Abstract
Previous studies have shown that nitrous oxide (N2O)-induced antinociception is sensitive to antagonism by blockade of opioid receptors and also by inhibition of nitric oxide (NO) production. The present study was conducted to determine whether these occur within the same brain site. Mice were stereotaxically implanted with microinjection cannulae in the periaqueductal gray (PAG) area of the midbrain. In saline-pretreated mice, exposure to 70% N2O resulted in a concentration-dependent antinociceptive effect in the mouse abdominal constriction test. Pretreatment with an opioid antagonist in the PAG significantly antagonized the antinociceptive effect. Pretreatment with an inhibitor of NO production in the PAG also significantly antagonized the antinociceptive effect. These findings suggest that N2O acts in the PAG via an NO-dependent, opioid receptor-mediated mechanism to induce antinociception.
Keywords: Nitrous oxide, antinociception, periaqueductal gray, opioid receptors, nitric oxide
1. Introduction
Nitrous oxide, also known as “laughing gas” or simply N2O, has gained clinical acceptance because of its anesthetic, analgesic and anxiolytic properties, which have been utilized in medicine and dentistry. How such a simple inorganic chemical structure can evoke this wide range of pharmacological effects is intriguing.
Exposure of test animals to N2O results in an antinociceptive effect that is sensitive to antagonism by naloxone and other opioid receptor blockers (Berkowitz et al., 1976; Quock and Graczak, 1988), indicating a key role for opioid receptors in the drug effect. We have previously reported that the antinociceptive effect of N2O is also antagonized by inhibition of nitric oxide (NO) production (McDonald et al., 1994; Ishikawa and Quock, 2003a; Li et al., 2004), implicating an involvement of NO in the drug effect as well. The present study was conducted to ascertain whether the opioid receptors and NO function that are involved in N2O-induced antinociception are co-localized in the periaqueductal gray (PAG) of the midbrain, which has been shown to be involved in N2O-induced antinociception (Zuniga et al., 1987; Hodges et al., 1994; Fang et al., 1997).
2. Experimental procedures
2.1. Subjects
A total of 265 male NIH Swiss mice, 18−22 g body weight, were obtained from Harlan Laboratories (Indianapolis, IN) and were housed five per cage in an ALAAAC-accredited animal facility. Food and water were available ad libitum. The facility was maintained on a 12-h light/dark cycle (lights on 0700−1900) under standard conditions (22 ± 1°C room temperature, 33% humidity). Mice were kept in the holding room for at least four days following arrival in the facility prior to use. The animals were tested in control and experimental groups of 8−12 mice per group.
The experimental protocol was approved by the Institutional Review Committee for the Use and Care of Animal Subjects of Washington State University and is in compliance with The Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996). All care was taken to minimize pain and discomfort in the experimental animals.
2.2. Surgery
Under isoflurane anesthesia, mice were mounted in a digital stereotaxic system (Cartesian Research, Inc., Sandy, OR). Using aseptic technique, 26-G stainless steel external guide cannulae (Plastics One, Roanoke, VA) were stereotaxically directed at the PAG of the midbrain at coordinates − 3.0 mm AP, ± 0.2 mm ML, and − 3.0 mm DV (Franklin and Paxinos, 2001). The tips of the external guide cannulae were positioned 1.0 mm dorsal to the target sites. Cannulae were secured to the calvarium using stainless steel screws and dental cement. Each cannula was plugged with a solid 33-gauge dummy cannula. After surgery, mice were allowed a minimum of five days recovery time before testing.
2.3. Histological verification of microinjection site
At the end of the experiments, animals were anesthetized with 2.5% isoflurane, and 0.5 l 10% India ink dye was microinjected into the PAG over 15 min to mark the microinjection site. Thirty minutes after dye injections, mice were perfused with cold phosphate-buffered saline followed by 4% formaldehyde. The brains were dissected out and cryoprotected in 25% sucrose. Forty-μm coronal sections were cut on a cryostat and processed for staining with 0.5% cresyl violet. The sections were later observed under a stereomicroscope and photographed (Fig. 1).
Fig. 1.
