cells stably expressing CXCR4 with a plectin specific siRNA (siRNA-1) or a scramble siRNA as control and determined CXCL12-induced chemotaxis by Boyden chamber assay. We observed a CXCL12 concentration-dependent chemotactic response in the cells transfected the scramble siRNA (Fig. 6A). Strikingly, cells transfected with the plectin specific siRNA exhibited a significantly reduced chemotactic response to CXCL12 gradients (Fig. 6A). Western blot analysis confirmed that transfection of the plectin specific siRNA resulted in significant knockdown of the plectin expression (Fig. 6B).
Fig. 6.
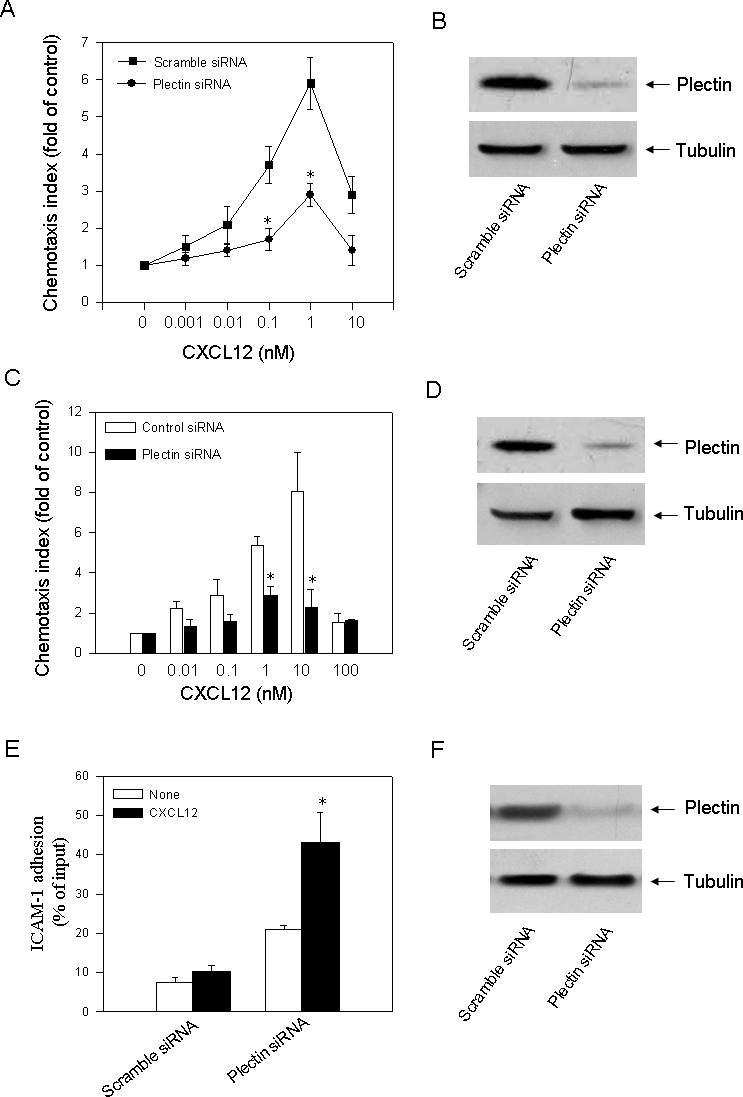
Effect of plectin RNAi on CXCR4–mediated chemotaxis. (A) HEK293 cells stably expressing CXCR4 were transiently transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1). After transfection, chemotactic migration of the cells toward CXCL12 gradients was assessed. Values represent the mean ± S.E. of three independent experiments. *P<0.05, compared to the control. (B) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells. (C) Jurkat T cells endogenously expressing CXCR4 were transiently transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1). After transfection, chemotactic migration of the cells toward CXCL12 gradients was assessed. Values represent the mean ± S.E. of three independent experiments. *P<0.05, compared to the control. (D) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells. (E) Equal numbers of transfected HeLa cells were plated onto ICAM-1-coated coverslips for 30 min, followed by four washes with adhesion medium to remove nonadhered cells. The number of adhered cells was determined using CyQUANT Cell Proliferation Kit (Molecular Probes, Eugene, OR, USA). Fifteen randomly chosen optical fields were counted in two independent experiments. Bars represent mean ± S.E.M. The data were analyzed using Student's paired t test. *P < 0.05 compared to the control. (F) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells.
