Abstract
Biologics targeting TNF have brought about a paradigm shift in the treatment of rheumatoid arthritis (RA) and infliximab, anti-TNF-α chimeric monoclonal antibody, was marketed in 2003 in Japan. We previously reported on the RECONFIRM study, a retrospective clinical study on the efficacy of infliximab therapy in a RA management group in Japan, where we evaluated the clinical response after 22 weeks of the therapy in 258 patients. The study reported here was aimed at reconfirming the clinical efficacy of the infliximab therapy and demographic factors related to the efficacy over a 54-week study period in 410 RA patients in the same study group. Infliximab was infused according to the domestically approved method, and the clinical response was evaluated following 54 weeks of infliximab therapy using the European League Against Rheumatism (EULAR) response criteria. Disease activity was assessed by DAS28-CRP (Disease Activity Score including a 28-joint count/C-reactive protein). Infliximab was discontinued in 24.4% of the 410 patients at 54 weeks and 9.3% and 8.1% discontinued the therapy due to adverse events and inefficiency, respectively. Average DAS28-CRP decreased from 5.5 at week 0 to 3.1 at week 54 after the therapy. Patients in remission and those showing low-, moderate-, and high-disease activity changed from 0.0, 1.0, 9.0 and 90.0%, respectively, at the start of the study to 27.6, 11.7, 34.4 and 26.3%, respectively, at week 54. Younger age, RF-negativity and low scores of DAS28-CRP showed significant correlations with remission at week 54. EULAR response criteria—good, moderate, and no response to infliximab—were 37.0, 41.7 and 21.2%, respectively. In conclusion, we reconfirmed the clinical efficacy of infliximab and demographic factors related to the efficacy over a 54-week study period in 410 Japanese patients with RA using DAS28-CRP and EULAR response criteria.
Keywords: Rheumatoid arthritis, Infliximab, EULAR response, Retrospective study
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease that causes significant morbidity and mortality. RA patients should be started with DMARDs as early as possible, and among multiple DMARDs methotrexate (MTX) is considered the anchor drug and should be used first of all. However, even the use of MTX often fails to control disease activity and to prevent structural damage, and so more effective treatment strategies are needed. TNF-α plays a pivotal role in the pathological processes of RA through the accumulation of inflammatory cells and the self-perpetuation of inflammation, leading to cartilage and bone destruction. The combinational use of biologics targeting TNF-α and MTX has revolutionized the treatment of RA, producing significant improvements in clinical, radiographic, and functional outcomes that were not previously observed [1–5].
Infliximab, anti-TNF-α chimeric monoclonal antibody, has been marketed since July 2003 in Japan and currently provides high efficacy and potential adverse events. Although global evidence of the efficacy and safety of infliximab has accumulated, including the ATTRACT study, ASPIRE study and many others [6–10], there is no well-established firm evidence of the efficacy of this agent in Japan. Only the RECONFIRM study, a retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a RA management group in Japan, has reported clinical evidence on its efficacy and safety; this study was performed in several major rheumatology centers in Japan [11]. However, the RECONFIRM study evaluated the clinical response following only 22 weeks of infliximab therapy in 258 patients.
In this RECONFIRM-2 study, we assessed the clinical efficacy and safety of infliximab and MTX over a 54-week study period when used in 410 RA patients in the same group, in order to reconfirm not only its clinical efficacy but also demographic factors related to the efficacy.
Patients and methods
Data and information on RA patients that fulfilled the diagnostic criteria of the American College of Rheumatology (ACR) were collected from three major rheumatology centers in Japan, including the First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health Japan, Kitakyushu; the Division of Rheumatology and Clinical Immunology, Department of Internal Medicine, Saitama Medical Center, Saitama Medical University, Saitama; and the Institute of Rheumatology, Tokyo Women’s Medical University. All patients that received infliximab treatment in each institution by December 2005 were registered with this retrospective study. Demographic data, including disease duration and concomitant therapy, were collected from medical charts. The following parameters were evaluated before and at 54 weeks after the initial infliximab infusion: tender joint count (TJC) 28, swollen joint count (SJC) 28, patient’s assessment of pain on a visual analog scale (patient’s pain VAS), patient’s global assessment of disease activity (patient’s global VAS), physician’s global assessment of disease activity (physician’s global VAS), and C-reactive protein (CRP).
