Summary
Nerves and blood vessels often follow parallel trajectories as they course through the body to their distal targets. Proteins that regulate the process of axon guidance have likewise been shown to play a critical role in blood vessel migration. With the recent description of the endothelial tip cell as an analog of the axonal growth cone, the nerve-vessel analogy seems complete. Notwithstanding these considerable similarities, one critical difference remains between neural and vascular guidance. While a navigating axon is but a single cell, a sprouting vessel is composed of multiple cells that must be coordinately regulated. Recent studies of the Dll4-Notch1 signaling pathway have provided valuable insight into how the vasculature accomplishes this critical task.
Keywords: angiogenesis, vascular guidance, axon guidance cues, blood vessels
Introduction
Formation of the vertebrate vasculature is a complex process that is orchestrated by a constellation of growth factors and guidance cues [1]. During vasculogenesis, the initial phase of vascular development, endothelial cells differentiate, migrate and coalesce to form the central axial vessels, the dorsal aortae and cardinal veins. The second phase, called angiogenesis, is characterized by the sprouting of new vessels from the nascent plexus to form a mature circulatory system. Following this angiogenic remodeling, the endothelium secretes platelet-derived growth factor (PDGF), which induces the recruitment and differentiation of vascular smooth muscle cells [2]. Subsequently, the vascular smooth muscle cells secrete angiopoietins, which ensure proper interaction between endothelial and vascular smooth muscle cells [3,4]. Finally, the vascular smooth muscle cells deposit matrix proteins, such as elastin, that inhibit vascular smooth muscle cell proliferation and differentiation, thereby stabilizing the mature vessel [5,6]. Thus, to establish and maintain a mature vascular network, the endothelial and smooth muscle compartments of a vessel must interact via autocrine and paracrine signaling.
Significant strides have been made in deciphering the molecular mechanisms underlying vasculogenesis and angiogenesis. However, we are only beginning to appreciate the guidance programs utilized by vertebrates to generate the highly stereotypical pattern of the mature vascular network.
Neural guidance pathways
Our knowledge of the way attractive and repulsive cues mediate organ system patterning has emerged from studies of the nervous system. At the cellular level, growing axons must navigate through a complex microenvironment to enervate a distal target tissue. The ability of the axon to reach this destination is predicated upon its ability to sense and respond to an array of guidance cues, both long-range diffusible factors and short-range membrane–bound proteins [7]. It is difficult to strictly classify these factors as attractive or repulsive due to the fact that a given molecule can elicit either response under different circumstances. The ultimate choice is dependent upon the complement of receptors expressed by the axon, the intracellular state of the growth cone, and the presence of other guidance molecules in the extracellular milieu. Thus, the context in which an axon encounters this relatively small number of factors can define the path choices required to establish complex neural connections [8,9].
There are four major classes of guidance cues, which include the secreted Semaphorins, Netrins, Slits, and the membrane-bound Ephrins. Each of these ligands activates cognate transmembrane receptors to affect attraction or repulsion of growing axons. Semaphorins primarily function as short-range inhibitory cues by stimulating receptors from the plexin and neuropilin families [10]. Netrins interact with Unc5, Neogenin, and DCC (deleted in colorectal cancer) receptor families, or combinations thereof, and may repel or attract axons depending on the type of neuron and complement of receptors that are expressed on the surface of the growth cone. For example, Unc5 signaling exclusively specifies repulsion, while DCC can mediate either repulsion or attraction to netrin [11,12]. The Slit proteins activate the Roundabout (Robo) family of transmembrane receptors to predominantly mediate chemorepulsion of growing axons [13]. The Ephrins are membrane-bound ligands for the Eph family of receptor tyrosine kinases and mediate short-range repulsive juxtacrine signaling [14,15,16]. In addition, Notch signaling, although principally associated with cell-fate determination, also regulates neurogenesis through inhibition of neurite outgrowth [17,18,19]. Together, these ligand-receptor pairs establish the precise pattern of synapses that are required for a functional neural network.
Neural and vascular guidance pathways share common signaling mechanisms
Over the past decade it has become apparent that molecular mechanisms underlying development of the nervous system have been co-opted by the vasculature. In fact, each of the aforementioned classes of axon guidance molecules has been shown to regulate some facet of vascular patterning, although the precise role of some of these proteins remains controversial.
The Ephrin/Eph signaling pathway appears to play an important role in regulating endothelial cell migration, analogous to its task of guiding axons in the nervous system. Studies in both mice and frogs have revealed that intersomitic blood vessels express Eph receptors, while the somites express Ephrin ligands. Juxtracrine interactions between this ligand-receptor pair mediate repulsive guidance signaling that prevents the intersomitic vessel from invading the somitic compartment [20,21].
