Abstract
Topoisomerase II (topo II) is required for chromosome segregation and for reprogramming replicons. Here, we show that topo II couples DNA replication termination with the clearing of replication complexes for resetting replicons at mitosis. Topo II inhibition impairs completion of DNA replication, accounting for replication protein A (RPA) stabilization onto ssDNA. Topo II inhibition does not affect the caffeine-sensitive ORC1 degradation found upon origin firing, but it impairs the cdk-dependent degradation/chromatin dissociation of an ORC1/2 reservoir at mitosis. Our results show that ORC1 degradation is rescued by Pin1 depletion and that this topo II-dependent clearing of ORC1/2 from chromatin involves the APC.
[Keywords: DNA replication origins, DNA replication foci, checkpoint, ICRF, ORC, DNA combing]
Higher-order chromatin organization is thought to play an important role in defining DNA replication origins (DePamphilis 1997; Berezney et al. 2000; Gilbert 2001; Mechali 2001). An advantage of such a mode of selection is that replication origins would be adapted to developmental stages. Embryonic extracts can reprogram differentiated nuclei so that they become competent for accelerated replication (Lemaitre et al. 2005), a characteristic of the early stages of development. This involves a reprogramming of the pattern of replication origins and requires both passage through mitosis and topoisomerase II (topo II) activity. Topo II is an enzyme required to regulate DNA decatenation, a topological function that may be involved in the resolution of converging replication forks (Wang 2002). It is also involved in chromosome assembly at mitosis (Adachi et al. 1991; Hirano and Mitchison 1993; Cuvier and Hirano 2003), raising the possibility that topo II-mediated chromosome assembly is required to reset replicon organization.
Here, we investigated how topo II might be involved in the reorganization of replicons at the S-to-M-phase transition using in vitro systems derived from Xenopus eggs. We find that topo II activity couples the completion of DNA replication with the clearing of replication complexes from chromatin. This step involves the interaction of topo II with Pin1 and regulates the degradation/dissociation from chromatin of an ORC1/2 reservoir.
Results and Discussion
Topo II is required to dissociate ORC and replication protein A (RPA) from chromatin at mitosis
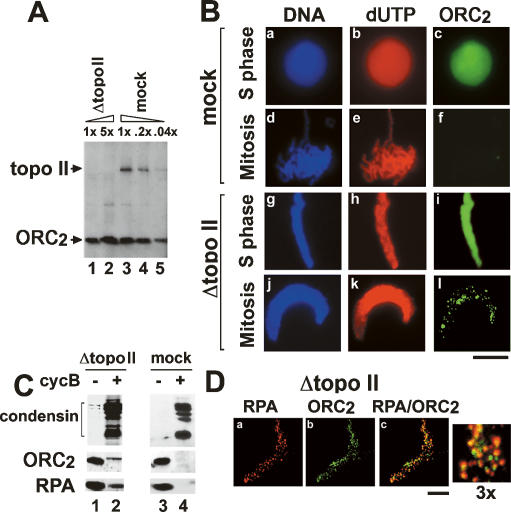
To investigate the potential role of DNA topo II in replicon organization, we depleted topo II from Xenopus egg extracts. More than 99% of the topo II was depleted from egg extracts (Fig. 1A), whereas ORC2 levels remained unchanged (Fig. 1A, lanes 1,3).
Figure 1.
Topo II is required for the dissociation from chromatin of ORC and RPA. (A) Interphase egg extracts were immunodepleted with anti-topo II antibodies and loaded (lanes 1,2) in parallel with a dilution series of mock-depleted extracts (1×, 0.2×, or 0.04×; lanes 3–5). Immunoblotting analyses were performed with the indicated antibodies. (B) Sperm chromatin was incubated with interphase topo II- or mock-depleted egg extracts before inducing mitosis. Chromatin was purified and stained with anti-biodUTP (panels b,e,h,k) or anti-ORC2 (panels c,f,i,l). (Panels a,d,g,j) DNA was counterstained with Hoechst 33258. Bar, 10 μm. (C) Four-fifths of the reaction in B was used to analyze chromatin-associated proteins by immunoblotting. (D) Confocal microscopy of mitotic ORC and RPA foci in topo II-depleted chromatin as described in B. Chromatin was stained with anti-RPA (panel a) and anti-ORC2 (panel b). Panel c shows the merged image with enlarged section (3×). Bar, 5 μm.
Sperm chromatin was added to mock or topo II-depleted extracts, and entry into mitosis was induced (Fig. 1B). The absence of topo II led to an absence of chromosome condensation in mitosis, with nuclei that remain elongated (Fig. 1B, cf. panels j and d), typical of topo II-depletion (Hirano and Mitchison 1993; Cuvier and Hirano 2003). Immunostaining analysis shows that ORC2 was removed from chromatin upon mitotic entry in mock-depleted extracts (Fig. 1B, panel f), in agreement with previous results (Romanowski et al. 1996; Cuvier et al. 2006). In contrast, chromatin removal of ORC2 was strongly impaired upon topo II depletion (Fig. 1B, panel l), as confirmed by Western blotting (Fig. 1C), and a similar result was obtained with the ORC1 subunit (data not shown).
