Abstract
Neural function is dependent upon the proper formation and development of synapses. We show here that Wnt5 regulates the growth of the Drosophila neuromuscular junction (NMJ) by signaling through the Derailed receptor. Mutations in both wnt5 and drl result in a significant reduction in the number of synaptic boutons. Cell-type specific rescue experiments show that wnt5 functions in the presynaptic motor neuron while drl likely functions in the postsynaptic muscle cell. Epistatic analyses indicate that drl acts downstream of wnt5 to promote synaptic growth. Structure–function analyses of the Drl protein indicate that normal synaptic growth requires the extracellular Wnt inhibitory factor domain and the intracellular domain, which includes an atypical kinase. Our findings reveal a novel signaling mechanism that regulates morphology of the Drosophila NMJ.
Keywords: Wnt, Drl, Ryk, NMJ, synapse, boutons
Introduction
The development and maintenance of synapses is of critical importance for nervous system development and higher order processes such as learning and memory. Once established, synapses undergo continuous remodeling in response to genetic and environmental cues. The molecular machinery that regulates synaptic growth and synaptic remodeling is not well understood.
The Drosophila larval neuromuscular junction (NMJ) provides an excellent system for dissecting the molecular basis of synapse formation, growth, and remodeling. These synapses are similar to mammalian central synapses in that they are glutamatergic and remodel in response to activity (Gramates and Budnik, 1999; Collins and Diantonio, in press). The terminals of the presynaptic motor neuron arrive at its target muscle midway through embryogenesis. After the initial contact, the motor neuron growth cone collapses and forms nascent presynaptic processes, termed prevaricosities. Seventeen to 22 h after egg laying (AEL), sections of the prevaricosities begin to swell and/or constrict such that presynaptic terminals (known as synaptic boutons) are formed (Johansen et al., 1989; Halpern et al., 1991; Sink and Whitington, 1991; Yoshihara et al., 1997; Rose and Chiba, 1999). During larval development (24–120 h AEL), the presynaptic arborization grows dramatically to accommodate the rapidly growing larval muscles. This growth includes increasing the number of boutons, branches, and active zones per bouton (Schuster et al., 1996a,b; Zito et al., 1999). The coordinated growth of the presynaptic motor neuron and postsynaptic muscle requires transsynaptic signaling between cells (for reviews see Koh et al., 2000; Budnik and Ruiz-Canada, 2006).
A number of molecular regulators of synaptic growth and plasticity have been identified. Mutations in ion channels proteins (Cacophony, GluRIIA, and Shaker), cytoplasmic signaling proteins (Nervous Wreck), or a cell adhesion molecule (FasII) lead to perturbations in bouton numbers (Budnik et al., 1990; Schuster et al., 1996a,b; Rieckhof et al., 2003; Schmid et al., 2006). Of particular interest are the discoveries that the secreted signaling proteins, Wingless (Wg) and Glass Bottom Boat (Gbb), reduces the size of the NMJ with mutations in Wg, reducing the number of synaptic boutons by ∼25% (Packard et al., 2002; McCabe et al., 2003). Wg and Gbb are evolutionary conserved proteins involved in a number of developmental processes including cell fate specification, axis patterning, and recently, neural development (for reviews see Logan and Nusse, 2004; Zou, 2004; Ille and Sommer, 2005; Marques, 2005).
We now report a novel anterograde signaling pathway that regulates the development of the Drosophila NMJ. We show that Wnt5 regulates growth of the NMJ by signaling through Derailed (Drl), a transmembrane receptor of the atypical receptor tyrosine kinase (RYK) family. Mutations in either wnt5 or drl result in a significant reduction in the number of synaptic boutons. Epistatic analyses indicate that wnt5, which is expressed by the presynaptic motor neuron, signals through drl, which is expressed by the postsynaptic muscle, to regulate synaptic growth. Our study is the first to demonstrate that a RYK protein regulates synapse development.
Methods
Antibodies and Immunocytochemistry
For staining and microscopy, animals were dissected and fixed for 30–60 min in either Bouin's fixative (when GluRIIA or nc82 antibodies were used) or 4% paraformaldehyde (for all other staining). First and second instar larvae were dissected and fillet preparations were glued down using Sylgard-coated coverslips. Third instar larvae were dissected and fillet preparations were pinned down in Sylgard-lined Petri dishes. All dissections were done in Drosophila standard saline (135 mM NaCl, 5 mM KCl, 4 mM MgCl, 1.8 mM CaCl, 5 mM TES, 72 mM sucrose) with 2 mM glutamate to preserve neuronal morphology (Augustin et al., 2007) at RT. Mouse monoclonal anti-GluRIIA and nc82 (Iowa Developmental Studies Hybridoma Bank, Iowa City, IA) were used at 1:100 and 1:50, respectively. Rabbit polyclonal anti-Drl and anti-Wnt5 (Fradkin et al., 2004) were used at 1:100. Fluorescently conjugated anti-HRP (Jackson Immunoresearch Labs, West Grove, PA) was used at 1:100. Goat anti-rabbit or goat anti-mouse fluorescent (FITC or TRITC) secondary antibodies (Jackson Immunoresearch Labs) were used at 1:400. The 6/7 NMJ of abdominal hemisegments A3 or A4 were used for all studies. Confocal images were obtained using a Zeiss LSM 510 laser-scanning confocal microscope. Image analysis and quantification was performed using ImageJ and Adobe Photoshop software.
The anti-Drl antiserum was raised in rabbits against a GST fusion protein, including Drl amino acids 123–222, and was affinity-purified against the same protein coupled to a column.
Molecular Biology
Full-length drl cDNA was obtained from Open Biosystems (Huntville, AL) and subcloned into the pUAST vector to generate the UAS-drl transgene. To generate the UAS-drlΔWIF construct, nucleotides 472–1830 were amplified by PCR from drl cDNA and ligated to the first 60 nucleotides of drl cDNA. This construct was then subcloned into pUAST. All constructs were subcloned into pUAST using the EcoRI and XbaI sites. The sequence was verified for all constructs. Fly transformation was performed by DNA microinjection of embryos using standard methods. To verify transcription of these constructs, total RNA was isolated using trizol extraction, and reverse-transcribed using cDNA-specific primers.
