Abstract
We recently reported the presence of ACE2 in brain regions controlling cardiovascular function; however, the role of ACE2 in blood pressure (BP) regulation remains unclear due to the lack of specific tools to investigate its function. We hypothesized that ACE2 could play a pivotal role in the central regulation of cardiovascular function by regulating other renin-angiotensin system components. To test this hypothesis, we generated an adenovirus expressing the human ACE2 cDNA upstream of an eGFP reporter gene (Ad-hACE2-eGFP). In vitro characterization shows that neuronal cells infected with Ad-hACE2-eGFP (10−100 MOI), but not Ad-eGFP (100 MOI), exhibit dose-dependent ACE2 expression and activity. In addition, an active secreted form was detected in the conditioned medium. In vivo, Ad-hACE2-eGFP infection (2×106 pfu icv) produced time-dependent expression and activity (with a peak at 7 days) in the mouse subfornical organ (SFO). More importantly, 7 days after virus infection, the pressor response to Ang-II (200 pmol icv) was significantly reduced in Ad-hACE2-eGFP-treated mice compared to controls. Furthermore, SFO-targeted ACE2 over-expression dramatically reduced the Ang-II-mediated drinking response. Interestingly, ACE2 over-expression was associated with down-regulation of the Ang-II type 1 (AT1) receptor expression both in vitro and in vivo. These data suggest that ACE2 over-expression in the SFO impairs Ang-II-mediated pressor and drinking responses at least by inhibiting the AT1 receptor expression. Taken together, our results show that ACE2 plays a pivotal role in the central regulation of BP and volume homeostasis, offering a new target for the treatment of hypertension and other cardiovascular diseases.
Keywords: adenovirus, carboxypeptidase, brain, blood pressure, gene therapy
Introduction
The renin-angiotensin system (RAS) is well known for its effects on the cardiovascular system and fluid homeostasis. Classically, these effects were thought to result primarily from the systemic production of angiotensin-II (Ang-II).1 Circulating Ang-II stimulates AT1 receptors present in the kidney and the vasculature to produce vasoconstriction but also water and salt reabsorption. Although kidneys and liver are the major endocrine sites of renin and angiotensinogen synthesis, respectively, this view of the RAS has been challenged in the last decade as both genes were detected in extra-renal and extra-hepatic tissues.2, 3 For instance, it has become clear that a local RAS is present in several tissues, for example the heart, adipose, vasculature and bone marrow, with similar effects to the endocrine RAS, but also more specific functions depending on the individual system.1 One of these local systems, the brain RAS, has long been considered pivotal in cardiovascular regulation and important in the pathogenesis of hypertension and heart failure.2 Yet the brain RAS remains poorly understood, due to the difficulty in experimentally dissecting the brain RAS at the cellular, regional and whole organism levels.
In the year 2000, a new member of the ACE family was identified and named ACE2.4 This carboxypeptidase was first sequenced and cloned from human heart failure ventricle and human lymphoma cDNA libraries. These studies reported major expression of ACE2 mRNA in heart and kidneys but failed to detect it in the brain. Later, studies reported ACE2 mRNA in rat medulla oblongata5 and ACE2 activity in mouse brain.6 Recently, we showed for the first time, the presence of ACE2 protein and mRNA in the mouse brain, including in regions involved in the central regulation of cardiovascular function.7 In addition to identifying ACE2 as a new member of the brain RAS, we showed that this carboxypeptidase is highly regulated by other components of this system, both in normotensive and hypertensive mice. ACE2 has been reported to degrade Ang-II into the vasodilatory peptide angiotensin-(1−7) (Ang-(1−7)) with an affinity 400-fold greater than for Ang-I.8, 9 Although central Ang-(1−7) has been shown to enhance baroreceptor reflex sensitivity and exert pressor or depressor responses depending on the targeted region,9, 10 the role of ACE2 in central cardiovascular regulation remains unclear due to the lack of specific tools to investigate its function.
We hypothesized that ACE2 could play a major role in the central regulation of the autonomic nervous system by increasing Ang-(1−7) and buffering the effects of enhanced Ang-II levels in cardiovascular diseases. To test this hypothesis and selectively manipulate ACE2 expression in specific tissues or cells, we developed an adenovirus coding for human ACE2 (hACE2). Our data demonstrate that Ad-hACE2-eGFP induces high levels of ACE2 mRNA, protein and activity in neurons. More importantly, icv Ad-hACE2-eGFP infection resulted in high ACE2 activity levels in the mouse subfornical organ (SFO), associated to a reduction in AT1 receptor expression, and leading to significant reductions in Ang-II-mediated pressor and drinking responses. Consequently, our results establish a role for ACE2 in the central regulation of cardiovascular function, offering a new target for the treatment of hypertension and other cardiovascular diseases.
Material and Methods
A detailed description of methods and experimental protocols can be found in the online data supplement available at http://www.circresaha.org.
Adenovirus Generation
The Ad-hACE2-eGFP virus was developed in collaboration with the University of Iowa Gene Transfer Vector Core. Briefly, the ACE2 pcDNA3.1 vector (kind gift of Dr Curt D. Sigmund, University of Iowa) was digested with XbaI and PmeI to excise the 2418 bp hACE2 fragment (accession number: AF291820). This fragment was then cloned into a pacAd5 CMV IRES eGFP pA shuttle (online data supplement 1A). The resulting construct was then used to generate the hACE2-eGFP adenovirus as described.11
Cell culture and adenovirus infection
Neuro-2A cells (mouse neuroblastoma, ATCC Manassas, VA) were grown in 6-well plates at a density of 2×105 cells/well. After 24 hr, cells were incubated in a low FBS medium (2%) in the presence of Ad-hACE2-eGFP (10, 50, 100 MOI) virus or Ad-eGFP control virus (100 MOI) for 6 hr, then returned to a 10% FBS medium. On the third day post infection, cells were examined using a fluorescence microscope (Olympus, IX81), then media and cells were collected and assayed as described below.
