Abstract
Cannabinoid drugs differ in their rank order of potency to produce analgesia versus other central nervous system effects. We propose that these differences are due to unique agonist-bound cannabinoid CB1 receptor conformations that exhibit different affinities for individual subsets of intracellular signal transduction pathways. In order to test this hypothesis, we have used plasmon-waveguide resonance (PWR) spectroscopy, a sensitive method that can provide direct information about ligand-protein and protein-protein interactions, and can detect conformational changes in lipid-embedded proteins. A recombinant epitope-tagged human cannabinoid CB1 receptor was expressed in insect Sf9 cells, solubilized and purified using two-step affinity chromatography. The purified receptor was incorporated into a lipid bilayer on the surface of the PWR resonator. PWR spectroscopy demonstrated that cannabinoid agonists exhibit high affinity (KD = 0.2 ± 0.03 nM and 2 ± 0.4 nM for CP 55,940 and WIN 55,212-2, respectively) for the purified epitope tagged hCB1 receptor. Interestingly however, these structurally different cannabinoid agonists shifted the PWR spectra in opposite directions, indicating that CP 55,940 and WIN 55,212-2 binding leads to different hCB1 receptor conformations. Furthermore, PWR experiments also indicated that these CP 55,940- and WIN 55,212 - bound hCB1 receptor conformations exhibit slightly different affinities to an inhibitory G protein heterotrimer, Gi1 (KD = 27 ± 8 nM and KD = 10.7 ± 4.7 nM, respectively), whereas they strikingly differ in their ability to activate this G protein type.
Keywords: trafficking, G proteins, PWR spectroscopy, functional selectivity
1. Introduction
Cannabis sativa (marijuana) has been used as a pharmacological agent and as a recreational drug for centuries. After identification of Δ9-tetrahydocannabinol as the major active ingredient of marijuana (Gaoni et al., 1964), numerous structurally different cannabinoid agents have been synthesized and characterized. Cannabinoids may be useful in clinical practice as analgesics, antiemetics, appetite stimulants, antiglaucoma agents and muscle relaxants (Pertwee, 2001; Tomida et al., 2004; Ben Amar, 2006). Unfortunately however, their central nervous system side effects are a fundamental barrier to widespread medicinal use. Cannabinoids produce their effects by activation of cannabinoid CB1 (Matsuda et al., 1990) or CB2 (Munro et al., 1993) receptors, members of Family A of the G-protein coupled receptors. Since the cannabinoid CB1 receptors are mainly found in the central nervous system (while the cannabinoid CB2 receptor type is preferentially expressed in immune cells and non-neuronal tissues) the central nervous system side effects of the cannabinoid agents are presumably due to cannabinoid CB1 receptor-mediated signal transduction processes.
The cannabinoid CB1 receptors interact with a broad group of structurally diverse ligands (Howlett et al., 2002). Interestingly, consistent and pronounced disparities have been observed between the potencies and efficacies of structurally different cannabinoid agonists in rodents in vivo (Wiley et al., 1998) in the measures of the cannabinoid tetrad (antinociception, hypothermia, catalepsy and reduction of spontaneous activity), suggesting fundamental differences in their mechanism of action. Since previous investigations have indicated that structurally different ligands may interact differently with the cannabinoid CB1 receptors (Song and Bonner, 1996; Shim et al., 2003; Shim and Howlett, 2006), we hypothesized that these disparities may be the consequence of unique agonist-bound receptor conformations that interact with distinct G protein types, and thus promote ligand-specific activation of unique intracellular second messenger pathways.
To test this hypothesis, we have employed a highly sensitive biophysical method, plasmon-waveguide resonance (PWR) spectroscopy (Salamon et al., 1997). PWR has been successfully used previously to evaluate ligand-mediated changes in the conformation of G-protein coupled receptors, to determine the affinities of G protein subtypes to ligand-bound receptors and to assess the efficacies of ligands to activate individual G proteins (Salamon et al., 2000; Alves et al., 2003; Alves et al., 2004a; Alves et al., 2004b).
The goal of the present study was to investigate whether two structurally distinct cannabinoid agonists (CP 55,940 and WIN 55,212-2, Fig. 1) interact with different human cannabinoid CB1 receptor conformations that may have differential effects on G protein activation. In order to perform these experiments we partially purified a fully glycosylated, G-protein-free, epitope-tagged human cannabinoid CB1 receptor. The results reported here provide evidence that the binding of CP 55,940 and WIN 55,212-2 shifts the PWR spectra of human cannabinoid CB1 receptor-containing proteolipid bilayers in opposite directions, indicating that these agonists produce unique human cannabinoid CB1 receptor conformations. Importantly, although CP 55,940- and WIN 55,212-2-bound human cannabinoid CB1 receptor conformations exhibited only slightly different affinities to a representative G protein heterotrimer Gi1, they markedly differed in their ability to activate this G protein type, consistent with the hypothesis that these agonists may produce different downstream intracellular effects. Direct demonstration of agonist-specific differences in activation of G protein-mediated signal transduction pathways should aid the design of novel cannabinoid pharmaceuticals that exhibit high potency towards desired subsets of signal transduction pathways (such as analgesia), but low potency toward signaling pathways that mediate undesirable side effects.
Fig. 1.
Structure of bicyclic (CP-55,940) and aminoalkylindole (WIN 55,212-2) cannabinoid receptor agonists.
2. Materials and Methods
2.1. Construction of expression vectors
The full-length cDNA encoding the human cannabinoid CB1 receptor in a pcDNA3.1 vector was purchased from the University of Missouri cDNA Resource Center (Rolla, MO). Site-directed mutagenesis (QuickChange Site-directed Mutagenesis Kit, Stratagene) was performed in order to mutate the stop codon of the human cannabinoid CB1 receptor cDNA and to introduce a unique SfuI restriction site at the 3′ end of the open reading frame. The cDNA fragment containing the human cannabinoid CB1 receptor open reading frame was digested from the mutant plasmid using HindIII and SfuI and ligated in frame into an epitope tagging pcDNA3.1mycHis expression vector. The human cannabinoid CB1 cDNA carrying myc- and His6 epitope tags at its C-terminus (human cannabinoid CB1-myc-His6 receptor) was subsequently inserted into BamHI and SphI - digested pFastBack donor plasmid. Recombinant bacmid was subsequently obtained through site-specific transposition according to the Bac-to-Bac Baculovirus Expression System instruction manual (Invitrogen, Carlsbad, CA).
All constructs were verified for their integrity by sequencing (University of Arizona Sequencing Facility).