During S phase, ORC2 staining is detected on chromatin in a diffuse manner (Fig. 1B, panel c), as an excess of ORC may be loaded onto chromatin in this system. However, in the absence of topo II, we observed that ORC staining was localized in discrete foci on mitotic chromatin (Fig. 1B, panel l). We asked whether these ORC2 foci were related to replication foci, and we analyzed by confocal microscopy the immunostaining of ORC2 in parallel with RPA. RPA colocalizes with replication foci throughout S phase and leaves chromatin at mitosis upon its hyperphosphorylation by cdc2 kinase (Adachi and Laemmli 1994; Françon et al. 2004; Cuvier et al. 2006). Figure 1C shows that RPA removal from chromatin was impaired in the absence of topo II. Confocal microscopy showed that ORC foci were colocalized with RPA foci for 80%–90% of the chromatin sites (Fig. 1D). The persisting RPA foci correspond to the unphosphorylated form of RPA in S phase, which is specifically recognized by our monoclonal antibodies (Supplemental Fig. S1; data not shown). These data suggested that in the absence of topo II, the stabilization of ORC and RPA at replication foci might be due to a failure to activate cdk. However, H1 kinase activity was not changed in the absence of topo II (Lemaitre et al. 2005) and the cdk-dependent chromatin binding/dissociation of condensin/cohesin was not drastically affected, supporting previous data using topo II inhibitors (Fig. 1C; Losada et al. 2002). Furthermore, we did not observe changes in chromatin assembly, as judged by measurements of nucleosome assembly and nucleosome spacing (Supplemental Fig. S2), suggesting that failure to disassemble RPA/ORC foci may not be due to a global effect on chromatin.
The inhibition of RPA/ORC dissociation is linked to the completion of replication
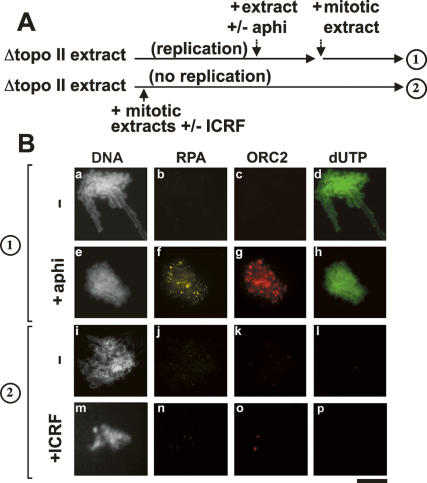
The sustained binding of RPA suggested that topo II inhibition interfered with the completion of replication, since this would leave unreplicated ssDNA regions, which may explain why RPA remains bound to chromatin as this factor tightly binds to ssDNA. In order to address this point, we performed the experiment described in Figure 2A. DNA replication was first allowed to proceed in the absence of topo II. Then, fresh extract containing topo II was added back with or without the replication inhibitor aphidicolin and mitosis was induced by addition of a mitotic extract (protocol 1). If DNA synthesis was not completed in the topo II-depleted extract, then aphidicolin should inhibit RPA/ORC dissociation from chromatin when the fresh extract was added. Figure 2B shows that aphidicolin prevented the dissociation of RPA from chromatin, indicating that failure to complete DNA replication in topo II-depleted extracts was preventing the removal of RPA.
Figure 2.
Topo II is critical for ORC/RPA removal after initiation of DNA replication. (A) Scheme of the experiments. The dissociation of RPA and ORC from chromatin was measured after replicating chromatin in topo II-depleted extracts (protocol 1), or without prior initiation of DNA replication (protocol 2). (B) Sperm chromatin was incubated with topo II-depleted S-phase egg extracts to allow DNA replication to proceed for 120 min. Fresh S-phase egg extracts were added in the absence (panels a–d) or in the presence (panels e–h) of aphidicolin for an additional 30 min, and a mitotic extract was added to induce mitosis. As an additional control, the same amounts of fresh mitotic egg extracts were added after only 15 min. In this condition, mitosis is induced without initiation of DNA replication (Cuvier et al. 2006). The topo II-specific inhibitor ICRF was also added to further inhibit topo II activity at mitosis. Chromatin was purified and stained with the indicated antibodies. (Panels a,e,i,m) DNA was counterstained with Hoechst. Bar, 10 μm.
