Abstract
Coexisting plants that share pollinators can compete through interspecific pollen transfer. A long-standing idea holds that divergence in floral morphology may reduce this competition by placing pollen on different regions of the pollinator's bodies. However, surprisingly little empirical support for this idea exists. Burmeistera is a diverse neotropical genus that exhibits wide interspecific variation in the degree to which the reproductive parts are exserted outside the corolla. Coexisting Burmeistera share bats as their primary pollinators, and the degree of exsertion determines the site of pollen deposition on the bats' heads. Here we study the mechanism, process and pattern of floral character displacement for assemblages of coexisting Burmeistera. Flight cage experiments with bats and pairs of Burmeistera species demonstrate that the greater the divergence in exsertion length, the less pollen transferred interspecifically. Null model analyses of exsertion lengths for 19 species of Burmeistera across 18 sites (each containing two to four species) demonstrate that observed assemblage structure is significantly overdispersed relative to what would be expected by chance. Local evolution, rather than ecological sorting, appears to be the primary process driving this pattern of overdispersion because local adaptation of the nine widespread species accounts for a large portion of the observed pattern. Taken together, results of this study provide strong support for the idea that competition through interspecific pollen transfer can drive character displacement in plants.
Keywords: community-wide character displacement, ecological sorting, Ecuador, floral morphology, pollen placement, flight cage experiments
1. Introduction
Many well-documented examples demonstrate that competitive mechanisms can select for evolutionary divergence in phenotype (reviewed by Schluter 2000 and Dayan & Simberloff 2005). This evolutionary process, termed character displacement (Brown & Wilson 1956), causes interacting species to diverge in resource use, which reduces competition. Competitive mechanisms could also lead to another process, termed ecological sorting, in which competitive exclusion prevents the invasion and/or causes the local extinction of ‘incompatible’ species. In a community of many interacting species, either or both of these processes may lead to an overall pattern of phenotypic overdispersion (or ‘community-wide character displacement’; sensu Strong et al. 1979).
Simply observing differences in the phenotypes of sympatric species is not sufficient to invoke a pattern of overdispersion; observed assemblages must be shown to differ significantly from randomly generated assemblages (Strong et al. 1979; Simberloff & Boecklen 1981). Such non-random patterns of assemblage structure have been found in a diverse array of taxa (e.g. Losos 1990; Kingston et al. 2000); however, relatively few studies also show the mechanisms/processes underlying these patterns (Schluter 2000; Dayan & Simberloff 2005). Furthermore, the overwhelming majority of cases focus on animals.
For plants, competition for pollination might be expected to impose strong selective pressures on coexisting species, given that pollination is critical to reproduction and thus to fitness (Rathcke 1983; Waser 1983). In support of this idea, a recent meta-analysis of pollination studies demonstrated a global pattern in that plants in regions with the greatest species richness face the highest levels of pollen limitation (Vamosi et al. 2006). When multiple species share the same guild of pollinators, visitation patterns can lead to competition through interspecific pollen transfer (sensu Waser 1983), a form of reproductive interference (sensu Armbruster et al. 1994) that imposes fitness costs, such as pollen loss to foreign stigmas and stigma blockage by foreign pollen (Campbell & Motten 1985; Caruso & Alfaro 2000; Brown & Mitchell 2001). Competition through interspecific pollen transfer differs from traditional forms of competition in that the pollinator ‘resource’ need not be limiting for the negative effects to be manifested. Such competition can be reduced by specializing on different pollinators (Armbruster & Herzig 1984; Sargent & Otto 2006) or by flowering at different times of the day or the year (Pleasants 1980; Ashton et al. 1988; Stone et al. 1998). Alternatively, competing species that share pollinators and overlap in flowering time can diverge in floral morphology in order to partition pollinator's bodies spatially. When such differences in floral morphology are observed for sympatric plants, character displacement is often inferred (Dressler 1968; Howell 1977; Brown & Kodric-Brown 1979; Miyake & Inoue 2003; Tschapka et al. 2006); however, such patterns have only rarely been examined statistically (Armbruster 1986; Armbruster et al. 1994; Murray et al. 1987; Hansen et al. 2000).