2.2. Cell culture and expression of human cannabinoid CB1 -myc-His6 receptor
Sf9 cells (Invitrogen, Carlsbad, CA) from army fall worm (Spodoptera frugiperda) were grown as suspension cultures in a shaker incubator at 27° C in Sf900 II medium (Invitrogen, Carlsbad, CA). Cells were infected by adding 4 ml of amplified viral stock (multiplicity of infection=2) to 50 ml of logarithmically growing cells (2 × 106 cells/ml). After 96 h incubation the cells were harvested by centrifugation (1000 × g, 15 min, 4° C). Cell pellets were used immediately or stored at -20° C. Virus amplification and transfection procedures were performed according to the Bac-to-Bac Baculovirus Expression System instruction manual (Invitrogen, Carlsbad, CA).
2.3. Membrane preparation and radioligand binding studies
Cell pellets were homogenized in 10 ml of assay buffer (20 mM HEPES, 5 mM MgCl2, 1 mM EDTA, 0.3% BSA, pH 7.4, containing 50 μg/ml bacitracin, 30 μM bestatin, 10 μM captopril and 0.1 mM phenylmethylsulfonyl fluoride) using a Teflon-glass tissue grinder. The homogenates were centrifuged at 40,000 × g for 20 min at 4° C. The membrane pellet was resuspended in ice-cold assay buffer and used for radioligand binding studies. In saturation binding experiments membrane suspensions (0.04 mg/ml protein content) were incubated with [3H] SR141716A (2.1 - 40 nM) for 90 min at 30° C in assay buffer. The reaction was terminated by rapid filtration through Whatman GF/B glass fiber filters. The filters were rinsed 9 times with 4 ml ice-cold assay buffer containing 0.01% BSA. Filter-bound radioactivity was measured in a liquid scintillation counter. Nonspecific binding was determined in the presence of 10 μM AM 251 (Tocris, Ellisville, MO). Protein concentrations in membrane preparations were measured by the method of Bradford (1976).
2.4. Solubilization and purification of the human cannabinoid CB1-myc-His6 receptor
Membrane pellets, derived from 50 ml suspension of baculovirus/ human cannabinoid CB1-myc-His6 receptor, infected Sf9 cells, were homogenized in 25 mM HEPES buffer (pH 7.4), containing 0.5 M KCl, 1% dodecyl-β-D-maltoside and a protease inhibitor cocktail (Complete Mini, Roche Diagnostics, Indianapolis, IN), solubilized on ice for 30 min and centrifuged at 130,000 × g for 20 min at 4° C. The supernatant was collected and incubated with 2 ml WGA-agarose (Vector Laboratories, Burlingame, CA) for 18 h at 4° C. The column was washed with three column volumes of 25 mM HEPES (pH 7.4) buffer containing 0.5 M KCl and 0.1 % dodecyl-β-D-maltoside. Glycosylated proteins were eluted with a stepwise gradient (0.125 M, 0.25 M and 0.5 M) of N-acetyl-glucosamine. The final eluate was incubated with 1ml His-Select Nickel affinity gel (Sigma, St. Louis, MO) for 1 h at 4° C. The gel suspension was washed with 3-5 column volumes of 25 mM HEPES (pH 7.4) buffer, containing 0.15 M KCl and 30 mM octyl glucoside. The column was washed sequentially with buffers containing 10 mM imidazole and 25 mM imidazole, respectively. The epitope-tagged human cannabinoid 1 receptor was eluted in 25 mM HEPES (pH 7.4) buffer, containing 0.15 M KCl, 30 mM octyl glucoside and 200 mM imidazole. Dodecyl maltoside was used in the first step of the purification process to extract the protein with higher efficiency from the cell membranes since it is a stronger detergent. The same detergent was not used in the PWR chamber because dodecyl maltoside interacts strongly with lipid bilayers and thus we needed to exchange the detergent for a milder one, octylglucoside in this case. High ionic strength buffer (0.5 M KCl) has been chosen in the process of purification to keep the protein closer to physiological condition. In PWR studies the 0.5M KCl has been changed to 10mM in order to prevent prism damage. The purified protein was never stored in a frozen state. Since we found that the purified protein is not stable upon long-term storage, we purified a new batch for each experiment. A bicinchoninic acid assay (Pierce, Rockford, IL) was used to determine protein concentration in the purified samples.
2.5. Radioligand binding studies with the purified human cannabinoid 1-myc-His6 receptor
The quality of the purified human cannabinoid CB1-myc-His6 receptor protein was assessed by measuring the inhibition of [3H]SR 141716A binding by the cannabinoid CB1 receptor agonist, CP 55,940 (Tocris, Ellisville, MO). Briefly, aliquots (100 μl) of the final protein eluate were diluted with equal amount of 25 mM HEPES (pH 7.4) buffer, containing 10 mM KCl, 30 mM octylglucoside and protease inhibitors (Complete Mini, Roche Diagnostics, Indianapolis, IN) and incubated with 2 nM [3H]SR 141716A for 1 h at room temperature. Unbound ligand was separated from the ligand-receptor complex by ultrafiltration (Centicon YM 30,000, Gelman Labs, Ann Arbor, MI). After washing two times with a dilution buffer (see above), CP 55, 940 (10-7 to 10-10M) was added to the [3H]SR141716A - cannabinoid CB1 receptor complex and the mixture was incubated for 1 h at room temperature. Three washes have been performed after the incubation with the competitive drug in order to remove any unbound ligand. Remaining [3H] SR141716A binding was determined by liquid scintillation spectroscopy. Binding results were plotted using Graph Pad Prism (San Diego. CA).
2.6. SDS/PAGE and Western analysis
Samples of the purified receptor eluted from the nickel column in 25 mM HEPES (pH 7.4) buffer, containing 0.15 M KCl, 30 mM octyl glucoside and 200 mM imidazole, were pre-incubated at room temperature for 20-30 min with NuPage reducing sample buffer (Invitrogen, Carlsbad, CA), and resolved on 10% NuPage Bis-Tris gels. For detection of the total protein content, the gels were silver stained using FASTsilver staining kit (GBiosciences, St.Lous, MO). For immunodetection, the proteins were transferred to nitrocellulose membranes and incubated with either a goat anti-human cannabinoid CB1 receptor antibody (Santa Cruz Biotech, Santa Cruz, CA; 1:1000 dilution) or a mouse anti-myc antibody (Cell Signaling, Inc, Danvers, MA; 1:1000 dilution). The specificity of anti-human cannabinoid CB1 antibodies was confirmed earlier in rat brain tissue. It was also shown in preliminary experiments that the antibody does not label non-specific bands in non-infected Sf9 cells. In order to assess G protein binding to the purified receptor, the blots were incubated with nonselective anti-G protein α-subunit antisera (A 569, 1:1000 dilution, a generous gift form Dr. Susan Mumby (Mumby et al., 1986)). Immunoreactive bands were labeled with horseradish peroxidase-conjugated secondary antibodies and visualized using the SuperSignal WestDura Extended Duration Substrate system (Pierce, Rockford, IL).