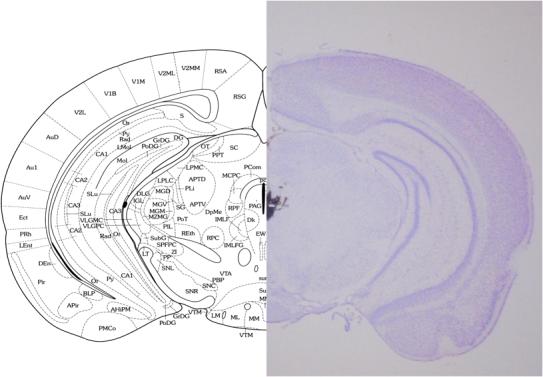
Photomicrograph of cresyl violet-stained coronal mouse brain section and corresponding atlas plate (Franklin and Paxinos, 2001) indicating the typical site and spread of microinjection as assessed by stain injections. All doses of β-CNA and TRIM were injected at a volume of 0.5 ml into the PAG.
2.4. Agents
Nitrous Oxide, U.S.P. and Oxygen, U.S.P. (A&L Welding, Spokane, WA) were mixed and delivered using a dental-sedation system (Porter, Hatfield, PA) at a total flow rate of 10 l/min. Mice were individually exposed in a clear Plexiglas® exposure chamber (35 cm L × 20 cm W × 15 cm H) with gas inlet and outlet ports. The concentrations of N2O and O2 delivered into the box were monitored using a POET II® anesthetic monitoring system (Criticare, Milwaukee, WI). Exhausted gases were routed by polyethylene tubing to a nearby fume hood.
β-Chlornaltrexamine was purchased from the Sigma Chemical Company (St. Louis, MO) and TRIM (1-[2-trifluoromethylphenyl]imidazole) was purchased from the Alexis Biochemical Company (San Diego, CA). Both agents were prepared in 0.9% physiological saline and microinjected in an intra-PAG (i.PAG) volume of 0.5 μl. The pre-treatment times for β-chlornaltrexamine and TRIM were 24 h and 30 min, respectively.
2.5. Antinociception assessment
Antinociceptive responsiveness to N2O was assessed by the abdominal constriction test. Mice were treated i.p. with 0.6% acetic acid (0.1 ml/10 g body weight); exactly 5 min later, the number of abdominal constrictions—lengthwise stretches of the torso with concave arching of the back—in each animal was counted for a 6-min period while in the clear Plexiglas® exposure chamber under conditions of room air or 25%, 50% or 70% N2O in oxygen (O2).
The degree of antinociception produced by N2O in various treatment groups of mice was calculated as:
Raters were trained extensively in evaluation of glacial acetic acid-induced abdominal constrictions during preliminary experiments. While raters were not blinded to drug conditions, multiple raters were used for some but not all experiments; however, the number of abdominal constrictions counted was very consistent between the raters.
2.6. Experimental design
To ascertain dose-dependent antagonism of N2O by β-CNA, different groups of mice were pretreated i.PAG with 0 (vehicle control), 0.1 or 0.5 μg β-CNA 24 hr prior to being assessed for antinociceptive responsiveness to 70% N2O (Fig. 2).
Fig 2.
Dose-related antagonism of N2O-induced antinociception by β-CNA. Different groups of mice received i.PAG microinjection of β–CNA or vehicle 24 hr prior to being assessed for antinociceptive responsiveness to 70% N2O in the abdominal constriction test. The height of each bar indicates the mean percent antinociceptive response ± s.e.m. of 8−12 mice per group. Significance of difference: *, p < 0.05 and **, p < 0.01, compared to vehicle (0 μg) control group (Bonferroni test).