We noticed that HEK293 cells do not naturally express CXCR4 and results obtained in this artificial system may not reflect the physiological conditions, so we also examined the effect of plectin knockdown on CXCR4-mediated chemotaxis of Jurkat T cells which naturally express CXCR4. We observed that cells transfected with the plectin siRNA exhibited significantly reduced chemotactic response to CXCL12 gradients compared to the cells transfected with the scramble siRNA (Fig. 6C), and Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific but not the scramble siRNA transfected cells (Fig. 6D).
Previous studies have shown that plectin isoform 1 deficient T cells exhibited reduced chemotaxtic response to CXCL12 but increased adhesion to ICAM-1 [30]. Based on the present data that knockdown of plectin in CXCR4 expressing cells results in decrease in chemotaxis, we propose that cell adhesion to ICAM-1 may be affected. To test this hypothesis, we transfected HeLa cells with a scramble siRNA or plectin specific siRNA, and examined the adhesion of HeLa cells to immobilized ICAM-1. We observed that the control HeLa cells could adhere to immobilized ICAM-1. This adherence was significantly enhanced by coimmobilized CXCL12 (Fig. 6E). Transfection of the plectin specific siRNA resulted in significant enhancement of adhesion of the HeLa cells to ICAM-1 with or without coimmobilized CXCL12. Western blot analysis confirmed a remarkable knockdown of the plectin expression in the plectin specific but not the scramble siRNA transfected cells (Fig. 6F). These data suggest that the enhanced cell adhesion by knockdown of plectin may play a role in inhibition of chemotaxis.
Plectin is involved in CXCR4 endocytosis
The complex formation between plectin and the internalized CXCR4 in internal vesicles suggests that plectin may play a role in CXCR4 endocytosis. To test this hypothesis, we examined the effect of plectin RNAi on CXCL12-induced internalization of CXCR4. HEK293 cells were transfected with a scramble siRNA which does not result in knockdown of any protein or a plectin specific siRNA (siRNA-1), and ligand-induced CXCR4 internalization was assessed by FACS analysis. As shown in Fig. 7, in the cells transfected with the scramble siRNA, CXCL12 treatment for 30 min induced a significant internalization of CXCR4 (approximately 42% reduction of mean fluorescence intensity (MFI) of CXCR4). In contrast, in the cells transfected with the plectin specific siRNA, CXCR4 expression appeared to be slightly reduced as exhibited by the slightly left-shifted curve, and more strikingly, CXCL12-induced CXCR4 internalization was significantly inhibited (approximately 9.8% MFI reduction). These data suggest that plectin plays a role in CXCL12-independent expression of CXCR4 and is involved in CXCR4 endocytosis.
Fig. 7.
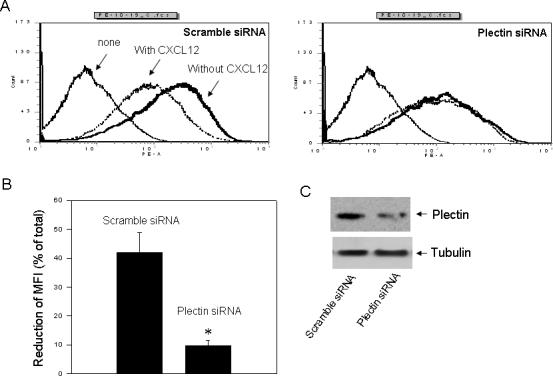
Effect of plectin RNAi on ligand-induced CXCR4 internalization. (A) HEK293 cells stably expressing CXCR4 were transiently transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1). Cells were incubated in HEPES (20 mM)-buffered DMEM at 37°C for 30 min in the presence (dotted lines) or absence of CXCL12 (10 nM) (thick solid lines) before being fixed in 2% formaldehyde in PBS. Cells were incubated with a PE-conjugated monoclonal anti-CXCR4 antibody at 4°C for 60 min. As a control, the parental HEK293 cells without expressing CXCR4 were incubated with the PE-conjugated CXCR4 antibody (thin solid lines). The cell surface CXCR4 receptors were analyzed by flow cytometry equipped with CellQuest software (BD Biosciences). (B) The mean fluorescence intensity (MFI) was quantified and CXCL12-induced reduction of MFI was calculated. *P<0.05, compared to the control. (C) Western blot analysis revealed a remarkable knockdown of the plectin expression in the plectin specific siRNA but not the scramble siRNA transfected cells.