Protein bands size were determined by the use of MagicMark XP Western Standards (Invitrogen, Carlsbad, CA).
2.7. PWR Spectroscopy
The principles and practice of PWR spectroscopy have been described extensively elsewhere (Salamon et al., 1999; Salamon and Tollin, 2001; Tollin et al., 2003). The PWR spectrometer (Proterion Corp.) used in these experiments had an angular resolution of 1 mdeg. Polarized light from a He-Ne laser (543.5 nm) was used to generate plasmons in the PWR resonator. PWR spectra were obtained by varying the incident angle of the perpendicular (p) or parallel (s) polarized laser light, and measuring the intensity of the light reflected from the plasmon resonator. Prior to receptor incorporation, lipid bilayers were formed on the silica surface of the PWR resonator from a 3:1 mixture (7 mg/ml) of egg phosphatidylcholine (PC) and 1-palmitoyl-2-oleyl-sn-glycero-3-phosphoglycerol (POPG) (Avanti Polar Lipids, Alabaster, AL), dissolved in squalene/ butanol/methanol (0.005:0.95:0.05, v/v). The partially purified human cannabinoid CB1-myc-His6 receptor was incorporated into the lipid bilayer by introducing aliquots of the solubilized protein into the aqueous compartment of the PWR resonator, under conditions that diluted the detergent concentration to below the critical micelle concentration (cmc = 25 mM). Unincorporated receptor and excess detergent were removed after the receptor incorporation reached equilibrium (about 2 hours) by washing the proteolipid membrane with 25 mM HEPES buffer (pH=7.4), containing 10 mM KCl.
To determine agonist-mediated conformational changes in the lipid-incorporated epitope-tagged human cannabinoid CB1 protein, small (microliter) amounts of CP 55,940 (Tocris, Ellisville, MO) or WIN 55, 212-2 (Sigma RGB, St. Louis, MO) solutions were added to the PWR chamber in a cumulative fashion and PWR spectra were acquired in regular time intervals until an equilibrium was reached - i.e. no further changes occurred in PWR spectra (approximately 15-20 minutes for each ligand concentration). Non-specific binding of the cannabinoid CB1 receptor agonists to the lipid bilayer was determined by measuring ligand dose-PWR spectral response curves for the lipid bilayer in the absence of any incorporated receptor in a similar fashion. KD values were obtained from plotting the resonance minimum position of the PWR spectra as a function of ligand concentration using GraphPad Prism software. Note that ligand binding to the receptor did not significantly decrease the total ligand concentration in the aqueous compartment due to the approximately 1000-fold difference in the bilayer and aqueous volumes. Since the PWR spectral shifts are directly proportional to the bound ligand concentration, KD values obtained in this way are true thermodynamic dissociation constants.
In order to determine the affinity of CP 55,940- or WIN 55,212-2-bound human cannabinoid CB1-myc-His6 receptor conformations to G-proteins, the detergent solubilized receptor was pre-incubated with saturating amounts of the appropriate agonist (50 nM), and the resulting agonist/eptope-tagged human cannabinoid CB1 receptor complex was incorporated into the lipid bilayer, using the method described above. Since it is known that GPCR receptor incorporation proceeds bidirectionally (Alves et al., 2003, 2004a,b), in this way a fraction of ligand-bound receptors will have their G-protein binding site accessible to the external aqueous medium. Subsequently, small aliquots of recombinant rat Giα1 (Calbiochem, San Diego,CA) and a βγ subunit mixture purified from bovine brain (Calbiochem, San Diego,CA) (1:1 ratio) were added to the equilibrated proteolipid system and PWR spectral changes were determined. After G protein saturation has been reached, GTPγS (Calbiochem, San Diego, CA) was added, and PWR spectral changes were again monitored. The KD values of G protein heterotrimer and GTPγS binding to the agonist bound receptor conformations were calculated using the Prism software, as described above for the agonists.
3. Results
3.1. Expression of an epitope-tagged human cannabinoid CB1 receptor in Sf9 insect cells
The optimal conditions of human cannabinoid CB1-myc-His6 receptor expression in Sf9 insect cells were determined by Western blots probed with anti-human cannabinoid CB1 receptor- and anti-myc tag antibodies. Membrane preparations were isolated from Sf9 cells 96 hours after infection with the secondary viral stock (multiplicity of infection=2). Human cannabinoid CB1- myc-His6 receptor expression levels in infected Sf9 cell membranes were assessed by saturation binding assays using a specific cannabinoid CB1 receptor antagonist, [3H]SR141716A. Saturation binding studies indicated that [3H] SR141716A exhibits high affinity (KD = 2.9 ± 0.8 nM) to infected Sf9 cell membrane preparations with Bmax values ranging from 1.5-5 pmol/mg protein (Fig. 2), depending on the number of cell passages and the freshness of the viral stock used for cell infection. Non-specific binding was determined in the presence of 10 μM AM251. Specific binding was 72% of total binding at 2 nM [3H] SR1417161A.
Fig. 2. Saturation isotherm for [3H] SR141716A specific binding to human cannabinoid CB1- myc-His6 receptor /Sf9 cell membranes.
Infection of Sf9 cells with the cannabinoid CB1- myc-His6 receptor -containing bacmid led to the expression of 2.4 ± 0.3 pmol/mg protein (mean ± S.E.M., n=4) [3H] SR141716A specific binding sites, with a KD value of 2.9 ± 0.8 nM. The figure is a representative of measurements (done in duplicates) performed for four independent infections.
3.2. Purification of the epitope tagged human cannabinoid CB1 receptor protein
The epitope tagged human cannabinoid CB1 receptor was solubilized from infected Sf9 cells using 1% dodecyl-β-D-maltoside as a detergent. Crude solubilized Sf9 cell membrane preparations were purified by two-step affinity chromatography using wheat germ agglutinin (WGA) and metal-affinity columns. WGA chromatography utilizes agarose-immobilized lectin that selectively binds membrane proteins containing complex oligosaccharides, such as the cannabinoid receptor. Bound glycoproteins are eluted with excess N-acetyl-D-glucosamine. Nickel Affinity Gel is effective for the purification of polyhistidine-tagged proteins.