In-vivo adenovirus intracerebroventricular (icv) injection
Male C57Bl/6J mice, 8−10 weeks old, were anesthetized and Ad-hACE2-eGFP or Ad-eGFP were injected icv (2×106 pfu, 200 nL) using a pressure injector (PicospritzerII). Mice were sacrificed 7 days post infection. Brains were either 1) sectioned coronally in a cryostat, then processed for GFP fluorescence visualization and hACE2 or AT1 immunohistochemistry, or 2) frozen at −80°C before the SFO being dissected and used for the following assays.
AT1 receptor binding
The AT1 receptor density was determined in enriched plasma membrane preparations12 from mouse SFOs (n=20 per group) and neuro-2A cells following infection with Ad-hACE2-eGFP or Ad-eGFP. The membrane suspension (100 μg/reaction) was incubated with 100 pM 125I-[Sar1,Ile8]Ang-II (Perkin Elmer, specific activity: 2200 Ci/mmol) for 2 hr at room temperature. Non-specific binding was determined in the presence of 5 μM of non-radioactive Ang-II.
Western blot
Cell culture media (10 μL), cell lysates (10 μg), and purified SFO membranes (5 μg) were collected separately and processed using a standard Western blot protocol against hACE2 or mouse AT1 antibodies. Specific bands were detected by chemiluminescence according to the manufacturer's instructions (ECL®, Perkin Elmer, Boston, MA) and quantitated by laser densitometry (FujiFilm, ImageReader version 1.2).
ACE2 activity
Cells and culture media were collected 3 days after infection and mouse SFOs were collected 7 days after Ad-hACE2-eGFP (n=15) or Ad-eGFP (n=15) (2×106 pfu icv). ACE2 activity was measured in cell lysates, cell culture media and SFO lysate, as described.13 Data (arbitrary fluorescence units, AFU) are presented as amounts of substrate FPSVI converted to product per minute and are normalized for total protein or volume of medium.
Immunohistochemistry
Seven days after infection with Ad-hACE2-eGFP (n=5) or Ad-eGFP (n=5), brains were perfused, postfixed overnight and cryo-sectioned, as described.7 Brain sections and cell cultures were processed for hACE2 or AT1 receptor detection (1:50 dilution for 48 hr). Immunostaining was detected using fluorescence (Olympus, IX81) and brightfield (Nikon Eclipse E600) microscopes.
Physiological recordings
Male C57Bl/6J mice (n=25) were anesthetized and instrumented with an icv canula and a radiotelemetry probe, as described.14, 15 Conscious mice were then injected icv with Ad-hACE2-eGFP (n=13) or Ad-eGFP (n=12) (2×106 pfu, 200 nL). After 7 days, BP was recorded on baseline and following icv injection of Ang-II (200 ng, 200 nL), carbachol (100 ng, 200 nL) or the Ang-(1−7) receptor blocker, D-Ala7-Ang-(1−7) (200 fmol, 200 nL) prior to icv Ang-II. Water intake was monitored by recording the time spent drinking during the 15 min following icv Ang-II administration. From baseline recordings, spontaneous baroreceptor reflex sensitivity (SBRS) was calculated using the sequence method as described.16
Statistical Analysis
Data are expressed as mean ±SEM. Data were analyzed by Student's t test or ANOVA (after Bartlett test of homogeneity of variance) followed by Newman-Keuls correction for multiple comparisons between means. Statistical comparisons were performed using Prism4 (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P<0.05.
Results
In vitro characterization of the adenovirus
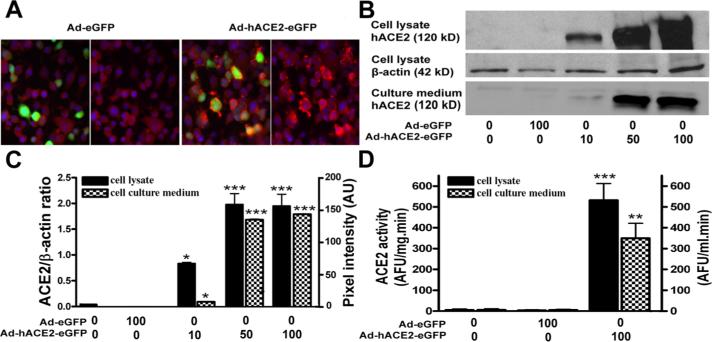
To overcome the lack of tools to investigate the role of the brain RAS in hypertension and particularly the involvement of ACE2 in the central regulation of BP, we developed a new adenovirus coding for the hACE2 carboxypeptidase. First, we investigated if the adenovirus coding for the full length hACE2 upstream of an eGFP reporter gene could be expressed properly in neuronal cells. Neuro-2A cells were infected with Ad-hACE2-eGFP or Ad-eGFP and infection efficiency was evaluated after 3 days with regard to GFP fluorescence expression, ACE2 protein and activity levels in both cell lysate and culture medium. Despite the short term exposure to the virus, highly efficient infection was achieved as evidenced by visualization of eGFP fluorescence (online data supplement 1B). In addition, in cells infected with Ad-hACE2-eGFP, GFP fluorescence was co-localized with hACE2 expression (Figure 1A), confirming that GFP fluorescence is a reliable index of hACE2 expression. As shown previously,17 virus infection did not significantly affect cell viability for titers ranging from 10 to 100 MOI (data not shown). Human ACE2 protein expression was dose-dependently increased in neuro-2A cells following Ad-hACE2-eGFP infection (Figure 1B and 1C). Additional experiments using an antibody targeting the hACE2 signal peptide identified hACE2 in cell lysates but not in cell culture medium (online data supplement 1C), suggesting normal excision of the signal peptide thus permitting hACE2 release into the surrounding milieu.