Electrophysiology
All electrophysiology was performed on the ventral body wall muscle 6. Larval recordings were performed on third instar larvae 110–120 h AEL. Muscle 6 was voltage-clamped at −60 mV. Standard two-electrode voltage clamp techniques were used, as previously described (Liebl et al., 2005). Data were acquired and analyzed using an Axopatch amplifier and pClamp9 (Axon Instruments, Union City, CA). All dissections and recordings were done in standard Drosophila saline at 19°C.
Data Acquisition and Statistics
All animals were raised at 25°C in standard fly vials with corn meal molasses medium. The total number of boutons was acquired from 6/7 NMJs of hemisegments A3 or A4. The density of nc82 labeling was quantified by counting the total number of nc82 puncta and dividing by the total NMJ area using ImageJ (NIH) software. Statistics were performed using GraphPad Prism (v. 4.01). All statistical comparisons were made using unpaired students t-tests. Control animals used were WT-Berlin, elav-Gal4, 24B-Gal4, Actin-Gal4, UAS-wnt5, and UAS-drl. There was no significant difference in bouton numbers between groups (WT-Berlin = 56.36 ± 3.609 boutons, n = 11; elav-Gal4 = 57.50 ± 4.586 boutons, n = 8, p = 0.8459; 24B-Gal4 = 52.11 ± 3.080 boutons, n = 9, p = 0.4157; actin-Gal4 = 57.38 ± 4.496 boutons, n = 8, p = 0.8614; UAS-wnt5 = 55.11 ± 3.438 boutons, n = 9, p = 0.8263; UAS-drl = 55.20 ± 3.286 boutons, n = 10, p = 0.8178). Therefore, the data were combined into one control group. Statistical significance in figures is represented as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. All error bars represent SEM.
Results
Wnt5 and Drl Are Expressed at the 6/7 NMJ
We first investigated whether Drl and Wnt5 were expressed at the NMJ using immunolocalization. Drl [Fig. 1(B,D)] and Wnt5 [Fig. 1(A,C)] are each localized to the area of boutons in third instar larvae (Fig. 1). Wnt5 and Drl antibody immunoreactivity was almost undetectable in homozygous wnt5400 null mutants and dramatically reduced in drl2 null mutants (Fig. 1, right panels), indicating that these antibodies are specific. We were unable to distinguish whether the Wnt5 and Drl immunoreactivity were pre- or postsynaptic by colocalization experiments with the presynaptic marker CSP and the postsynaptic marker DLG (data not shown). The pre- and postsynaptic membranes of the NMJ are closely apposed (Atwood et al., 1993; Prokop et al., 1996) making it difficult to determine the cellular locations of proteins using confocal microscopy. The Drl labeling is consistent with previous reports, which showed that Drl is expressed in subsets of CNS axons (Yoshikawa et al., 2003) and ventral body wall muscles (Callahan et al., 1996). Wnt5 is expressed in posterior commissural axons of the CNS (Fradkin et al., 2004) but has not been localized to the NMJ. Our immunlocalization experiments suggest that Drl and Wnt5 may function at the NMJ.
Figure 1.

Wnt5 and Drl are localized to the NMJ. (A) Confocal fluorescent images showing NMJs on muscles 6 and 7 in third instar larvae. Animals were labeled with antibodies against HRP (magenta), which recognizes presynaptic membranes and Wnt5 (green). (B) Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta) and Drl (green). Scale bar: 20 μm. (C) High magnification view of an area from A. (D) High magnification view of an area from B. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Wnt5 Is a Positive Regulator of NMJ Size and Active Zone Formation
To investigate the role of wnt5 at the NMJ, we examined the NMJs of the null mutant, wnt5400. Homozygous wnt5400 mutants are adult-viable as previously described (Fradkin et al., 2004). Mutant larvae exhibit no visible defects in muscle patterning or muscle size (muscle 6: WT = 26,490 ± 1526 μm2, n = 6; wnt5400 = 26,430 ± 1077 μm2, n = 7, p = 0.9750; muscle 7: WT = 20,020 ± 764.6 μm2, n = 6; wnt5400 = 20,370 ± 1313 μm2, n = 7, p = 0.8214). To examine NMJ morphology, we visualized presynaptic motor neurons with HRP to label the neuronal membranes. Our analysis revealed that wnt5 mutants have significantly fewer boutons compared with control animals [Fig. 2(A,C), control = 55.85 ± 3.059 boutons, n = 13; wnt5400 = 45.36 ± 3.459 boutons, n = 14, p = 0.0329]. This reduction correlated with a reduction in NMJ size (WT = 526.2 ± 42.66 μm2, n = 10; wnt5400 = 382.0 ± 37.80 μm2, n = 10, p = 0.0209). There was no significant difference in the number of branches at the 6/7 NMJ (control = 4.938 ± 0.4784 branches, n = 16; wnt5400 = 4.688 ± 0.6240 branches, n = 16, p = 0.7527).
Figure 2.

wnt5 mutant animals exhibit a reduction in the number of boutons and active zones. (A) Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta) and the presynaptic protein Brp (nc82, green). Scale bar: 20 μm. (B) Magnification of a portion of the NMJ shown in A. (C) Quantification of active zones density (nc82 puncta/μm2, left) and bouton number (per NMJ, right). (D) Representative two-electrode voltage clamp recordings from muscle 6 of control and wnt5400 mutant third instar larvae, showing evoked endplate junctional currents (EJCs). (Right) Quantification of EJC amplitudes. (E) Representative voltage clamp recordings from control and wnt5400 third instar larvae, showing spontaneous excitatory junctional currents (mEJCs) in the muscle 6 NMJ. Quantification of mEJC frequency (middle) and amplitude (right). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We examined the synaptic expression and localization of the presynaptic protein, Bruchpilot/nc82 (Brp), and the postsynaptic glutamate receptor subunit, GluRIIA, by immunolabeling. Expression of Brp, which promotes the assembly of presynaptic active zones (Kittel et al., 2006; Wagh et al., 2006), is significantly reduced in wnt5 mutants compared with controls [Fig. 2(B,C); WT = 0.5585 ± 0.03462 puncta/μm2, n = 10; wnt5400 = 0.3904 ± 0.02506 puncta/μm2, n = 7, p = 0.0026]. The expression and localization of GluRIIA appeared normal in wnt5 mutant animals (data not shown, normalized fluorescence: WT = 1.000 ± 0.1006, n = 11; wnt5400 = 0.8977 ± 0.06632, n = 9, p = 0.4288). We placed wnt5400 in trans with the deficiency, Df (1) N19, which uncovers the wnt5 gene to exclude the possibility that the NMJ defects are caused by extraneous mutations in the wnt5 mutant background. wnt5400/Df (1) N19 animals exhibited a significant reduction in both the number of boutons and density of Brp (boutons: WT = 55.85 ± 3.059, n = 13; wnt5400/Df (1) N19 = 42.50 ± 2.141, n = 10, p = 0.003; Brp: WT = 0.5585 ± 0.0346 puncta/μm2, n = 10; wnt5400/Df (1) N19 = 0.3766 ± 0.0246 puncta/μm2, n = 9, p = 0.0006). These data indicate that wnt5 mutant animals exhibit a significant reduction in the number of boutons and suggest that the density of active zones in the presynaptic terminals is also significantly reduced.