After elution of the bound glycoproteins from the WGA column with 0.5M N-acetyl-D-glucosamine, Western blots with human cannabinoid CB1 receptor protein specific (recognizing the first 14 amino acid residues on the N-terminus, Santa Cruz Biotech, Santa Cruz, CA, Fig. 3) or epitope-tag-specific (recognizing the myc-tag on the C-terminus, Cell Signaling, Inc, Danvers, MA, data not shown) antibodies detected several immunoreactive bands with molecular weights of ∼ 48, 50, 55 and 64 kDa. The majority of the immunoreactive protein migrated with molecular weights considerably lower than the molecular weight of the human cannabinoid CB1- myc-His6 receptor expressed in mammalian (CHO) cells (64 and 55 kDa) (Georgieva et al., unpublished results, reported at the INRC meeting, 2005). Interestingly, it was found earlier that infection of Sf21 insect cells with a cannabinoid 1 receptor led to the expression of an N-terminally truncated form of the receptor (Xu et al., 2005). In our case however, this is not likely to be the problem, since the anti-human cannabinoid CB1 receptor antibody used for Western blots was raised against the extreme N-terminus of the human cannabinoid CB1 protein. Therefore, this antibody would not recognize any N-terminally truncated human cannabinoid CB1 receptor protein. On the other hand, it is well known that Sf9 cells express relatively low amounts of glycosyltransferase enzymes that catalyze the formation of complex, terminally sialylated N-glycans (Altmann et al., 1999). Consequently, the observed lower molecular weight immunoreactive bands are presumably due to partially glycosylated forms of the human cannabinoid 1 receptor. Therefore, in order to enrich the preparation in the desired, fully glycosylated protein a stepwise gradient elution was employed with increasing concentrations of N-acetylglucosamine. As seen in Fig. 3, elution with lower N-acetyl-D-glucosamine concentrations eliminated a large fraction of the lower molecular weight (presumably partially glycosylated) immunoreactive bands.
Fig. 3. Enrichment of the fully glycosylated form (64 kDa) of human cannabinoid CB1- myc-His6 receptor by lectin affinity chromatography and gradient elution.
The figure shows a representative Western blot probed with an antibody raised against the N-terminus of the human cannbinoid CB1 receptor. 1. solubilized Sf9/ human cannabinoid CB1- myc-His6 receptor membrane preparation. Glycosylated proteins were eluted from the WGA column with increasing concentrations of 2. 0.125 M, 3. 0.25 M and 4. 0.5 M N-acetylglucosamine; 5. molecular weight markers.
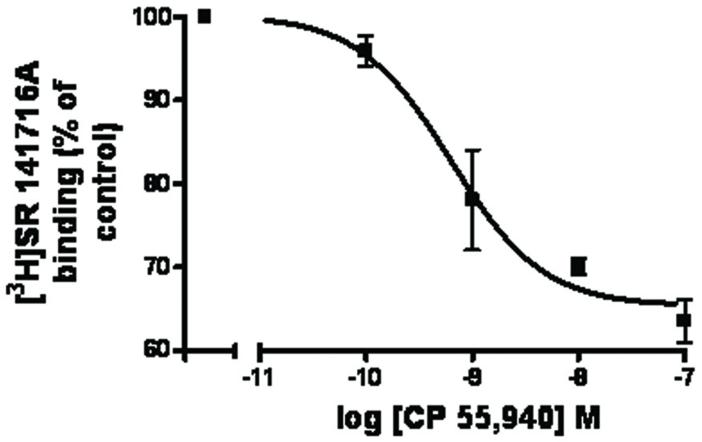
The final eluate (with 0.5 M N-acetyl-D-glucosamine) from the WGA column was further purified using metal affinity chromatography. The final purified protein was eluted from the nickel column with a buffer containing 200 mM imidazole. Silver stained gels (Fig. 4A) and Western blots (Fig. 4B) were used to assess the purity of the final protein preparation. As shown in Fig 4B, the major immunoreactive bands visible in Western blots using either anti-human cannabinoid CB1 receptor or anti-myc antibodies exhibited apparent molecular weights of ∼64 kDa and 55 kDa, and that is similar to the human cannabinoid CB1 receptor molecular weight obtained from transfected mammalian cells (Georgieva et al., unpublished results, reported at the INRC meeting, 2005). Importantly, [3H] SR141716A inhibition binding studies have demonstrated that the purified human cannabinoid CB1-myc-His6 receptor preparation retained its high affinity to the cannabinoid 1-selective agonist, CP 55,940 (IC50 = 0.6 ± 0.2 nM) (Fig. 5).
Fig. 4. Purification of the WGA column eluate using metal affinity chromatography.
A. Silver stained SDS-polyacrylamide gel: 1. solubilized membrane preparation, 2. partially purified human cannabinoid CB1- myc-His6 receptor, 3. MW markers. B. Western blot of the final, partially purified human cannabinoid CB1- myc-His6 receptor preparation probed with: 1. an anti-human cannabinoid CB1 antibody, 2. an anti- myc antibody. 1 μg protein has been loaded on each lane.
Fig. 5. Inhibition of specific [3H]SR 141716A binding to the partially purified human cannabinoid 1- myc-His6 receptor receptor by a cannabinoid 1 selective agonist, CP 55,940.
The data represent the mean value of two independent experiments done in duplicates. IC50 = 0.6 ± 0.2 nM (mean ± range).
In order to investigate whether the human cannabinoid CB1-myc-His6 receptor co-purify with constitutively bound G proteins from the infected Sf9 insect cells, we also performed Western blots with a non-selective anti-G protein α-subunit antibody, recognizing a highly homologous region of G proteins (A 569, a generous gift from Dr. Susan Mumby). This region shares 100% sequence identity between the cloned Drosophila G proteins and their mammalian counterparts. As seen in Fig. 6 the antiserum cross-reacted with insect G proteins in solubilized Sf9 cell preparations. On the other hand, no immunoreactive bands were detected in the partially purified human cannabinoid CB1 receptor preparation, indicating that the receptor does not co-purify with insect G proteins from the Sf9 cells.
Fig. 6. The human cannabinoid 1- myc-His6 receptor does not co-purify with insect G proteins from infected Sf9 cells.
A representative Western blot of the 1. solubilized and 2. partially purified human cannabinoid CB1- myc-His6 receptor preparations, probed with a nonselective anti-G protein α-subunit antibody (A569). Equal amounts of 2 μg total protein have been loaded on lanes 1 and 2.