Like the Ephrins, the Semaphorins and their cognate receptors have been implicated in the regulation of vascular development. It was reported that knockdown of Sema3a1 in zebrafish [22] or targeted ablation in mice [23] resulted in defective formation of the intersomitic vessels. More recently, positional cloning of the zebrafish out-of-bounds (OBD) mutation, which causes highly arborized intersomitic vessels, identified the semaphorin receptor Plexin-D1 [24]. In a companion report, gene targeting of the Plexin-D1 locus in mice resulted in blood vessel and cardiovascular defects [25]. Interestingly, independent knockdown of the presumptive Plexin-D1 ligands, Sema3a1 and Sema3a2, caused only moderate intersomitic vessel phenotypes when compared to OBD mutants or Plexin-D1 morphants, suggesting either functional redundancy or an alternative ligand [24]. Indeed, a subsequent study showed that another Semaphorin family member, Sema3e, but not Sema3a, formed a high affinity complex with Plexin-D1, and targeted inactivation of Sema3e caused intersomitic vessels defects that phenocopied a null allele of Plexin-D1 [26]. These data suggest that Sema3e-Plexin-D1 signaling is critical for mammalian vascular patterning.
There is ample, albeit contradictory evidence that the protypical axonal attractant Netrin is involved in vascular development. Our laboratory has demonstrated that, similar to their role in the nervous system, Netrins stimulate migration of endothelial cells [27,28]. Furthermore, we have shown that knockdown of Netrin1a in zebrafish embryos prevents formation of the parachordal vessel, and overexpression of Netrin1 ameliorates defects in a murine model of ischemia [27]. However, Lu et al. [29] has reported that deletion of the Netrin receptor Unc5b, which is expressed in the vasculature of the mid-gestational mouse, causes excessive branching in multiple arterial vascular beds. They argue that this increased peripheral resistance in the arterial system leads to heart failure and lethality in Unc5b−/− animals [29]. These data suggest that Netrin-Unc5b signaling specifies a repulsive cue to the endothelium. Future studies will be needed to precisely define the role of Netrin and Unc5b in regulating vascular development.
The most recent ligand-receptor pair to be implicated in regulating endothelial cell behavior is Slit-Robo. Three Robo family members have been described in the nervous system, and we and others, identified a fourth member of the family, named Magic Roundabout or Robo4 [30,31]. Unlike Robo1-3, Robo4 is expressed in the endothelium of the embryonic and adult mouse, and appears to be up regulated in the vessels of human neoplastic lesions [32]. Studies from our laboratory showed that Robo4 interacts with Slit2 to suppress migration of HEK cells ectopically expressing the receptor, and primary human endothelial cells, which express endogenous Robo4 [31]. These data have been corroborated by a recent report showing that recombinant Slit2, or overexpression of Robo4 is sufficient to inhibit the migration of endothelial cells [32]. Cumulatively, these observations suggest that Slit-Robo signaling mediates chemorepulsion in the vascular endothelium. There are, however, several studies that imply an alternate function for Robo4. Suchting et al. [33] showed that Robo4 was unable to interact with recombinant Slit1-3 using BiaCore analysis and immunoprecipitation, indicating that Robo4 is not a receptor for Slit proteins. They also provided evidence that excess soluble Robo4 ectodomain could inhibit the migration and tube formation of endothelial cells, suggesting that Robo4 promotes angiogenesis via an unknown or unappreciated ligand. Along these lines, Ramchandran and colleagues have proposed that Robo4 induces angiogenesis in the zebrafish through activation of the Rho GTPases [34]. These contradictory findings illuminate the need for additional analysis of Robo4 signaling in the vascular system. Perhaps characterization of Robo4 null mice will provide necessary insight into this interesting paradox.
Dll4-Notch1 signaling and the tip cell
Although functionally distinct, the nervous and vascular systems are remarkably similar at the anatomical level. The information presented thus far has elaborated upon this likeness to include the molecular mechanisms underlying neural and vascular guidance. These similarities can be further extended based on the recent characterization of the endothelial tip cell [36]. Analogous to the axonal growth cone, the tip cell is a highly dynamic structure that uses filipodial protrusions to sample the extracellular environment and dictate the direction in which the vascular plexus will expand [36]. Unlike the growth cone, which must initiate extension of a single axon, the tip cell must coordinate the expansion of the proliferating stalk cells that comprise the vascular plexus. A series of elegant experiments led to a model where heparin generated gradients of immobilized vascular endothelial growth factor (VEGF-A) induce tip cell formation and direct cell migration, whereas local concentrations of VEGF-A control stalk cell proliferation and growth of the plexus [35,36].
