Abstract
Information about sensory stimuli is represented by spatiotemporal patterns of neural activity. The complexity of the central nervous system, however, frequently obscures the origin and properties of signals and noise that underlie these activity patterns. We minimized this constraint by examining mechanisms governing correlated activity in mouse retinal ganglion cells (RGCs) under conditions where light-evoked responses traverse a specific circuit - the rod bipolar pathway. Signals and noise in this circuit produced correlated synaptic input to neighboring On and Off RGCs. Temporal modulation of light intensity did not alter the degree to which noise in the input to nearby RGCs was correlated, and action potential generation in individual RGCs was largely insensitive to differences in network noise generated by dynamic and static light stimuli. Together, these features enable noise in shared circuitry to diminish simultaneous action potential generation in neighboring On and Off RGCs under a variety of conditions.
Differences in the responses of neurons in parallel pathways to sensory stimuli depend on many features of network input (e.g.,1–4 ), including: (1) differences in the source and properties of excitatory and inhibitory synaptic input; (2) the prevalence of noise in shared circuitry that causes correlated variability in synaptic input; and (3) the source and properties of noise that limits the fidelity of action potential generation in individual neurons. Ambiguities in the signals that anatomically-defined circuits convey and/or the sources of input that parallel pathways receive frequently obscure the contribution of these factors to the functional properties of specific neurons.
The mammalian retina provides an excellent opportunity to identify the contribution of distinct and shared circuitry to the functional properties of neurons in parallel pathways (reviewed in 5–7 ). Activity in On and Off RGCs is influenced by distinct features of light stimuli, and dim fluctuating light stimuli modulate action potential generation in RGCs via specific and tractable circuitry 8,9 - the rod bipolar pathway (rod -> rod bipolar -> AII amacrine -> cone bipolar terminal -> RGC; reviewed in 10–12 ). The AII amacrine cell plays a fundamental role in this circuitry by segregating signals into On and Off pathways; light-evoked depolarization of AII amacrine increases excitatory synaptic input to On RGCs through gap junctions with the synaptic terminals of On cone bipolar cells and decreases excitatory synaptic input to Off RGCs via glycinergic inhibition of the synaptic terminals of Off cone bipolar cells (Fig. 1a ).
Figure 1.
Synaptic input to AII amacrine cells and Off transient RGCs exhibits similar sensitivity to light stimuli and AMPA/Kainate receptor antagonists. a, Schematic of the mammalian retina, with elements of the rod bipolar pathway highlighted in gray. RBC=rod bipolar cell; CBC = cone bipolar cell; RGC = retinal ganglion cell; mGluR = metabotropic glutamate receptor; GluR = ionotropic glutamate receptor; GlyR = glycine receptor; GapJ = gap junction. Convergence (∼20 rods -> 1 RBC; ∼25 RBCs -> 1 AII; ∼20 AIIs -> RGC) is not represented. b, Family of synaptic currents evoked simultaneously in an AII amacrine (−70 mV) and Off transient RGC (+10 mV) by a 10 ms light stimulus (λ) producing 0.075 –1.2 photoisomerizations per rod (Rh*/rod). c, Charge transfer for synaptic currents evoked in AII amacrine cells (right axis, open circles) and Off transient RGCs (left axis; filled circles) by light stimuli of varying intensity (n=4 pairs; Mean ± SEM). d, Latency of postsynaptic current onset in AII amacrine cells and Off transient RGCs. e–g, Same as b–d, but in the presence of the AMPA/Kainate receptor antagonist NBQX (10 μM).
We find that AII amacrine cells, in addition to modulating excitatory synaptic input to On and Off RGCs, provide direct inhibitory synaptic input to Off RGCs. This circuitry enables light-evoked signals in AIIs to generate anti-correlated action potentials in On and Off RGCs in two fundamentally distinct ways: (1) via negatively correlated excitatory synaptic input to On and Off RGCs, and (2) positively correlated excitatory synaptic input to On RGCs and inhibitory synaptic input to Off RGCs. Noise arising in the rod bipolar circuitry, like signals evoked by changes in light intensity, produces significant correlations in synaptic input to neighboring On and Off RGCs, and these correlations depend only slightly on the degree to which light stimuli fluctuate about the mean intensity. This correlated noise in synaptic input reinforced differences in the patterns of action potentials generated in nearby On and Off RGCs. Together, these features of specific, shared circuitry dictate the temporal precision of action potential generation in individual neurons and highlight how common sources of network input can accentuate differences in the output of parallel pathways.
RESULTS
The experiments described here explore network properties governing temporal patterns of action potential generation in individual and neighboring mouse RGCs. The manuscript is divided into three sections: (1) identification of the synaptic circuitry by which dim light stimuli modulate excitatory and inhibitory synaptic input to neighboring On and Off RGCs; (2) the implications of this circuitry for correlated noise in the network input nearby RGCs receive; and (3) the impact of correlated and uncorrelated noise in synaptic input on the temporal pattern of action potential generation in On and Off RGCs.
Circuitry governing synaptic input to On and Off RGCs
Modulation of excitatory synaptic input alone is sufficient to produce the pattern of action potentials elicited by fluctuating dim light stimuli in On RGCs 8. The same light stimuli, however, produce large (>50 nS) inhibitory postsynaptic conductances in Off RGCs, and this inhibitory synaptic input plays a critical role in modulating spike generation in these cells 8. Several features make the glycinergic AII amacrine cell an attractive candidate for the source of this inhibitory input: these cells are exquisitely sensitive to dim light stimuli 13–17 and they synapse with the dendrites of Off RGCs 18-22. Functional data supporting this hypothesis includes the presence of inhibitory synaptic input to some Off RGCs under conditions where light-evoked signals in amacrines other than the AII amacrine should be abolished 23 and the similarity of excitatory synaptic input to On RGCs and inhibitory synaptic input to Off RGCs 8. This existing data, however, is equally consistent with an amacrine cell downstream of On cone bipolar cells providing inhibitory synaptic input to Off RGCs; indeed, inhibitory synaptic input to RGCs is frequently attributed to feedforward inhibitory circuits of this type 6. The following three lines of evidence indicate that the AII amacrine cell is, indeed, the source of inhibitory synaptic input evoked in Off RGCs by dim light stimuli.