Plectin is involved in HIV infection in CXCR4-expressing cells
CXCR4 is one of the major HIV co-receptors that mediate the infection of X4-tropic HIV-1 in target cells. Endocytosis of CXCR4 has been considered as a major component of the mechanism of chemokine inhibition of viral infection [15]. The inhibitory effect of pelctin-1 RNAi on CXCR4 endocytosis suggests that knockdown of plectin may affect X4-tropic HIV-1 infection. To test this hypothesis, MAGI-CXCR4 cells were transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1), and X4-trpoic HIV-1 infection was assessed. As shown in Fig. 8, cells transfected with the plectin specific siRNA exhibited a significant inhibition of HIV-1 infection compared to the cells transfected with the scramble siRNA. Western blot analysis confirmed a remarkable knockdown of the plectin expression and a remarkably reduced viral protein (gp120) expression in the plectin specific siRNA transfected cells relative to the scramble siRNA transfected cells (Fig. 6F). However, transfection of the plectin siRNA did not affect cell growth as evidenced by the equal amount of tubulin immunoblots between the scramble and the plectin siRNA transfected cells. These data indicate that plectin is involved in CXCR4-mediated HIV infection.
Fig. 8.
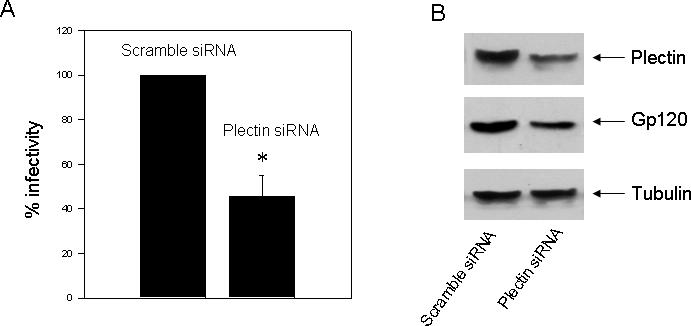
Effect of plectin RNAi on HIV-1 infectiuon in MAGI-CXCR4 cells. MAGI-CXCR4 cells CXCR4 were transiently transfected with a scramble siRNA or a plectin specific siRNA (siRNA-1). The cells were incubated with 20 ng of p24 antigen of a luciferase reporter virus, NL-Luc-HXB (X4-tropic), for 60 min. Luciferase activities in cell lysates were determined 48 h post-infection. The data are represented as percent infection efficiency, which is the total relative light units measured in control or untreated cells. Data are the means ± S.E. of three different experiments. *P<0.05, compared to the control. (B) Western blot analysis revealed a remarkable knockdown of the plectin expression and reduced gp120 expression in the plectin specific siRNA but not the scramble siRNA transfected cells.
Discussion
The targeting of chemokine receptor signaling to certain intracellular sites is an effective way to achieve precise biological responses. Signaling scaffolds, through which this spatial and temporal regulation occurs, have, therefore, gained more and more attention over the past years. One protein emerging as an important player in this scenario is plectin. Here we provide evidence that plectin forms a complex with CXCR4 and plays a role in the receptor signaling and trafficking.
Our in vitro and in vivo experiments have demonstrated an interaction between CXCR4 and plectin. At this moment, it remains unclear whether or not this interaction is direct and is isoform specific. Since the GST-CXCR4 C-terminal fusion protein interacted with the N-terminal ABD containing domain of plectin 1, which contains the longest isoform-specific sequence (60 amino acid residues), it is postulated that plectin isoforms containing the ABD domain may interact with CXCR4. This interaction was further confirmed by confocal microscopic reconstructions showing the co-localization of these two proteins in internal vesicles after ligand induced internalization of CXCR4. Although it has been demonstrated that CXCR4 is internalized into endosomal compartments [38], our previous results revealed that the internalized CXCR4 receptors are partially co-localized with cortactin [39], an invadopodia marker [40]. Thus, it remains a question whether the co-localization between CXCR4 and plectin occurs in the endosomal compartments or in the invadopodia.