The genus Burmeistera (Campanulaceae) provides an ideal system to explore the effects of competition for pollination on assemblage structure of coexisting plants. It is highly diverse and multiple species typically co-occur within a given site. The potential for competition between sympatric species appears high as populations flower throughout the year and, with the exception of the hummingbird-adapted Burmeistera rubrosepala, all species use bats as their primary pollinators (Muchhala 2003, 2006b). Bats indiscriminately visit all local species of Burmeistera, as evidenced by the fact that individuals were typically carrying the pollen of multiple Burmeistera species on their fur at the time of capture (Muchhala & Jarrin-V 2002; Muchhala in press). This low flower fidelity leads to interspecific pollen transfer; a detailed study of nine Burmeistera species found that approximately 20% of the pollen grains a flower received were heterospecific and the majority of these belonged to other species of Burmeistera (Muchhala 2006b).
Although bat-pollinated Burmeistera display little interspecific variation in aspects of floral morphology such as corolla width and length, the degree to which the anthers and stigmas are exserted outside the corolla varies widely across the genus (Muchhala 2006b). This raises the possibility that sympatric species may reduce competition for pollination by diverging in exsertion length. In support of this idea, previous work has shown that differences in exsertion length do indeed correspond to differences in the location of pollen placement on the heads of bats (Muchhala in press).
Here we use both ‘bottom-up’ and ‘top-down’ approaches to explore the effects of competition on the assemblage structure of co-occurring Burmeistera. First, we conducted flight cage experiments with bats and flowers to study the competitive mechanism. We predicted that the greater the divergence in exsertion length between a pair of flowers, the less pollen that bats would transfer interspecifically. Second, we measured exsertion lengths for co-occurring Burmeistera in 18 different sites to study the pattern of assemblage structure. We predicted that observed differences in exsertion length between sympatric species would be significantly greater than those of randomly generated null assemblages. By using two different types of null models, we were also able to estimate the relative importance of evolutionary versus ecological processes (i.e. character displacement versus ecological sorting) in generating the observed pattern.
2. Material and methods
(a) Study system
Burmeistera (Campanulaceae) is a neotropical genus with 102 known species distributed from Guatemala to Peru (Lammers 2007). It reaches its highest diversity and endemism in the cloud forests of the Andes of northern South America. Thirty-six species occur in Ecuador alone. For this study, we visited 18 cloud forest sites (table 1, electronic supplementary material); 17 in Ecuador and 1 in Costa Rica (Monteverde; see Muchhala 2003). For the flight cage experiments, we focused on two of these sites: Yanayacu on the eastern slopes of the Andes and Bellavista on the western slopes.
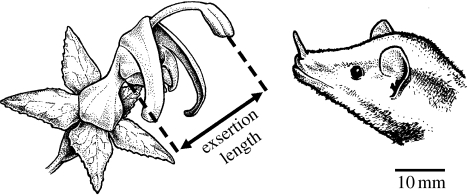
Flowers of Burmeistera are zygomorphic (bilaterally symmetrical) with tubular corollas. A staminal column positions reproductive parts above the corolla opening, and the length of this column determines the degree of exsertion of both the anthers and the stigma (figure 1). Anthers are fused together to form a tube into which pollen is shed. Flowers are protandrous (i.e. sexes are temporally separated; first male, then female). During the male phase, pollen is released gradually through the open end of the anther tube via a ‘pump mechanism’ as the style elongates within the tube (Erbar & Leins 1995). When the stigma emerges from the tube, its dorsal and ventral lobes open in a manner that prevents self-pollination, and the female phase begins. Sympatric species overlap in flowering time, as flowers remain open for several days, plants flower for several months and populations remain in flower throughout the year (with somewhat lower flowering levels during the dry season). Flowers are primarily pollinated by nectarivorous bats of the subfamily Glossophaginae (Phyllostomidae), with occasional secondary pollination by hummingbirds (Muchhala 2003, 2006b). In Ecuadorian cloud forests, Anoura geoffroyi and Anoura caudifer are the most abundant nectar bats and indiscriminately visit all local species of Burmeistera (Muchhala & Jarrin-V 2002; Muchhala in press). Anoura fistulata also occurs in these forests but was never found carrying the pollen of Burmeistera (Muchhala 2006a).
Figure 1.
Illustration of a typical Burmeistera flower (B. borjensis) and a bat head (A. geoffroyi). Exsertion length was measured as the distance from the constriction of the corolla tube to the distal portion of the staminal column. Modified from fig. 1A of Muchhala (2006b).