(1) The amplitude and latency of light-evoked currents in AII amacrines and Off transient RGCs exhibit similar dependence on light intensity
If AII amacrine cells provide inhibitory synaptic input to Off RGCs, dim light flashes should generate a light-evoked signal in AII amacrine cells that is followed quickly by an inhibitory postsynaptic current (IPSC) in Off RGCs. Moreover, the amplitude and latency of light-evoked postsynaptic currents of AII amacrine cells and Off RGCs should exhibit a similar dependence on light intensity.
We tested these predictions by simultaneously measuring synaptic input to an AII amacrine cell (held at the reversal potential for inhibitory synaptic input) and an Off RGC (held at the reversal potential for excitatory synaptic input) evoked by steps in light intensity from darkness; we focused on Off transient RGCs, a class of RGC distinguished by its large soma and transient bursts of action potentials at light offset 8,16,24. The amplitude of excitatory synaptic input to AII amacrine cells and the IPSC in Off transient RGCs differed by more than an order of magnitude (Fig. 1b ), however relative changes in the area (time integral or charge transfer; Fig. 1c ) and onset latency (Fig. 1d ) of light-evoked postsynaptic currents in the two cells exhibited a near-identical dependence on light intensity.
(2) Light-evoked currents in AII amacrines and Off transient RGCs persist in the presence of AMPA/kainate receptor antagonists
Antagonists that block excitatory synaptic signaling between bipolar and amacrine cells fail to abolish responses evoked by bright light stimuli in AII amacrine cells 17,25,26. Under these conditions, signals transmitted from cone photoreceptors to On cone bipolar cells (via metabotropic glutamate receptor-mediated synaptic signaling) can be transmitted to AII amacrine cells via electrical (gap junction-mediated) synaptic transmission.
Signals generated in rod photoreceptors could also be transmitted to On cone bipolar cells, either directly 27 or via cone photoreceptors through rod-cone gap junctions (reviewed in 10,12 ). If AII amacrine cells are a source of inhibitory synaptic input elicited in Off RGCs by dim light stimuli, and signals propagated to AII amacrine cells via either alternative pathway are sufficient to elicit glycine release, IPSCs in Off RGCs should persist in the presence of the AMPA- and kainate-receptor antagonist 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline -7-sulfonamide (NBQX; 10 μM) under conditions in which the alternative pathway is active (see ref. 23 ). Consistent with this hypothesis, signals evoked in AII amacrines and Off transient RGCs by brief, dim steps in light intensity from darkness persisted in the presence of NBQX (Fig. 1e–g ). Persistent IPSCs were not due to partial block of AMPA/kainate receptors, as NBQX abolished spontaneous and light-evoked excitatory postsynaptic currents in RGCs (data not shown); NBQX-insensitive currents in AII amacrine cells were also insensitive to the NMDA receptor antagonist D-APV (30 μM, data not shown; 26 ).
(3) Time course of excitatory input to On RGCs and inhibitory input to Off transient RGCs is nearly identical
If the AII amacrine cell is the source of inhibitory input to Off RGCs, signals in the rod bipolar pathway should produce excitatory synaptic input to On RGCs and inhibitory synaptic input to Off RGCs by traversing the same number of chemical synapses (Fig. 1a ). By the same logic, signals in the rod bipolar pathway produce changes in excitatory synaptic input to On RGCs via one fewer chemical synapse than changes in excitatory synaptic input to Off RGCs. These features of the circuitry make two predictions: (1) signals in the rod bipolar pathway should generate roughly coincident changes in inhibitory synaptic input to Off RGCs and excitatory synaptic input to On RGCs if the AII amacrine is the source of inhibitory synaptic input; and (2) changes in excitatory synaptic input to Off RGCs should lag behind changes in excitatory synaptic input to On RGCs. To test these predictions we simultaneously measured responses in nearby On and Off transient RGCs elicited by fluctuating light stimuli that produced, on average, 2–3 Rh*/rod/sec; at this (and lower) light intensity, blocking synaptic signaling between rod photoreceptors and rod bipolar cells with the mGluR6 agonist L-APB largely abolishes responses in Off RGCs8, indicating that these responses are transmitted via the rod bipolar pathway.
Figure 2a shows the mean stimulus-evoked excitatory postsynaptic conductance (gExc) - the measured excitatory postsynaptic current (EPSC) divided by the driving force for EPSCs - in an On RGC and the mean inhibitory postsynaptic conductance (gInh) in a neighboring Off transient RGC. Fluctuations in light intensity generated larger changes in Off gInh than On gExc(Fig. 2a; 8 ), but the time course of the two postsynaptic conductances was nearly identical. The amplitude of the peak of the cross correlation function between these two conductances (0.85 ± 0.01; n=5; Fig. 2a ), and the lack of a significant offset in the peak of this function (0.9 ± 0.7 ms; p>0.25; Fig. 2a, inset), demonstrate that, on average, changes in light intensity produce proportional and nearly simultaneous changes in On gExc and Off gInh. The offset of the negative peak of the cross correlation function between gExc in neighboring On RGCs and Off transient RGCs (Fig. 2b ), on the other hand, indicates that glycinergic signaling between the AII amacrine and Off cone bipolar terminal introduces a ∼5 ms delay in light-evoked changes in Off gExc relative to On gExc. Together with the data presented in Figure 1, and established anatomical features of the rod bipolar pathway 18–22, these results strongly support the idea that the AII amacrine is the source of most (if not all) inhibitory synaptic input evoked in Off transient RGCs by dim light stimuli. In addition, these results indicate that signals in AII amacrine cells govern the opposite responses of On and Off RGCs via dynamic regulation of both excitatory and inhibitory synaptic input.
Figure 2.
Fluctuating light stimuli generate correlations in excitatory and inhibitory synaptic input to neighboring On and Off RGCs. a, Mean excitatory postsynaptic conductance (gExc; black trace; holding potential −70 mV) in an On RGC and inhibitory postsynaptic conductance (gInh; blue trace; holding potential 10 mV) in an Off transient RGC evoked by repeated presentations of the same pattern of time-varying changes in light intensity (λ; shown above). Peak-normalized postsynaptic conductances are superimposed below. Mean cross correlation function between On gExc and Off gInh (n=5 pairs, Mean ± SEM). Inset shows the peak of the cross correlation function on an expanded time scale. b, Same as a, except with gExc in the Off transient RGC (red trace).