In agreement, we also found that the dissociation of RPA from chromatin was not dependent on topoisomerase when chromatin was proceeded in mitosis in absence of S phase (protocol 2) (Fig. 2B, panel j). Addition of the topo II-specific inhibitor ICRF (Tanabe et al. 1991) confirmed that topo II activity was not involved. This result is supported by previous observations showing that RPA dissociation is independent of topo II for extracts that are not competent to replicate DNA (Adachi and Laemmli 1994). We conclude that the dissociation of RPA from chromatin is dependent on topo II, most likely because DNA replication cannot be completed in the absence of topo II, leaving ssDNA regions where RPA tightly associates.
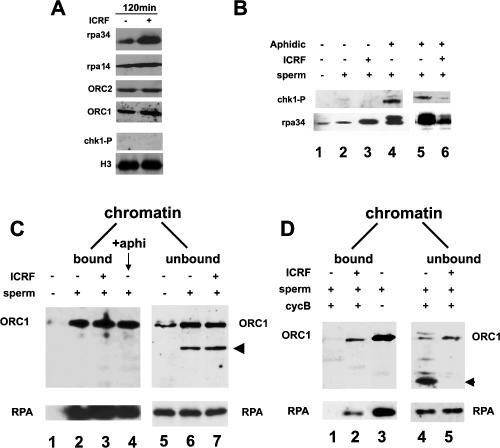
Although ssDNA regions at blocked replication forks could explain the stabilization of RPA onto chromatin, it was unclear why topo II is required to dissociate ORC (Fig. 2B, panel g), which does not target ssDNA regions. A checkpoint response might impair the dissociation of ORC, if cdc2 kinase was inhibited, as phosphorylation of RPA and/or ORC by this kinase reduces its binding to chromatin (Romanowski et al. 1996; Cuvier et al. 2006). However, ICRF did not lead to detectable levels of phosphorylated chk1 or RPA on chromatin (Fig. 3A), as detected after induction of a checkpoint response by the replication inhibitor aphidicolin (Fig. 3B; Shechter et al. 2004). Interestingly, ICRF inhibits the accumulation of both phosphorylated RPA and chk1 induced by addition of aphidicolin (Fig. 3B), possibly because catenation may prevent extensive unwinding by DNA helicases, thereby limiting the checkpoint response.
Figure 3.
ICRF blocks the complete degradation of ORC1 at mitosis. (A) Sperm chromatin was incubated with S-phase egg extracts to allow DNA replication to proceed for 120 min in the presence or absence of ICRF (added at t45). Chromatin-associated protein samples were analyzed by immunoblotting with anti-ORC1/2 or anti-RPA32/14 antibodies and nucleoplasmic fractions with anti-phosphorylated chk1 (chk1-P) or anti-H3 antibodies. (B) Sperm chromatin was assembled in extract after treatments with ICRF as in A and/or aphidicolin added after 1 h and analyzed by immunoblotting. (C) Sperm chromatin was incubated with S-phase egg extracts to allow DNA replication to proceed for 120 min in the presence or absence of ICRF (t45) or aphidicolin (t60) as indicated. Chromatin-associated proteins (bound) were purified through a sucrose cushion (left panels) after detergent extraction of soluble proteins (chromatin unbound; right panels). Samples were analyzed by immunoblotting with the indicated antibodies. The arrowhead indicates a degradation product of ORC1. (D) Same as described in C, except that cyclin B was added after purification of interphase chromatin to drive mitosis for 120 min. Chromatin-associated proteins (bound) were purified after detergent extraction of soluble proteins (unbound) as in C. The arrowhead indicates a degraded form of ORC1 that is only seen at mitosis.
Topo II inhibition prevents the mitotic degradation/dissociation of an ORC1/2 reservoir
In Drosophila, as well as in mammals, a cell cycle-regulated binding of ORC1 to chromatin has been observed, which involves its degradation during S phase and at the S–M transition (Li and DePamphilis 2002; Mendez et al. 2002). In Xenopus, we also observed that upon origin firing, a subfraction of ORC1 is degraded, providing that chromatin is present in the extract and this fraction is released from chromatin (Fig. 3C, unbound fraction). Most of the ORC1 pool remains stable during S phase, however, and ICRF does not affect ORC1 stability or binding to chromatin at this stage (Fig. 3C, cf. lanes 6 and 7). Addition of caffeine, a checkpoint inhibitor that activates more origins to accelerate replication initiation (Shechter et al. 2004; Woodward et al. 2006), also increases the degradation of ORC1 in early S phase (Supplemental Fig. S3), which is not affected by the inhibition of topo II. Therefore, Xenopus ORC1 is degraded upon initiation of replication, yet topo II does not interfere with the binding/degradation of ORC at this stage. Upon mitotic entry, the bulk of ORC1 is released from chromatin (Fig. 3D, lane 1) and a massive degradation was observed in the unbound fraction (Fig. 3D, lane 4), unlike for RPA, which remained stable. The degradation of ORC1 was abolished upon addition of MG132 (data not shown). These data show that in Xenopus, ORC1 is subject to cell cycle-regulated modifications affecting its stability, as observed in Drosophila and mammals (Mendez et al. 2002; DePamphilis 2003). Strikingly, addition of ICRF impaired the mitotic degradation of ORC1, leaving a fraction (5%–10%) of both ORC1 and ORC2 bound to chromatin (Fig. 3D, lane 2; data not shown). We concluded that topo II inhibition prevents the complete degradation of ORC1 and the complete dissociation of an ORC1/2 reservoir that remains bound to chromatin until mitosis.