Infliximab therapy
Infliximab was infused to patients at zero, two, and six weeks and thereafter every eight weeks at a dose of 3 mg/kg according to the drug labeling and the guidelines of the Infliximab Study Group in the Ministry of Health, Welfare and Labor in Japan [12]. Concomitant use of MTX was instituted in all cases, although the dose of MTX was determined by each attending physician.
Therapeutic response
Disease activity was assessed by Disease Activity Score, including a 28-joint count (DAS28)-CRP that was calculated according to the authorized formula (http://www.das-score.nl/). The value of DAS28-CRP is reported to be less than the original DAS28 using the erythrocyte sedimentation rate (ESR), and we used a threshold of 4.1 instead of the original 5.1 as the cut-off for high activity and 2.7 instead of 3.2 as the cut-off for low activity. Thus, we defined a value of DAS28-CRP >4.1 as high activity, 2.7–4.1 as moderate activity, <2.7 as low activity, with <2.3 being defined as remission [9]. The response to infliximab therapy at 22 weeks was evaluated by the European League Against Arthritis (EULAR) response criteria using 4.1 and 2.7 as the thresholds for the high and low disease activities, respectively [13]. Secondary insufficiency to infliximab was defined as patients with good or moderate EULAR response at week 22, and whose DAS28 increased to >0.6 or >1.2 from week 22 to 54.
Discontinued subjects
The continuation rate of infliximab therapy was calculated by all causes of discontinuation. Cumulative hazards and associated factors were analyzed in relation to three types of discontinuation: adverse events, inefficacy, and remission.
Statistical analysis
Baseline characteristics of patients are summarized in Table 1 using the mean, standard deviation, median, 25%, and 75% values for continuous variables. The continuation rate of infliximab therapy was calculated using the Kaplan–Meier estimator in pooled samples of three institutes. The discontinuation-cause specific hazards were estimated using the Nelson–Aalen estimator. Next, risk factors for the discontinuations were evaluated using the stratified proportional hazards model in order to adjust for differences among institutions. The clinical efficacies of infliximab were evaluated using a multivariate logistic regression to adjust for institutional effects. In this analysis, the last observed DAS28-CRP values were used for discontinued patients. All multivariate analyses were conducted using the variables gender, age, duration of disease, positive/negative rheumatoid factor (RF), concomitant MTX dose, concomitant prednisolone (PSL) dose, and DAS28-CRP at baseline. All reported P-values are two-sided and are not adjusted for multiple testing. The significance level was set at a P value of <0.05.
Table 1.
Baseline characteristics of patients in three institutions of rheumatology in Japan
| Mean | SD | Min | 25% | Median | 75% | Max | |
|---|---|---|---|---|---|---|---|
| Female (%) | 87.6 | – | – | – | – | – | – |
| Age | 53.1 | 12.7 | 19 | 46 | 55 | 62 | 80 |
| Duration | 9.4 | 8.8 | 0 | 3 | 6.6 | 13 | 54 |
| Stage | 3.0 | 1.0 | 1 | 2 | 3 | 4 | 4 |
| Class | 2.2 | 0.5 | 1 | 2 | 2 | 2 | 4 |
| RF positive (%) | 87.6 | – | – | – | – | – | – |
| RF (titer) | 213 | 331 | 1 | 39 | 96 | 241 | 2980 |
| MTX dose | 7.8 | 2.0 | 0 | 6 | 8 | 8 | 20 |
| PSL dose | 3.8 | 3.7 | 0 | 0 | 4 | 5 | 22.5 |
| CRP | 3.3 | 2.8 | 0 | 1.18 | 2.7 | 4.7 | 13.7 |
| TJC28 | 10.5 | 7.3 | 0 | 5 | 9 | 15 | 28 |
| SJC28 | 10.6 | 6.1 | 0 | 6 | 10 | 14 | 28 |
| GH | 63.1 | 21.9 | 0 | 49.3 | 66 | 80 | 100 |
| DAS28-CRP | 5.5 | 1.1 | 1.9 | 4.8 | 5.6 | 6.3 | 8.0 |
MTX methotrexate, PSL prednisolone, RF rheumatoid factor, CRP C-reactive protein, GH general health, TJC tender joint count, SJC swollen joint count, DAS disease activity score
Results
Baseline demographic and clinical characteristics
The baseline demographic and clinical characteristics of 410 patients receiving infliximab therapy are summarized in Table 1. Patients had active disease at baseline as evidenced by high mean counts of tender and swollen joints, CRP levels, and the DAS28. Age, sex, and disease duration were similar among these three institutes, while the %user and dose of MTX or PSL were divergent and the disease activities as assessed by TJC, SJC, GH, and DAS28 was also different.