The interaction between plectin and CXCR4 appears to be critical for the receptor endocytosis as evidenced by the result that knockdown of plectin remarkably reduced ligand-induced CXCR4 internalization. Although little is known about the underlying mechanism, increasing lines of evidence indicate the involvement of the actin cytoskeleton in endocytosis. Actin microfilaments are thought to function during early stages of clathrin-coated pit invagination [41], and an actin-binding protein, cortactin, has been shown to be critical for CXCR4 endocytosis [42]. As plectin serves as an important binding partner of actin filament, we propose that plectin might be involved in the formation of a multiprotein complex linking CXCR4 to actin filaments, thereby playing a role in the endocytosis of CXCR4.
The major evidence for the involvement of plectin in CXCR4 signaling is that knockdown of plectin attenuated CXCR4-mediated activation of ERK1/2 pathway. However, since knockdown of plectin also reduced CXCR4-mediated calcium mobilization, an immediate signal of the receptor-G protein coupling, it is likely that plectin plays a role in the CXCR4-G protein coupling rather than directly interferes with the ERK1/2 signaling cascades. Moreover, we demonstrated that knockdown of plectin attenuated CXCR4-mediated chemotaxis in both HEK293 cells stably expressing CXCR4 or in Jurkat T cell endogenously expressing CXCR4. These data are consistent with the previous report that plectin 1 deficient T lymphocytes exhibited reduced migration towards CXCL12 gradients [30]. As several signaling pathways, including MEK-ERK1/2 and phosphatidylinositol 3-kinase-Akt signaling cascades [43-45], are involved in chemotactic migration, we postulate that plectin may be involved in chemotaxis through regulating some (if not all) of these signaling pathways. However, since endocytosis of chemokine receptors is also involved in the receptor-mediated chemotaxis [46-50], we can not exclude the possibility that regulation of CXCR4 endocytosis is one of the mechanisms for its involvement in chemotaxis. Nevertheless, these findings may be of particular significance because CXCR4 is one of the major chemokine receptors involved in tumorigenesis and metastasis [51-53], and plectin is upregulated in certain cancer types [54-56].
Finally, we demonstrated that plectin was involved in HIV-1 infection. CXCR4 is one of the key components of the receptor/fusion complexes of HIV-1 and influences virus entry and infection. Although the underlying mechanisms remain not fully understood, part of the mechanism through which the CXCR4 ligand protects cells from HIV-1 infection might be through ligand-induced internalization of the co-receptors. In addition, mechanisms may exist to regulate the trafficking of newly synthesized receptors to the cell surface [57]. However, a recent study showed that inhibition of the chemokine receptor internalization may also play a role in preventing HIV-1 infection [58]. Consistently, we observed that knockdown of plectin by RNAi attenuated CXCR4 endocytosis and reduced HIV-1 infection, thereby suggesting that plectin influences HIV-1 infection through regulating CXCR4 endocytosis.