Correlated and uncorrelated noise in synaptic input to RGCs
The degree to which patterns of action potentials in neighboring On and Off RGCs are distinct depends on the intrinsic mechanisms governing action potential generation in each cell, the mean light-evoked postsynaptic conductances in each cell (‘light-evoked signals’; Fig. 2 ), and the degree to which variability (or ‘noise’) in synaptic input to RGCs is correlated. The experiments in this section characterize noise in synaptic input that neighboring On and Off RGCs receive.
Correlated noise in synaptic input to nearby On and Off RGCs
To determine whether noise in shared circuitry - i.e. in or upstream of the AII amacrine - produced correlated trial-to-trial variability in synaptic input to neighboring On and Off RGCs, we subtracted the mean postsynaptic conductance (data not shown) evoked by repeated presentations of the same stimulus from the response on each individual trial (for example, Fig. 3a ). We then computed the cross-correlation function between the resulting conductance residuals (Fig. 3b ) measured in neighboring RGCs. The cross correlation function between the residuals of gExc measured in On RGCs and the residuals of gInh measured simultaneously in Off transient RGCs (Fig. 3d, gold line) exhibited a positive peak without a significant time offset (−0.1 ± 0.1 ms; p>0.5; n=4), like the cross correlation function between the full On gExc and Off gInh (Fig. 3d, black line). Shuffling trials abolished structure in the cross correlation functions for residuals of postsynaptic conductances evoked by time-varying stimuli (Fig. 3d, dashed gold line) but had little effect on the cross correlation function between the total gExc in On RGCs and gInh in Off transient RGCs (Fig. 3d, dashed black line). Thus both signals and noise in the rod bipolar pathway produce correlations in synaptic input to neighboring RGCs.
Figure 3.
Correlated noise in network input to nearby RGCs. a1, Simultaneous recording of the excitatory postsynaptic conductance (gExc; holding potential −70 mV) in an On RGC and inhibitory postsynaptic conductance (gInh; holding potential 10 mV) in a neighboring Off transient RGC elicited by fluctuations in light intensity (λ). b, Residuals - the conductance measured on a single trial minus the mean conductance across trials - of the conductances in a1. c, Residuals of conductances evoked in the same pair of RGCs by a fixed-intensity stimulus. d4, Cross correlation functions between On gExc and Off gInh (black) and residuals of On gExc and Off gInh evoked by dynamic (50% contrast; gold) and static (0% contrast; blue) stimuli (Mean ± SEM, n=4 pairs). Dashed lines represent the cross correlation function computed between responses measured on shuffled - i.e., non-coincident - trials. Inset; Width at half max for cross correlation functions.
To determine how properties of light stimuli influenced correlated noise in network input, we compared the cross correlation function for residuals of postsynaptic conductances evoked in neighboring RGCs by stimuli that differed only in the degree to which light intensity varied about the mean. Cross correlation functions were normalized to represent the fraction of the total variance in synaptic input accounted for by correlated noise. The peak and width at half max of the cross correlation function between residuals of On gExc and Off gInh evoked by dynamic (50% contrast; Fig. 3d, gold) and static (0% contrast; Fig. 3d, blue) light stimuli were indistinguishable (p> 0.11 and p> 0.47). The total input variance was also insensitive to the stimulus (see Fig. 4 ). Thus the noise that generated correlated variability in synaptic input to neighboring On and Off RGCs was insensitive to modulations in light intensity.
Figure 4.
Network noise exhibits similar properties in the presence and absence of fluctuations in light intensity. a, Mean gExc evoked in an On RGC by repeated presentations of the same light stimulus (λ; shown above). Bottom, residual of gExc from the areas denoted by gray boxes. b, Mean power spectra for gExc evoked by fluctuations in light intensity (black) and the residuals of gExc evoked by dynamic (gold) and static (blue) stimuli in 8 On RGCs. c, Mean auto correlation function for residuals of gExc evoked by dynamic (gold) and static (blue) stimuli. d, Mean amplitude distribution of residuals evoked by dynamic (gold) and static (blue) stimuli. e, Relationship between variance and mean of responses evoked by repeated presentations of the same stimulus - the variance and mean were calculated across trials for each time point. f, Distribution of synaptic conductance amplitudes elicited by dynamic (gold) and static (blue) light stimuli. g–l, same as a–f, except for gInh in Off transient RGCs (n=6).
Where does noise giving rise to correlated variability in the synaptic input to neighboring RGCs arise? Data presented in Figure 3 provides two constraints. First, the positive correlation between residuals of On gExc and Off gInh(Fig. 3d ) and the negative correlation between residuals of On gExc and Off gExc (see supplementary figure 1 ) indicate that correlated noise in synaptic inputs to RGCs likely arises in, or upstream of, AII amacrine cells. The difference in the width of cross correlation functions between (1) residuals in neighboring RGCs and (2) the total light-evoked postsynaptic conductances in the same RGCs (Fig. 3d, inset) also constrains the source of the noise generating correlated network variability. Noise associated with responses of rod photoreceptors to dim light stimuli has a similar time course as the response itself 28,29, and the slow kinetics of phototransduction produce a broad (50–100 ms) window over which light-modulated synaptic input to neighboring RGCs is correlated (e.g., Fig. 3d, black lines). Thus the narrow width of the cross correlation function between conductance residuals must reflect a noise source downstream of phototransduction.
Noise in network input to individual On and Off RGCs
Focusing on correlated network input to neighboring RGCs enabled us to examine the properties of signals and noise originating in shared circuitry. However, the cross correlation function between the residuals of On gExc and Off gInh had a peak amplitude near 0.35, indicating that ∼65% of the variance in synaptic input to individual RGCs was uncorrelated. We therefore compared the properties of correlated and uncorrelated network noise.