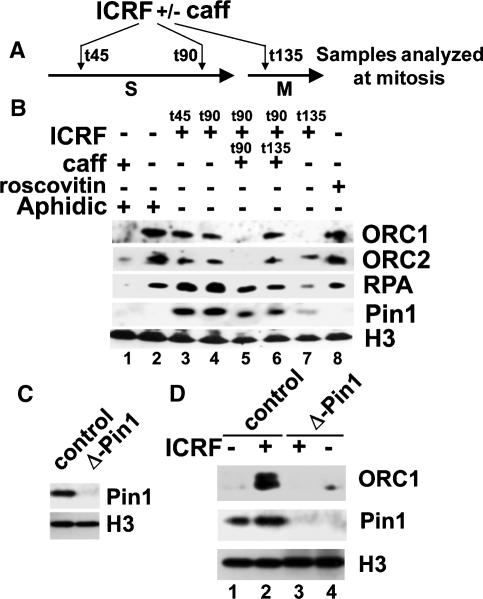
We further asked whether the topo II requirement for ORC dissociation was an early or late S-phase event. ICRF was added in early (t45) and late S phase (t90) or after mitotic entry (t135) (Fig. 4A). Addition of ICRF in early or late S phase similarly impairs ORC1/2 dissociation from chromatin at mitosis (Fig. 4B, lanes 3,4; data not shown), as observed when ICRF was added at t0 (Fig. 3). In contrast, addition of ICRF in mitosis does not significantly impair the ORC1/RPA dissociation from chromatin (Fig. 4B, lane 7). These data clearly show that topo II is required in late S phase for clearing ORC1 and RPA from chromatin. Blocking DNA replication with aphidicolin also prevents the dissociation of ORC and RPA at mitosis, and caffeine overrides this block (Fig. 4B, cf. lanes 1 and 2). The ICRF block is also overridden when caffeine is added prior to (Fig. 4B, lane 5) but not after (Fig. 4B, lane 6) mitotic entry (see Supplemental Fig. S4). In contrast, the cdk-inhibitor roscovitine prevents ORC1 degradation at mitotic entry (Fig. 4B, lanes 7,8). Our data suggest that there are two ORC degradation pathways. One occurs upon origin firing and is not affected by ICRF, and the second occurs on an ORC1 reservoir still bound on chromatin that will be degraded when cdk levels increase at mitotic entry.
Figure 4.
The topo II-dependent block of ORC1 degradation at mitosis is caffeine-insensitive but cdk- and Pin1-dependent. (A) Experimental scheme of the experiment shown in B. (B) Sperm chromatin was incubated with S-phase egg extracts for 120 min in the presence or absence of ICRF added in S phase (t45min; lane 3) or 15 min before (t90min, lanes 4–6) or after (t135min, lane 7) mitotic entry. (Lanes 1,2) Aphidicolin was added at 45 min, and caffeine was added at the time indicated, before or after addition of cyclin B (t120) to drive mitosis. Chromatin-associated proteins were purified at mitosis and analyzed by immunoblotting. (C) Interphase egg extracts were mock-depleted (left) or immunodepleted with anti-Pin1 antibodies (right) and analyzed with the indicated antibodies. (D) Immunodepletion of Pin1 impairs ORC1 stability in ICRF-treated extracts. Sperm chromatin was incubated with Pin1-depleted or mock-depleted S-phase extracts and driven into mitosis as described in B. Chromatin-associated proteins were analyzed by immunoblotting.
The topo II-regulated ORC1/2 degradation/dissociation involves the APC and Pin1
Given the reduced intra S-phase checkpoint after topo II inhibition (Fig. 3), we asked whether other mechanisms might account for this inhibition of ORC1 degradation. Recently, it was shown that Pin1, a peptidyl-prolyl isomerase, is recruited to chromatin by topo II for proper chromosome condensation at mitosis (Xu and Manley 2007). In agreement, we found that ICRF, which traps Topo II onto chromatin, stabilizes the recruitment of Pin1 to chromatin (Fig. 4B). Pin1 functions as an isomerase to prevent the precocious activation of the APC (Reimann et al. 2001; Bernis et al. 2007; Di Fiore and Pines 2007; Xu and Manley 2007) and it may regulate ORC1 degradation. Supporting this view, immunodepletion of the cdc27 subunit of APC impaired the mitotic ORC1 degradation (Supplemental Fig. S5). We therefore asked whether Pin1 might regulate this ORC1 degradation. Figure 4, C and D, shows that depletion of Pin1 clearly impaired the stabilization of ORC1 by ICRF treatments (cf. lanes 2 and 3; data not shown). Taken together, these results support that the dissociation of ORC involves topo II function in recruiting Pin1 to chromatin to prevent APC-dependent degradation of ORC1 at mitosis.