Continuation of infliximab therapy
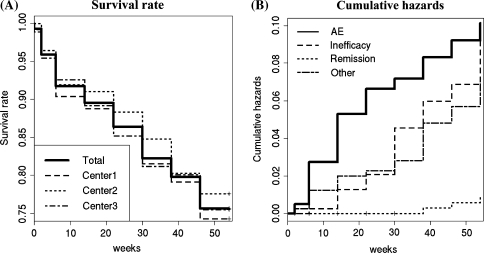
Infliximab was discontinued in 100 cases (24.4%) among 410 patients during a 54-week period and the survival rate for infliximab use was comparable among three institutes by week 54 after the treatment according to Kaplan–Meier analysis (Fig. 1a). Cumulative hazards of the discontinuation during the 54-week infliximab therapy were different among the causes of discontinuation; discontinuation due to adverse events, inefficiency, remission and other causes (such as change of hospitals/clinics and economic reasons), were 0.093, 0.081, 0.007 and 0.063, respectively (Fig. 1b). Although the cause of the discontinuation was similar among three institutes, adverse events were higher in Center 1 than in the other two institutes, and remission and other causes including economic problems of the patient were greater in Center 3 than in the other two (data not shown). In 100 patients who terminated infliximab treatment, stratified Cox regression was performed to analyze factors associated with the discontinuation of the infusion. Male, older age and RF-negativity were significantly associated with the discontinuation of infliximab due to adverse reactions, whereas there was no significant factor responsible for the discontinuation due to maintained remission or a lack of efficacy (Table 2).
Fig. 1.
Continuation of the infliximab therapy in RA patients for 54 weeks. a Survival rate of RA patients treated with infliximab (n = 410, total and three institutes) during the 54-week therapy. b Cumulative hazards of the discontinuation of infliximab therapy by week 54 of the treatment
Table 2.
Results from a Cox regression analysis performed to examine the factors related to the discontinuation of infliximab therapy
| Variable | AE (n = 38) | Inefficacy (n = 33) | Remission (n = 3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | HR | CL | CU | P | Coef. | HR | CL | CU | P | Coef. | HR | CL | CU | P | |
| Age | 0.037 | 1.038 | 1.006 | 1.070 | 0.020 | −0.014 | 0.987 | 0.957 | 1.017 | 0.38 | −0.021 | 0.980 | 0.888 | 1.081 | 0.68 |
| Gender | −0.940 | 0.391 | 0.177 | 0.863 | 0.020 | 0.160 | 1.173 | 0.352 | 3.910 | 0.79 | – | – | – | – | – |
| RA duration | 0.018 | 1.018 | 0.983 | 1.054 | 0.320 | 0.005 | 1.005 | 0.964 | 1.046 | 0.83 | −0.114 | 0.893 | 0.622 | 1.280 | 0.54 |
| RF (±) | −0.949 | 0.387 | 0.171 | 0.878 | 0.023 | 1.545 | 4.686 | 0.635 | 34.573 | 0.13 | −1.150 | 0.317 | 0.023 | 4.291 | 0.39 |