Figure 1. Expression of hACE2 in Neuro-2A cells.
Neuro-2A cells were infected with Ad-hACE2-eGFP (10−100 MOI) or the control Ad-eGFP virus (100 MOI) then protein expression and ACE2 activity were measured in cell lysates and culture medium, as described in Material and Methods. Typical immunostaining (A) showing the co-localization of GFP fluorescence (green) with ACE2 expression (red). Representative Western Blot (B) and quantified data (C) show a dose-dependent increase in hACE2 protein expression in cell lysate (normalized to β-actin) and culture medium (pixel intensity). Similarly, ACE2 activity (D) was dramatically increased in cell lysate (AFU/mg.min) and culture medium (AFU/ml.min) following Ad-hACE2-eGFP, but not Ad-eGFP infection. Statistical significance: *P<0.05, **P<0.01 and ***P<0.001 vs. baseline. AFU: arbitrary fluorescence units.
To determine the functionality of the protein, ACE2 activity was measured. All Ad-hACE2-eGFP virus titers, but not Ad-eGFP, induced a significant increase in ACE2 activity in both cell lysate (532.4±80.1 vs. 5.5±3.4 AFU/mg.min for 100 MOI of Ad-hACE2-eGFP and Ad-eGFP, respectively; P<0.001) and culture medium (349.6±71.7 vs. 4.2±2.7 AFU/mL.min for 100 MOI of Ad-hACE2-eGFP and Ad-eGFP, respectively; P<0.01) (Figure 1D), confirming the ability of hACE2 to efficiently hydrolyze target peptides. Taken together, these data provide evidence that Ad-hACE2-eGFP can be used to express a functional and active hACE2 protein in neurons in an efficient and easily traceable manner.
SFO-targeted expression of hACE2 in mice
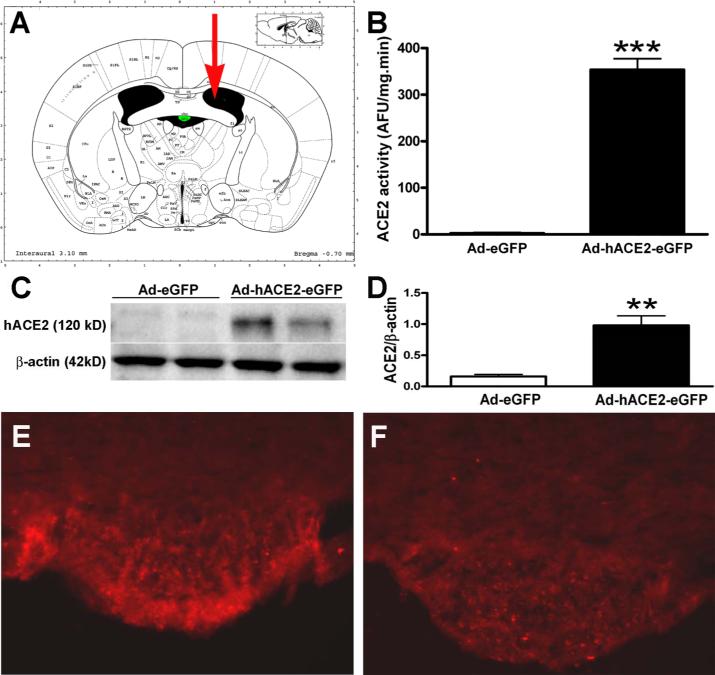
To determine whether ACE2 participates in the central regulation of blood pressure, we over-expressed the enzyme in the SFO, a brain region known for its involvement in the regulation of cardiovascular function. C57Bl/6J mice were infected icv with the virus in order to determine the course of expression and activity in the brain. Figure 2A shows the localization of the icv injection site relative to the SFO, on a coronal section of the mouse brain. The viral infection efficiency was determined by using eGFP as a reporter gene.
Figure 2. SFO-targeted ACE2 activity and protein expression.
Seven days after virus infection, SFOs were collected and processed for Western blot and ACE2 activity assay. ICV-delivered (A) Ad-hACE2-eGFP induced a significant increase in hACE2 activity (B) and protein expression (C,D) in the SFO. ACE2 immunofluorescence was dramatically increased in the SFO following Ad-hACE2-eGFP (E), but not Ad-eGFP (F) infection. Statistical significance: **P<0.01 and ***P<0.001 vs. Ad-eGFP.