Topo II is involved in late stages of DNA replication
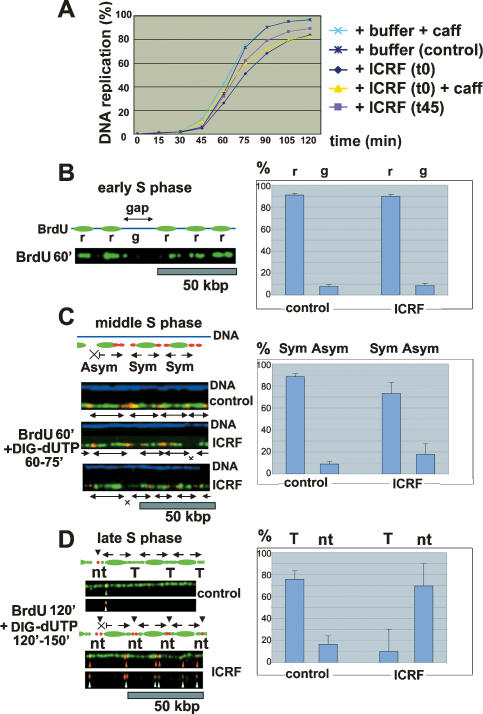
Our data show that topo II allows the clearing of ORC and RPA from chromatin at the S–M-phase transition, and this activity is distinct from intra-S-phase checkpoints, which may limit the firing of origins in replication factories (Shechter et al. 2004) rather than disassembling all factories. Our data further suggest that this disassembly may be coupled to the completion of DNA replication (Fig. 2). However, there is no experimental evidence supporting a role of topo II in DNA replication (Wang 2002). Rather, we observed that inhibition of topo II activity by adding ICRF does not change the initial rates of DNA synthesis (Fig. 5A; Supplemental Fig. S6A; data not shown), as previously suggested (Takasuga et al. 1995). Supporting this result, ICRF did not impair the ability of caffeine to accelerate the initial rates of DNA replication (Fig. 5A) when it does not interfere with ORC1 degradation. However, replication was not completed, as confirmed by the accumulation of partially replicated DNA molecules at the end of the reactions (see Supplemental Fig. S6B). In addition, caffeine could not override the topo II inhibition of the final stage of replication (Fig. 5A). Furthermore, addition of ICRF at 45 min also inhibited the completion of DNA replication (Fig. 5A), showing that the absence of complete DNA replication is independent to the topo II-dependent decondensation of sperm chromatin, which occurs within the first minutes of chromatin assembly (Supplemental Fig. S7; Hirano and Mitchison 1993;Cuvier and Hirano 2003). Taken together, these results strongly suggest that topo II activity is, in fact, required for late stages of DNA replication.
Figure 5.
Inhibition of Topo II activity prevents the completion of DNA replication. (A) Sperm chromatin was incubated in a Xenopus egg extract and 20 μM ICRF was added either at time 0 or after 45 min with or without caffeine. DNA synthesis was measured by the incorporation of 32PdATP (Materials and Methods). Percentage replication relative to input sperm chromatin is indicated. (B) Sperm chromatin was incubated with egg extracts treated with ICRF or a buffer control. The incorporation of BrdU (green) was monitored after 1 h for visualization of actively replicating regions (r). The graph shows the proportion of actively versus inactively replicating regions marked by “gaps” (g; >15 kb), measured along >10 Mb of combed DNA. (C) Same as in B, except that a second nucleotide analog (DIG-dUTP, in red) was added after 1 h of incubation to mark the progression of forks. The graph shows the proportion of symmetric forks (Sym) versus asymmetric forks (Asym), which harbor DIG-dUTP at only one side of the BrdU track. (D) Same as in C, except that BrdU was present during the whole S phase (2 h; ±ICRF) and DIG-dUTP (red) was added together with a fresh egg extract, after removal of ICRF, to mark unreplicated sites. The graph shows the proportion (along >13 Mb of combed DNAs) of terminated (T) or nonterminated (nt) replicons, where incorporation of the second nucleotide was visible. Note: Each dot represents ∼2 kb of unreplicated DNA (see Supplemental Figs. S8, S9).