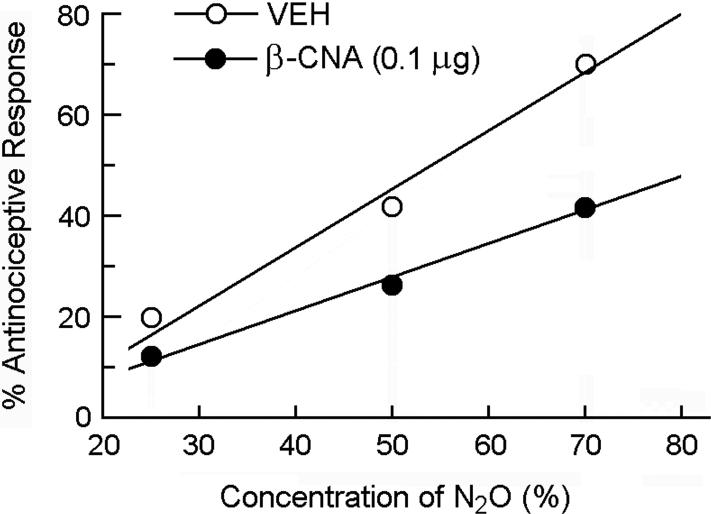
To determine the influence of β-CNA pretreatment on the dose-response curve for N2O-induced antinociception, different groups of mice were pretreated i.PAG with 0 (vehicle control) or 0.1 μg β-CNA 24 hr prior to being assessed for antinociceptive responsiveness to 0% (room air control), 25%, 50% or 70% N2O (Fig. 3).
Fig 3.
Rightward shift of the dose-response curve for N2O-induced antinociception by i.PAG microinjection of 0.1 μg β-CNA 24 hr prior to being assessed for antinociceptive responsiveness to N2O in the abdominal constriction test. Each symbol represents the mean percent antinociceptive response of 8−12 mice per group.
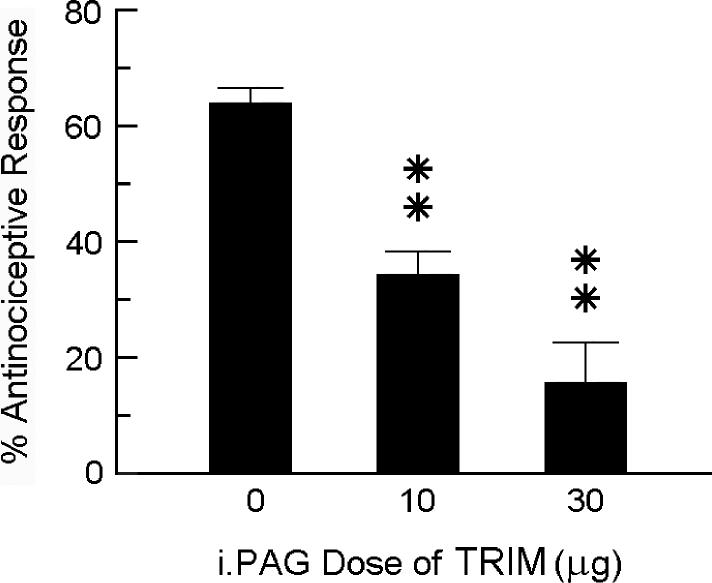
To ascertain dose-dependent antagonism of N2O by TRIM, different groups of mice were pretreated i.PAG with 0 (vehicle control), 10 or 30 μg TRIM 30 min prior to being assessed for antinociceptive responsiveness to 70% N2O (Fig. 4).
Fig 4.
Dose-related antagonism of N2O-induced antinociception by TRIM. Different groups of mice received i.PAG microinjection of TRIM or vehicle 30 min prior to being assessed for antinociceptive responsiveness to 70% N2O in the abdominal constriction test. The height of each bar indicates the mean percent antinociceptive response ± s.e.m. of 8−12 mice per group. Significance of difference: **, p < 0.01, compared to vehicle (0 μg) control group (Bonferroni test).