Measurements of ACE2 activity in the whole hypothalamus, at different time points showed a significant increase as early as 3 days post infection (24.9±1.4 vs. 2.7±3.3 AFU/mg.min for the control virus, P<0.001) and achieved a maximum 7 days post infection (33.3±5.9 vs. 2.7±3.3 AFU/mg.min for the control virus, P<0.001). Expression of hACE2 in the mouse brain correlates with the activity time-course, as evidenced by cells expressing GFP as early as day 3 (data not shown) and very high fluorescence at 7 days post infection (online data supplement 2). High hACE2 expression (visualized by GFP fluorescence) following icv injection of Ad-hACE2-eGFP, was essentially restricted to the SFO. GFP expression in the SFO, 7 days post Ad-hACE2-eGFP infection, was identified in the core and horns of the organ as well as in the surrounding tissue. Regions exhibiting weaker fluorescence included the wall of the lateral ventricle, the dorsal third ventricle, the fimbria of the hippocampus and the ventral hippocampal commissure (data not shown). Despite the persistence of few cells still harboring GFP fluorescence in the wall of the lateral ventricle (data not shown), ACE2 activity had returned to baseline (below detection levels) at 14 and 28 days post Ad-hACE2-eGFP infection (online data supplement 2). The time-course and pattern of expression obtained with Ad-hACE2-eGFP are identical to previous data using similar constructs.15, 17, 18
To confirm that our activity measurements in the hypothalamus, and the use of eGFP as a reporter gene, are representative of ACE2 activity and expression in the SFO, we performed these measurements directly in the SFO. ACE2 activity was significantly increased in the SFO (Figure 2B) in Ad-hACE2-eGFP-infected mice (353.4±23.7 AFU/mg.min) compared to Ad-eGFP-treated mice (2.4±1.7 AFU/mg.min) 7 days post infection. Similarly, hACE2 protein expression was detected only in the SFO of Ad-hACE2-eGFP-infected mice (Figure 2C and 2D). As observed previously with eGFP, immunohistochemistry for hACE2 shows SFO-restricted expression of the carboxypeptidase (Figure 2E), 7 days after icv injection of Ad-hACE2-eGFP. In Ad-eGFP-infected mice, hACE2 expression was undetectable (Figure 2F). Higher magnification showed eGFP and hACE2 expression in both neurons and glia, consistent with the adenovirus lack of cell tropism. However, hACE2 expression to glial cells was extremely weak compared to neurons (online data supplement 3). Moreover, consistent with our previous observations, hACE2 cellular expression was localized to the cytoplasm and the cell membrane.4, 7
Taken together, these data indicate that Ad-hACE2-eGFP induces a functional hACE2 expression in the brain for at least 7 days, allowing its use to investigate the role of ACE2 in regulating central cardiovascular function.
ACE2 expression and Ang-II-mediated pressor and drinking responses
To assess the ability of ACE2 to counter the effects of Ang-II in the SFO, conscious freely moving mice, previously infected with Ad-hACE2-eGFP (7 days), were injected icv with Ang-II (200 ng in 200 nL) and its effects recorded on BP and water intake. Baseline mean arterial pressure (MAP) and heart rate (HR), 7 days after infection, were not significantly different between Ad-eGFP (MAP: 111±3 mmHg; HR: 605±9 bpm) and Ad-hACE2-eGFP (MAP: 113±3 mmHg; HR: 617±26 bpm; P>0.05) groups. Figure 3A illustrates the typical pressor response induced by icv Ang-II in a conscious mouse previously infected with Ad-eGFP, showing a rapid increase in BP, developing in the minutes following icv injection and slowly returning to baseline. Ad-eGFP pretreatment did not affect the peak effect and duration of the icv Ang-II pressor response.14 However, mice infected with Ad-hACE2-eGFP exhibit a significant reduction in Ang-II-mediated pressor response with decreases in both amplitude (ΔMAP: Ad-hACE2-eGFP 8±2 vs. Ad-eGFP 18±2 mmHg, P<0.01) and duration of the effect (Ad-hACE2-eGFP 9±2 vs. Ad-eGFP 16±2 min, P<0.05)(Figure 3B and 3C). This reduction in the Ang-II pressor response was not prevented by pre-treatment with D-Ala7-Ang-(1−7) (ΔMAP: Ad-hACE2-eGFP 5±3 vs. Ad-eGFP 14±2 mmHg) suggesting that hACE2 expression in the SFO inhibits Ang-II-induced pressor response by decreasing Ang-II levels rather than involving an Ang-(1−7)-mediated mechanism. In addition, Ad-hACE2-eGFP pre-treatment had no effect on the pressor response mediated by the muscarinic agonist carbachol (100 ng icv in 200 nL) (ΔMAP: Ad-hACE2-eGFP 14±3 vs. Ad-eGFP 18±7 mmHg, P>0.05) confirming the selectivity of hACE2 for angiotensin peptides. Finally, the decrease in Ang-II-mediated pressor response observed in the Ad-hACE2-eGFP group was correlated to an increase in enzyme activity in these mice (Figure 3D).
Figure 3. ACE2 over-expression reduces Ang-II pressor response in conscious mice.
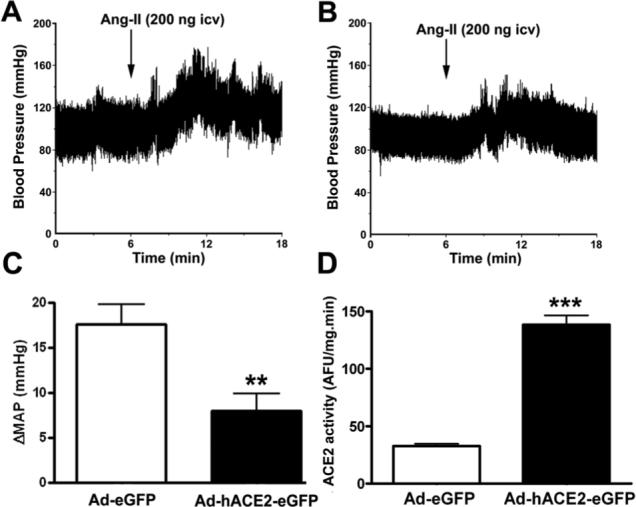
Mice were anesthetized and surgically instrumented with an icv canula and a radiotelemetry probe for continuous BP recording. Seven days after icv infection, Ang-II (200 ng, 200 nL icv) was delivered centrally and BP recorded for 30 min. Following euthanasia, the hypothalamus was rapidly dissected and processed for ACE2 activity assay. Panel A shows a typical pressor response following icv Ang-II in Ad-eGFP-treated mice. The amplitude and duration of this response was significantly blunted in mice pre-treated with Ad-hACE2-eGFP (B,C). In addition, the reduction in Ang-II pressor response was correlated with a significant increase in ACE2 activity in mice treated with Ad-hACE2-eGFP (D). Statistical significance: **P<0.01; ***P<0.001.
