To the editor:
Sirolimus is an immunosuppressant commonly used in solid organ transplantation as an alternative to corticosteroids or calcineurin inhibitors. Common side effects include hyperlipidemia, arthralgias, and non-cardiac peripheral edema. Pulmonary toxicity is increasingly recognized after solid organ transplants.(1–3) Here we report the first case of sirolimus associated pneumonitis (SAP) in a peripheral blood stem cell transplant (PBSCT) patient.
A 21-year-old Puerto Rican woman with homozygous sickle cell disease (SCD) had a history of stroke as a child and frequent vaso-occlusive crises while on hydroxyurea. She met inclusion criteria and enrolled in an IRB approved non-myeloablative peripheral blood stem cell transplant study at the Clinical Center of the National Institutes of Health. She tolerated a low dose radiation based conditioning regimen and received a T-replete graft with 10×106 per kilogram CD34+ cells from her HLA-matched sister. She experienced no sickle related complications and achieved prompt hematopoietic recovery. She was maintained on sirolimus only, targeting a trough level of 15–20 ng/mL. One year later, she was free of SCD: hemoglobin was 12 g/dL, hemoglobin electrophoresis was stable and compatible with her sickle trait donor, and 100% of peripheral blood CD14/15 leukocytes were of donor origin. She was regularly phlebotomized to correct transfusional iron overload.
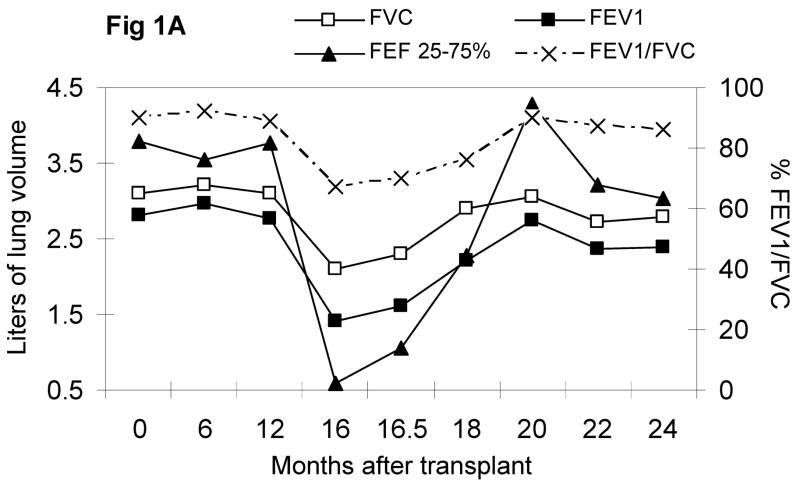
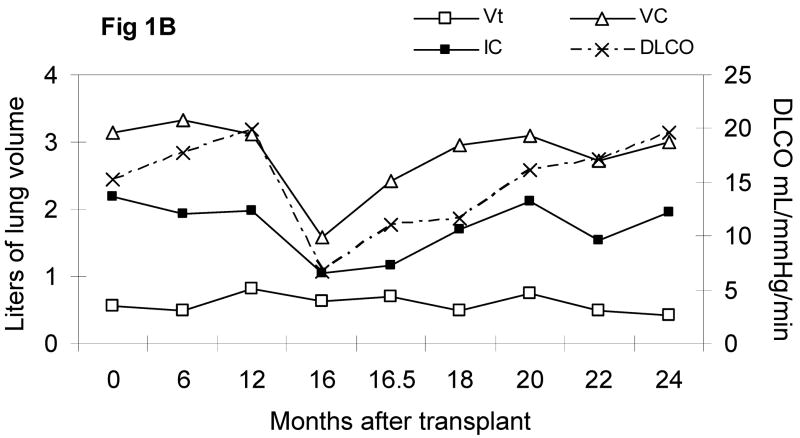
Sixteen months after the transplant, she developed cough, dyspnea on exertion, and progressed to debilitating dyspnea over a 4 week interval, but never required supplemental oxygen. There were no antecedent infections, CMV reactivation, acute or chronic GVHD, or other transplant related complications. Upon admission to evaluate her dyspnea, she was afebrile with normal vital signs; her resting pulse oximetry was 99–100% on room air, which decreased to 95% after ambulating 100 meters. Her echocardiogram showed a normal ejection fraction, normal valvular function, and no pulmonary hypertension. Pulmonary function tests showed a severe obstructive and restrictive defect. Computerized tomography (CT) of the chest showed fine, hazy, bilateral, lower lobe infiltrates; no bronchial dilatation or thickening, peribronchial nodules or consolidations were observed. Broncho-alveolar lavage recovered numerous mononuclear and few granulocytic cells; studies for bacteria, atypical organisms, acid fast organisms, fungi, pneumocystis jiroveci (PCP), CMV, and other viruses, were all negative. Sirolimus associated pneumonitis was suspected, sirolimus was tapered off over 3 days, and oral cyclosporine was initiated; systemic corticosteroid was not administered. Albuterol, ipratroprium, and corticosteroid inhalers were administered. One week later, she had a dramatic response: her subjective dyspnea improved and the distance of her 6-minute walk increased from 195 to 330 meters. She was discharged to home after 2 weeks of hospitalization. 6–8 months later, her respiratory status, exercise tolerance, PFTs, and CT scans all returned to pre-transplant levels (Figure 1A and 1B).
Figure 1.
Figure 1A. Pulmonary forced spirometry
FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 sec, FEF = forced expiratory flow in liters/sec
Figure 1B. Pulmonary slow spirometry
VC = vital capacity, IC = inspiratory capacity, Vt = tidal volume, DLCO = lung diffusing capacity of carbon monoxide
Late non-infectious pulmonary complications following allogeneic PBSCT often require aggressive treatment including corticosteroids and monoclonal antibodies, and are associated with significant morbidity and mortality.(4) While our patient demonstrated classic features of bronchiolitis obliterans (BO) and bronchiolitis obliterans with organizing pneumonia (BOOP),(5) her symptoms resolved in 1–2 weeks without intensifying immunosuppression. Her follow-up PFTs steadily improved and imaging studies showed no indication of relapse or progression, which are uncharacteristic of BO and BOOP.
Though our patient demonstrated both restrictive and obstructive changes, obstructive pulmonary function tests are less commonly observed in SAP (6). Our patient’s underlying SCD may have been a predisposing factor for the observed obstruction, since SCD is commonly associated with asthma(7–9). This case also highlights that with the increase use of sirolimus in stem cell transplantations,(10) SAP may be increasingly recognized as in solid-organ recipients. Furthermore, the clinical presentation may differ in this setting. Finally, sirolimus withdrawal can be utilized as a diagnostic test before committing to long-term aggressive corticosteroid and/or monoclonal antibody therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Champion L, Stern M, Israel-Biet D, et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Ann Intern Med. 2006;144(7):505–9. doi: 10.7326/0003-4819-144-7-200604040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Weiner SM, Sellin L, Vonend O, et al. Pneumonitis associated with sirolimus: clinical characteristics, risk factors and outcome--A single-centre experience and review of the literature. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm420. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RJ, Wells AC, Unitt E, et al. Sirolimus-induced pneumonitis following liver transplantation. Liver Transpl. 2007;13(6):853–6. doi: 10.1002/lt.21141. [DOI] [PubMed] [Google Scholar]
- 4.Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43(1):42–52. doi: 10.1053/j.seminhematol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(7):749–59. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Gupte GL, Mahadevan S, Clarke JR, et al. Sirolimus-related pulmonary toxicity mimicking ‘asthma like’ symptoms. World J Gastroenterol. 2007;13(38):5151–3. doi: 10.3748/wjg.v13.i38.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassberg J, Spivey JF, Strunk R, et al. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol. 2006;28(8):481–5. doi: 10.1097/01.mph.0000212968.98501.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–7. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koumbourlis AC, Lee DJ, Lee A. Longitudinal changes in lung function and somatic growth in children with sickle cell disease. Pediatr Pulmonol. 2007;42(6):483–8. doi: 10.1002/ppul.20601. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2006 doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]