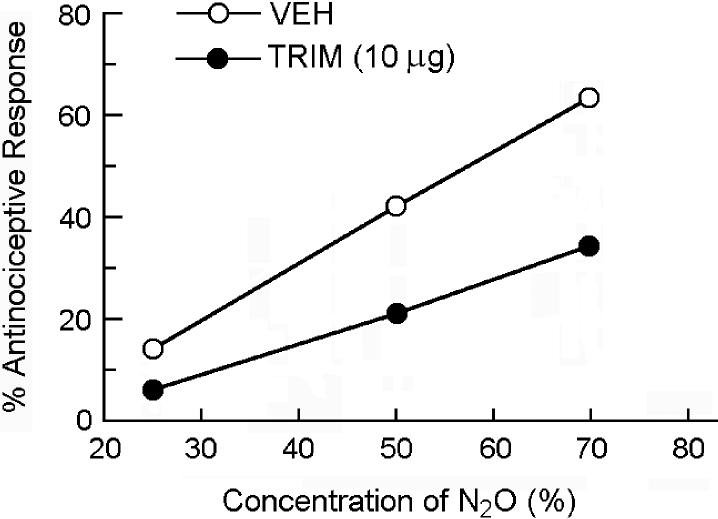
To determine the influence of TRIM pretreatment on the dose-response curve for N2O-induced antinociception, different groups of mice were pretreated i.PAG with 0 (vehicle control) or 10 μg TRIM 30 min prior to being assessed for antinociceptive responsiveness to 0% (room air control), 25%, 50% or 70% N2O (Fig. 5).
Fig 5.
Rightward shift of the dose-response curve for N2O-induced antinociception by i.PAG microinjection of 10 μg TRIM 30 min prior to being assessed for antinociceptive responsiveness to N2O in the abdominal constriction test. Each symbol represents the mean percent antinociceptive response of 8−12 mice per group.
2.7. Statistical analysis
The percent antinociceptive responses of control and experimental groups were compared using one-way analysis of variance (ANOVA) and post-hoc Bonferroni test. Dose-response curves were constructed for N2O-induced antinociception for each treatment group. The probit of the percent antinociceptive response (percent inhibition of abdominal constrictions) was plotted against the log concentration of N2O, and the antinociceptive dose, 50% (AD50) values and 95% confidence intervals were determined by the method of Litchfield and Wilcoxon (1949), as described by Dewey et al [1970].
3. Results
On average, i.p. treatment with 0.6% glacial acetic acid evoked 25.6 ± 2.3 abdominal constrictions over a 6-min period starting 5 min following injection (N=12). Following exposure to increasing concentrations of N2O (25%, 50% and 70%), the mean number of glacial acetic acid-induced abdominal constrictions was reduced to 23.2 ± 1.8 (9.0% antinociceptive response), 14.9 ± 1.4 (41.8% antinociceptive response) and 4.6 ± 1.3 (70.1% antinociceptive response), respectively.
In animals that were pretreated i.PAG with saline vehicle 24 h earlier, exposure to 70% N2O evoked a 70.1% antinociceptive response. In mice that were pretreated with one of two doses of β-CNA (0.1 or 0.5 μg) 24 h earlier, the peak antinociceptive response was reduced in a dose-related manner to 33.5% and 15.6%, respectively (Fig. 2). Intra-PAG treatment with β-CNA alone had no influence on the number of glacial acetic acid-induced abdominal constrictions. Comparison of dose-response curves in the absence and presence of 0.1 μg β-CNA show that opioid receptor blockade in the PAG caused a rightward shift of the dose-response curve for N2O-induced antinociception (Fig. 3). The AD50 value (with 95% confidence intervals) was 53.7% (37.6−76.8%) for the vehicle control group, whereas, in the β-CNA-pretreated group, the AD50 value was increased to 89.4% (66.9−119.4%).
In another experiment, mice that received an i.PAG microinjection of saline vehicle 30 min beforehand responded to 70% N2O with a 64.6% antinociceptive response. In mice that were pretreated with one of two doses of TRIM (10 or 30 μg) 30 min earlier, the intensity of the N2O antinociceptive response was reduced to 37.6% and 12.2%, respectively (Fig. 4). Intra-PAG treatment with TRIM alone had no influence on the number of glacial acetic acid-induced abdominal constrictions. Comparison of dose-response curves in the absence and presence of 10 μg TRIM show that inhibition of NO production in the PAG shifted the dose-response curve for N2O-induced antinociception to the right (Fig. 5). The AD50 value of the vehicle control group was 54.8% (32.5−92.4%), while that of the TRIM pretreatment group was 102.7% (64.4−163.8%).