We used DNA combing to investigate how the inhibition of topo II might affect DNA replication during early, middle, or late S phase (Fig. 5B,D; see Materials and Methods). In early S phase, ICRF treatment did not increase the frequency of unreplicated regions where origins have not yet fired (Fig. 5B, marked by gaps [g] of BrdUTP incorporation). Origins were found every 18–20 kb in both control and ICRF-treated extracts (Supplemental Fig. S6C,D), confirming that ICRF does not significantly affect the initiation of DNA replication.
The progression of forks was estimated by adding a second nucleotide analog in middle S phase (DIG-dUTP; time 60–75 min). No defect in fork progression was scored for the majority of forks (Fig. 5C, graph) suggesting that the ICRF-mediating defect was mainly at later stages. We observed a slight increase in asymmetric forks, where incorporation of the second nucleotide analog occurs at one side of the origin (Fig. 5C, “x”), indicating that a minor proportion of replication forks started to be blocked in middle S phase.
Finally, DNA was replicated in the presence of BrdUTP during the whole of S phase, and the second nucleotide analog (DIG-dUTP) was added after ICRF removal in late S phase to mark the regions where DNA replication had been inhibited by ICRF (Fig. 5D; Supplemental Fig. S8). ICRF did not lead to an increase in the frequency of large unreplicated regions (>10-kb gaps) (Supplemental Fig. S8, BrdU label, top panels), suggesting that the completion of replication by topo II inhibition was not impaired, because few large regions remained unreplicated. A fourfold to fivefold increase in the number of incorporation sites was observed upon ICRF removal compared with untreated control extracts. This incorporation was limited over small DNA regions <5 kb (Fig. 5D; Supplemental Fig. S8, each dot corresponds to ∼2 kb of DNA or less). Since geminin and cycloheximide were present, these small dots were unlikely to be due to reinitiation of DNA replication, but instead, because it blocks/delays replication over short regions near terminations of most replicons (Fig. 5D). Supporting this view, the distances between these incorporation sites exhibits a peak distribution and a mean value of 20–25 kb (Supplemental Fig. S8C), corresponding to the replicon sizes in this system (Walter and Newport 1997; Lemaitre et al. 2005). Similar experiments using HeLa cells showed that the incorporation sites following the release of inhibition by ICRF were between 40 and 140 kb apart (Supplemental Fig. S9), in agreement with the replicon sizes in human cells (40–250 kb) (Berezney et al. 2000). Altogether with our previous observations, these results show that topo II links the completion of DNA replication with the clearing of replication factories from chromatin, and they further highlight a new discrete termination step for DNA replication, which is blocked by ICRF.
During the G1 phase of the cell cycle, ORC is the earliest known factor to recognize DNA replication origins and it serves as a landing platform for other proteins of the replication initiation complex (Gavin et al. 1995). Most of the ORC remains on chromatin during S phase and is removed from bulk chromatin at mitosis (Prasanth et al. 2004; Cuvier et al. 2006). Our data suggest that ORC is regulated through APC-dependent degradation of the ORC1 subunit, as shown in mammals (Mendez et al. 2002; DePamphilis 2003). In Xenopus, a fraction of ORC1 is required and degraded during S phase in a reaction that may depend on origin usage, as it is caffeine-sensitive. The partial degradation of ORC in S phase suggests that there is an ORC reservoir whose degradation is only completed upon increased cdk levels at mitosis, thus providing a unique cell cycle window to reset origins (Supplemental Fig. S10). The ORC reservoir may account for the repeated observation that ORC does not bind only to prereplication complexes (pre-RCs) at origins, but also binds chromatin in a diffuse manner during S phase, not limited to active replication foci.
Our data also show that DNA topo II is required to disassemble both ORC and RPA at the S–M transition, and that this disassembly could be important for changes in chromatin organization of replicons on newly condensed chromosomes. Upon topo II inhibition, DNA replication cannot be completed and topo II-DNA clamps are likely to be responsible for the blocking of both replication forks and of helicases, which would explain the persistence of unphosphorylated RPA bound to ssDNA regions in the absence of ATM/ATR checkpoint. Live microscopy analyses show that synthesized DNA moves along factories (Kitamura et al. 2006). Thus, although progressing replication forks move away from replication origins, ORC and RPA may be stabilized within the same replication factories, possibly explaining why ORC is maintained on chromatin until DNA replication is completed.
Our data support that topo II activity is required for completion of DNA replication at converging replication forks, supporting its role in linking DNA replication to chromosome condensation (Cuvier and Hirano 2003). As ORC1 and ORC2 are part of the same complex (Gavin et al. 1995), this may account for the sustained chromatin association of both proteins after topo II inhibition. Moreover, post-translational modifications of either subunit perturb the localization of both subunits (Saha et al. 2006). ORC2 further associates with centromeres and centrosomes upon mitotic entry, and it plays an important role in chromosome segregation (Prasanth et al. 2002, 2004), and late ICRF inhibition might also stabilize ORC2 in these loci (Fig. 4B, lane 7; data not shown). Interestingly, the interaction of topo II with Pin1 (Xu and Manley 2007) may prevent or delay the activation of the APC (Bernis et al. 2007) implicated in the topo II checkpoint (Andrews et al. 2006) and APC-dependent ORC1 degradation.