| MTX dose | 0.096 | 1.101 | 0.920 | 1.318 | 0.290 | −0.142 | 0.867 | 0.709 | 1.060 | 0.16 | 0.339 | 1.403 | 0.799 | 2.465 | 0.24 |
| PSL dose | −0.066 | 0.936 | 0.848 | 1.033 | 0.190 | 0.003 | 1.003 | 0.908 | 1.109 | 0.95 | −0.636 | 0.529 | 0.187 | 1.500 | 0.23 |
| DAS (0 week) | 0.205 | 1.227 | 0.890 | 1.692 | 0.210 | 0.113 | 1.120 | 0.815 | 1.537 | 0.49 | −0.474 | 0.623 | 0.164 | 2.367 | 0.49 |
Coef. coefficient, HR hazard ratio, CL 95% confidence lower limit of HR, CU 95% confidence upper limit of HR
Efficacy of infliximab therapy
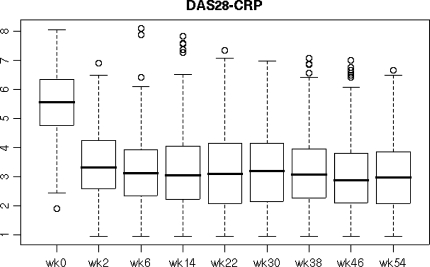
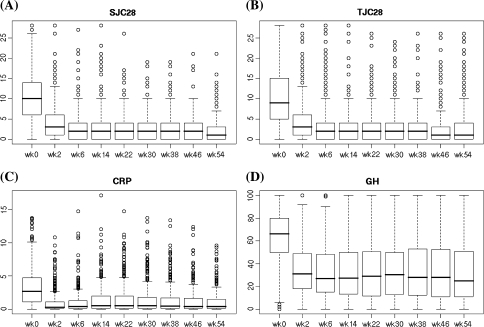
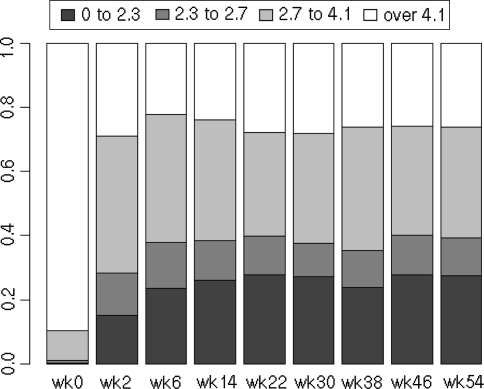
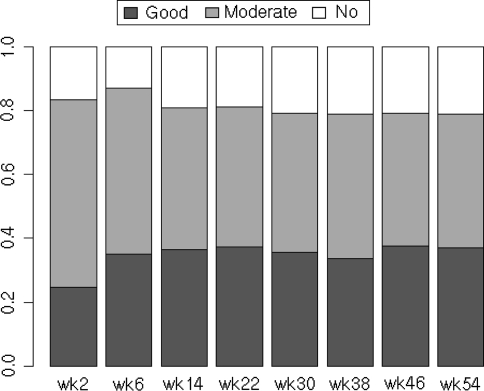
The average DAS28-CRP before starting infliximab was 5.5 ± 1.1, and this decreased to 3.4 ± 1.2, 3.2 ± 1.4, 3.1 ± 1.3 at weeks 2, 22 and 54, respectively, after the infliximab therapy (Fig. 2). Disease characteristics at baseline and after 2, 22 and 54 weeks of the inflilximab therapy were as follows: tender joints count (10.5, 4.2, 3.2 and 2.9), swollen joints count (10.6, 4.2, 2.7 and 2.3), GH (63, 34, 33 and 33 mm), and CRP (3.3, 1.0, 1.5 and 1.1 mg/dl) (Fig. 3). Before starting infliximab, the proportions of patients showing low, moderate, and high disease activity were 1.0, 9.0 and 90.0%, respectively. At week 22, patients in remission (defined as DAS28-CRP <2.3) and those showing low (<2.7), moderate (2.7–4.1), and high disease activity (>4.1) had changed to 27.8, 12.0, 32.4 and 27.8%, respectively, and at week 54 patients in remission and those showing low, moderate, and high disease activity were 27.6, 11.7, 34.4 and 26.3%, respectively (Fig. 4). Thus, approximately 27–28% of the patients satisfied the remission criteria at week 22 and still remained at week 54 after the infliximab therapy. Also, when the responses were evaluated by the EULAR response criteria, the proportions for good, moderate, and no response to infliximab as measured by DAS28-CRP were 37.3, 43.7 and 19.0%, respectively, at week 22 and 37.0, 41.7 and 21.2%, respectively, at week 54 (Fig. 5).
Fig. 2.
Longitudinal analysis of DAS28 values during the 54-week study of patients using infliximab. Line in the box represents the median and the upper and lower ends of the box show the 25th and 75th percentiles of the population
Fig. 3.
Longitudinal analysis of a SJC28, b TJC28, c CRP, and d GH values during the 54-week study of patients using infliximab. Line in the box represents the median, and the upper and lower ends of the box show the 25th and 75th percentiles of the population
Fig. 4.