(b) Competition experiments
We conducted flight cage experiments with wild-caught bats in Yanayacu and Bellavista during four visits to each site in 2004 (Bellavista: 4–12 February, 24–31 March, 3–12 May, 19–28 September; Yanayacu: 1–8 March, 21–25 April, 29 August–7 September, 9–16 November). We captured Anoura with mist nets and placed individuals singly in one of two 3×3 m flight cages. On the first night following capture, we allowed the bat to acclimate to the cage and to feed freely from a test tube filled with honey water (approx. 20% honey). Bats that failed to learn to feed were released within 3 hours. Experiments were conducted over the following 2–3 days, after which all bats were released. In total, we conducted experiments for five A. geoffroyi and three A. caudifer in Bellavista, and for five A. caudifer and one A. geoffroyi in Yanayacu.
For each experimental trial, we placed four fresh flowers in the flight cage: one male and one female from two different species of Burmeistera. Flowers were positioned approximately 1 m above the ground and at 45° angles relative to the horizon, which mimics the natural positioning of Burmeistera flowers (Muchhala 2006b). We randomized the relative order of the four flowers between trials. In order to collect pollen transferred to female flowers, we wrapped the stigma in parafilm and affixed a 10×5 mm rectangle of double-sided tape to its tip. Before each trial, we filled corollas with honey water. After allowing the bat to visit for 30 min, we collected the squares of double-sided tape from the two female flowers, placed them on a slide and covered them with a layer of single-sided tape. We stained these samples with gelatin cubes containing fuchsin dye (Kearns & Inouye 1993) and used a light microscope to count and identify all pollen present along two transects through the centre.
Pairs of Burmeistera were chosen such that pollen was identifiable to species. We also elected to focus on Burmeistera sodiroana because this species exhibits different exsertion lengths in the two reserves. Before experimental trials, we measured the exsertion length of each flower as the distance from the constricted part of the corolla tube (which corresponds to the deepest that a visiting bat can insert its head into the flower) to the distal tip of the anther tube (figure 1). Species pairs, with mean exsertion lengths and study sites in parentheses, included: (i) B. sodiroana (15.8 mm) and Burmeistera ceratocarpa (15.2 mm; Yanayacu), (ii) B. sodiroana (19.9 mm) and Burmeistera cylindrocarpa (14.3 mm; Bellavista), (iii) Burmeistera borjensis (23.7 mm) and B. sodiroana (15.6 mm; Yanayacu), and (iv) B. sodiroana (20.5 mm) and Burmeistera succulenta (11.2 mm; Bellavista). Thus, the mean difference in exsertion length for these four pairs was (i) 0.6 mm, (ii) 5.6 mm, (iii) 8.1 mm, and (iv) 9.3 mm. We ran 10 replicates of each experiment for each bat, for a grand total of 280 experimental trials. For statistical analyses, we treated each bat as an independent sample and used the average of the 10 replicates as the response variable. We used paired t-tests to determine whether female flowers received more conspecific or heterospecific pollen during the experiments.
(c) Assemblage structure analysis
If competition for pollination has played a role in structuring assemblages of Burmeistera, local divergence in exsertion length should be greater than that expected by chance. In order to test this prediction, we measured the mean exsertion length of all locally occurring populations of Burmeistera in 18 different cloud forest sites. We did not include a species in our analyses if we encountered only one individual in the site, as these probably do not represent locally established populations. We preserved samples of flowers and fruits in alcohol, and deposited voucher herbarium sheets for all species in the herbarium of the Pontificia Universidad Católica del Ecuador. Sample sizes for each population ranged from 1 to 126; the larger sample sizes were obtained for species intensively studied elsewhere (Muchhala 2006b).
To determine whether the observed assemblage structure is different from what would be expected by chance, we developed two null models that generate random assemblages. Two competitive processes may generate a pattern of trait overdispersion, and differences in null model design can provide clues as to the importance of each. The first process, termed ecological sorting, occurs when incompatible species either become locally extinct or fail to colonize a community. This process does not involve evolution; species are static entities, which are either preadapted or not preadapted for the community. The second process is the local evolution of interacting species through character displacement; that is, sympatric populations diverge morphologically to reduce competition. Our ‘combined’ null model is designed to test for a pattern of overdispersion irrespective of the process responsible, while our ‘evolutionary’ null model is designed to test specifically for local evolution of sympatric populations.