3.3. PWR spectral shifts upon binding of two structurally different agonists
PWR spectra correspond to plots of light intensity reflected from the inner surface of a plasmon-generating silica-coated silver film deposited on a glass prism vs. the incident angle of a polarized He-Ne laser light (wavelength = 543.5 nm). In order to study the interaction of membrane proteins (such as G-protein coupled receptors) with their ligands, proteins are inserted into a lipid bilayer, formed on the hydrated surface of the silica layer across a small orifice in a Teflon space that separates the resonator surface from the aqueous compartment. PWR spectra are obtained with laser light polarized in either perpendicular (p-polarization) or parallel (s- polarization) directions relative to the plane of the lipid bilayer. Molecules immobilized on the silica surface alter the resonance characteristics by changing the optical properties (thickness and/or refractive index) of the system. This property allows the measurement of ligand-induced changes in the conformation and orientation of lipid-incorporated membrane proteins, such as G-protein coupled receptors (Salamon et al., 1999; Salamon and Tollin, 2001; Tollin et al., 2003). Briefly, the position, width and depth of the PWR spectrum are determined by the refractive index (n), the thickness (t), and the optical absorption coefficient (k) of the proteolipid bilayer. Since the refractive index (n) is dependent on the molecular polarizability, and thus the surface mass density, differences in the refractive index values measured using p- and s- polarized light (np and ns) will reflect the anisotropic structure of the proteolipid bilayer, i.e. the distribution of mass on the resonator surface. Thus, for anisotropic molecules inserted into the lipid bilayer with their long axes perpendicular to the surface plane (such as the cylindrically shaped cannabinoid 1 receptor), the refractive index in the p-direction will be larger than that measured in the s-direction. This refractive index anisotropy provides information about molecular orientation and/or conformation, as well as changes in these properties induced by bimolecular interactions occurring at the surface (e.g. ligand binding to an incorporated receptor).
Interestingly, we found that two structurally different cannabinoid agonists produced qualitatively distinct changes in the amplitude and direction of the PWR spectra of the human cannabinoid CB1-myc-His6 receptor - containing proteolipid bilayer (Figs. 7 and 8). Thus, whereas the bicyclic cannabinoid CB1 agonist, CP 55,940 induced a saturable rightward shift in the position of the plasmon resonance minimum, the aminoalkylindole ligand, WIN 55,212-2 caused a saturable leftward shift. These differences in the spectral consequences of binding of the two agonists are a direct indication of the formation of different receptor conformations. Spectral shifts due only to mass increases would generate rightward shifts in both p- and s-polarized resonances. Since both agonists have comparable molecular weights, opposite PWR shifts can only result from differences in mass distribution (i.e. structure), not total mass density.
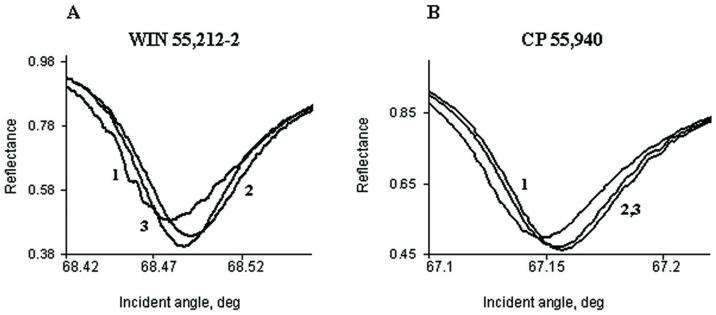
Fig. 7. Two structurally different cannabinoid agonists, CP 55,940 (A and B) and WIN 55,212-2 (C and D) shift the PWR angle in different directions.
PWR spectra for the: 1. empty PC/POPG lipid bilayer; 2. the proteolipid bilayer after incorporation of the partially purified human cannabinoid CB1- myc-His6 receptor; and 3. human cannabinoid 1- myc-His6 receptor -containing lipid bilayer after the addition of saturating concentrations of CP 55,940 (3 nM) or WIN 55,212-2 (10 nM). The spectra were obtained with laser light polarized in p- (A and C) or s- (B and D) directions. Arrows indicate the directions of the PWR angle shifts upon agonist addition. The spectra are representative from three independent experiments.
Fig. 8. Agonist dose-response curves for CP 55,940 (A) and WIN 55,212-2 (B).
PWR angle shifts (deg) were measured after addition of increasing concentrations of the appropriate agonist into the PWR chamber, containing either an empty PC/POPG lipid bilayer (dashed lines) or the human cannabinoid CB1– myc-His6 receptor -containing proteolipid system (solid lines). PWR spectral shifts (representative experiments from three independent experiments are shown) were obtained using p- (open symbols) or s- (solid symbols) polarized laser light. Lines through the data points were obtained by a hyperbolic fit using Graph Pad Prism; KD values are given in the text.
Although it is well known that the highly lipophilic cannabinoid ligands interact with lipid bilayers, the PWR shifts noted above were not due to non-specific cannabinoid-lipid interactions, since control experiments (Fig. 8) demonstrated that addition of CP 55,940 or WIN 55,212-2 to a receptor-free PC/POPG lipid bilayer causes much smaller rightward shifts (3.5 mdeg at 3 nM or 4 mdeg at 10 nM ligand concentration, respectively) in the PWR spectrum for both ligands, consistent with small mass increases. This directly confirms that the data obtained in the presence of the receptor reflect the formation of different proteolipid membrane structures upon ligand binding to human cannabinoid 1 receptor.
Fitting of agonist dose-PWR response curves provided KD values (0.2 ± 0.03 nM and 2 ± 0.4 nM, for CP 55,940 and WIN 55,212-2, respectively; Fig. 8A, B) that are in excellent agreement with those from radioligand binding studies. As expected, measurements using p- or s-polarized light resulted in similar KD values for both ligands. The calculated KD values are also in excellent agreement with our data from PWR experiments using an human cannabinoid CB1-myc-His6 receptor preparation, purified from recombinant mammalian cells (human cannabinoid 1- myc-His6 receptor /CHO) (KD = 0.3 ± 0.1 nM for CP 55,940, and KD = 4.5 ± 1.9 nM for WIN 55,212-2) (Georgieva et al., unpublished results, reported at the INRC meeting, 2005). These results indicate that the bilayer-incorporated receptors retained native ligand binding properties.
3.4. CP 55,940 and WIN 55,212-bound human cannabinoid CB1-myc-His6 receptor conformations exhibit different affinities and potencies toward a representative G protein type (Gi1)
Previous investigations have demonstrated (Alves et al., 2003; Alves et al., 2004a,b) that ligand-bound G-protein coupled receptors incorporate into the lipid bilayer randomly, with either their extracellular (ligand binding) or their intracellular (G protein binding) surfaces facing the aqueous compartment of the PWR cell. Thus, upon addition of G proteins into the aqueous compartment we can directly measure the affinity of ligand-bound receptor conformations to individual G protein types. Addition of G proteins to the PWR cell is expected to increase the resonance angle and change the resonance depth for both p- and s-polarized exciting light due to the large mass increase resulting from the interaction of the incorporated receptor with the G-protein. Fig. 9 shows that indeed, addition of a Gi protein heterotrimer (1:1 mixture of recombinant myristoylated GDP-bound Giα1 subunit + Gβγ subunit mixture purified from bovine brain) to CP 55,940- or WIN 55,212-2-bound human cannabinoid CB1-myc-His6 receptor-containing lipid bilayers caused saturable rightward shifts in the PWR spectra. Fitting of Gi1 protein dose-PWR response curves to a hyperbolic function indicated that CP 55,940 or WIN 55,212-2 bound human cannabinoid CB1-myc-His6 receptors exhibit slightly different affinities (KD values: 27 ± 8 nM for CP 55,950 and 10.7 ± 4.7 nM WIN 55,212-2) for Gi1.