Changes in DAS28 values during the 54-week study of patients using infliximab. The ratios of patients who demonstrated high disease activity (defined as DAS28-CRP >4.1), moderate activity (2.7–4.1), low activity (<2.7) and remission (<2.3) at each observation point during the 54-week study are shown
Fig. 5.
The response to infliximab therapy during the 54-week study. The ratios of patients whose responses were evaluated by the European League Against Arthritis (EULAR) response criteria are shown
Demographic factors related to the clinical efficacy of infliximab therapy
In order to clarify demographic factors related to the clinical efficacy of infliximab therapy, we performed a multivariate analysis adjust for institutional differences. Younger age, RF-negativity and lower levels of DAS28-CRP were significantly associated with the clinical remission induced by infliximab therapy at week 54, whereas gender, duration of the disease, dose of MTX and dose of PSL did not show a significant association (Table 3). On the other hand, most of the demographic factors, except for older age, did not affect secondary insufficiency from week 22 to 54 after the infliximab therapy according to the logistic regression analysis (Table 4).
Table 3.
Results from the logistic regression analysis used to examine the factors related to clinical remission at weeks 22 and 54 induced by infliximab therapy
| Variables | Week 22 (n = 113) | Week 54 (n = 112) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | OR | SE | CL | CU | P | Coef. | OR | SE | CL | CU | P | |
| Intercept | 1.530 | 4.617 | 1.034 | 0.608 | 35.067 | 0.139 | 1.770 | 5.868 | 1.010 | 0.811 | 42.445 | 0.080 |
| Center 2 vs. Center 1 | 0.174 | 1.190 | 0.359 | 0.588 | 2.405 | 0.629 | 0.564 | 1.758 | 0.350 | 0.886 | 3.488 | 0.107 |
| Center 3 vs. Center 1 | 1.072 | 2.922 | 0.324 | 1.549 | 5.510 | 0.001 | 0.989 | 2.689 | 0.325 | 1.423 | 5.082 | 0.002 |
| Age | −0.014 | 0.986 | 0.010 | 0.967 | 1.006 | 0.168 | −0.024 | 0.976 | 0.010 | 0.957 | 0.995 | 0.014 |
| Gender | 0.563 | 1.755 | 0.392 | 0.813 | 3.787 | 0.152 | 0.154 | 1.166 | 0.370 | 0.565 | 2.409 | 0.677 |
| RA duration | −0.007 | 0.993 | 0.015 | 0.964 | 1.023 | 0.640 | 0.010 | 1.010 | 0.014 | 0.982 | 1.039 | 0.491 |
| RF (±) | −0.413 | 0.662 | 0.339 | 0.340 | 1.287 | 0.224 | −0.742 | 0.476 | 0.329 | 0.250 | 0.908 | 0.024 |
| MTX dose | 0.042 | 1.043 | 0.068 | 0.913 | 1.193 | 0.535 | 0.005 | 1.005 | 0.066 | 0.884 | 1.143 | 0.936 |
| PSL dose | −0.063 | 0.939 | 0.035 | 0.876 | 1.005 | 0.070 | −0.058 | 0.944 | 0.034 | 0.882 | 1.009 | 0.092 |
| DAS28-CRP | −0.464 | 0.629 | 0.116 | 0.501 | 0.789 | 0.000 | −0.276 | 0.759 | 0.112 | 0.609 | 0.945 | 0.014 |
Coef. coefficient HR, OR odds ratio, SE standard error, CL 95% confidence lower limit of OR, CU 95% confidence upper limit of OR
Table 4.