(i) Combined null model
The combined model randomizes the co-occurrences of species observed across the sites. It starts with the original site×species matrix of observed assemblage structure and randomly permutes each column of the matrix independently to generate new matrices. This preserves a large amount of the observed structure by not changing the number and identity of the populations, but changing only their relative positions in the matrix. Species that were widespread in nature remain widespread in the null models (thus addressing a concern raised by Stone et al. 2000). Furthermore, this method prevents different populations of the same widespread species from ‘co-occurring’ in the random matrices. We imposed an additional constraint in that all randomly generated matrices matched the observed pattern in terms of the number of sites with two, three or four co-occurring species.
(ii) Evolutionary null model
The evolutionary model focuses on species that occur in more than one site, asking what would be the expected assemblage structure if they had evolved local exsertion lengths at random (rather than in response to the local community). It starts with the observed matrix and randomly permutes the non-zero entries for each column of the widespread species. Thus, this model preserves even more of the observed structure than the combined model in that it does not change the position of the blank cells nor the position and identity of those species with only one population. A drawback of this approach is its relatively low statistical power, as it only uses a portion of the full species×site matrix. Both types of null models were created using original functions written in R (R Development Core Team, 2006). The R code used and the data file are available as electronic supplementary material 1 and 2.
(iii) Statistical analyses
For each of the above models, we generated 1000 null matrices and analysed their local divergence in two ways. First, we simply calculated the mean difference in exsertion length between all ‘adjacent’ pairs of species. For each site, we sorted species from the shortest to the longest exsertion length, and then calculated the difference between adjacent pairs; for example, for a habitat with three species, we calculated the difference between the first and the second and the second and the third. For each matrix (both the observed and the nulls), there are 32 differences. We calculated the mean difference for each matrix and generated a frequency distribution of this value for the 1000 null matrixes. We then compared the observed mean difference with this frequency distribution; if statistically significant, less than 5% of the nulls will show higher average difference.
The previous analysis assumes that there is a linear relationship between the difference in exsertion length for adjacent species pairs and the competitive consequences in terms of interspecific pollen transfer. For the second analysis, we transformed the exsertion difference to a more accurate estimate of interspecific pollen transfer using data obtained from the flight cage experiments. We used the function that best fits the relationship between mean difference in exsertion for the Burmeistera species pair used in each experiment and the resulting mean proportion of conspecific pollen transferred (relative to interspecific pollen transfer). A number of different functions were explored (linear and nonlinear), with two constraints to increase biological realism: (i) a y-intercept at 0.5 (when there is no difference in exsertion length, each female flower receives 50% heterospecific and 50% conspecific pollen) and (ii) an asymptote at 1 (the proportion of conspecific pollen transfer cannot exceed 100%). Models were fitted using nonlinear least squares, and the most parsimonious model was selected using Akaike's information criterion (Akaike 1974). All analyses were performed in R (R Development Core Team, 2006). We applied this function to the 32 adjacent differences in each matrix and calculated the mean value for each matrix. Again, if the observed mean is statistically significant, less than 5% of the nulls will have larger means.