If a gradient of VEGF-A is all that is required to form a tip cell, what prevents all endothelial cells from adopting this phenotype? The answer to this question has emerged from analysis of Delta-like ligand 4 (Dll4)-Notch1 signaling in the murine retina and zebrafish embryo [37–41]. Dll4 is an endothelial-specific ligand that interacts with its cognate receptor Notch1 on the surface of endothelial cells. The outcome is the γ–secretase-dependent proteolysis of the Notch1 intracellular domain (NICD), resulting in translocation of the NICD to the nucleus and subsequent changes in gene expression [42]. The fundamental significance of Dll4 function in vivo was established by gene targeting experiments, which showed that ablation of a single copy of Dll4 resulted in severe vascular defects and embryonic lethality [43,44]. Additionally, global or vascular-specific deletion of the Dll4 receptor Notch1 also resulted in vascular anomalies and lethality [45,46], indicating the fundamental requirement for Dll4-Notch1 signaling during vascular development.
The haploinsufficient phenotype of Dll4 is reminiscent of the extreme dosage sensitivity for VEGF-A during murine embryogenesis [47,48], and studies have determined that VEGF is epistatic to Notch signaling in a genetic pathway that regulates arterial identity [49]. In agreement with this idea, Lobov et al. [39] found that Dll4 is up regulated in murine retinas following intraocular injection of VEGF-A. Using an ICR mouse strain to decrease embryonic lethality associated with Dll4 happloinsufficiency, they and others demonstrated that Dll4+/− animals exhibit increased numbers of filipodial protrusions at the sprouting front of the retinal vascular plexus, and in the peripheral vascular plexus, which is normally devoid of such structures [37,39,41]. As filipodial protrusions are a unique characteristic of the endothelial tip cell, these data indicate that loss of Dll4 leads to an increased number of tip cells. Accordingly, Siekmann and Lawson [40] reported that loss of an essential Notch signaling component, recombining binding protein suppressor of hairless (Rbpsuh) in zebrafish, caused all of the endothelial cells in the segmental arteries to adopt tip cell behavior. The consequence, in both the murine retina and embryonic zebrafish, is an abnormally patterned vascular bed that is characterized by excessive branching [37–41]. Intraocular injection of γ-secretase inhibitors, which suppress activation of the Dll4 receptor Notch1, caused a similar increase in filipodial extensions [37,41], suggesting that a principal function of the Dll4-Notch1 pathway is to restrict the tip cell phenotype to a precise number of cells at the sprouting front of the vascular plexus. This role of Notch1 in the vasculature is similar to its role in limiting axon sprouting in the nervous system, providing further evidence that the neural and vascular networks utilize similar molecular mechanisms.
Conclusions
The identification of distinct endothelial cell populations (tip and stalk cell) within the retinal vascular plexus, along with the demonstration that the Dll4-Notch1 pathway acts to prevent the stalk cell from adopting a tip cell fate heralds a new beginning in our ongoing quest to understand endothelial biology. These seminal discoveries suggest that signal transduction specifically within the stalk cells has an important role in regulating the patterning, and perhaps function of the vascular system. In the future it will be important to identify and characterize signaling networks that operate within the stalk cells. Of particular interest will be determining whether the neural/vascular guidance molecules and their cognate receptors can regulate stalk cell-specific signaling.
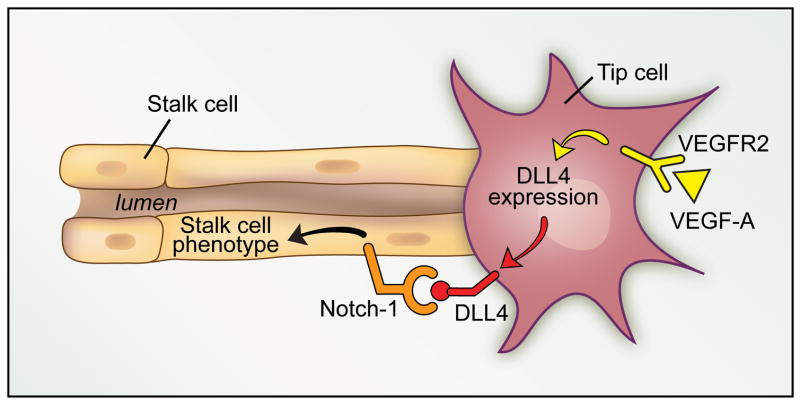
Figure 1.
DLL4-Notch1 signaling enforces the stalk cell phenotype. In the developing murine retina, activation of VEGFR2 by VEGF-A initiates expression of DLL4 in the tip cell. As DLL4 is membrane restricted, juxtracrine signaling between DLL4 and Notch1 on an adjacent cell leads to induction of gene expression programs that enforce the stalk cell phenotype. The consequence is precise control of the number of tip cells at the sprouting front of the vascular plexus.