Correlated noise in network input was insensitive to stimulus contrast and exhibited fluctuations too rapid to explain from events in rod phototransduction (Fig. 3d ). Several measures of the total noise in network input to individual RGCs shared these properties. First, power spectra of residuals measured in the presence and absence of fluctuations in light intensity were similar (Fig. 4b,h ). Second, the width of autocorrelation functions for residuals of On gExc (Fig. 4c ) and Off gInh (Fig. 4i ) evoked by time-varying (gold) and time-invariant (blue) stimuli were only slightly different (On gExc = 8.9 ± 1.0 ms vs. 9.9 ± 1.1 ms, p<0.01; Off gInh = 11.1 ± 0.7 ms vs. 17.5 ± 3.1 ms, p<0.05). These two observations indicate that the time course of fluctuations in synaptic input was relatively insensitive to temporal modulation of light intensity. Third, the width of the autocorrelation function for residuals was much less than the width of the autocorrelation of the full response to time-varying stimuli (data not shown), indicating that much of the noise in the total network input originated downstream of rod phototransduction.
The amplitude of fluctuations in synaptic input to RGCs, unlike the time course, was sensitive to temporal modulation of light intensity. Thus the amplitude distribution of residuals evoked by dynamic light stimuli (‘modulated network noise’) exhibited a higher proportion of small and large values compared to the distribution evoked by static stimuli (‘background network noise’; Fig. 4d, j ). This distinction likely reflects differences in the degree to which dynamic and static stimuli modulated synaptic input; variance in synaptic input increased as the amplitude of signals increased (Fig. 4e, k ), and fluctuating stimuli increased dramatically the proportion of small and large amplitude signals (Fig 4f, l ). The next section tests the sensitivity of RGCs to these differences.
Impact of noise in synaptic input on spike generation
To determine whether RGCs are sensitive to differences in noise during static and dynamic stimuli, we blocked synaptic input to RGCs pharmacologically and compared the trial-to-trial variability of action potential generation elicited by dynamic (conductance) clamp injection 30,31 of three unique sets of light-evoked postsynaptic conductances: (1) the mean gExc and gInh evoked in On or Off transient RGCs by repeated presentations of the same 50% contrast stimulus (Fig. 5a, b; top); (2) the mean gExc and gInh evoked by the 50% contrast stimulus plus background network noise (Fig. 5a, b; middle); and (3) the individual gExc and gInh evoked by single presentations of a time-varying stimulus (which by definition contain the mean conductances plus modulated network noise; Fig. 5a,b; bottom). If RGCs are sensitive to differences in network noise associated with responses to dynamic and static stimuli, trial-to-trial variability in action potential generation should differ for action potentials elicited by somatic injection of the 2nd and 3rd set of conductances. Somatic injection of exactly the same gExc and gInh waveforms from one trial to the next generated highly reproducible patterns of spikes in the presence of AMPA/kainate, NMDA, GABA, and glycine receptor antagonists (Fig. 5a,b; top traces; 8 ). Background network noise (Fig. 5a, b; middle traces) or modulated network noise (Fig. 5a,b; bottom traces) increased considerably the variability of action potential generation in both On and Off transient RGCs.
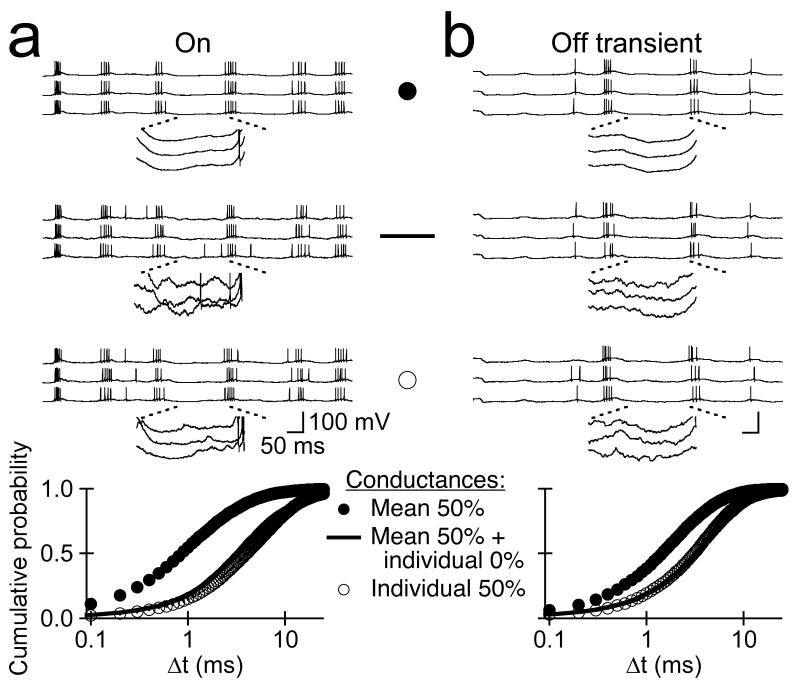
Figure 5.
Network noise measured in the presence and absence of fluctuations in light intensity impacts the temporal precision of AP generation similarly. a, Top, AP trains generated on three successive trials by dynamic (conductance) clamp injection of the mean gExc and gInh waveforms evoked by time-varying stimuli in On RGCs. Middle, AP trains elicited by dynamic clamp injection of the mean postsynaptic conductances evoked by time-varying stimuli plus background network noise measured on three separate trials. Bottom, AP trains generated by dynamic clamp injection of postsynaptic conductances evoked on three separate trials by the same sequence of fluctuations in light intensity. Insets show an expanded view of the subthreshold membrane potential for a section of each trace; scale bar values for insets is 1/10 of that for the full traces. Graph at bottom shows the cumulative probability distribution of Δt values for APs evoked by dynamic clamp injection of distinct sets of light-evoked postsynaptic conductances (n=6, Mean ± SEM). b, same as a, for Off transient RGCs (n=7). Dynamic clamp experiments were performed in the presence of NBQX (5–10 μM), D-APV (10–20 μM), and strychnine (10–20 μM) to block AMPA/Kainate, NMDA, GABA, and glycine receptor-mediated synaptic input.
To compare quantitatively the temporal variability of action potential generation we employed a spike distance metric 32. The algorithm underlying this metric generates a unique pairing of >90% of the action potentials on two different trials, and the temporal offset (Δt) of paired action potentials provides a measure of temporal precision (see Methods and 8 ). In On RGCs, the median Δt of action potentials evoked by conductances containing background network noise was slightly greater than that of action potentials elicited by conductances with modulated network noise (3.9 ± 0.1 ms, 3.0 ± 0.2 ms; n=6, p<0.003); in Off transient RGCs, trial-to-trial variability in the timing of action potential generation with background network noise was slightly less than that with modulated network noise (3.0 ± 0.1 ms, 3.2 ± 0.1 ms; n=7, p<0.04). Repeated injection of the mean light-evoked synaptic conductances, however, produced a much more dramatic change in precision (p<0.0001). These data indicate that noise in background and modulated synaptic input produces a similar degradation of the temporal precision of action potential generation. This result, in turn, has implications for the properties of network input that limit the temporal precision of action potential generation (see Discussion).