Fig. 9. Dose-PWR response curves for Gi1 protein binding to agonist-bound human cannabinoid CB1–myc-His6 receptor conformations.
PWR spectra were measured after addition of increasing concentrations of GDP-bound Gi1 heterotrimer (1:1 mixture of recombinant myristoylated Giα1 and purified βγ-subunits from bovine brain) to the aqueous compartment of the PWR resonator containing WIN 55,212-2 (■) or CP 55,940 (▲)-bound epitope tagged CB1 receptors. PWR spectra were obtained with p-polarized light; similar dose-response curves were obtained using s-polarization (not shown). Lines through the data points were obtained by a hyperbolic fit; KD values are given in the text. The spectra are representative from three independent experiments.
According to the ternary complex theory, interaction of a G protein with an agonist-bound receptor leads to the dissociation of GDP from the α-subunit of the G protein followed by the formation of a high affinity agonist/receptor/empty Gα-subunit ternary complex. Addition of a GTP molecule results in the dissociation of the high affinity ternary complex and the activation of the G protein. Therefore, measuring the affinity of a non-hydrolyzable GTP-analogue (GTPγS) to the agonist/receptor/G protein ternary complex can provide information about the potency of the given agonist-bound receptor conformation to activate G protein types (Alves et al., 2003; Alves et al., 2004b; Subramaniam et al., 2005). As seen in Fig 10A, addition of GTPγS to WIN 55,212-2 bound human cannabinoid CB1-myc-His6 receptor led to a decrease in the resonance angle, indicating a decrease in the proteolipid mass, presumably due to the dissociation of the agonist/ human cannabinoid CB1-myc-His6 receptor /Giα1 ternary complex and release of the alpha subunit from the bilayer. This is similar to results obtained with the Δ-opioid receptor (Alves et al. 2003). The PWR shift was GTPγS dose-dependent and reached saturation at 10 nM GTPγS concentration. Fitting of the GTPγS dose-PWR response curve provides a direct measure of the affinity of the WIN 55,212-2-bound human cannabinoid CB1-myc-His6 receptor ternary complex to GTPγS (KD = 4.5 ± 0.01 nM; not shown), directly reflecting the potency of the agonist-bound human cannabinoid CB1-myc-His6 receptor to activate Giα1. Interestingly however, addition of GTPγS (up to 200 nM) failed to produce any measurable leftward shift in the PWR spectrum of the CP 55,940 bound human cannabinoid CB1-myc-His6 receptor conformation (Fig.10B), indicating that CP 55,940 binding to the human cannabinoid CB1 receptor does not promote the dissociation of the agonist/ human cannabinoid CB1-myc-His6 receptor /Gi1 ternary complex, and thus it is presumably unable to activate Gi1-mediated signal transduction pathways.
Fig. 10. The effect of saturating concentrations of GTPγS on the PWR spectra of cannabinoid agonist/ human cannabinoid CB1-myc-His6 receptor /Gi1 ternary complexes.
PWR spectra of (A) WIN 55,212-2- or (B) CP 55,940-bound human cannabinoid CB1 receptor-containing proteolipid bilayers in the absence (1) or presence (2) of saturating concentrations of Gi1 protein (80 nM). (3) PWR spectra after addition of saturating concentration (200 nM) of GTPγS to the agonist/receptor/Gi1 ternary complexes. The figure shows only PWR spectra obtained with s-polarized light; similar results were obtained with p-polarization (not shown). The spectra are representative from three independent experiments.
4. Discussion
The theory of agonist directed trafficking (Kenakin, 2002) hypothesizes that structurally different ligands may show preference for different activated receptor conformations. If, in turn, such individual agonist-bound receptor conformations exhibit preference for G protein types, structurally different agonists may direct the signaling of a specific receptor type to distinct subsets of intracellular pathways and thus, produce different physiological effects. The cannabinoid receptors are of particular interest in this regard because there is an increasing amount of data in the literature showing consistent and pronounced disparities among the potencies and efficacies of the major cannabinoid classes in the cannabinoid tetrad (antinociception, hypothermia, catalepsy and reduction of spontaneous activity), used to evaluate central cannabinoid effects in rodents, in vivo.
Thus, a 7-fold difference was observed for WIN 55,212-2 between its ability to reduce mobility and its potency to produce antinociception (Martin et al., 1993), whereas CP 55,940 was almost 10 times more potent in reducing motor activity than in catalepsy (Fan et al., 1994). A separation in the efficacies of anandamide in the individual cannabinoid tetrad responses has also been observed: anandamide and classical cannabinoids were equally efficacious in antinociception and hypomobility assays, but anandamide had significantly less effect on body temperature (Ryan et al., 1997). These data strongly suggest that structurally different cannabinoid agonists may activate different subsets of intracellular signal transduction cascades to produce their physiological effects.
Interestingly, earlier experimental data indicate that structurally different cannabinoid ligands indeed may bind to different epitopes in the cannabinoid CB1 receptor. Thus, mutation of a lysine residue (Lys192) in the third transmembrane domain of the human cannabinoid CB1 receptor led to complete loss of HU-210, CP 55,940 and anandamide binding whereas the mutation had no effect on the binding of WIN 55,212-2 (Song and Bonner, 1996). Even more importantly, recent data (Shim et al., 2003; Shim and Howlett, 2006) indicate that structurally distinct agonists, such as CP 55,940 and WIN 55,212-2 interact differently with a hydrophobic pocket of the cannabinoid 1 receptor that is believed to be essential for G protein activation (Reggio, 1999).
Based on these data we hypothesized that structurally distinct ligands may stabilize different active conformations of the cannabinoid CB1 receptor that, in turn, can exhibit different efficacies toward unique subsets of intracellular second messenger pathways. To evaluate our hypothesis, we have used a sensitive biophysical technique, PWR spectroscopy. This technique has already provided novel insights into the interaction of other G-protein coupled receptors - such as the human δ-opioid (Salalmon et al., 2000; Alves et al., 2003; Alves et al., 2004a; Alves et al., 2004b) and the β-adrenergic (Devanathan et al., 2004) receptors and the visual receptor rhodopsin (Subramaniam et al., 2005) - with ligands, G proteins, GTPγS and/or lipids.