We performed electrophysiology to determine whether the reduction in the number of active zones affected synaptic function. Muscle 6 was voltage clamped at −60 mV and the presynaptic segmental nerve was stimulated (1 Hz, 5 V) to induce synaptic activity. The amplitude of evoked excitatory junctional currents (EJCs) was reduced 40% in wnt5 mutant animals [Fig. 2(D), WT = 207.2 ± 18.51 nA, n = 8; wnt5400 = 114.7 ± 17.94 nA, n = 6, p = 0.0044]. Similarly, the frequency of spontaneous miniature EJCs (mEJCs) was significantly reduced in wnt5 mutants [Fig. 2(E), WT = 2.030 ± 0.3035 s, n = 9; wnt5400 = 1.231 ± 0.1985 s, n = 8, p = 0.0491]. These data suggest that presynaptic function is impaired in wnt5 mutants and is consistent with the reduction in the number of boutons and active zones observed immunocytochemically. Postsynaptic function, however, appeared normal, as there was no significant difference in mEJC amplitudes [Fig. 2(E), WT = 0.8677 ± 0.05136 nA, n = 9; wnt5400 = 0.8034 ± 0.04789 nA, n = 8, p = 0.3781], an indicator of postsynaptic glutamate receptor function. Taken together, our results suggest that wnt5 promotes synaptic growth and the assembly of presynaptic active zones.
The Reduction in NMJ Size Is the Result of Underdevelopment
Growth of the NMJ involves the addition of new boutons during larval development to correspond with the increase in muscle size (Schuster et al., 1996a,b; Zito et al., 1999). To ascertain whether the deficit in bouton number resulted from a failure to coordinate growth of the presynaptic motor neuron and postsynaptic muscle, we also examined wnt5 mutant NMJs during both first and second instar larval stages (24- and 48-h AEL, respectively). There was no significant difference in the number of boutons between mutant and control first instar larvae [Supp. Fig. (A,B), WT = 11.13 ± 1.093 boutons, n = 8; wnt5400 = 9.429 ± 1.232 boutons, n = 7, p = 0.3199]. However by second instar, there was a significant reduction in the number of boutons in wnt5 mutants compared with controls [Fig. Supp. Fig. (C,D), WT = 19.44 ± 0.9146 boutons, n = 9; wnt5400 = 15.13 ± 1.217 boutons, n = 8, p = 0.0115]. There was no significant difference in the density of active zones during first or second instar larval stages (Fig. Supp. Fig., 1st instar: WT = 0.6530 ± 0.0534 puncta/μm2, n = 8; wnt5400 = 0.6084 ± 0.0516 puncta/μm2, n = 7, p = 0.5613; 2nd instar: WT = 0.5846 ± 0.0716 puncta/μm2, n = 7; wnt5400 = 0.4614 ± 0.0378 puncta/μm2, n = 7, p = 0.1383). These data suggest that mutant NMJs fail to keep pace with postsynaptic muscle development.
Restoration of wnt5 in Neurons But Not Muscle Rescues the Null Phenotype
To identify the cell type in which wnt5 functions, we performed cell-type specific gene rescue experiments by expressing the UAS-wnt5 transgene specifically in neurons (using the elav-Gal4 driver) or muscle (using the 24B-Gal4 driver) in the wnt5400 mutant background. Expression of wnt5 in neurons but not in muscles restored the number of boutons to slightly greater than control levels [Fig. 3(A,B), wnt5400 = 45.36 ± 3.459 boutons, n = 14; neuron expression = 59.64 ± 3.123 boutons, n = 11, p = 0.007, muscle expression = 50.31 ± 4.531 boutons, n = 13, p = 0.3893; control; compared with control animals the p values were 0.3978 and 0.3211 for neuronal and muscle expression, respectively]. Similarly, expression of wnt5 in neurons but not muscles restored the NMJ size to slightly greater than control levels (wnt5400 = 382.0 ± 37.80 μm2, n = 10, neuron expression = 595.8 ± 29.72 μm2, n = 10, p = 0.0003; muscle expression = 437.3 ± 28.30 μm2, n = 8, p = 0.2794; compared with control animals the p values were 0.6837 and 0.0415 for neuronal and muscle expression, respectively). Expression of the transgene in either neurons or muscle, however, restored the number of active zones/μm2 to near control levels [Fig. 3(A,C), wnt5400 = 0.3904 ± 0.02506, n = 7; neuron expression = 0.5140 ± 0.03132, n = 8, p = 0.0099, muscle expression = 0.5364 ± 0.03560, n = 8, p = 0.0062]. These data indicate that wnt5 functions in the presynaptic motor neuron to promote synaptic growth, but may function in either the presynaptic neuron or the postsynaptic muscle cell to regulate active zone formation. The differential requirement for wnt5 suggests that synaptic growth and active zone development may be governed by distinct wnt5-dependent signaling mechanisms.
Figure 3.