3. Results
(a) Competition experiments
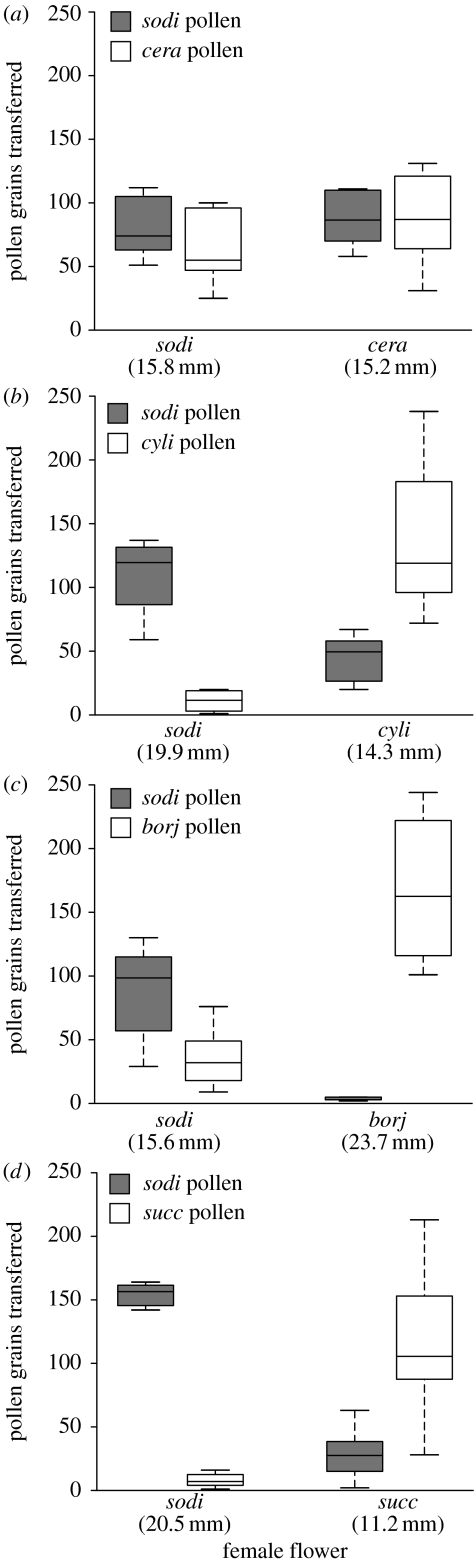
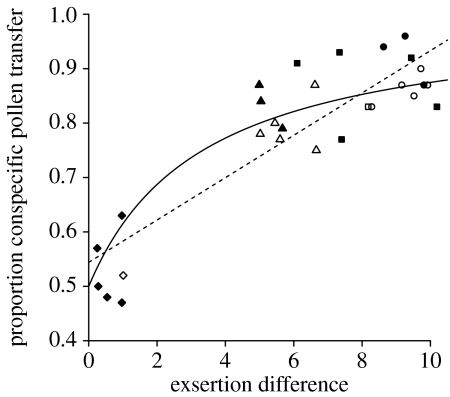
Taken together, the experimental results strongly support the prediction that divergence in exsertion length reduces the amounts of interspecific pollen flow between pairs of sympatric Burmeistera (and thus decreases competition for pollination). For B. sodiroana and B. ceratocarpa, the species pair with virtually identical exsertion lengths, the amounts of conspecific and heterospecific pollen that female flowers received were statistically indistinguishable (figure 2a). For the other three species pairs, which each differed more than 5 mm in exsertion length, female flowers always received significantly more conspecific than heterospecific pollen (figure 2b–d; see figure legend for statistics). Figure 3 combines data from all experiments, with each data point representing one experiment for one bat individual (i.e. the mean of the 10 replicates). Linear regression showed a highly significant relationship; the proportion of conspecific pollen flow increased with increasing divergence in exsertion length (R2=0.78, d.f.=26, F=90.7, p<0.0001; figure 3). The best-fit function for this relationship was of the form y=(ax+1)/(ax+2), with a=0.5985 (figure 3).
Figure 2.
Box plots of pollen transfer experiments. The height of the box is equal to the interquartile distance (IQD), which is the difference between the third and first quartile of the data. The whiskers extend to the extreme values of the data or to 1.5 IQD from the centre (whichever is less). The solid line inside the box indicates the median. Mean exsertion lengths are given in parentheses. (a) The experiment with B. sodiroana and B. ceratocarpa (N=8 bats). For each female flower, there was no statistical difference in the amount of conspecific vs. heterospecific pollen grains received (B. sodiroana: t=1.02, d.f.=5, p=0.35; B. ceratocarpa: t=−0.01, d.f.=5, p=0.99). (b) The experiment with B. sodiroana and B. cylindrocarpa (N=8 bats). Each female received significantly more conspecific than heterospecific pollen (B. sodiroana: t=5.19, d.f.=7, p=0.0012; B. cylindrocarpa: t=−4.23, d.f.=7, p=0.0039). (c) The experiment with B. sodiroana and B. borjensis (N=6 bats). Each female received significantly more conspecific than heterospecific pollen (B. sodiroana: t=3.45, d.f.=5, p=0.0183, B. cylindrocarpa: t=−6.45, d.f.=5, p=0.0013). (d) The experiment with B. sodiroana and B. succulenta (N=6 bats). Each female received significantly more conspecific than heterospecific pollen (B. sodiroana: t=16.93, d.f.=7, p≤0.0001; B. succulenta: t=−5.67, d.f.=7, p=0.0008).
Figure 3.
The proportion of conspecific pollen deposited by each bat in each experiment graphed against the average difference in exsertion length for the experiment. The four experimental treatments are marked by diamonds (B. sodiroana and B. ceratocarpa), triangles (B. sodiroana and B. cylindrocarpa), squares (B. sodiroana and B. borjensis) and circles (B. sodiroana and B. succulenta). Filled symbols, A. caudifer individuals; open symbols, A. geoffroyi individuals; dashed line, the linear regression (y=0.0389x+0.5441; R2=0.78, F=90.7, p<0.0001); solid line, the best-fit function (y=(ax+1)/(ax+2), with a=0.5985; see §2).