To confirm the ability of SFO-targeted hACE2 expression in preventing local Ang-II-mediated effects, we assessed the Ang-II-mediated drinking response following adenovirus pre-treatment. Ad-hACE2-eGFP significantly blunted the water intake behavior resulting from central administration of Ang-II (Ad-hACE2-eGFP: 5±3 vs. Ad-eGFP: 41±6 sec, P<0.001)(Figure 4A).
Figure 4. ACE2 over-expression reduces Ang-II water intake but not SBRS in conscious mice.
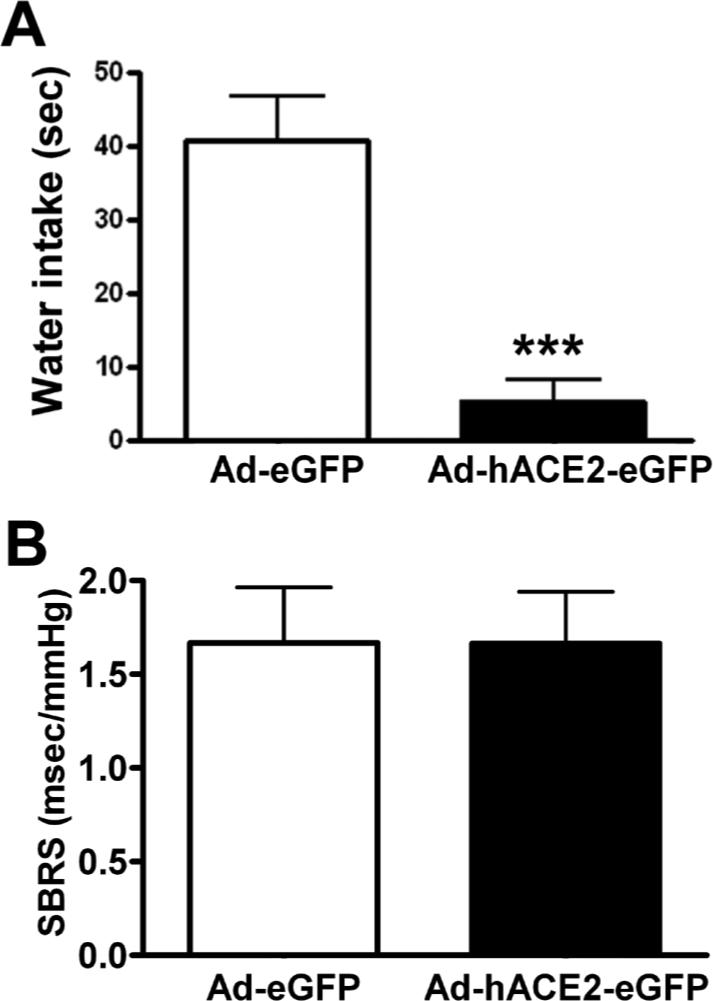
Seven days after icv infection, baseline BP was recorded (2000 Hz sampling rate) in conscious freely moving mice and SBRS analyzed using the sequence method. Following Ang-II (200 ng, 200 nL) icv injection, water intake was recorded for 15 min. Panel A shows a significant reduction in Ang-II-mediated drinking behavior in mice pre-treated with Ad-hACE2-eGFP. However, virus infection did not significantly altered SBRS (B). Statistical significance: ***P<0.001.
To assess whether disruption of local Ang-II levels by hACE2 in the SFO is able to modify distant physiological mechanisms, we analyzed baroreflex sensitivity in mice following virus infection. No significant changes were observed in SBRS 7 days post infection (up-sequences: Ad-hACE2-eGFP 2.0±0.5 vs. Ad-eGFP 2.2±0.4; down sequences: Ad-hACE2-eGFP 1.5±0.2 vs. Ad-eGFP 1.6±0.2 msec/mmHg, P>0.05, Figure 4B) suggesting that disruption of Ang-II signaling in the SFO is unable to alter baroreflex mechanisms originating in the brainstem.
ACE2 and AT1 receptor expression
To gain insight of the mechanism by which ACE2 prevents Ang-II-mediated pressor and drinking responses, we investigated the effects of ACE2 over-expression on AT1 receptors regulation. This choice was motivated by the inability of D-Ala7-Ang-(1−7) to block Ad-hACE2-eGFP-mediated effects and by previous studies showing that Ang-(1−7) has hypotensive effects in hypertensive19, 20 but not in normotensive21 animals.