The similar properties of correlated network noise for dynamic and static stimuli (Fig. 3 ), and the similar impact of background and modulated network noise on the temporal precision of spike generation in individual RGCs (Fig. 5 ), suggest that action potentials generated in neighboring On and Off RGCs may not be independent under a variety of lighting conditions. However, most of the noise in synaptic input to neighboring RGCs is uncorrelated, and this uncorrelated noise could minimize or obscure the impact of correlated network input on the output of neighboring neurons. To determine under what conditions correlated noise in synaptic input influenced correlations in the output of neighboring RGCs we examined the correspondence between patterns of action potentials evoked in neighboring On and Off transient RGCs by repeated presentations of the same stimulus; stimulus contrast for separate blocks of (∼10–20) trials was 0%, 10%, or 50% (Fig 6a-c ).
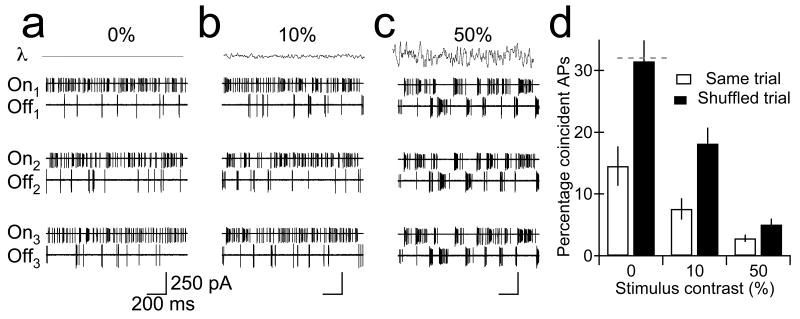
Figure 6.
Correlated network noise accentuates differences in AP generation between neighboring On and Off transient RGCs. APs generated in neighboring On and Off transient RGCs on three successive trials in the presence of 0% (a), 10% (b), and 50% (c) contrast stimuli. d, Percent of APs in Off RGC that occur within ±5 ms of an AP in a nearby On RGC on the same trial (open bars) or a different (shuffled; closed bar) trial (Mean ± SEM). Differences in the percentage of coincident APs on the same and different trials were highly significant under all conditions (p<0.001; n=6–7). Gray dashed line indicates prediction for two cells generating APs independently (see Methods).
The impact of correlated noise in synaptic input to neighboring RGCs was most obvious in the absence of fluctuations in light intensity (Fig. 6a ); under these conditions, coincident action potentials in neighboring On and Off transient RGCs were rare, while it was more common to observe coincident action potentials in spike trains recorded on separate trials - e.g., spikes generated in an On RGC on the second trial (On2) and spikes generated in the Off RGC on the third trial (Off3). We quantified the impact of correlated noise in synaptic input by comparing the percentage of spikes in an Off transient RGC that occurred within ±1,5, or 10 ms of a spike in a neighboring On RGC (1) on the same trial or (2) a different trial. In the presence of 0% contrast stimuli, 14.5% of the action potentials in an Off transient RGC occurred within ±5 ms of an action potential in an On RGC (Fig. 6d, left open bar); decreasing or increasing the window over which action potentials were considered coincident produced corresponding changes in this percentage (data not shown). Shuffling the trials abolished correlated noise in synaptic input to neighboring RGCs (Fig. 3d ) and increased the percentage of action potentials that occurred at the same time (Fig. 6d, left black bar); indeed, under these conditions, the percentage of action potentials that occurred within ±5 ms on different trials in On and Off transient RGCs (31.5%) was similar to that predicted by modeling action potential generation in neighboring RGCs as independent, Poisson processes (32%; see Methods). Thus correlated synaptic input to On and Off RGCs produces anti-correlated action potentials in the absence of light-modulated synaptic input.
What is the impact of correlated noise in synaptic input when fluctuations in light intensity produce complementary patterns of action potentials in On and Off RGCs (Fig. 6b,c3 )? Under these conditions, uncorrelated sources of network and/or intrinsic noise could reduce the extent to which action potential generation in parallel On and Off RGCs is distinct; correlated noise in the synaptic input On and Off RGCs, on the other hand, could help to reinforce and/or accentuate the distinct temporal pattern of action potentials generated in On and Off RGCs by changes in light intensity. We found that action potentials evoked in Off transient and On RGCs on the same trial by changes in light intensity were less likely to occur at the same time as action potentials evoked by the same stimulus on separate trials whether the temporal window over which action potentials were considered coincident was ±5 ms (Fig. 6d, middle/right bars) or ± 1 or 10 ms (data not shown). These results indicate that correlated noise in synaptic input - i.e., negative correlations in excitatory synaptic input to On and Off RGCs and/or positive correlations in excitatory synaptic input to On RGCs and inhibitory synaptic input to Off RGCs - accentuates differences in the pattern of action potentials evoked in On and Off RGCs by static and dynamic light stimuli.
DISCUSSION
We used light stimuli that modulate action potential generation via a specific, tractable circuit in mammalian retina - the rod bipolar pathway - to identify the source and properties of synaptic input governing patterns of spikes in single and neighboring RGCs. These experiments revealed that both signals and noise in the rod bipolar pathway generate correlated synaptic input to neighboring On and Off RGCs.
Source of synaptic input to RGCs
Identifying the source of inhibitory synaptic input to specific neurons, and the temporal relationship between excitatory and inhibitory synaptic input, is critical to understanding spatiotemporal patterns of neural activity (reviewed in 33,34 ). Indeed, these properties of neural circuits have attracted considerable interest in light of recent evidence that diverse origins and/or properties of synaptic input can give rise to distinct patterns and/or precision of spike generation in populations of neurons (reviewed in 35,36 ). The complexity of the central nervous system, however, has complicated efforts to identify the source and/or properties of synaptic input elicited by physiological stimuli.