4. Discussion
The PAG has long been known to be the site of action of opioid-induced antinociception (Jacquet and Lajtha, 1974; Sharpe et al., 1974). It was also reported that kainic acid-induced lesions of the ventral and caudal PAG antagonized the antinociceptive effect of N2O in rats (Zuniga et al., 1987). We reported that i.PAG microinjection of the μ opioid antagonist CTOP caused a dose-dependent antagonism of N2O-induced antinociception in the rat hot plate test (Hodges et al., 1994). The antinociceptive response of rats to N2O in the tail-flick test was also sensitive to antagonism by naloxone administered directly into the ventrolateral part of the PAG (Fang et al., 1997), from which N2O would activate descending noradrenergic pain-modulatory pathways to the spinal cord (Fujinaga and Maze, 2002). Consistent with these earlier studies, the results of the present investigation demonstrate that i.PAG pretreatment with the non-equilibrium opioid antagonist β-CNA caused a dose-dependent attenuation of the antinociceptive response of mice to N2O.
We have previously demonstrated that exposure of mice to N2O increased the level of nitric oxide synthase (NOS) enzyme activity in the brain (Li et al., 2003). NOS activity is apparently critical to the antinociceptive response to N2O since N2O-induced antinociception in rats and mice is blocked by pretreatment with any of a variety of NOS-inhibiting arginine analogs (McDonald et al., 1994; Ishikawa and Quock, 2003a) and also by pretreatment with an antisense oligodeoxynucleotide directed against the neuronal NOS enzyme (Li et al., 2004). Moreover, DBA/2 inbred mice that are poorly sensitive to N2O-induced antinociception failed to exhibit an increase in NOS enzyme activity during exposure to N2O when compared to N2O-sensitive C57BL/6 mice (Ishikawa and Quock, 2003b; Henry et al., 2005). Mice that were selectively bred for poor responsiveness to N2O likewise did not exhibit an increase in NOS activity during exposure to N2O (Henry et al., 2005).
The results of the present study demonstrate clearly that microinjection of the NOS-inhibitor TRIM into the PAG also caused a dose-related antagonism of N2O-induced antinociception. Since we have proposed in earlier investigations that N2O owes its antinociceptive effect in animals to stimulated neuronal release of endogenous opioid peptides (Branda et al., 2000; Cahill et al., 2000), the importance of the present findings is the localization of both opioid and NO mechanisms in the PAG of the midbrain. In as much as inhibition of NOS can significantly attenuate the increase in release of methionine-enkephalin in the rat spinal cord in response to centrally-administered β-endorphin (Hara et al., 1995), one explanation of these findings is that NO may play a key regulatory role in the N2O-stimulated neuronal release of endogenous opioid peptides that then activate opioid receptors in the PAG. Consistent with this hypothesis is our recent observation that exposure to N2O induces an NO-dependent increase in β-endorphin and NO metabolites in diasylate collected from the rat arcuate nucleus (Ohgami et al., 2007).
Other possibilities for NO modulation of N2O-induced antinociception must also be acknowledged. While there is evidence that NO appears to be involved in the development of opioid tolerance (Kolesnikov et al., 1993; Majeed et al., 1994), most studies report a lack of involvement of NO in morphine-antinociception (Duarte and Ferreira, 1992; Xu and Tseng, 1993; McDonald et al., 1994; Heinzen and Pollack, 2004). There are some exceptions, however, where inhibition of NO production with different NOS-inhibitors altered antinociceptive responsiveness to morphine. Pretreatment with L-NG-nitro arginine methyl ester (L-NAME) or L-NG-monomethyl nitro arginine (L-NMMA) reportedly attenuated morphine-induced analgesia in mice (Pataki and Telegdy, 1998; Abacioglu et al., 2001); on the other hand, pretreatment with L-NG-nitro arginine (L-NOARG) significantly enhanced pain threshold and potentiated morphine-induced antinociception in mice (Ozek et al., 2003) and potentiated morphine-induced antinociception in dogs (Pelligrino et al., 1996). Yet another possibility is NO modulation of the functionality of opioid receptors as increased NO (through L-arginine loading) was found to elevate constitutive activation of the μ opioid receptor (Heinzen et al., 2005).