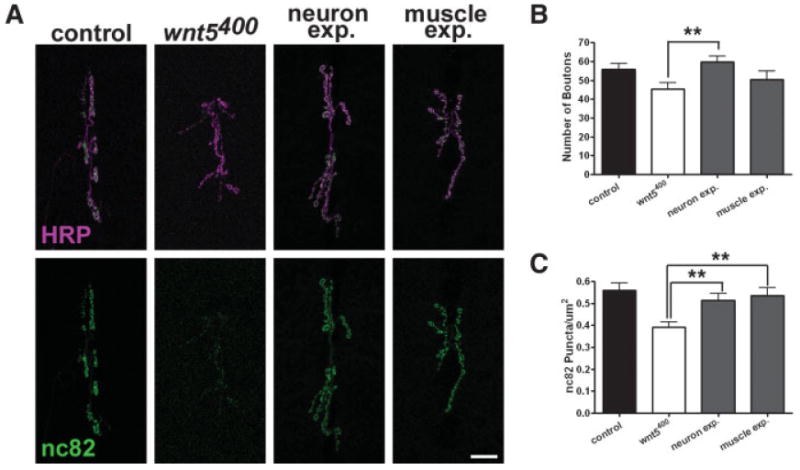
wnt5 expression in neurons rescues the mutant phenotype. (A) Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta) and the presynaptic protein Brp (nc82, green). For rescue experiments, a UAS-wnt5 transgene was expressed in the wnt5 mutant background in neurons using the elav-Gal4 driver (neuron exp.) or in muscle using the 24B-Gal4 driver (muscle exp.). Scale bar: 20 μm. (B) Quantification of the number of boutons per NMJ. (C) Quantification of active zone density (nc82 puncta/μm2). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Overexpression of wnt5 in Neurons Increases Synaptic Growth
If wnt5 promotes NMJ development, we would expect that increasing the dose of wnt5 in wild-type animals would alter NMJ development. We therefore overexpressed wnt5 by driving the UAS-wnt5 transgene with cell-type specific Gal4 drivers in wild-type animals. Driving UAS-wnt5 using either a ubiquitous (Actin-Gal4) or neuron-specific drivers (elav-Gal4 and OK6-Gal4) significantly increased the number of boutons of the 6/7 NMJ [Fig. 4(A,B), control = 55.85 ± 3.059 boutons, n = 13; Act>wnt5 = 78.07 ± 5.274 boutons, n = 14, p = 0.0015; elav>wnt5 = 77.75 ± 4.254 boutons, n = 8, p = 0.0004; OK6>wnt5 = 68.50 ± 4.323 boutons, n = 12, p = 0.0239]. Driving UAS-wnt5 in postsynaptic muscle cells, using the 24B-Gal4 driver, significantly reduced the number of boutons (control = 55.85 ± 3.059 boutons, n = 13; 24B>wnt5 = 42.92 ± 4.087 boutons, n = 12, p = 0.0176). Similar results were obtained when we overexpressed wnt5 using the GSV line, wnt51192, and used the Actin-Gal4, elav-Gal4, and 24B-Gal4 drivers (data not shown). The overexpression results support our rescue experiments, which show that wnt5 functions in the presynaptic neuron to positively regulate NMJ size. The inhibition of NMJ growth by postsynaptic wnt5 overexpression might be due to a deleterious effect of high levels of wnt5 on muscle physiology. Indeed, muscle defects are observed in wnt5-overexpressing muscle (R.W. Wouda, L.G.F. and J.N.N., in preparation). Our overexpression results support the idea that wnt5 functions in motor neurons to promote NMJ growth.
Figure 4.

Overexpression of wnt5 in neurons produces an overgrowth of the NMJ. (A) Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta) and the presynaptic protein Brp (nc82, green). For overexpression experiments, a UAS-wnt5 transgene was expressed ubiquitously (using Actin-Gal4, act>wnt5), or in neurons (elav>wnt5) or in muscle (24B>wnt5). Scale bar: 20 μm. (B) Quantification of the number of boutons per NMJ.
drl Mutant Animals Exhibit a Similar Morphology as wnt5 Mutants
The observation that wnt5 acts presynaptically to regulate NMJ morphology raises the question as to the downstream signaling mechanism involved. Our immnunolocalization experiments show that similar to Wnt5, the Drl receptor is also localized to the boutons of the NMJ [Fig. 1(B)]. To test whether Drl also functions in NMJ development, we examined the phenotype of drl2 mutant animals, which are protein nulls and homozygous-viable (Dura et al., 1995). Although the lateral ventral body wall muscles (specifically, muscles 21, 22, and 23) are abnormal and sometimes missing in mutant animals as previously described (Callahan et al., 1996), the medial ventral body wall muscles exhibited no visible defects in muscle size (muscle 6: WT = 26,490 ± 1526 μm2, n = 6; drl2 = 26,300 ± 1392 μm2, n = 6, p = 0.9257; muscle 7: WT = 20,020 ± 764.6 μm2, n = 6; drl2 = 20,860 ± 1020 μm2, n = 6, p = 0.5188). Similar to wnt5 mutants, drl mutant animals exhibited a significant reduction in the number of boutons [Fig. 5(A,B) and data not shown, control = 55.85 ± 3.059 boutons, n = 13; drl2 = 43.17 ± 2.458 boutons, n = 12, p = 0.0040] and this correlated with a reduction in the size of the 6/7 NMJ (WT = 526.2 ± 42.66 μm2, n = 10; drl2 = 382.6 ± 49.34 μm2, n = 10, p = 0.0410). Also similar to the wnt5 mutant phenotype, there was no significant difference in the number of branches at the 6/7 NMJ (control = 4.938 ± 0.4784 branches, n = 16; drl2 = 4.167 ± 0.8424, n = 12 branches, n = 12, p = 0.4888) and the synaptic growth defect became apparent only during the second larval instar (Supp. Fig., first instar: WT = 11.13 ± 1.093 boutons, n = 8; drl2 = 9.000 ± 0.9512 boutons, n = 7, p = 0.1720; second instar: WT = 19.44 ± 0.9146 boutons, n = 9; drl2 = 13.29 ± 1.539 boutons, n = 7, p = 0.0028).
Figure 5.