In the retina, the distinct modulation of action potential generation in On and Off RGCs by dim light stimuli is frequently attributed to the opposite effect of light-evoked signals in AII amacrine cells on excitatory synaptic input to On and Off RGCs (reviewed in 10,12 ); indeed, fluctuating dim light stimuli produce negatively correlated modulation of excitatory synaptic input to neighboring On and Off RGCs (Fig. 2b ). However, the same stimuli also generate large inhibitory postsynaptic conductances in Off RGCs, and this conductance contributes substantially to light-evoked patterns of action potentials 8.
Our results indicate that the AII amacrine cell is the source of this inhibitory input. By providing direct glycinergic synaptic input to RGCs, and modulating excitatory synaptic input to RGCs via interactions with cone bipolar terminals, activity in AII amacrine cells can give rise to anti-correlated action potential generation in neighboring On and Off RGCs via two fundamentally different mechanisms: (1) negatively correlated modulation of excitatory synaptic input to On and Off RGCs and/or (2) nearly simultaneous, positively correlated modulation of inhibitory synaptic input to Off RGCs and excitatory synaptic input to On RGCs. Although additional experiments will be required to resolve the relative impact of these two mechanisms on anti-correlated AP generation, these results (1) provide powerful insight into the source and properties of synaptic input that govern activity in nearby RGCs and (2) identify principles that could influence activity in parallel circuits throughout the CNS.
Source and properties of noise in network input to RGCs
Positive or negative correlations in action potential generation between neighboring RGCs persist when changes in light intensity are small or non-existent 37–39 (reviewed in 5 ). Under these conditions, correlations in action potential generation must reflect correlated variability in the synaptic input to neighboring RGCs - i.e., correlated noise. We found that the correlated noise in the input to neighboring RGCs arose in (or upstream) of the AII amacrine cell. This result was neither expected nor required based on existing knowledge of the rod bipolar circuitry; uncorrelated noise arising downstream of the AII amacrine cell could have masked noise arising in shared upstream circuitry.
The degree to which noise in the synaptic input to neighboring RGCs was correlated did not depend on the degree to which light stimuli fluctuated about the mean intensity. However, there was a strong relationship between the variance and mean of synaptic input to individual RGCs elicited by temporal modulations in light intensity (Fig. 4e, k ). How can correlations in synaptic input remain constant when the total variance in the input to individual cells varies with the amplitude of light-evoked signals? Our data are consistent with a model in which noise in the rod bipolar circuitry is largely constant over the range of light stimuli we delivered, signals and noise in the AII amacrine are independent and additive, and the relationship between responses in AII amacrine cells and synaptic input to RGCs is nonlinear. Below we highlight data that support this hypothesis.
Noise in the input to AII amacrine cells exhibits only a weak dependence on light intensity across a broad range of background light intensities (0–40 Rh*/rod/sec; 14 ). Our results demonstrate that correlated noise in the synaptic input to adjacent RGCs is insensitive to fluctuations about a mean light intensity that produces, on average, 2–3 Rh*/rod/sec. These results are consistent with the idea that noise in the input to AII amacrine cells is constant for the dynamic and static stimuli we used (Fig. 7, Input) and that this noise contributes to correlated variability in the network input to neighboring RGCs. Consistent with this view, most of the noise in ganglion cell synaptic inputs appears to be inherited from noise in the AII synaptic inputs across a large range of mean light intensities 14.
Figure 7.
Schematic representation of input and output properties of AII amacrine cells. Sinusoidal changes in light intensity modulate signals (black line) in the input to AII amacrine cells; noise (red lines) about the input signal, however, remains constant. a, Parallel outputs of AII amacrine cells are linear transformations of signals and noise in the input. b, Parallel outputs of AII amacrine cells are identical to each other but nonlinearly related to signals and noise in the input.
If signals and noise in AII amacrine cells are independent and additive the degree to which noise in parallel outputs of the AII amacrine cell are correlated should be independent of the amplitude of the light-evoked signal. This prediction holds whether input to RGCs exhibits a linear (Fig. 7a ) or nonlinear (Fig. 7b ) dependence on responses in the AII amacrine cell. Although both of these scenarios could explain the properties of correlated noise in synaptic input that we measured, the latter provides a more likely explanation for our results. In particular, conductances measured during modulated light stimuli exhibit a clear asymmetry consistent with synaptic rectification (Fig. 2 ). Release at many synapses throughout the CNS exhibits a nonlinear dependence on Ca2+, and this (or another) nonlinearity likely underlies the observed relationship between the variance and mean of synaptic input evoked in RGCs by fluctuating light stimuli (Fig. 4e,k ).
Impact of network noise on spike generation
The degree to which noise in the input to neighboring RGCs was correlated did not depend on stimulus contrast, suggesting that correlated noise in the input to RGCs could influence correlations in the output of RGCs under a variety of conditions. On the other hand, the temporal precision and correlation of action potential generation depends critically (and nonlinearly) on the properties of the input that neurons receive (40–43 ), and noise in the input to individual RGCs varied with the degree to which light stimuli fluctuated about the mean intensity. These considerations prompted us to determine (1) whether correlated network noise in the input to neighboring RGCs influences correlations in the output of RGCs across a range of stimulus conditions and (2) if RGCs are sensitive to differences in noise measured in the presence and absence of stimulus contrast - i.e., modulated and background network noise.
We found that background and modulated network noise impacts the temporal precision of AP generation in On and Off RGCs similarly, indicating that RGCs are largely insensitive to differences in these sources of noise. One possible explanation for this result is that the differences in noise are small compared to the signal. Furthermore, noise in the synaptic input elicited by static and dynamic light stimuli could be similar over the range of input amplitudes that influence action potential generation 44. In an On RGC, for example, differences in the noise associated with very small and very large excitatory synaptic conductances would be unlikely to impact action potential generation: at one extreme the neuron is generating few, if any, action potentials; at the other extreme the pattern of action potentials is dictated largely by intrinsic properties of the RGC that govern the absolute and/or relative refractive period of action potential generation. This result and interpretation highlight an important general principle: differences in the properties of synaptic input do not necessarily generate differences in patterns of action potentials (reviewed in 45 ).