Altogether, our results show that topo II activity, by regulating the completion of replication, may couple the chromatin dissociation of RPA and ORC involved in replicon organization to the initiation of mitotic chromosome assembly and segregation.
Materials and methods
Preparation of Xenopus egg extracts, immunodepletions, and chromosome assembly
Preparation of Xenopus egg extracts, immunodepletions, chromosome assembly, biochemical analysis of chromatin-associated proteins, and immunostaining were performed as described previously (Cuvier et al. 2006) with the indicated antibodies. Immunostaining was performed using anti-RPA-34 or RPA14, and anti-ORC2 antibodies rose in the laboratory. DNA replication was inhibited with 5–10 μg/mL (Supplemental Fig. S6C) or 50 μg/mL aphidicolin (Figs. 2, 3; Supplemental Figs. S1, S4). The topo II block by ICRF (10–20 μM) was controlled by decatenation assays as described previously (Cuvier and Hirano 2003). Checkpoint override was induced by addition of 5 mM caffeine and cdk inhibition by 10 μM roscovitine. Mitosis was induced by adding 1/2 vol of mitotic extracts or a quantity of purified recombinant cyclin BΔ90 (Solomon et al. 1990) similar to endogenous levels. See the Supplemental Material for further details.
DNA replication analyses and DNA combing
DNA replication kinetics and denaturing gels to analyze the extent of DNA replication were performed as described (Michalet et al. 1997; Lemaitre et al. 2005). The distances between interdot incorporation sites were measured with MetaMorph (Universal Imaging Corp.) using adenovirus DNA molecules as a size standard (1 pixel = 340 base pairs). See the Supplemental Material for further details.
Acknowledgments
We thank S. Bocquet for antibody production, T. Hirano for the anti-topo II antibodies, and T. Lorca for anti-Pin1/cdc27 antibodies. We thank Philippe Pasero, Dan Fisher, Philippe Coulombe, and Emma Ralph for critical reading of the manuscript; Eldon Emberly for computational analyses; the Montpellier DNA combing facility for providing silanized surfaces; and the CRIC and N. Lautredou for help with confocal microscopy. O.C. was supported by INSERM. This work was supported by the CNRS, the Association pour la Recherche sur le Cancer, the Ligue contre le Cancer, and the Human Frontier Science Program Organization.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.445108.
References
- Adachi Y., Laemmli U.K. Study of the cell cycle-dependent assembly of the DNA pre-replication centres in Xenopus egg extracts. EMBO J. 1994;13:4153–4164. doi: 10.1002/j.1460-2075.1994.tb06733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y., Luke M., Laemmli U.K. Chromosome assembly in vitro: Topoisomerase II is required for condensation. Cell. 1991;64:137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Andrews C.A., Vas A.C., Meier B., Gimenez-Abian J.F., Diaz-Martinez L.A., Green J., Erickson S.L., Vanderwaal K.E., Hsu W.S., Clarke D.J. A mitotic topoisomerase II checkpoint in budding yeast is required for genome stability but acts independently of Pds1/securin. Genes & Dev. 2006;20:1162–1174. doi: 10.1101/gad.1367206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Dubey D.D., Huberman J.A. Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma. 2000;108:471–484. doi: 10.1007/s004120050399. [DOI] [PubMed] [Google Scholar]
- Bernis C., Vigneron S., Burgess A., Labbe J.C., Fesquet D., Castro A., Lorca T. Pin1 stabilizes Emi1 during G2 phase by preventing its association with SCF(βtrcp) EMBO Rep. 2007;8:91–98. doi: 10.1038/sj.embor.7400853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier O., Hirano T. A role of topoisomerase II in linking DNA replication to chromosome condensation. J. Cell Biol. 2003;160:645–655. doi: 10.1083/jcb.200209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier O., Lutzmann M., Mechali M. ORC is necessary at the interphase to mitosis transition to recruit cdc2 kinase and disassemble RPA foci. Curr. Biol. 2006;16:516–523. doi: 10.1016/j.cub.2006.01.059. [DOI] [PubMed] [Google Scholar]
- DePamphilis M.L. The search for origins of DNA replication. Methods. 1997;13:211–219. doi: 10.1006/meth.1997.0521. [DOI] [PubMed] [Google Scholar]
- DePamphilis M.L. The ‘ORC cycle’: A novel pathway for regulating eukaryotic DNA replication. Gene. 2003;310:1–15. doi: 10.1016/s0378-1119(03)00546-8. [DOI] [PubMed] [Google Scholar]
- Di Fiore B., Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Françon P., Lemaître J.-M., Dryer C., Maiorano D., Cuvier O., Méchali M. A hypophosphorylated form of RPA 34 is a specific component of pre-replication centers. J. Cell Sci. 2004;117:4909–4920. doi: 10.1242/jcs.01361. [DOI] [PubMed] [Google Scholar]