Results from the logistic regression analysis used to examine the factors related to the secondary inefficiency from week 22 to 54 during infliximab therapy
| Variables | Week 54–22 >0.6 (n = 85) | Week 54–22 >1.2 (n = 47) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | OR | SE | CL | CU | P | Coef. | OR | SE | CL | CU | P | |
| (Intercept) | −1.461 | 0.232 | 1.079 | 0.028 | 1.923 | 0.176 | −4.331 | 0.013 | 1.439 | 0.001 | 0.221 | 0.003 |
| Center 2 vs. Center 1 | −0.008 | 0.992 | 0.350 | 0.499 | 1.970 | 0.981 | 0.074 | 1.077 | 0.440 | 0.455 | 2.549 | 0.866 |
| Center 3 vs. Center 1 | 0.071 | 1.073 | 0.333 | 0.559 | 2.060 | 0.831 | 0.054 | 1.056 | 0.426 | 0.458 | 2.433 | 0.899 |
| Age | 0.019 | 1.019 | 0.011 | 0.998 | 1.042 | 0.079 | 0.032 | 1.032 | 0.014 | 1.004 | 1.062 | 0.028 |
| Gender | −0.283 | 0.753 | 0.358 | 0.373 | 1.520 | 0.429 | 0.250 | 1.284 | 0.511 | 0.471 | 3.500 | 0.625 |
| RA duration | −0.029 | 0.972 | 0.017 | 0.941 | 1.004 | 0.083 | −0.020 | 0.980 | 0.020 | 0.942 | 1.019 | 0.313 |
| RF (±) | −0.233 | 0.792 | 0.366 | 0.387 | 1.622 | 0.524 | 0.164 | 1.178 | 0.512 | 0.432 | 3.212 | 0.749 |
| MTX dose | −0.001 | 0.999 | 0.069 | 0.873 | 1.144 | 0.990 | 0.039 | 1.040 | 0.086 | 0.879 | 1.230 | 0.649 |
| PSL dose | −0.015 | 0.985 | 0.036 | 0.918 | 1.057 | 0.682 | 0.019 | 1.019 | 0.045 | 0.934 | 1.112 | 0.676 |
| DAS28-CRP (0 week) | −0.033 | 0.967 | 0.118 | 0.767 | 1.219 | 0.779 | −0.011 | 0.989 | 0.148 | 0.739 | 1.323 | 0.941 |
Coef. coefficient HR, OR odds ratio, SE standard error, CL 95% confidence lower limit of OR, CU 95% confidence upper limit of OR
Discussion
The RECONFIRM-2 study was designed to fully evaluate the effect of infliximab used in combination with MTX on the clinical results during a 54-week study period in DMARD-resistant RA patients of a RA management group in Japan. The safety profile of infliximab therapy for a total of 5,000 cases was investigated in Japan using an all-case registered post-marketing surveillance system, and the entire profile of adverse events related to infliximab therapy was clearly identified [14]. The assessment of efficacy, however, was based only on the physician’s general evaluation and not on quantitative measures such as EULAR criteria. In this report, therefore, efficacy data based on DAS28-CRP and EULAR improvement criteria were intensively assessed. The average DAS28-CRP before starting infliximab was 5.5, it decreased to 3.2 at week 22 and it remained steady at 3.1 by week 54 after the infliximab therapy. At week 22 after the therapy, about 28% and 40% of patients satisfied the remission and low disease activity criteria, respectively, and these effects remained (28 and 39%, respectively) by week 54. Also, about 37% of the patients exhibited good response according to EULAR criteria continuously from week 22 to 54. These results reconfirmed that the clinical efficacy of infliximab at week 22 was maintained until week 54 in most of the patients treated with infliximab. Thus, appropriate treatment with infliximab and MTX in this study group could minimize secondary insufficiency of the therapy for at least one year.
On the other hand, the discontinuation of infliximab was observed in approximately 24% of the patients during the 54-week period. This rate of discontinuation of the therapy was comparable to that obtained in other countries: 26.7% in the Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy (ATTRACT) international study carried out in the USA and the EU, 34.6% in a German domestic study and 26% in a French group study during the first one-year period [7, 15, 16]. Cumulative hazards differed among causes of discontinuation; discontinuations due to adverse events, inefficiency, remission and other causes (including changes of hospital/clinic and economic problems) were 0.093, 0.081, 0.007 and 0.063, respectively. Male gender, older age and RF-negativity were significantly correlated with discontinuation of infliximab due to adverse reactions, including infusion reactions (N = 7), toxicoderma (4), bacterial pneumonia (3), pneumocystis jirovecii pneumonia (3), but more than two-thirds of the withdrawals due to adverse events were observed within the first 14 weeks after the therapy.