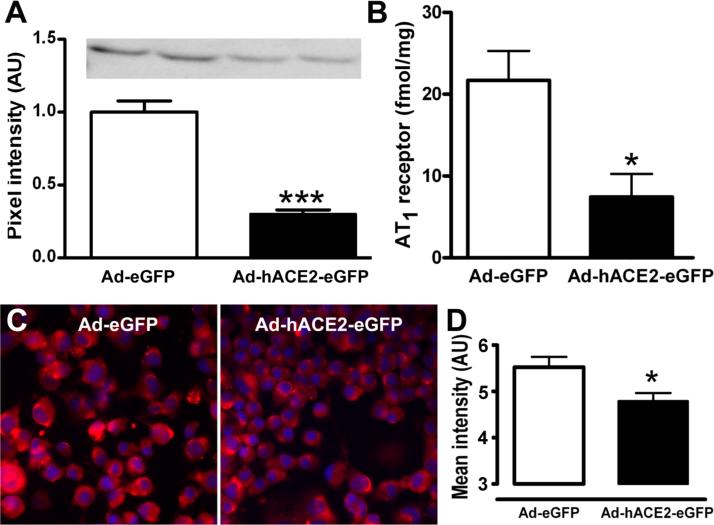
In vitro, over-expression of ACE2 in neuro-2A cells resulted in a 70% reduction (0.30±0.03) of AT1 protein expression compared to Ad-eGFP (1.00±0.08; P<0.001)(Figure 5A). Similarly, AT1 receptor binding (Figure 5B) was dramatically decreased in Ad-hACE2-eGFP versus Ad-eGFP-treated cells (7.5±2.8 vs. 21.7±3.6 fmol/mg of proteins, P<0.05). These observations were further confirmed by AT1 immunofluorescence (Figure 5C and 5D), suggesting that in vitro, ACE2 down-regulates AT1 receptor expression.
Figure 5. Regulation of AT1 receptor expression by ACE2 in vitro.
Neuro-2A cells were harvested 48 hr post virus infection. Ad-hACE2-eGFP infection induced a significant reduction of AT1 protein expression in Neuro-2A cells compared to Ad-eGFP (A). Similarly, hACE2 over-expression in neuro-2A cells significantly reduced the Ang-II receptor binding (B) and immunostaining (C,D) compared to Ad-eGFP treated cells. *P<0.05, and ***P<0.001 vs. Ad-eGFP.
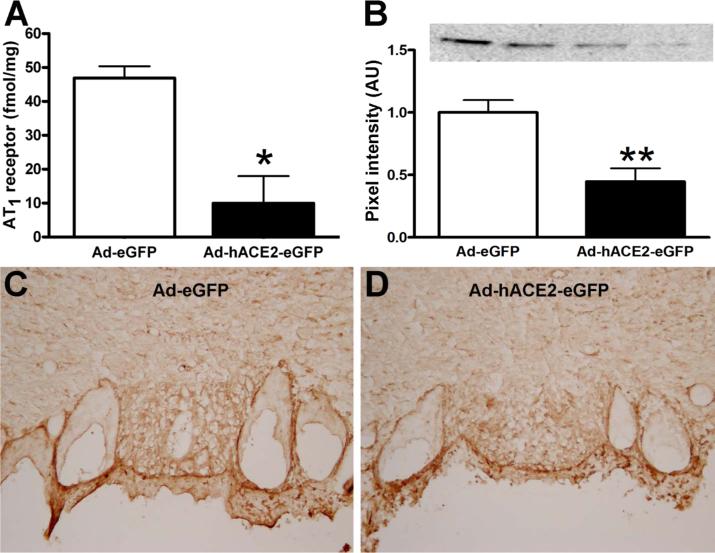
We then addressed ACE2-AT1 interaction in vivo. Mice (n=50) were injected with Ad-hACE2-eGFP or Ad-eGFP and after 7 days, SFOs were collected and processed for AT1 receptor binding, western blot and immunohistochemistry. As observed in vitro, AT1 receptor binding was significantly decreased in the SFO of Ad-hACE2-eGFP-treated mice compared to Ad-eGFP (10.0±8.0 vs. 36.9±10.7 fmol/mg of proteins; P<0.05)(Figure 6A). A similar reduction in AT1 receptor level was observed in SFO plasma membrane extracts from Ad-hACE2-eGFP-treated mice (Figure 6B). Finally, AT1 receptor immunostaining (Figure 6C) was significantly reduced in the core of the SFO following ACE2 over-expression. Altogether, our data suggest that ACE2 exerts its modulatory effects partly through down-regulation of the AT1 receptor expression, thus preventing Ang-II-mediated pressor and drinking responses.
Figure 6. ACE2 down-regulates AT1 receptor expression in the SFO.
Seven days after virus infection, SFOs were collected and processed for Ang-II receptor binding, Western blot and immunofluorescence. Ad-hACE2-eGFP over-expression in the SFO significantly reduced the Ang-II receptor binding (A) compared to Ad-eGFP. Typical Western blot and quantitated data (B) showed a reduction in AT1 receptor expression in the SFO, following Ad-hACE2-eGFP infection. This result was further confirmed by immunostaining against the mouse AT1 receptor (C).
Discussion
Over-activity of the RAS has been implicated in the development and maintenance of several cardiovascular diseases, and participation of the brain RAS to such diseases is now well recognized.1, 22 However, studying the brain RAS has been limited by the difficulty of unmasking brain-specific mechanisms and avoiding systemic RAS-mediated effects.2 Nevertheless, important data have emerged, such as the role of key forebrain and brainstem regions in which activation of angiotensin pathways leads to an increase in sympathetic tone. The difficulty in understanding how these pathways may interact has recently been increased by the discovery of new players in the RAS, both in the periphery and central nervous system.7, 9 Consequently, new tools are needed to face the challenge of an evolving brain RAS. In this study, we developed a new viral vector expressing hACE2 and unmasked a role for ACE2 in buffering the Ang-II-mediated pressor and drinking responses originating in the SFO, suggesting a role for this enzyme in the central regulation of cardiovascular function and volume homeostasis.