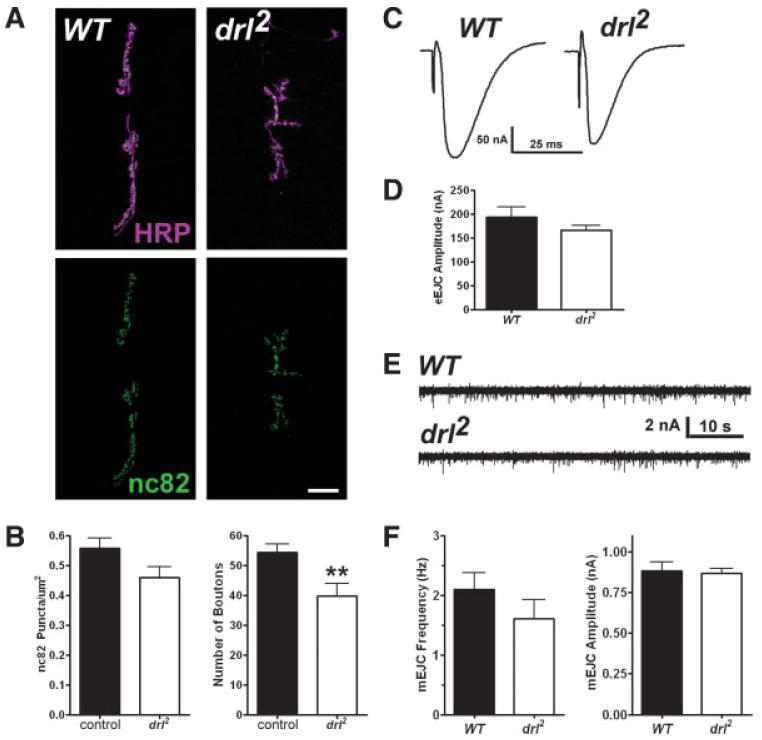
drl mutant animals exhibit a reduction in the number of boutons but not active zones. (A) Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta) and the presynaptic protein Brp (nc82, green). Scale bar: 20 μm. (B) Quantification of active zone density (nc82 puncta/μm2, left) and bouton numbers (per NMJ, right). (C) Representative two-electrode voltage clamp recordings from muscle 6 of control and drl2 third instar larvae, showing evoked endplate junctional currents (EJCs). (D) Quantification of EJC amplitudes. (E) Representative voltage clamp recordings from control and drl2 third instar larvae, showing spontaneous excitatory junctional currents (mEJCs) in the muscle 6 NMJ. (F) Quantification of mEJC frequency (left) and amplitude (right). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We examined the synaptic expression and localization of Brp and the postsynaptic glutamate receptor subunit, GluRIIA, by immunolabeling third instar mutant animals. Unlike the wnt5 mutant, the expression and localization of both Brp and GluRIIA were not significantly different than controls in drl mutants [Fig. 5(A,B), Brp: WT = 0.5585 ± 0.03462 puncta/μm2, n = 10; drl2 = 0.4739 ± 0.03352 puncta/μm2, n = 8, p = 0.1035; GluRIIA normalized fluorescence: WT = 1.000 ± 0.1006, n = 11; drl2 = 1.155 ± 0.1140, n = 12, p = 0.3240]. In addition, drl mutant animals exhibited small statistically insignificant reductions in evoked amplitude and mini frequency [Fig. 5(C–F), evoked amplitude: WT = 207.2 ± 18.51 nA, n = 8; drl2 = 166.4 ± 9.015 nA, n = 7, p = 0.3080; mini amplitude: WT = 0.8677 ± 0.05136 nA, n = 9; drl2 = 0.8665 ± 0.02951 nA, n = 10, p = 0.9832; mini frequency: WT = 2.030 ± 0.3035 Hz, n = 9; drl2 = 1.605 ± 0.3264 Hz, n = 11, p = 0.2677]. To exclude the possibility of secondary mutations in the drl2 mutant background, we placed the drl2 mutation in trans to the deficiency, Df(2R) lio2, pigeon2, drl2. These animals also exhibited a significant reduction in the number of NMJ boutons, with no significant difference in the density of active zones (control = 55.85 ± 3.059 boutons, n = 13; drl2/Df(2R) lio2, pigeon2, drl2 = 43.78 ± 4.390 boutons, n = 9, p = 0.030; Brp: WT = 0.5585 ± 0.03462 puncta/μm2, n = 10; drl2/Df(2R) lio2, pigeon2, drl2 = 0.5240 ± 0.0336 puncta/μm2, n = 9, p = 0.486). Taken together, these results indicate that drl regulates presynaptic neuronal morphology, but is not likely to regulate the formation of active zones.
drl Likely Functions in Postsynaptic Muscle
To delineate the cell type in which drl functions, we sought to restore drl functions in specific cell types in the drl2 mutant background. Neither the presence of the UAS-drl transgene alone nor the expression of the transgene under the control of the pan-glial driver, Repo-Gal4, rescued the growth phenotype [Fig. 6(A,C), drl2 = 43.17 ± 2.458 boutons, n = 12, drl2; UAS-drl = 40.80 ± 2.476 boutons, n = 10, p = 0.509; glia expression = 41.67 ± 3.322 boutons, n =12, p = 0.7201]. Expression of the transgene in muscle strongly rescued the drl2 mutant phenotype [Fig. 6(A,C), drl2 = 43.17 ± 2.458 boutons, n = 12, muscle expression = 56.83 ± 3.303 boutons, n = 12, p = 0.0031] as the number of boutons is restored to slightly greater than control levels. Interestingly, drl expression in neurons produced an increase in the number of NMJ branches [Fig. 6(C), control = 5.846 ± 0.5644 branches, n = 13; neuron expression = 8.500 ± 0.7638 branches, n = 12, p = 0.0096], a phenotype not seen in the wild type. The increased arborization produces an increase in bouton number [Fig. 6(A,C), drl2 = 43.17 ± 2.458 boutons, n = 12, neuron expression = 59.55 ± 4.288 boutons, n = 11, p = 0.0028], leading to ambiguity about whether drl functions in the presynaptic motor neuron or postsynaptic muscle cell. Examination of NMJ size produced similar results (drl2 = 382.6 ± 49.34 μm2, n = 10, neuron expression = 564.8 ± 49.61 μm2, n = 9, p = 0.019; muscle expression = 559.6 ± 37.99 μm2, n = 9, p = 0.012, glial expression = 465.3 ± 36.04 μm2, n = 9, p = 0.202).
Figure 6.