In order to apply PWR spectroscopy, an epitope-tagged human cannabinoid CB1 receptor protein was partially purified. Since one of the goals is to examine the effect of ligand structure on the affinity and potency of G protein types to ligand-bound human cannabinoid CB1 receptor conformations, particular care was taken to obtain a G protein-free human cannabinoid CB1 receptor preparation. It was found earlier that the cannabinoid CB1 receptors exhibit an unusually high affinity for mammalian G proteins, leading to co-purification of the receptor with G proteins from mammalian cells (Mukhopadhyay and Howlett, 2005). Since insect G proteins exhibit much lower affinity to heterologously expressed mammalian G-protein coupled receptors (Parker et al., 1991), we selected Sf9 insect cells as the surrogate cell line to express an epitope-tagged human cannabinoid CB1 receptor. Radioligand binding studies indicated that after infection with the human cannabinoid CB1-myc-His6 receptor containing bacmid, Sf9 cells gained high affinity toward the radiolabeled SR141716A (KD = 2.9 ± 0.8 nM) and that is in excellent agreement with previous results obtained for the human cannabinoid CB1-myc-His6 receptor expressed in mammalian (CHO) cells (KD = 3.14 nM).
The recombinant human cannabinoid CB1-myc-His6 receptor protein was purified from infected Sf9 cell lysates by two consecutive affinity chromatography steps. In radioligand binding assays, the purified cannabinoid CB1-myc-His6 receptor protein preparation exhibited high affinities for cannabinoid ligands indicating that the purified receptor protein retained its active conformation.
By incorporating the partially purified human cannabinoid CB1 protein preparation into a lipid bilayer (PC:POPG, 3:1) and employing the advantages of the PWR technique, we were able to show that the addition of two structurally different cannabinoid agonists, CP 55,940 and WIN 55,212-2, produced distinct spectral changes (PWR shifts in opposite directions) upon binding to the human cannabinoid CB1-myc-His6 receptor. The observed spectral changes can be the result of mass density and/or structural alterations occurring within the proteolipid system (Salamon et al., 1999). The contribution of the ligand itself must be relatively small due to its small size and mass compared to the receptor and surrounding lipid molecules.
Moreover, the differences in the PWR shifts upon CP 55,940 and WIN 55,212-2 binding cannot be attributed to differences in adsorbed mass since these ligands are quite similar in their molecular masses (MW = 385.59 and 531.62 for CP 55,940 and WIN 55,212-2, respectively). Therefore, it is evident that the demonstrated PWR changes are caused by different structural changes in the receptor itself upon interaction with structurally different agonists. This can be visualized as CP binding of a receptor conformation resulting in a net increase in mass density in the membrane, whereas the WIN-bound conformation causes a net decrease. Most likely, such mass density changes are partly due to lipid molecules moving into or out of the bilayer in response to changes in receptor volume. The results reported here are the first to show directly that two cannabinoid agonists, CP 55,940 and WIN 55,212-2, produce qualitatively distinct active conformations of the human cannabinoid CB1 receptor.
In order to prove that the receptor incorporates into the lipid bilayer in a functionally active state, we also investigated whether a representative inhibitory G protein subtype (Gi1) is able to interact with and to be activated by the CP 55,940 and WIN 55,212-2-bound human cannabinoid CB1-myc-His6 receptor. We selected Giα1 for this study, since previous research (Mukhopadhyay and Howlett, 2005; Glass and Northup, 1999; Prather et al., 2000) showed that this G protein type exhibits high affinity toward WIN 55,212-2-bound cannabinoid CB1 receptor. Indeed, our PWR experiments indicated that the WIN 55,212-2-bound human cannabinoid CB1-myc-His6 receptor exhibited high affinity (10.7 ± 4.7 nM) toward Gi1. Interestingly however, the CP 55,950-bound conformation of the human cannabinoid CB1- myc-His6 receptor revealed slightly lower affinity (27 ± 8 nM).
Even more importantly, PWR measurements also demonstrated that while the WIN 55,212-2/ human cannabinoid CB1- myc-His6 receptor /Gi1 protein ternary complex exhibits high affinity to GTPγS, the CP 55,940-bound ternary complex has no or only very low (>>200 nM) affinity to GTPγS. These data indicate that while WIN 55,212-2 may serve as a highly efficacious agonist toward Giα1 mediated signal transduction pathways, a structurally different cannabinoid agonist, CP 55,940, may not be able to activate the same pathways. It is critical to note that both cannabinoids act as full agonists in stimulation of [35S] GTPγS binding to rodent brain membrane preparations (Darmani et al., 2003). Since brain membrane preparations contain multiple types of G proteins, we hypothesize that a higher efficacy of the CP 55,940-bound cannabinoid 1 receptor toward other G protein types may compensate for its inability to activate Gi1.
Other investigators have also found agonist-specific differences in the potency and/or intrinsic activity of the CB1 receptor toward different G protein types. Thus, Glass and Northup (1999) have shown that WIN 55,212-2 behaves as a full agonist toward Gi proteins, but it is a partial agonist for the activation of Go proteins. Additionally, Prather and colleagues (2000) found that WIN 55,212-2 exhibits different potencies to catalyze nucleotide exchange in different G protein types in rat cerebellar membranes. Similar to our data, Mukhopadhyay and Howlett (2005) have found that while WIN 55,212-2 was very potent in promoting the dissociation of the CB1 receptor- Gi1 protein complexes, achieving a maximal dissociation at 10nM, a structurally different cannabinoid agonist, DALN was only a partial agonist toward this G protein type (50% dissociation at 100 nM ligand concentration). Finally, the eicosanoid cannabinoid 1 agonist, (R)-methanandamide failed to promote the dissociation of the cannabinoid 1 receptor- Gi1 complex.
The stability of the ternary complex depends on the dissociation rate of the interacting G proteins. The agonist-receptor-G protein complex could require a sequence of transitions that must overcome a series of energy barriers to achieve release of G proteins from the receptor and GDP-GTP exchange. Chemically distinct ligands may allow these transitions to progress by multiple pathways because of their differential ability to provide the activation energy for microisomerization to unique conformations that can direct the activation of selected G protein subtypes (Kenakin and Onaran, 2002), and consequently different downstream effectors.
In summary, the present investigation used PWR spectroscopy to study agonist-mediated conformational changes in a partially purified, fully glycosylated and G protein free epitope-tagged human cannabinoid CB1 receptor protein. The results reported here show in a direct way that two structurally different cannabinoid agonists, CP 55,940 and WIN 55,212-2, produce qualitatively different conformations of the human cannabinoid CB1 receptor. The data also demonstrate that CP 55940- and WIN 55,212-2-bound human cannabinoid CB1 receptor conformations exhibit different affinities and efficacies toward a representative inhibitory G protein type, Gi1, indicating possible agonist-directed trafficking in human cannabinoid CB1 receptor signaling. By exploring the correlation between agonist structure and G protein selectivity, we hope to identify structural properties in cannabinoid CB1 receptor ligands that preferentially direct cannabinoid receptor signaling to desired intracellular pathways.