To overcome the limitations associated with studying the brain RAS, scientists have used brain-targeted over-expression of RAS genes, by generation of transgenic animals14, 23, 24, gene delivery18, 25, 26 or combination of both15, 27. These approaches have been successful in unmasking physiologic responses otherwise below detection levels. Classic methods of gene therapy for the brain include the use of adeno-associated virus28, lentivirus22, 26 or adenovirus.18, 25, 29 Unlike lentivirus, targeting essentially neurons when administered in the brain, adenovirus vectors have no tissue tropism, thus allowing transduction of both glia and neurons.17, 30 Although we recently demonstrated that central ACE2 was expressed primarily on neurons,7 others reported astrocytic expression in vitro.31 In addition, adenoviral transgenes do not integrate into the genome, therefore preventing random insertional mutagenesis. Although we did not assess inflammation resulting from virus injection, previous work using similar vectors and titers in the brain,15, 27 added to the lack of abnormal behavior in our infected mice, ruled out this possibility. Finally, the rapid (as early as 3 days) and efficient expression of hACE2 makes Ad-hACE2-eGFP an attractive tool to manipulate ACE2 expression and activity in vitro and in vivo.
The main goal of this study was to assess the effects of ACE2 over-expression on Ang-II-mediated physiological responses. Our data provide the first evidence that ACE2 over-expression in the forebrain counters Ang-II-mediated responses partly by down-regulating AT1 receptors expression. Icv administration of Ang-II is responsible for powerful pressor and bradycardic responses preceding water intake, all resulting from the stimulation of AT1 receptors located in the SFO and other nuclei of the lamina terminalis.1, 32
Our data show that ACE2 over-expression to the forebrain, essentially the SFO, inhibited both pressor and drinking responses resulting from icv administration of Ang-II. This could result from: 1) a decrease in Ang-II levels due to ACE2-mediated conversion of Ang-II into Ang-(1−7), thus leading to a lesser stimulation of AT1 receptors or 2) a decrease in Ang-II levels, associated with increased Ang-(1−7) levels leading to the activation of an Ang-(1−7) receptor. Although, Ferreira et al. observed a slight decrease in water intake in transgenic rats with high plasma Ang-(1−7) levels,33 and while Ang-(1−7) was shown to alter the bradycardic component of the baroreflex,21 the participation of Ang-(1−7) in the reduction of Ang-II-mediated BP and water intake in our experiments seems unlikely. Indeed, the vasoactive properties of Ang-(1−7) in vivo have been primarily reported in pathophysiologic conditions, such as hypertension or myocardial infarction and this peptide has minimal effects in normotensive animals.9 Accordingly, failure to restore Ang-II-mediated pressor and drinking responses following pre-treatment with an Ang-(1−7) receptor blocker might be expected. Moreover, the fact that we observed no change in either arm of the baroreflex curve, suggests that decreased Ang-II-mediated pressor and drinking responses only results from lessened stimulation of the Ang-II signaling pathways. These observations are consistent with the ability of ACE2 to hydrolyze Ang-II with a high catalytic efficiency (kcat/Km=1.9×106 M−1s−1).8 Finally, our conclusions are supported by a recent study, where a lentivirus coding for the murine ACE2 was delivered to the RVLM of spontaneously hypertensive rats, resulting in a transient decrease in BP and HR, 4 weeks post-infection.26 Since injection of Ang-(1−7) in the RVLM has previously been shown to increase BP,34 these data suggest that the BP decrease was not mediated by Ang-(1−7) and is likely to result from a reduction in Ang-II signaling pathway.
Although the unaltered baroreflex sensitivity following hACE2 over-expression in the SFO probably suggests that modulation of the RAS is restricted to the region infected by the virus, our data show that hACE2 is also a target for sheddases, thus releasing the enzyme in the cell environment. In the case of the brain, because the secreted form conserves its activity, we cannot rule out the possibility that secreted ACE2 could travel from the interstitial to the cerebrospinal fluid, allowing the enzyme to modulate Ang-II levels and central signaling pathways throughout the brain.
The SFO exerts a key role in the central regulation of BP and volume homeostasis.15, 27, 32 Indeed, the lack of a blood brain barrier renders it sensitive to circulating peptides, such as angiotensins, that can reach the brain and stimulate local receptors to exert central effects in addition to their peripheral effects. Not only the SFO is sensitive to systemic peptides, but it is also a pivotal site for synthesis of Ang-II involved in generating cardiovascular and drinking responses.15, 27 In addition, the SFO projects and receive projections from several brain regions, including paraventricular and supraoptic nuclei, nucleus of tractus solitarius and ventrolateral medulla.32 Over-expression of ACE2 in the SFO could potentially result in the alteration of several downstream and upstream neuronal networks. For example, as a result to ACE2 over-expression in the SFO, neuronal activation could be reduced in the PVN, thus participating in the reduction of both pressor and drinking responses. However, lack of hACE2 expression in the PVN rules out a direct involvement.
Interestingly, we observed that prevention of Ang-II-mediated pressor and drinking responses, by ACE2 over-expression, was associated with a down-regulation of AT1 receptor expression in the SFO. These data suggest that not only ACE2 over-expression reduces the amount of Ang-II available to stimulate its receptors, but it also further impairs Ang-II downstream signaling by limiting the number of AT1 receptors mediating these signaling pathways. Whether this decrease in AT1 receptor expression on the cell membrane resulted from internalization or gene regulation remains to be determined. This could be mediated by Ang-(1−7) as suggested previously,35 although our experiments seem incompatible with formation of the micromoles of Ang-(1−7) necessary to produce this response. Alternatively, it is conceivable that longer stimulation of Ang-(1−7) release could lead to such down-regulation.36 More recent studies reported an inhibitory effect of AT1 receptors on ACE2 gene and protein expression,37, 38 however, assessing the role of ACE2 on other RAS components was so far limited by the lack of tools available to modulate ACE2 expression. Ad-hACE2-eGFP was determinant in showing for the first time that ACE2 is able to regulate other components of the RAS.