Acknowledgement
We thank Dr. Arnoud Sonnenberg in the Division of Cell Biology at The Netherlands Cancer Institute for providing us the plectin constructs. We thank Dr. Gang Pei in Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences for the generous gifts of the CXCR4 plasmids. We thank the Imaging Core in the Institutes for Biological Sciences, Chinese Academy of Sciences for the technical help of confocal microscopy. We also thank Catherine Allen, Lynn Butler, and Melanie Smith (Nashville VA Medical Center) for assistance with FACS analysis. We thank Dr. Lee Limbird in the Department of Biomedical Sciences at Meharry Medical College for helpful discussion. This work was supported by a VA Merit Award (G.-H. Fan), a grant from Science and Technology Commission of Shanghai Municipality (project 04DZ14902), a Specialized Neuroscience Research Program (SNRP) grant (U54NS041071-06) from the National Institute of Health, and was supported in part by NIH grants U54NS041071-06, RR03032-19, and U54RR019192-04.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev . 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T. An overview of the determinants of CCR5 and CXCR4 co-receptor function. J. Gen. Virol. 2001;82:1807–1814. doi: 10.1099/0022-1317-82-8-1807. [DOI] [PubMed] [Google Scholar]
- 3.Maekawa T, Ishii T. Chemokine/receptor dynamics in the regulation of hematopoiesis. Intern. Med. 2000;39:90–100. doi: 10.2169/internalmedicine.39.90. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 6.Zou YR, Kottmann A. h., Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 7.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev. Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 10.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 11.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 12.Aiuti A, Webb LJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur. J. Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 14.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 15.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J. Biol. Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 16.Zaitseva M, Peden K, Golding H. HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors. Biochim. Biophys. Acta. 2003;1614:51–61. doi: 10.1016/s0005-2736(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 17.Guinamard R, Signoret N, Ishiai M, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J. Exp. Med. 1999;189:1461–1466. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezniczek GA, Abrahamsberg C, Fuchs P, Spazierer D, Wiche G. Plectin 5'-transcript diversity: short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Hum. Mol. Genet. 2003;12:3181–3194. doi: 10.1093/hmg/ddg345. [DOI] [PubMed] [Google Scholar]
- 19.House CM, Frew IJ, Huang HL, Wiche G, Traficante N, Nice E, Catimel B, Bowtell DD. binding motif for Siah ubiquitin ligase. Proc. Natl. Acad. Sci. USA. 2003;100:3101–3106. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwig JH. Actin-binding proteins 1: spectrin superfamily. Protein Profile. 1994;1:706–778. [PubMed] [Google Scholar]
- 21.Van Troys M, Vandekerckhove J, Ampe C. Structural modules in actin-binding proteins: towards a new classification. Biochim. Biophys. Acta. 1999;1448:323–348. doi: 10.1016/s0167-4889(98)00152-9. [DOI] [PubMed] [Google Scholar]
- 22.Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A A. Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foisner R, Leichtfried FE, Herrmann H, Small JV, Lawson D, Wiche G. Cytoskeleton-associated plectin: in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J. Cell Biol. 1998;106:723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolic B, Mac Nulty E, Mir B, Wiche G. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J. Cell Biol. 1996;134:1455–1467. doi: 10.1083/jcb.134.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J. Biol. Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- 26.Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 28.Osmanagic-Myers S, Wiche G. Plectin-RACK1 (receptor for activated C kinase 1) scaffolding: a novel mechanism to regulate protein kinase C activity. J. Biol. Chem. 2004;279:18701–18710. doi: 10.1074/jbc.M312382200. [DOI] [PubMed] [Google Scholar]
- 29.Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S. Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin. J. Immunol. 2001;167:641–645. doi: 10.4049/jimmunol.167.2.641. [DOI] [PubMed] [Google Scholar]