In this study, to clarify how predisposing factors from the demographic characteristics of RA patients were related to the clinical efficacy of infliximab therapy, a multivariate analysis using a logistic regression was performed. In this study, multiple variables including sex, age, duration of disease, stage, class, positive/negative RF, concomitant MTX dose, concomitant PSL dose, and initial levels of CRP, TJC, SJC and GH were assessed. It is worth noting that, among multiple variables, younger age, RF-negativity and lower levels of DAS28-CRP at the baseline were significantly correlated with the clinical remission induced by infliximab therapy at week 54. These results imply that the timely use of MTX and infliximab can be strongly recommended for younger RA patients that show RF-negativity in order to efficiently achieve clinical remission. On the other hand, male gender, older age and RF-negativity were significantly correlated with discontinuation of infliximab due to adverse reactions, whereas there was no significant factor responsible for discontinuation as a result of inefficacy, and only older age affected secondary insufficiency from week 22 to 54 after the infliximab therapy. Although it is intriguing that male gender predisposes for discontinuation as a result of adverse events, these results provide the first information that can be used to facilitate the more efficacious use of infliximab and MTX in the daily practice of rheumatologists.
Taken together, this REOCNFIRM-2 study reconfirms the clinical efficacy of treatment with infliximab and MTX in Japanese RA patients using the DAS28-CRP and EULAR response criteria. Among 410 patients with active RA, approximately 28% and 39% of the patients satisfied the remission and low disease activity criteria, and good response according to the EULAR criteria was achieved in 37% of the patients treated with infliximab plus MTX during the 54-week study period. The clinical efficacy of infliximab was maintained from week 22 to 54 after the treatment, and secondary insufficiency after week 22 was marginal after the appropriate treatment in this study group. Several demographic factors, including male gender, RF-negativity and lower scores of DAS28-CRP, were significant predisposing factors for remission. The promising effectiveness of infliximab at improving measures of disease activity in RA patients has led to this therapy becoming one of the key advances in the management of RA. Thus, this study is important because it provides obvious and invaluable evidence concerning the efficacy of the combinational use of infliximab and MTX, and can guide the real clinical use of infliximab in the future.
Acknowledgments
The authors thank all medical staff in the three institutions for providing the data. This work was supported in part by a Research Grant-In-Aid for Scientific Research by the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the University of Occupational and Environmental Health, Japan.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. [DOI] [PubMed]
- 2.Weaver AL. Efficacy and safety of the anti-TNF biologic agents. Mod Rheumatol. 2004;14:101–12. [DOI] [PubMed]
- 3.American College of Rheumatology Subcommittee on Rheumatoid Arthritis. Guidelines for the management of rheumatoid arthritis, 2002 update. Arthritis Rheum 2002;46:328–46. [DOI] [PubMed]
- 4.Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:34–45. [DOI] [PMC free article] [PubMed]
- 5.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet 2007;370(9602):1861–74. [DOI] [PubMed]
- 6.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumor necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–9. [DOI] [PubMed]
- 7.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594–602. [DOI] [PubMed]
- 8.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. [DOI] [PubMed]
- 9.Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement—a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52:1020–30. [DOI] [PubMed]
- 10.Smolen JS, van Der Heijde DM, St Clair EW, et al. Active-controlled study of patients receiving infliximab for the treatment of rheumatoid arthritis of early onset (ASPIRE) study group. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10. [DOI] [PubMed]
- 11.Yamanaka H, Tanaka Y, Sekiguchi N, Inoue E, Saito K, Kameda H, Iikuni N, Nawata M, Amano K, Shinozaki M, Takeuchi T. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007;17:28–32. [DOI] [PubMed]
- 12.Miyasaka N, Takeuchi T, Eguchi K. Offi cial Japanese Guidelines for the use of infl iximab for rheumatoid arthritis. Modern Rheum. 2005;15:4–8. [DOI] [PubMed]
- 13.Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of DAS28-ESR and DAS28-CRP on threshold values. Ann Rheum Dis. 2007;66:407–9. [DOI] [PMC free article] [PubMed]
- 14.Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Post-marketing surveillance of the safety profile of infliximab in 5,000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008;67(2):189–94. [DOI] [PubMed]
- 15.Zink A, Listing J, Kary S, Ramlau P, Stoyanova-Scholz M, Babinsky K, von Hinueber U, Gromnica-Ihle E, Wassenberg S, Antoni C, Herzer P, Kekow J, Schneider M, Rau R. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis. 2005;64:1274–9. [DOI] [PMC free article] [PubMed]
- 16.Wendling D, Materne GE, Michel F, Lohse A, Lehuede G, Toussirot E, Massol J, Woronoff-Lemsi MC. Infliximab continuation rates in patients with rheumatoid arthritis in everyday practice. Joint Bone Spine. 2005;72:309–12. [DOI] [PubMed]