Acknowledgments
The authors thank Magda Kaczmarska and Carol Haussler for maintaining the cell cultures. We thank Takashi Yamamoto for providing the cannabinoid agonist structures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann F, Staudacher E, Wilson IBH, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Alves ID, Ciano KA, Boguslavski V, Varga E, Salamon Z, Yamamura HI, Hruby VJ, Tollin G. Selectivity, cooperativity, and reciprocity in the interactions between the delta-opioid receptor, its ligands, and G-proteins. J. Biol. Chem. 2004a;279:44673–44682. doi: 10.1074/jbc.M404713200. [DOI] [PubMed] [Google Scholar]
- Alves ID, Cowell SM, Salamon Z, Devanathan S, Tollin G, Hruby VJ. Different structural states of the proteolipid membrane are produced by ligand binding to the human delta-opioid receptor as shown by plasmon-waveguide resonance spectroscopy. Mol. Pharmacol. 2004b;65:1248–1257. doi: 10.1124/mol.65.5.1248. [DOI] [PubMed] [Google Scholar]
- Alves ID, Salamon Z, Varga E, Yamamura HI, Tollin G, Hruby V. Direct observation of G-protein binding to the human delta-opioid receptor using plasmon-waveguide resonance spectroscopy. J. Biol. Chem. 2003;278:48890–48897. doi: 10.1074/jbc.M306866200. [DOI] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;7:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Sim-Selly LJ, Martin BR, Janoyan JJ, Crim JL, Parekh B, Breivogel CS. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur. J. Pharmacol. 2003;459:83–95. doi: 10.1016/s0014-2999(02)02815-7. [DOI] [PubMed] [Google Scholar]
- Devanathan S, Yao Z, Salalmon Z, Kobilka B, Tollin G. Plasmon-waveguide resonance studies of ligand binding to the human beta 2-adrenergic receptor. Biochemistry. 2004;43:3280–3288. doi: 10.1021/bi035825a. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol. Exp. Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Ame.r Chem. Soc. 1971;93:217–24. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol. Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Onaran O. The ligand paradox between affinity and efficacy: can you be there and not make a difference? Trends Pharmacol. Sci. 2002;23:275–280. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Semus SF, Lin S, Marciniak G, Grzybowska C, Charalambous A, Makriyannis A. Pharmacological evaluation of iodo and nitro analogs of delta 8-THC and delta 9-THC. Pharmacol. Biochem. Behav. 1993;46:295–301. doi: 10.1016/0091-3057(93)90356-x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol. Pharmacol. 2005;67:2016–2024. doi: 10.1124/mol.104.003558. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Kahn RA, Manning DR, Gilman AG. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc. Nat. Acad. Sci. 1986;83:265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Noyes R, Jr., Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J. Clin. Pharmacol. 1975;18:139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- Parker EM, Kameyama K, Higashijima T, Ross EM. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J. Biol. Chem. 1991;266:519–527. [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog. Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Prather PL, Martin NA, Breivogel CS, Childers SR. Activation of cannabinoid receptors in rat brain by WIN 55212-2 produces coupling to multiple G protein alpha-subunits with different potencies. Mol. Pharmacol. 2000;57:1000–1010. [PubMed] [Google Scholar]
- Reggio PH. Ligand-ligand and ligand-receptor approaches to modeling the cannabinoid CB1 and CB2 receptors: achievements and challenges. Curr. Med. Chem. 1999;6:665–683. [PubMed] [Google Scholar]
- Ryan WJ, Banner WK, Wiley JL, Martin BR, Razdan RK. Potent anandamide analogs: the effect of changing the length and branching of the end pentyl chain. J. Med. Chem. 1997;40:3617–3625. doi: 10.1021/jm970212f. [DOI] [PubMed] [Google Scholar]
- Salamon Z, Tollin G. Optical anisotropy in lipid bilayer membranes: coupled plasmon-waveguide resonance measurements of molecular orientation, polarizability, and shape. Biophys. J. 2001;80:1557–67. doi: 10.1016/S0006-3495(01)76128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon Z, Brown MF, Tollin G. Plasmon resonance spectroscopy: probing molecular interactions within membranes. Trends Biochem. Sci. 1999;24:213–219. doi: 10.1016/s0968-0004(99)01394-8. [DOI] [PubMed] [Google Scholar]
- Salamon Z, Cowell S, Varga E, Yamamura HI, Hruby VJ, Tollin G. Plasmon resonance studies of agonist/antagonist binding to the human delta-opioid receptor: new structural insights into receptor-ligand interactions. Biophys. J. 2000;79:2463–2472. doi: 10.1016/S0006-3495(00)76489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon Z, Macleod HA, Tollin G. Coupled plasmon-waveguide resonators: a new spectroscopic tool for probing proteolipid film structure and properties. Biophys. J. 1997;73:2791–2797. doi: 10.1016/S0006-3495(97)78308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JY, Howlett AC. WIN55212-2 docking to the CB1 cannabinoid receptor and multiple pathways for conformational induction. J. Chem. Inf. Model. 2006;46:1286–1300. doi: 10.1021/ci0504824. [DOI] [PubMed] [Google Scholar]
- Shim JY, Welsh WJ, Howlett AC. Homology model of the CB1 cannabinoid receptor: sites critical for nonclassical cannabinoid agonist interaction. Biopolymers. 2003;71:169–189. doi: 10.1002/bip.10424. [DOI] [PubMed] [Google Scholar]
- Song Z, Bonner TI. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol. Pharmacol. 1996;49:891–896. [PubMed] [Google Scholar]
- Subramaniam V, Alves ID, Saldago GF, Lau PW, Wysocki RJ, Jr., Salamon Z, Tollin G, Hruby VJ, Brown MF, Saavedra SS. Rhodopsin reconstituted into a planar-supported lipid bilayer retains photoactivity after cross-linking polymerization of lipid monomers. J. Am. Chem. Soc. 2005;127:5320–6321. doi: 10.1021/ja0423973. [DOI] [PubMed] [Google Scholar]
- Tollin G, Salamon Z, Hruby HJ. Techniques: plasmon-waveguide resonance (PWR) spectroscopy as a tool to study ligand-GPCR interactions. Trends. Pharm. Sci. 2003;24:655–569. doi: 10.1016/j.tips.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Tomida I, Pertwee RG, Azuara-Balnco A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004;88:708–713. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol. Exp. Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Xu W, Filppula SA, Mercier R, Yaddanapudi S, Pavlopoulos S, Cai J, Pierce WM, Makriyannis A. Purification and mass spectroscopic analysis of human CB1 cannabinoid receptor functionally expressed using the baculovirus system. J. Pept. Res. 2005;66:138–150. doi: 10.1111/j.1399-3011.2005.00283.x. [DOI] [PubMed] [Google Scholar]