- 30.Abrahamsberg C, Fuchs P, Osmanagic-Myers S, Fischer I, Propst F, Elbe-Burger A, Wiche G G. Targeted ablation of plectin isoform 1 uncovers role of cytolinker proteins in leukocyte recruitment. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18449–18454. doi: 10.1073/pnas.0505380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol. Biol. Cell. 2004;15:1211–1223. doi: 10.1091/mbc.E03-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Parseval A, Elder JH. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 2001;75:4528–4539. doi: 10.1128/JVI.75.10.4528-4539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokunaga K, Greenberg ML, Morse MA, Cumming RI, Lyerly HK, Cullen BR. Molecular basis for cell tropism of CXCR4-dependent human immunodeficiency virus type 1 isolates. J. Virol. 2001;75:6776–6785. doi: 10.1128/JVI.75.15.6776-6785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochizuki N, Otsuka N, Matsuo K, Shiino T, Kojima A, Kurata T, Sakai K, Yamamoto N, Isomura S, Dhole TN, Takebe Y, Matsuda M, Tatsumi M. An infectious DNA clone of HIV type 1 subtype C, AIDS Res. Hum. Retrovir. 1999;15:1321–1324. doi: 10.1089/088922299310223. [DOI] [PubMed] [Google Scholar]
- 36.Malim MH, Hauber J, Fenrick R, Cullen BR. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988;335:181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- 37.Gregor M, Zeold A, Oehler S, Marobela KA, Fuchs P, Weigel G, Hardie DG, Wiche G. Plectin scaffolds recruit energy-controlling AMP-activated protein kinase (AMPK) in differentiated myofibres. J. Cell Sci. 2006;119:1864–1875. doi: 10.1242/jcs.02891. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Foudi A, Geay JF, Berthebaud M, Buet D, Jarrier P, Jalil A, Vainchenker W, Louache F. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells. 2004;22:1015–1029. doi: 10.1634/stemcells.22-6-1015. [DOI] [PubMed] [Google Scholar]
- 39.Luo C, Pan H, Mines M, Watson K, hang JZ, Fan GH. CXCL12 induces tyrosine phosphorylation of cortactin, which plays a role in CXC chemokine receptor 4-mediated extracellular signal-regulated kinase activation and chemotaxis. J. Biol. Chem. 2006;281:30081–30093. doi: 10.1074/jbc.M605837200. [DOI] [PubMed] [Google Scholar]
- 40.Webb BA, Jia L, Eves R, Mak AS. Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur. J. Cell Biol. 2007;86:189–206. doi: 10.1016/j.ejcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 42.Luo C, Pan H, Mines M, Watson K, Zhang J, Fan GH. CXCL12 induces tyrosine phosphorylation of cortactin which plays a role in CXCR4-mediated extracellular signal regulated kinase activation and chemotaxis. J. Biol. Chem. 2006;281:30081–30093. doi: 10.1074/jbc.M605837200. [DOI] [PubMed] [Google Scholar]
- 43.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 1999;163:5954–5963. [PubMed] [Google Scholar]
- 44.Tsai HR, Yang LM, Tsai WJ, Chiou WF. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur. J. Pharmacol. 2004;498:45–52. doi: 10.1016/j.ejphar.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 45.Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H, Sato M. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp. Cell Res. 2003;290:289–302. doi: 10.1016/s0014-4827(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Wang D, Richmond A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J. Biol. Chem. 1999;274:11328–11333. doi: 10.1074/jbc.274.16.11328. [DOI] [PubMed] [Google Scholar]
- 47.Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan GH, Yang W, Sai J, Richmond A. Hsc/Hsp70 interacting protein (hip) associates with CXCR2 and regulates the receptor signaling and trafficking. J. Biol. Chem. 2002;277:6590–6597. doi: 10.1074/jbc.M110588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 50.Ji LL, Wang Z, Dong F, Zhang WB, Wang ZT. Andrograpanin, a compound isolated from anti-inflammatory traditional Chinese medicine Andrographis paniculata, enhances chemokine SDF-1alpha-induced leukocytes chemotaxis. J. Cell Biochem. 2005;95:970–978. doi: 10.1002/jcb.20464. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J. Biol. Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 52.Helbig G, Christopherson KW, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 53.Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J. Dermatol. Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 55.Mewani RR, Tian S, Li B, Danner MT, Carr TD, Lee S, Rahman A, Kasid UN, Jung M, Dritschilo A, Gokhale PC. Gene expression profile by inhibiting Raf-1 protein kinase in breast cancer cells. Int. J. Mol. Med. 2006;17:457–463. [PubMed] [Google Scholar]
- 56.Bahadoran P, Perrin C, Aberdam D, Spadafora-Pisani A, Meneguzzi G, Ortonne JP. Altered expression of the hemidesmosome-anchoring filament complex proteins in basal cell carcinoma: possible role in the origin of peritumoral lacunae. Br. J. Dermatol. 1997;136:35–42. [PubMed] [Google Scholar]
- 57.Pelchen-Matthews A, Signoret N, Klasse PJ, Fraile-Ramos A, Marsh M. Chemokine receptor trafficking and viral replication. Immunol. Rev. 1999;168:33–49. doi: 10.1111/j.1600-065x.1999.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 58.Catusse J, Parry CM, Dewin DR, Gompels UA. Inhibition of HIV-1 infection by viral chemokine U83A via high-affinity CCR5 interactions that block human chemokine-induced leukocyte chemotaxis and receptor internalization. Blood. 20079;109:3633–3639. doi: 10.1182/blood-2006-08-042622. [DOI] [PubMed] [Google Scholar]