In summary, these results demonstrate that over-expression of ACE2 in the SFO plays a pivotal role in the regulation of BP and volume homeostasis by modulating both amounts of Ang-II and AT1 receptors available to stimulate downstream signaling pathways. Moreover, we speculate that ACE2-mediated formation of Ang-(1−7) may only be relevant in pathophysiological conditions. Finally, this study provides proof in principle of a therapeutic role for ACE2 gene therapy and more generally, validates ACE2 as a new target for the treatment of conditions involving a dysfunctional RAS, such as hypertension and other cardiovascular diseases.
Acknowledgements
The work described herein was partly funded by The American Heart Association Heartland Affiliate (BGIA 0560007Z) and the National Institutes of Health (NS052479 and P20 RR018766) to E. Lazartigues. The authors would like to thank Drs. Curt D. Sigmund and Carmen Halabi (University of Iowa) for providing the ACE2 pcDNA3.1 vector; Dr. Beverly Davidson and Maria Scheel at the University of Iowa Gene Transfer Vector Core; Dr. Kurt J. Varner and the Cardiac and Vascular Biology Core.
Footnotes
Publisher's Disclaimer: Disclosure: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures: None
Bibliography
- 1.Paul M, Poyan Mehr A, Kreutz R. Physiology of Local Renin-Angiotensin Systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 2.Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol - Regul Integr Comp Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie JL, Sigmund CD. Overview of the renin-angiotensin system-An endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 4.Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- 5.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1−7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 6.Elased KM, Cunha TS, Gurley SB, Coffman TM, Morris M. New Mass Spectrometric Assay for Angiotensin-Converting Enzyme 2 Activity. Hypertension. 2006;47:1010–1017. doi: 10.1161/01.HYP.0000215588.38536.30. [DOI] [PubMed] [Google Scholar]
- 7.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol - Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 9.Ferrario CM. Angiotensin-Converting Enzyme 2 and Angiotensin-(1−7): An Evolving Story in Cardiovascular Regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 10.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow:pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 12.Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the Cell Surface Expression and Function of Angiotensin II Type 1 Receptor by Rab1-mediated Endoplasmic Reticulum-to-Golgi Transport in Cardiac Myocytes. J. Biol. Chem. 2004;279:41077–41084. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- 13.Huentelman MJ, Zubcevic J, Katovich MJ, Raizada MK. Cloning and characterization of a secreted form of angiotensin-converting enzyme 2. Regul Pept. 2004;122:61–67. doi: 10.1016/j.regpep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- 15.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic Ablation of Angiotensinogen in the Subfornical Organ of the Brain Prevents the Central Angiotensinergic Pressor Response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- 16.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor Reflex Sensitivity Estimated by the Sequence Technique is Reliable in Rats. Am J Physiol - Heart Circ Physiol. 2006;291:H482–H483. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: comparison of two viral vector systems. Hypertension. 2002;39:603–608. doi: 10.1161/hy0202.103295. [DOI] [PubMed] [Google Scholar]
- 18.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of Cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25–32. doi: 10.1152/physiolgenomics.00048.2004. [DOI] [PubMed] [Google Scholar]
- 19.Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1−7) Hypertension. 1995;26:1138–1144. doi: 10.1161/01.hyp.26.6.1138. [DOI] [PubMed] [Google Scholar]
- 20.Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1−7). Peptides. 2001;22:927–933. doi: 10.1016/s0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 21.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- 22.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res. 2001;89:365–372. doi: 10.1161/hh1601.094988. [DOI] [PubMed] [Google Scholar]
- 24.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 25.Allen AM, Dosanjh JK, Erac M, Dassanayake S, Hannan RD, Thomas WG. Expression of Constitutively Active Angiotensin Receptors in the Rostral Ventrolateral Medulla Increases Blood Pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. [DOI] [PubMed] [Google Scholar]
- 26.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the. Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 27.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips MI. Antisense inhibition and adeno-associated viral vector delivery for reducing hypertension. Hypertension. 1997;29:177–187. doi: 10.1161/01.hyp.29.1.177. [DOI] [PubMed] [Google Scholar]
- 29.Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CX, Parker A, Menocal E, Xiang S, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5:2103–2109. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1−7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 32.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira AJ, Pinheiro SVB, Castro CH, Silva GAB, Simoes e Silva AC, Almeida AP, Bader M, Rentzsch B, Reudelhuber TL, Santos RAS. Renal function in transgenic rats expressing an angiotensin-(1−7)-producing fusion protein. Regul Peptides. 2006;137:128–133. doi: 10.1016/j.regpep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Fontes MAP, Silva LCS, Campagnole-Santos MJ, Khosla MC, Guertzenstein PG, Santos RAS. Evidence that angiotensin-(1−7) plays a role in the central control of blood pressure at the ventro-lateral medulla acting through specific receptors. Brain Res. 1994;665:175–180. doi: 10.1016/0006-8993(94)91171-1. [DOI] [PubMed] [Google Scholar]
- 35.Clark MA, Diz DI, Tallant EA. Angiotensin-(1−7) Downregulates the Angiotensin II Type 1 Receptor in Vascular Smooth Muscle Cells. Hypertension. 2001;37:1141–1146. doi: 10.1161/01.hyp.37.4.1141. [DOI] [PubMed] [Google Scholar]
- 36.Clark MA, Tallant EA, Tommasi E, Bosch S, Diz DI. Angiotensin-(1−7) reduces renal angiotensin II receptors through a cyclooxygenase-dependent mechanism. J Cardiovasc Pharmacol. 2003;41:276–283. doi: 10.1097/00005344-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1−7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]