If RGCs are largely insensitive to differences in the noise generated by static and fluctuating light stimuli and the strength of correlated synaptic noise to neighboring RGCs is constant then action potential generation in nearby RGCs should be correlated across a range of stimulus conditions. Indeed, many fewer action potentials occurred simultaneously in neighboring On and Off RGCs than expected by chance for a range of stimulus contrasts (Fig. 6 ). In the presence of static (0% contrast) light stimuli, correlated noise in synaptic input alone produces anti-correlated action potential generation - this anti-correlated action potential generation would have been small or non-existent if noise sources downstream of the AII amacrine cell were large compared to that arising in shared upstream circuitry. In the presence of dynamic (> 0% contrast) stimuli both correlated signal and noise in the synaptic input contribute to anti-correlated action potential generation. Thus the distinct temporal patterns of action potentials in neighboring On and Off RGCs depend on a combination of signals and noise generated in the rod bipolar pathway. Correlated noise may play a similar role in reinforcing differences in the patterns of activity produced by other parallel circuits in the central nervous system.
The number and diversity of inputs that neurons receive frequently frustrates attempts to identify the source and properties of synaptic input that govern correlations in AP generation. By examining network input and action potential generation under conditions in which light-evoked signals traversed a specific, well-studied neural circuit we were able to: (1) quantify correlations in action potential generation in the presence of a variety of stimuli; (2) relate properties of correlated action potential generation to the properties/patterns of synaptic input that RGCs received; and (3) demonstrate that the dynamic control of synaptic input responsible for correlations in action potential generation reflected signals and noise in a single type of glycinergic amacrine cell. A similar approach in other tractable circuits should provide precise and insightful information about the properties of signals and noise that govern the representation of sensory information throughout the CNS.
METHODS
Tissue Preparation
Mice (c57/BL6, 5–8 weeks old) were dark adapted overnight and sacrificed according to protocols approved by the Administrative Panel on Laboratory Animal Care at the University of Washington. After hemisecting each eye, we removed the vitreous mechanically and stored the eyecup in a light-tight container in warm (∼32° C), bicarbonate-buffered Ames Solution (280–285 mOsm; Sigma-Aldrich, St. Louis) equilibrated with 5% CO2 / 95% O2. These and all subsequent procedures were carried out under infrared light (>900 nm) to keep the retina dark adapted.
All experiments were performed in a flat mount preparation of the retina. Pieces of isolated retina were placed retina ganglion cell-side up on a triangular piece of filter paper (Anodisc 25; Whatman, Maidstone, England) and transferred into a recording chamber. The retina was secured by nylon wires stretched across a platinum ring and perfused with warm (30–34° C) equilibrated Ames solution flowing at 6–8 mL/min.
Light Stimuli and Data Collection
Light from a blue light-emitting diode (LED; peak output = 470 nm) was delivered to the recording chamber via a fiber optic cable. The spatially uniform light stimulus illuminated a circular area 0.65 mm in diameter centered on the recorded cell(s). Calibrated photon fluxes at the preparation were converted to photoisomerizations per rod (Rh*/Rod) from the spectral output of the LED, absorption spectrum of rhodopsin, and an assumed collecting area per rod of 0.5 μm2. Most experiments used repeated presentations of a stimulus with a temporal trajectory drawn from a Gaussian distribution (bandwith 0–60 Hz). We defined the contrast of this stimulus as the SD divided by the mean.
We performed electrophysiological recordings with pipettes fabricated from thin-wall borosilicate glass (Sutter Instruments, Novato, CA). To record the pattern of light-evoked spikes without disrupting the internal composition of RGCs we performed cell-attached (or “loose patch”) recordings with pipettes (∼10 MΩ) filled with Ames solution. To characterize light-evoked excitatory and inhibitory synaptic input to RGCs, we performed whole cell voltage clamp recordings with pipettes (∼4–6 MΩ) filled with an internal solution containing 105 mM CsCH3SO3, 10 mM TEA-Cl, 20 mM HEPES, 10 mM EGTA, 5 mM Mg-ATP, 0.5 mM Tris-GTP, and 2 mM QX-314 (pH ∼7.3 with CsOH, ∼280 mOsm). Series resistance for voltage clamp recordings (∼10–15 MΩ) was compensated electronically by 75–80%. We corrected for the ∼10 mV junction potential offline. We targeted AII amacrine cells by focusing on neurons at the inner boundary of the inner nuclear layer which projected a tapered primary dendrite into the inner plexiform layer. AII amacrines were first identified by their characteristic light responses; the kinetics and sensitivity of responses recorded in the flat mount preparation were similar to those recorded in slice preparations 14. Subsequent visualization of neurons recorded with an internal solution containing Alexa 488 or 544 revealed (1) a narrow, dense tuft of dendrites that projected to the inner border of the inner plexiform layer and (2) lobular appendages in the Off sublamina of the inner plexiform layer. RGCs were voltage clamped near the reversal potential for chloride-mediated conductances (∼ −67mV) to isolate excitatory postsynaptic currents; to isolate inhibitory postsynaptic currents, we clamped RGCs slightly positive to the reversal potential for excitatory postynaptic currents (∼0 mV) to compensate for the effect of uncompensated series resistance (see 8 ). Light steps, which temporally segregate excitatory and inhibitory currents, were used to check isolation. For whole cell current and dynamic (conductance) clamp recordings we used an internal solution containing 125 mM K Aspartate, 10mM KCl, 1 mM MgCl2, 10 mM HEPES, 5 mM NMDG-HEDTA, 0.5 mM CaCl2, 4 mM Mg-ATP, and 0.5 Tris-GTP (pH ∼7.2 with KOH, ∼280 mOsm).
Signals were amplified with Axoclamp 200B amplifiers (Axon Instruments; Foster City, CA), filtered at 3 kHz (8 pole Bessel low pass), and sampled at 10 kHz (ITC16 interface, Instrutech Corporation, Port Washington, NY). Dynamic clamp30,31 experiments employed custom software to inject current, where the current was calculated from the measured cell voltage, the reversal potentials for excitatory (0 mV) and inhibitory (−80 mV; 8 ) synaptic inputs, and the light-evoked conductance waveforms. To mimic the effect of an excitatory postsynaptic conductance, for example, we injected the current Iexc(t)= gExc(t) • (VRev–V), where VRev= 0 mV and V equals the membrane potential of the cell at time t. The feedback between measured voltage and injected current operated on each voltage sample - i.e. at 10 kHz. Dynamic clamp experiments were performed in the presence of 5-10 μM NBQX to block AMPA/Kainate receptors, 10–20 μM D-APV to block NMDA receptors, and high concentrations (10–20 μM) of strychnine to block both GABA and glycine receptors 46,47.