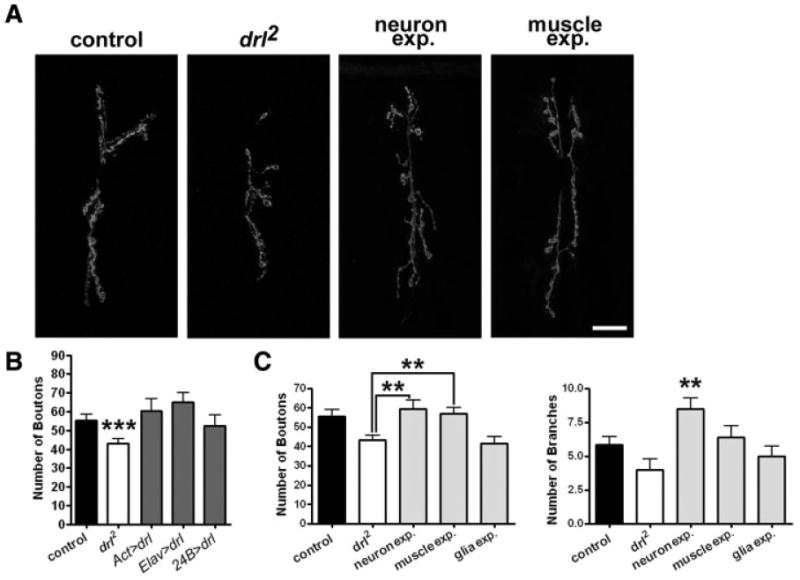
Expression of drl in muscle rescues the drl mutant phenotype. (A) Confocal images showing NMJs labeled with antibodies against HRP. For rescue experiments, a UAS-drl transgene was expressed in the drl mutant background in neurons using the elav-Gal4 driver or in muscle using the 24B-Gal4 driver. Scale bar: 20 μm. (B) For overexpression experiments, a UAS-drl transgene was expressed ubiquitously (act>drl), or in neurons (elav>drl) or muscle (24B>drl). Quantification of the number of boutons per NMJ for overexpression experiments. (C) Quantification of the number of boutons and branches for the rescue experiments.
We also overexpressed drl in an otherwise wild-type background. Overexpressing drl using various Gal4 drivers did not significantly affect NMJ morphology [Fig. 6(B), control = 55.85 ± 3.059, n = 13; Act>drl = 60.50 ± 6.425, n = 12, p = 0.5091; elav>drl = 64.93 ± 5.195, n = 14, p = 0.1521; 24B>drl = 52.50 ± 5.704, n = 12, p = 0.6023]. We conclude from the aforementioned rescue experiments that drl likely functions in the postsynaptic muscle to regulate NMJ growth, but may also function in the presynaptic motor neuron for other developmental processes.
The NMJ Overgrowth Produced by Overexpression of wnt5 in Neurons Is Suppressed in the drl Mutant Background
The reduction in bouton numbers in both wnt5 and drl mutants raised the possibility that the two genes may function in a signaling pathway to regulate synaptic growth. To test this possibility, we constructed animals bearing simultaneously the wnt5 and drl mutations. The NMJ morphology of wnt5400; drl2 double mutants is not statistically different than that of each of the single mutants [Fig. 7(A,B), drl2 = 43.17 ± 2.458 boutons, n = 12; wnt5400 = 45.36 ± 3.459 boutons, n = 14; wnt5400; drl2 = 49.55 ± 4.345, n = 11, p = 0.2056], supporting our hypothesis that wnt5 and drl act in the same signaling pathway to regulate synaptic growth.
Figure 7.

Epistatic analyses of the relationship between wnt5 and drl. (A) Confocal images showing NMJs labeled with antibodies against HRP. Scale bar: 20 μm. (B) Quantification of the number of boutons per NMJ. (C) Confocal images showing NMJs labeled with antibodies against HRP. For the epistatic analysis, the UAS-wnt5 transgene was overexpressed in neurons in the drl mutant background (drl2; elav>wnt5 and drl2/Cyo; elav>wnt5). Scale bar: 20 μm. (D). Quantification of the number of boutons per NMJ.
We postulate that wnt5 acts upstream of drl. This hypothesis makes the prediction that mutations in the drl gene would block the effects of wnt5. To test this possibility, we overexpressed the UAS-wnt5 transgene in the drl mutant background. Neuronal overexpression of wnt5 in the wild-type background leads to overgrowth of the NMJ with a significant increase in the number of boutons [Figs. 4(A,B) and 7(C,D)]. Removal of a single copy of the drl gene (drl2/CyO) in the wnt5 overexpressing animal partially, but significantly suppressed the NMJ overgrowth phenotype [Fig. 7(C,D) elav>wnt5 = 77.75 ± 4.254 boutons, n = 8; drl2/Cyo; elav>wnt5 = 60.33 ± 4.311 boutons, n = 9, p = 0.0119]. Removal of both copies of the drl gene in the wnt5 overexpressing animal results in a phenotype indistinguishable from that of the drl2 mutant [Fig. 7(C,D), drl2 = 43.17 ± 2.458 boutons, n = 12; drl2; elav>wnt5 = 47.55 ± 2.855 boutons, n = 11, p = 0.2560]. These data indicate that the effect of neuronal overexpression of wnt5 is highly sensitive to the dose of the drl gene and that drl is epistatic to wnt5. This result supports the idea that wnt5 functions through drl to promote the growth of the NMJ.
The Intracellular and WIF Domains of Drl Are Required for Normal Synaptic Growth
The observation that Drl acts downstream of Wnt5 during NMJ development raises intriguing questions about the mechanisms by which Drl transduces the Wnt5 signal. The Drl protein is a member of the evolutionarily conserved RYK receptor tyrosine kinase-related family. Members of this family possess a WIF motif in the extracellular domain and an intracellular domain that includes an “atypical” kinase. Our ability to rescue the drl mutant phenotype using transgenes afforded us the opportunity to probe the requirement of these domains of the Drl protein in synapse development. We expressed mutant drl constructs in the muscle of drl2 mutant animals to assess their ability to rescue the drl2 mutant phenotype. Deletion of the WIF motif completely abolished Drl's capacity to rescue the mutant phenotype, indicating that the WIF motif is necessary for Drl function in NMJ development (Fig. 8, drl2 = 43.17 ± 2.458 boutons, n = 12; drl2, 24B>drlΔWIF = 49.85 ± 3.123 boutons, n = 13, p = 0.1102; p-value when compared with control = 0.280). Expression of the truncated DrlΔcyt protein containing only the extracellular and transmembrane domains failed to rescue the drl2 mutant phenotype, indicating that the cytoplasmic domain is necessary for Drl function in NMJ development (Fig. 8, drl2 = 43.17 ± 2.458 boutons, n = 12, drl2, 24B>drlΔcyt = 49.09 ± 2.246 boutons, n = 11, p = 0.0915; p value when compared to control = 0.171). Examination of NMJ size yielded similar results (drl2 = 382.6 ± 49.34 μm2, n = 10, 24B>drlΔWIF = 361.6 ± 44.12 μm2, n = 10, p = 0.906; 24B>drlΔcyt = 361.9 ± 39.10 μm2, n = 11, p = 0.889). We conclude that the cytoplasmic and WIF domains of drl are required for normal growth of the presynaptic motor neuron.
Figure 8.