In summary, these findings indicate that N2O-induced antinociception in the mouse abdominal constriction test is dependent on both NO and opioid mechanisms that are co-localized in the PAG.
Acknowledgement
This research was supported in part by NIH Grant DA-10047 and by State of Washington Initiative Measure No. 171. A.D. was supported by a Summer Undergraduate Research Fellowship (SURF) from the American Society for Pharmacology and Experimental Therapeutics (ASPET) and the Washington State University College of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abacioglu N, Ozmen R, Cakici I, Tunctan B, Kanzik I. Role of L-arginine/nitric oxide pathway in the antinociceptive activities of morphine and mepyramine in mice. Arzneimittelforschung. 2001;51:977–983. doi: 10.1055/s-0031-1300148. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Ngai SH, Finck AD. Nitrous oxide “analgesia”: resemblance to opiate action. Science. 1976;194:967–968. doi: 10.1126/science.982058. [DOI] [PubMed] [Google Scholar]
- Branda EM, Ramza JT, Cahill FJ, Tseng LF, Quock RM. Role of brain dynorphin in nitrous oxide antinociception in mice. Pharmacol. Biochem. Behav. 2000;65:217–222. doi: 10.1016/s0091-3057(99)00202-6. [DOI] [PubMed] [Google Scholar]
- Cahill FJ, Ellenberger EA, Mueller JL, Tseng LF, Quock RM. Antagonism of nitrous oxide antinociception in mice by intrathecally administered opioid peptide antisera. J. Biomed. Sci. 2000;7:299–303. doi: 10.1007/BF02253248. [DOI] [PubMed] [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Nuite J. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J. Pharmacol. Exp. Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- Duarte ID, Ferreira SH. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992;221:171–174. doi: 10.1016/0014-2999(92)90789-7. [DOI] [PubMed] [Google Scholar]
- Fang F, Guo TZ, Davies MF, Maze M. Opiate receptors in the periaqueductal gray mediate analgesic effect of nitrous oxide in rats. Eur. J. Pharmacol. 1997;336:137–141. doi: 10.1016/s0014-2999(97)01219-3. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 2nd ed. Academic Press; San Diego: 2001. [Google Scholar]
- Fujinaga M, Maze M. Neurobiology of nitrous oxide-induced antinociceptive effects. Mol. Neurobiol. 2002;25:167–189. doi: 10.1385/MN:25:2:167. [DOI] [PubMed] [Google Scholar]
- Hara S, Kuhns ER, Ellenberger EA, Mueller JL, Shibuya T, Endo T, Quock RM. Involvement of nitric oxide in intracerebroventricular β-endorphin-induced neuronal release of methionine-enkephalin. Brain Res. 1995;675:190–194. doi: 10.1016/0006-8993(95)00065-x. [DOI] [PubMed] [Google Scholar]
- Heinzen EL, Booth RG, Pollack GM. Neuronal nitric oxide modulates morphine antinociceptive tolerance by enhancing constitutive activity of the mu-opioid receptor. Biochem. Pharmacol. 2005;69:679–688. doi: 10.1016/j.bcp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Heinzen EL, Pollack GM. The development of morphine antinociceptive tolerance in nitric oxide synthase-deficient mice. Biochem. Pharmacol. 2004;67:735–741. doi: 10.1016/j.bcp.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Henry ED, Ohgami Y, Li S, Chung E, Quock RM. Correlation of inbred mouse sensitivity to nitrous oxide antinociception with brain NOS activity following exposure to nitrous oxide. Pharmacol. Biochem. Behav. 2005;81:764–768. doi: 10.1016/j.pbb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Gagnon MJ, Gillespie TR, Breneisen JR, O'Leary DF, Hara S, Quock RM. Antagonism of nitrous oxide antinociception in the rat hot plate test by site-specific μ- and ε-opioid receptor blockade. J. Pharmacol. Exp. Ther. 1994;269:596–600. [PubMed] [Google Scholar]