(b) Assemblage structure analysis
A total of 50 populations of Burmeistera were found to occur in the 18 study sites (table 1, electronic supplementary material). These correspond to 19 different species. Of these, 10 occurred in only one site, while 9 widespread species occurred in 2–11 sites. Each site contained two to four species. Local exsertion lengths varied from 11.2 to 31.7 mm. The mean exsertion difference for the 32 adjacent pairs was 6.03 mm (±3.93 s.d.). When exsertion differences were transformed using the best-fit function for the experimental data (see §3a and figure 3), the resulting mean estimated proportion of conspecific pollen transfer was 0.786 (±0.098 s.d.).
(i) Combined null model
The mean exsertion difference for adjacent pairs for the 1000 combined null matrices was 5.09, with a range from 3.53 to 6.09 and a mean s.d. of ±4.03. Only one of the null matrices showed a greater exsertion difference than the observed value of 6.03. This represents a p-value of 0.001. When data were transformed using the best-fit function, the mean estimated proportion of conspecific pollen transfer was 0.755, with a range from 0.704 to 0.792 and a mean s.d. of ±0.12. Only six null matrices showed a higher value than the observed value of 0.786 (hence, p=0.006).
(ii) Evolutionary null model
The mean exsertion difference for the 1000 evolutionary null matrices was 5.66 (±4.08 s.d.), with a range from 4.84 to 6.30. Eighty null matrices had a higher average difference than the observed value of 6.03 (p=0.080). When data were transformed using the best-fit function, the mean estimated proportion of conspecific pollen transfer was 0.773 (±0.104 s.d.), with a range from 0.737 to 0.798. Seventy-two null matrices showed a higher value than the observed value of 0.786 (p=0.072).
4. Discussion
This study used a combination of bottom-up and top-down approaches to analyse character displacement in plants. Results provide strong support for the hypothesis that competition for pollination drives community assembly structure of co-occurring Burmeistera. Flight cage experiments demonstrated that divergence in the exsertion length of floral reproductive parts reduces interspecific pollen transfer between pairs of Burmeistera species. The analysis of assemblage structure shows that the exsertion lengths of sympatric species are overdispersed relative to what would be expected by chance. This is one of only a handful of studies to find evidence for a pattern of trait overdispersion in plants (Pleasants 1980; Armbruster 1986; Ashton et al. 1988; Armbruster et al. 1994; Stone et al. 1998), and the first we are aware of to also show the competitive mechanism underlying this pattern.
(a) Competition experiments
When bats were allowed to visit pairs of Burmeistera species that differed in the exsertion length of their reproductive parts, female flowers consistently received significantly higher levels of conspecific than heterospecific pollen (figure 2b–d). In contrast, when bats visited the pair of species with similar exsertion lengths, female flowers received statistically indistinguishable amounts of conspecific and heterospecific pollen (figure 2a). Thus, divergence in exsertion length reduces interspecific pollen transfer. Furthermore, when the results of the four experiments are taken together, an overall trend emerges in that the greater the divergence in exsertion length, the less interspecific pollen is transferred (figure 3).
These results provide important empirical support for the idea, termed the ‘sexual architecture hypothesis’ by Murcia & Feinsinger (1996), that divergence in the position of the reproductive parts of coexisting plants will reduce interspecific pollen transfer. This idea is intuitively satisfying and widely accepted; when differences in floral morphology are observed in sympatric species, they are often assumed to represent a means of reducing competition for pollination (Dressler 1968; Howell 1977; Brown & Kodric-Brown 1979; Armbruster et al. 1994; Miyake & Inoue 2003; Tschapka et al. 2006). However, empirical support to date has been limited. In fact, an experimental study of hummingbird-pollinated flowers found that those species with greater divergence in pollen placement did not receive lower levels of interspecific pollen (Murcia & Feinsinger 1996). While a few studies of reproductive isolation between pairs of species found that differences in flower morphology do appear to limit interspecific pollen transfer (Wolf et al. 2001; Kephart & Theiss 2004; Kay 2006), our study provides even stronger support by demonstrating that, in a continuum from identical to highly divergent exsertion lengths, the greater the divergence, the less pollen is transferred interspecifically.