Normal NMJ growth requires the WIF and intracellular domains of Drl. (A). Confocal fluorescent images showing NMJs labeled with antibodies against HRP (magenta). Scale bar: 20 μm. (B) Quantification of the number of boutons per NMJ. (C) Model of Wnt5-Drl signaling at the NMJ. (i) Wnt5 signals through Drl to regulate NMJ size. (ii, iii) In the absence of either Wnt5 (ii) or Drl (iii), the NMJ is reduced in size. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
After its establishment, the Drosophila NMJ exhibits as much as a 10-fold increase in growth between the first and third instar larval stages by adding additional boutons and branches to correspond with the increase in surface area of the postsynaptic muscle (Schuster et al., 1996a,1996b; Zito et al., 1999). This growth requires coordination between the presynaptic motoneuron and postsynaptic muscle. This process is likely mediated by a number of signaling mechanisms. Here we provide evidence that Wnt5 positively regulates NMJ growth by signaling through the Drl RYK receptor. This is the first study to demonstrate that a member of the RYK family plays a role in synapse formation.
Wnt5 Is an Anterograde Signal That Regulates NMJ Development
Several pieces of evidence support our conclusion that the wnt5 gene acts as a positive regulator of NMJ growth and synaptic transmission. Mutation of wnt5 leads to stunted NMJs with decreased number of boutons. In addition to the structural defects, the NMJ shows reduced number of active zones. Electrophysiological recordings showed that the amplitudes of evoked releases and frequency of spontaneous releases are both significantly decreased. Examination of the mutant NMJs early in development showed that they are normal, indicating that wnt5 is needed for NMJ growth to maintain pace with muscle growth. Cell-type specific cDNA expression showed that wnt5 functions in the motoneurons. In contrast to the reduced bouton numbers in the loss-of-function wnt5 mutant, animals overexpressing wnt5 in the motoneurons exhibit significant overgrowth of the NMJ, indicating that NMJ development is highly sensitive to the dose of wnt5. Our results therefore indicate that Wnt5 is an anterograde signal that promotes the development of the NMJ.
Our finding that Wnt5 apparently acts as a transsynaptic signal adds it to a growing list of Wnt proteins and Wnt signaling pathway components that play key roles in synaptogenesis (for review see Salinas, 2005). Most notably, Packard et al. (2002) observed that at the Drosophila NMJ, Wg is expressed by the presynaptic motor neuron where it governs the development of both pre- and postsynaptic structures (Packard et al., 2002; Mathew et al., 2005). In the mouse, Wnt3 and Wnt7a are necessary for the cessation of axon growth and the remodeling of the axons into presynaptic terminals, characterized by growth cone spreading and clustering of the synapsin I protein (Hall et al., 2000; Krylova et al., 2002). Dissection of the mechanisms by which these Wnt proteins regulate synapse development showed that they act through the Frizzled receptor (Packard et al., 2002; Mathew et al., 2005). However, our observations indicate that Wnt5 functions through the Drl RYK receptor to govern NMJ growth.
Drl Functions downstream of Wnt5 to Regulate Synapse Growth
Several lines of evidence indicate drl is necessary for the growth of the Drosophila NMJ. Mutation of the drl gene results in a significant reduction in NMJ size, a phenotype that resembles that of the wnt5 mutant. As with the wnt5 mutant, the NMJs of the drl mutant appear normal during the first instar larval stage, indicating that drl regulates NMJ growth. Cell-type specific gene rescue showed that drl likely functions in the postsynaptic muscle cell. Interestingly, despite the significant reduction in NMJ size in the drl mutant, this synapse displayed wild-type electrophysiology, and thus differs from wnt5 mutation in this respect. The density of active zones, the amplitudes of both evoked and spontaneous releases, and the frequency of miniature releases are normal compared with wild-type animals, suggesting that drl does not regulate the formation of active zones. Instead, drl appears to function specifically to regulate presynaptic growth. This observation raised the possibility that drl and wnt5 may function in the same pathway to regulate NMJ growth.
That drl functions downstream to mediate wnt5 signaling is reinforced by the following observations. First, the wnt5; drl double mutants exhibit reduced NMJ sizes, a phenotype that resembles both the single wnt5 and drl mutants. Second, the overgrowth phenotype produced by neuronal overexpression of wnt5 is completely suppressed in the drl mutant background, indicating that drl acts downstream of wnt5. We conclude that wnt5 signals through the drl receptor to regulate NMJ growth. Collectively, our results suggest a novel anterograde signaling pathway [Fig. 8(C)] where Wnt5, expressed in the presynaptic cell, acts through Drl, likely in the postsynaptic cell, to promote synaptic growth.
The Drl Cytoplasmic and WIF Domains Are Necessary for NMJ Growth
The capacity of the Drl RYK to mediate Wnt5 function in NMJ growth raises the question of the mechanism by which Drl transduces the Wnt5 signal. The Drl protein contains an extracellular WIF motif with homology to the Wnt-binding domain of the Wnt inhibitory factor-1 (WIF-1) protein (Yoshikawa et al., 2003) and an intracellular atypical kinase domain (Yoshikawa et al., 2001). To assess the requirements of these domains during NMJ development, we engineered Drl proteins lacking these domains and expressed them in muscle cells. Deletion of the extracellular WIF domain completely abolished NMJ function in NMJ development. Since the extracellular domain of Drl has been shown to bind to Wnt5 (Yoshikawa et al., 2003), our result suggests that Wnt5 may regulate growth of the NMJ by binding to the WIF motif of Drl. Deletion of the cytoplasmic domain of Drl also abolished its function in NMJ development. This result is consistent with observations in the embryonic ventral nerve cord, where the Drl cytoplasmic domain is needed for commissural axons to navigate properly in response to the Wnt5 signal (Yoshikawa et al., 2001). This domain is likely to play a role in signal transduction, although the mechanism by which it functions is unknown. Recently, the cytoplasmic tail of the mammalian RYK protein was shown to bind to the Dishevelled protein, raising the possibility that RYK proteins may act through the canonical pathway (Lu et al., 2004).