- Gavin K.A., Hidaka M., Stillman B. Conserved initiator proteins in eukaryotes. Science. 1995;270:1667–1671. doi: 10.1126/science.270.5242.1667. [DOI] [PubMed] [Google Scholar]
- Gilbert D.M. Making sense of eukaryotic DNA replication origins. Science. 2001;294:96–100. doi: 10.1126/science.1061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Mitchison T.J. Topoisomerase II does not play a scaffolding role in the organization of mitotic chromosomes assembled in Xenopus egg extracts. J. Cell Biol. 1993;120:601–612. doi: 10.1083/jcb.120.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E., Blow J.J., Tanaka T.U. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell. 2006;125:1297–1308. doi: 10.1016/j.cell.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre J.M., Danis E., Pasero P., Vassetzky Y., Mechali M. Mitotic remodeling of the replicon and chromosome structure. Cell. 2005;123:787–801. doi: 10.1016/j.cell.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Li C.J., DePamphilis M.L. Mammalian Orc1 protein is selectively released from chromatin and ubiquitinated during the S-to-M transition in the cell division cycle. Mol. Cell. Biol. 2002;22:105–116. doi: 10.1128/MCB.22.1.105-116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A., Hirano M., Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes & Dev. 2002;16:3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M. DNA replication origins: From sequence specificity to epigenetics. Nat. Rev. Genet. 2001;2:640–645. doi: 10.1038/35084598. [DOI] [PubMed] [Google Scholar]
- Mendez J., Zou-Yang X.H., Kim S.Y., Hidaka M., Tansey W.P., Stillman B. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell. 2002;9:481–491. doi: 10.1016/s1097-2765(02)00467-7. [DOI] [PubMed] [Google Scholar]
- Michalet X., Ekong R., Fougerousse F., Rousseaux S., Schurra C., Hornigold N., van Slegtenhorst M., Wolfe J., Povey S., Beckmann J.S., et al. Dynamic molecular combing: Stretching the whole human genome for high-resolution studies. Science. 1997;277:1518–1523. doi: 10.1126/science.277.5331.1518. [DOI] [PubMed] [Google Scholar]
- Prasanth S.G., Prasanth K.V., Stillman B. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science. 2002;297:1026–1031. doi: 10.1126/science.1072802. [DOI] [PubMed] [Google Scholar]
- Prasanth S.G., Prasanth K.V., Siddiqui K., Spector D.L., Stillman B. Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J. 2004;23:2651–2663. doi: 10.1038/sj.emboj.7600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann J.D., Freed E., Hsu J.Y., Kramer E.R., Peters J.M., Jackson P.K. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Romanowski P., Madine M.A., Rowles A., Blow J.J., Laskey R.A. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- Saha T., Ghosh S., Vassilev A., DePamphilis M.L. Ubiquitylation, phosphorylation and Orc2 modulate the subcellular location of Orc1 and prevent it from inducing apoptosis. J. Cell Sci. 2006;119:1371–1382. doi: 10.1242/jcs.02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter D., Costanzo V., Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- Solomon M.J., Glotzer M., Lee T.H., Philippe M., Kirschner M.W. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Takasuga Y., Andoh T., Yamashita J., Yagura T. ICRF-193, an inhibitor of topoisomerase II, demonstrates that DNA replication in sperm nuclei reconstituted in Xenopus egg extracts does not require chromatin decondensation. Exp. Cell Res. 1995;217:378–384. doi: 10.1006/excr.1995.1100. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Ikegami Y., Ishida R., Andoh T. Inhibition of topoisomerase II by antitumor agents bis(2,6-dioxopiperadine) derivatives. Cancer Res. 1991;51:4903–4908. [PubMed] [Google Scholar]
- Walter J., Newport J.W. Regulation of replicon size in Xenopus egg extracts. Science. 1997;275:993–995. doi: 10.1126/science.275.5302.993. [DOI] [PubMed] [Google Scholar]
- Wang J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Woodward A.M., Gohler T., Luciani M.G., Oehlmann M., Ge X., Gartner A., Jackson D.A., Blow J.J. Excess Mcm2–7 license dormant origins of replication that can be used under conditions of replicative stress. J. Cell Biol. 2006;173:673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.X., Manley J.L. The prolyl isomerase Pin1 functions in mitotic chromosome condensation. Mol. Cell. 2007;26:287–300. doi: 10.1016/j.molcel.2007.03.020. [DOI] [PubMed] [Google Scholar]