- Ishikawa M, Quock RM. Role of nitric oxide synthase isoforms in nitrous oxide antinociception in mice. J. Pharmacol. Exp. Ther. 2003a;306:484–489. doi: 10.1124/jpet.103.049551. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Quock RM. N2O stimulates NOS enzyme activity in C57BL/6 but not DBA/2 mice. Brain Res. 2003b;976:262–263. doi: 10.1016/s0006-8993(03)02654-4. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974;185:1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc. Natl. Acad. Sci. USA. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bieber AJ, Quock RM. Antagonism of nitrous oxide antinociception in mice by antisense oligodeoxynucleotide directed against neuronal nitric oxide synthase enzyme. Behav. Brain Res. 2004;152:361–363. doi: 10.1016/j.bbr.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Li S, Ohgami Y, Dai Y, Quock RM. Antagonism of nitrous oxide-induced behavior in mice by pharmacologic disruption of endogenous nitric oxide function. Psychopharmacology. 2003;166:366–372. doi: 10.1007/s00213-002-1363-0. [DOI] [PubMed] [Google Scholar]
- Majeed NH, Przewlocka B, Machelska H, Przewlocki R. Inhibition of nitric oxide synthase attenuates the development of morphine tolerance and dependence in mice. Neuropharmacology. 1994;33:189–192. doi: 10.1016/0028-3908(94)90006-x. [DOI] [PubMed] [Google Scholar]
- McDonald CE, Gagnon MJ, Ellenberger EA, Hodges BL, Ream JK, Tousman SA, Quock RM. Inhibitors of nitric oxide synthesis antagonize nitrous oxide antinociception in mice and rats. J. Pharmacol. Exp. Ther. 1994;269:601–608. [PubMed] [Google Scholar]
- Ohgami Y, Chung E, Quock RM. Pharmacol. Soc. Ann. Mtg. Banff, Alberta; Canada: 2007. Exposure to nitrous oxide causes a nitric oxide-dependent release of β-endorphin in the rat arcuate nucleus. Abstracts of Banff 2007, West. p. 45. [Google Scholar]
- Ozek M, Uresin Y, Gungor M. Comparison of the effects of specific and nonspecific inhibition of nitric oxide synthase on morphine analgesia, tolerance and dependence in mice. Life Sci. 2003;72:1943–1951. doi: 10.1016/s0024-3205(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Pataki I, Telegdy G. Further evidence that nitric oxide modifies acute and chronic morphine actions in mice. Eur. J. Pharmacol. 1998;357:157–162. doi: 10.1016/s0014-2999(98)00561-5. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Laurito CE, VadeBoncouer TR. Nitric oxide synthase inhibition modulates the ventilatory depressant and antinociceptive actions of fourth ventricular infusions of morphine in the awake dog. Anesthesiology. 1996;85:1367–1377. doi: 10.1097/00000542-199612000-00018. [DOI] [PubMed] [Google Scholar]
- Quock RM, Graczak LM. Influence of narcotic antagonist drugs upon nitrous oxide analgesia in mice. Brain Res. 1988;440:35–41. doi: 10.1016/0006-8993(88)91156-0. [DOI] [PubMed] [Google Scholar]
- Sharpe LG, Garnett JE, Cicero TJ. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav. Biol. 1974;11:303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Xu JY, Tseng LF. Increase of nitric oxide by L-arginine potentiates β-endorphin- but not μ-, δ or κ-opioid agonist-induced antinociception in the mouse. Eur. J. Pharmacol. 1993;236:137–142. doi: 10.1016/0014-2999(93)90236-b. [DOI] [PubMed] [Google Scholar]
- Zuniga J, Joseph S, Knigge K. Nitrous oxide analgesia: partial antagonism by naloxone and total reversal after periaqueductal gray lesions in the rat. Eur. J. Pharmacol. 1987;142:51–60. doi: 10.1016/0014-2999(87)90653-4. [DOI] [PubMed] [Google Scholar]