Wnt5 Signals Via Different Mechanisms to Regulate Structure and Function
We found that the Wnt5-Drl signaling pathway mediates a signal that passes from motor neurons to muscle cells. This raises the question as to how the pathway regulates NMJ development. One intriguing possibility is that the signaling pathway stimulates the production of a signal in the muscle cell, which acts in a retrograde fashion to influence synaptic growth. One known retrograde signal is the BMP signaling pathway (for reviews see Marques, 2005). Intriguingly, mutations in components of this pathway (Gbb, Saxophone, Thick Veins, Wishful thinking, and Mad) lead to a reduction in NMJ size with fewer boutons (Aberle et al., 2002; Marques et al., 2002; McCabe et al., 2003, 2004; Rawson et al., 2003), a phenotype that resembles that of the wnt5 and drl mutants. We are currently exploring the possibility that BMP signaling is downstream of drl.
Besides regulating NMJ structure, Wnt5 also regulates the function of the synapse. In this role, however, it does not appear to act via the Drl receptor. Wnts have been previously implicated in the development of presynaptic active zones (Hall et al., 2000; Krylova et al., 2002; Packard et al., 2002; Ahmad-Annuar et al., 2006). We show that in the absence of wnt5, mutant NMJs have fewer active zones and impaired presynaptic function. Both the amplitude of evoked currents and the frequency of spontaneous currents are significantly reduced. It is unclear how Wnt5, which is produced by the presynaptic motor neuron, acts on the same cell that produces it. However, our data demonstrate that Wnt5 does not signal through Drl to regulate active zone formation. Drl mutant animals are functionally normal with no detectable loss of Brp labeling. Further studies will hopefully identify the mechanisms by which Wnt5 and Drl govern NMJ structure and function.
Acknowledgments
We thank the Iowa Developmental Hybridoma Bank for antibodies and the Bloomington Stock Center for fly stocks.
Contract grant sponsor: NIH Institutional Training Grant (University of Illinois); contract grant number: HD07333.
Contract grant sponsor: NIH Individual NRSA (NIH/NIDCD); contract grant number: 1 F32 DC08443-01.
Contract grant sponsor: NIH-NINDS Grant; contract grant number: R01NS045628.
Contract grant sponsor: Pionier Grant from the NOW.
Contract grant sponsor: NIH/NIDCD; contract grant number: DC5408-01.
Contract grant sponsor: Roy J. Carver Charitable Trust; contract grant number: 03-27.
Footnotes
This article contains supplementary material available via the Internet at http://www.mrw.interscience.wiley.com/suppmat/1932-8451/suppmat/
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, et al. Signaling across the synapse: A role for Wnt and dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci. 2007;27:111–123. doi: 10.1523/JNEUROSCI.4770-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Canada The fly neuromuscular junction: Structure and Function. Int Rev Neurobiol. 2006;75 doi: 10.1016/S0074-7742(06)75010-3. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CA, Bonkovsky JL, Scully AL, Thomas JB. Derailed is required for muscle attachment site selection in Drosophila. Development. 1996;122:2761–2767. doi: 10.1242/dev.122.9.2761. [DOI] [PubMed] [Google Scholar]
- Collins CA, Diantonio A. Synaptic development: Insights from Drosophila. Curr Opin Neurobiol. 17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Dura JM, Taillebourg E, Preat T. The Drosophila learning and memory gene linotte encodes a putative receptor tyrosine kinase homologous to the human RYK gene product. FEBS Lett. 1995;370:250–254. doi: 10.1016/0014-5793(95)00847-3. [DOI] [PubMed] [Google Scholar]
- Fradkin LG, van Schie M, Wouda RR, de Jong A, Kamphorst JT, Radjkoemar-Bansraj M, Noordermeer JN. The Drosophila Wnt5 protein mediates selective axon fasciculation in the embryonic central nervous system. Dev Biol. 2004;272:362–375. doi: 10.1016/j.ydbio.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Gramates LS, Budnik V. Assembly and maturation of the Drosophila larval neuromuscular junction. Int Rev Neurobiol. 1999;43:93–117. doi: 10.1016/s0074-7742(08)60542-5. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Chiba A, Johansen J, Keshishian H. Growth cone behavior underlying the development of stereotypic synaptic connections in Drosophila embryos. J Neurosci. 1991;11:3227–3238. doi: 10.1523/JNEUROSCI.11-10-03227.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille F, Sommer L. Wnt signaling: Multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: Molecular components and mechanisms underlying synaptic plasticity. Microsc Res Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Liebl FL, Chen K, Karr J, Sheng Q, Featherstone DE. Increased synaptic microtubules and altered synapse development in Drosophila sec8 mutants. BMC Biol. 2005;3:27. doi: 10.1186/1741-7007-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB. The Drosophila BMP type II receptor wishful thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, Haerry TE, Wan H, et al. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron. 2004;41:891–905. doi: 10.1016/s0896-6273(04)00073-x. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Landgraf M, Rushton E, Broadie K, Bate M. Presynaptic development at the Drosophila neuromuscular junction: Assembly and localization of presynaptic active zones. Neuron. 1996;17:617–626. doi: 10.1016/s0896-6273(00)80195-6. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-β type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Rose D, Chiba A. A single growth cone is capable of integrating simultaneously presented and functionally distinct molecular cues during target recognition. J Neurosci. 1999;19:4899–4906. doi: 10.1523/JNEUROSCI.19-12-04899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC. Retrograde signalling at the synapse: A role for Wnt proteins. Biochem Soc Trans. 2005;33:1295–1298. doi: 10.1042/BST0331295. [DOI] [PubMed] [Google Scholar]
- Schmid A, Qin G, Wichmann C, Kittel RJ, Mertel S, Fouquet W, Schmidt M, et al. Non-NMDA-type glutamate receptors are essential for maturation but not for initial assembly of synapses at Drosophila neuromuscular junctions. J Neurosci. 2006;26:11267–11277. doi: 10.1523/JNEUROSCI.2722-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996a;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996b;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Sink H, Whitington PM. Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons. Development. 1991;112:307–316. doi: 10.1242/dev.112.1.307. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Rheuben MB, Kidokoro Y. Transition from growth cone to functional motor nerve terminal in Drosophila embryos. J Neurosci. 1997;17:8408–8426. doi: 10.1523/JNEUROSCI.17-21-08408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, Bonkowsky JL, Kokel M, Shyn S, Thomas JB. The Derailed guidance receptor does not require kinase activity in vivo. J Neurosci. 2001;21:RC119. doi: 10.1523/JNEUROSCI.21-01-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Zito K, Parnas D, Fetter RD, Isacoff EY, Goodman CS. Watching a synapse grow: Noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 1999;22:719–729. doi: 10.1016/s0896-6273(00)80731-x. [DOI] [PubMed] [Google Scholar]
- Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]