Data Analysis
All analyses were performed using procedures written in MATLAB (The MathWorks, Natick, MA). The charge transfer of currents evoked in AII amacrine and Off RGCs by brief steps (10 ms; Fig 1 ) in light intensity was measured as the peak of the absolute value of the integrated current. The onset latency of light-evoked currents was measured as the time at which the absolute amplitude of the current exceeded 10% of the peak amplitude of the response to the brightest flash; the criterion threshold was calculated independently under control conditions and in the presence of NBQX.
We calculated the cross correlation between postsynaptic conductances recorded in neighboring RGCs, and the auto correlation for conductances in individual RGCs, according to the following equation:
Where x and y are measured postsynaptic conductances (comprised of N points each), and xi and yi represent the difference between the response on a single trial and the mean response to repeated presentations of the same stimulus. The temporal relationship between the mean light-evoked postsynaptic conductances was computed by averaging the response across many (>10) trials and computing the cross correlation function between these averaged signals. To examine the cross correlation function between light-evoked postsynaptic conductances in neighboring RGCs on a trial-by-trial basis, we computed the cross correlation function between responses on each trial and then averaged across the cross correlation functions. Residuals of postsynaptic conductances evoked by time-varying (>0% contrast) and time-invariant (0% contrast) stimuli were isolated by subtracting the mean response to repeated presentations of the same stimulus from each individual response; the relationship between noise in postsynaptic condutances in neighboring RGCs was determined by calculating the cross correlation function between residuals on a trial-by-trial basis. One-sided power spectra of postsynaptic conductances and residuals were calculated as described in ‘Numerical Recipes in C’ 48.
To estimate the percentage of APs that would occur coincidentally in neighboring RGCs receiving independent synaptic input we modeled AP generation as a poisson processes. In the presence of 0% contrast stimuli, On RGCs exhibited a higher firing rate (38.8 ± 5.1 APs/sec) than Off RGCs (17.7 ± 2.5 APs/sec; see Fig 5A ). For any given ±5 ms window, the probability of 0 spikes in an On RGC is P0= e-m, where m equals the mean number of spikes in the window (0.388). Thus, under these conditions, the probability of coincident (within ±1 ms) APs in neighboring Off and On RGCs is 1-P0 = 0.32 (or 32%).
We used the spike distance metric 32 to quantify the trial-to-trial variability in the temporal pattern of AP generation. This metric defines the distance - or how different two AP trains are in a defined metric space - by converting one train into the other via two operations: (1) shifting an AP in time or (2) deleting and/or adding a new AP. For a given pair of AP trains, the algorithm underlying the spike distance metric identifies the unique set of AP pairings that minimize the total spike distance, and the temporal precision of APs that are aligned by shifting in time is quantified from histograms of the corresponding Δt values (see 8 ).
All data are presented as mean ± SEM; statistical significance was evaluated with the Student's t test.
Supplementary Material
Acknowledgments
We thank David Perkel, Kevin Briggman, Lindsey Glickfeld, and Barry Wark for comments on the manuscript, and Eric Martinson and Paul Newman for excellent technical assistance. Support for this research was provided by HHMI and NIH (EY-11850).
References
- 1.Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- 3.Gegenfurtner KR, Kiper DC. Color vision. Annu Rev Neurosci. 2003;26:181–206. doi: 10.1146/annurev.neuro.26.041002.131116. [DOI] [PubMed] [Google Scholar]
- 4.Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- 5.Field GD, Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 2007;30:1–30. doi: 10.1146/annurev.neuro.30.051606.094252. [DOI] [PubMed] [Google Scholar]
- 6.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 7.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52:511–524. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 11.Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 1999;22:497–504. doi: 10.1016/s0166-2236(99)01458-7. [DOI] [PubMed] [Google Scholar]
- 13.Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- 16.Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- 18.Chun MH, Han SH, Chung JW, Wassle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- 19.Famiglietti EVJ, Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- 20.Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- 21.Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- 22.Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- 23.Cohen ED. Interactions of inhibition and excitation in the light-evoked currents of X type retinal ganglion cells. J Neurophysiol. 1998;80:2975–2990. doi: 10.1152/jn.1998.80.6.2975. [DOI] [PubMed] [Google Scholar]
- 24.Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang JJ, et al. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp AA, O'Neil MB, Abbott LF, Marder E. Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol. 1993;69:992–995. doi: 10.1152/jn.1993.69.3.992. [DOI] [PubMed] [Google Scholar]
- 31.Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J Neurosci Methods. 1993;49:157–165. doi: 10.1016/0165-0270(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 32.Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol. 1996;76:1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- 33.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 34.Destexhe A, Contreras D. Neuronal computations with stochastic network states. Science. 2006;314:85–90. doi: 10.1126/science.1127241. [DOI] [PubMed] [Google Scholar]
- 35.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callway EM. Feedforward, feedback, and inhibitory connections in primate visual cortex. Neural Netw. 2004;17:625–632. doi: 10.1016/j.neunet.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Arnett D, Spraker TE. Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol. 1981;317:29–47. doi: 10.1113/jphysiol.1981.sp013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginsburg KS, Johnsen JA, Levine MW. Common noise in the firing of neighbouring ganglion cells in goldfish retina. J Physiol. 1984;351:433–450. doi: 10.1113/jphysiol.1984.sp015254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastronarde DN. Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. J Neurophysiol. 1983;49:325–349. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- 41.de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature. 2007;448:802–806. doi: 10.1038/nature06028. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 43.Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demb JB, Sterling P, Freed MA. How retinal ganglion cells prevent synaptic noise from reaching spike output. J Neurophysiol. 2004;92:2510–2519. doi: 10.1152/jn.00108.2004. [DOI] [PubMed] [Google Scholar]
- 45.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 46.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 47.Protti DA, Gerschenfeld HM, Llano I. GABAergic and glycinergic IPSCs in ganglion cells of rat retinal slices. J Neurosci. 1997;17:6075–6085. doi: 10.1523/JNEUROSCI.17-16-06075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes in C : the art of scientific computing. Cambridge University Press; Cambridge; New York: 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.