Acknowledgments
We thank Joshua Wythe and Kirk Thomas for critical reading of the manuscript. Our work is supported by grants from the American Cancer Society, American Heart Association, Burroughs Wellcome Fund, Flight Attendants Medical Research Institute and National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. •Describes the process of blood vessel maturation as it relates to both development and disease. [DOI] [PubMed] [Google Scholar]
- 2.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 3.Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 4.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 5.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–80. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 6.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–23. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 7.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 8.Culotti JG, Merz DC. DCC and netrins. Curr Opin Cell Biol. 1998;10:609–13. doi: 10.1016/s0955-0674(98)80036-7. [DOI] [PubMed] [Google Scholar]
- 9.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–41. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 10.Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 11.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 12.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–17. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 13.Hohenester E, Hussain S, Howitt JA. Interaction of the guidance molecule Slit with cellular receptors. Biochem Soc Trans. 2006 Jun;34(Pt 3):418–21. doi: 10.1042/BST0340418. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–81. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 15.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–70. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–64. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- 17.Franklin JL, Berechid BE, Cutting FB, Presente A, Chambers CB, Foltz DR, Ferreira A, Nye JS. Autonomous and non-autonomous regulation of mammalian neurite development by Notch1 and Delta1. Curr Biol. 1999;9:1448–57. doi: 10.1016/s0960-9822(00)80114-1. [DOI] [PubMed] [Google Scholar]
- 18.Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci. 2000;3:30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- 19.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–6. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 20.Helbling PM, Saulnier DM, Brandli AW. The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development. 2000;127:269–78. doi: 10.1242/dev.127.2.269. [DOI] [PubMed] [Google Scholar]
- 21.Adams RH. Molecular control of arterial-venous blood vessel identity. J Anat. 2003;202:105–12. doi: 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–36. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- 23.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhard C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–7. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 24.Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van N Pham, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004 Jul;7(1):117–23. doi: 10.1016/j.devcel.2004.06.008. •Proposed that plexin-D1-Sema3a signaling regulates patterning of the intersomitic vessels. [DOI] [PubMed] [Google Scholar]
- 25.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004 Jul;7(1):107–16. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005 Jan 14;307(5707):265–8. doi: 10.1126/science.1105416. •Demonstrated that a high affinity interaction between plexin-D1 and Sema3E, but not Sema3a, controls patterning of the intersomitic vessels. [DOI] [PubMed] [Google Scholar]
- 27.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004 Nov 6;101(46):16210–5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006 Aug 4;313(5787):640–4. doi: 10.1126/science.1124704. •Using zebrafish and murine models, provided the first direct evidence that the classical axon guidance molecule, Netrin1, promotes angiogenesis during both development and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004 Nov 11;432(7014):179–86. doi: 10.1038/nature03080. •Reported that UNC5B loss of function leads to embryonic lethality due to hyper-branching of arterial vessels. [DOI] [PubMed] [Google Scholar]
- 30.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–52. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 31.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003 Sep 1;261(1):251–67. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 32.Seth P, Lin Y, Hanai J, Shivalingappa V, Duyao MP, Sukhatme VP. Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem Biophys Res Commun. 2005 Jul 1;332(2):533–41. doi: 10.1016/j.bbrc.2005.03.250. [DOI] [PubMed] [Google Scholar]
- 33.Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005 Jan;19(1):121–3. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- 34.Kaur S, Castellone MD, Bedell VM, Konar M, Gutkind JS, Ramchandran R. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J Biol Chem. 2006 Apr 1;281(16):11347–56. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- 35.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002 Oct 15;16(20):2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003 Jun 23;161(6):1163–77. doi: 10.1083/jcb.200302047. ••Using high-resolution imaging, this study demonstrated the existence of two specialized endothelial cells types within the murine retina: tip cells are the guided compartment, while the stalk cells are the proliferative compartment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007 Feb 15;445(7129):776–80. doi: 10.1038/nature05571. ••Employing gain-of-function and loss-of-function strategies, this report described an essential role for Dll4-Notch1 signaling in specifying tip cell formation during murine retinal angiogenesis. [DOI] [PubMed] [Google Scholar]
- 38.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007 Mar;134(5):839–44. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 39.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007 Feb 12; doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007 Feb 15;445(7129):781–4. doi: 10.1038/nature05577. ••Demonstrated that morpholino-mediated knockdown of the Notch1 effector, Rbpsuh, leads to excessive tip cell formation in the segmental arteries of the embryonic zebrafish. [DOI] [PubMed] [Google Scholar]
- 41.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007 Feb 12; doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006;364:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 43.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004 Oct 15;18(20):2469–73. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000 Jun 1;14(11):1343–52. [PMC free article] [PubMed] [Google Scholar]
- 46.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005 Apr 12;111(14):1826–32. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996 Apr 4;380(6573):439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 49.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002 Jul;3(1):127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]