From the perspective of male flowers, the costs of interspecific pollen transfer are obvious, because every pollen grain lost to heterospecific stigmas represents a decrease in potential fitness (Waser 1983; Campbell & Motten 1985). For example, the high rates of interspecific pollen flow between B. sodiroana and B. ceratocarpa (figure 2a) essentially represent a 50% reduction in fitness for each male flower. From the female perspective, further study is required to fully understand fitness consequences. Foreign pollen may block the stigmatic surface or clog the staminal column (Armbruster & Herzig 1984; Caruso & Alfaro 2000; Brown & Mitchell 2001), or it may fertilize ovules, leading to the production of inviable seeds or unfit hybrid offspring (Arnold & Hodges 1995). It should be noted that, from either the male or the female perspective, competition through interspecific pollen transfer differs from more traditional forms of competition in that the fitness costs will be experienced regardless of whether or not pollinators are a limiting resource.
(b) Assemblage structure
Across the 18 cloud forest sites we studied, both the mean exsertion difference and the mean estimated proportion of conspecific pollen transfer were significantly greater than the values predicted by the combined null model. Thus, exsertion lengths of co-occurring Burmeistera show a statistically significant pattern of overdispersion.
The average exsertion difference observed in nature (6.03 mm) was 0.96 mm greater than that predicted by the combined null model (5.09 mm). Closer analysis suggests that local evolution of the nine widespread species accounts for a large proportion of this discrepancy. Two approaches can be taken to generate a null prediction for what the expected difference would be if the widespread species did not adapt to local communities. The first approach is to randomly shuffle their populations (which we did for the evolutionary null model); this predicts an average exsertion difference of 5.66. The second one is to replace the observed exsertion length for each of their populations with the mean for that species, which gives an average exsertion difference of 5.56. Thus, by these methods approximately half (40–51%) of the 0.96 discrepancy is due solely to local evolution of the nine widespread species. The other half must be due to either ecological sorting or evolution of the 10 remaining species. If local evolution has been so important for the widespread species, we must assume that it was also important for the others; perhaps even more so given that gene flow can retard local adaptation for widespread species. Thus, we argue that local evolution via character displacement has been the major process driving the observed pattern of trait overdispersion. Ecological sorting appears to have only played a minor role, if any, although these analyses cannot rule it out entirely.
(c) Mechanism, process and pattern
In conclusion, this study provides evidence for a mechanism, process and pattern of character displacement in plants. An overall pattern was found, in that co-occurring species of Burmeistera differ more from each other in exsertion length than would be expected by chance. Circumstantial evidence suggests that the primary process responsible for this pattern was local evolution, because local adaptation of the nine widespread species accounted for a large portion of the observed pattern. Experimental work suggests that the competitive mechanism that drives this local divergence is selective pressure to reduce interspecific pollen transfer by placing pollen in different portions of pollinator's bodies. Previous work with Burmeistera found no support for other potential mechanisms by which divergence in exsertion length might reduce interspecific pollen transfer; specifically, differences in exsertion length did not correspond to specialization on different bats or to the effectiveness of hummingbirds as secondary pollinators (Muchhala in press).
Similar research with a community of hummingbird-pollinated plants failed to find strong links between competitive mechanisms, processes and patterns (Feinsinger et al. 1991). Pollination experiments found that individual plants can compete through interspecific pollen transfer (Feinsinger & Tiebout 1991), and that such competition can affect population-level processes (Feinsinger et al. 1991), but the predicted pattern of overdispersion in floral phenology or morphology was not detected (Murray et al. 1987).
In fact, the only other study to find a statistically significant pattern of overdispersion in floral morphology was one examining assemblages of Stylidium (Stylidiaceae) in Western Australia (Armbruster et al. 1994). Stylidium is similar to Burmeistera in that it is a highly diverse genus, with up to five species co-occurring in any given site. Furthermore, its flowers show a similar high degree of integration of the various floral parts (sensu Armbruster et al. 2004), with pollen deposited and received from very precise portions of pollinator's bodies. The lack of other examples of significant overdispersion in floral morphology may be due in part to the fact that flowers with less precise modes of pollen deposition are less able to respond to interspecific pollen flow by diverging in pollen placement. Alternatively, sample sizes for other plant groups may simply be too low to detect significant overdispersion. For example, many instances exist of sympatric plants that place pollen on either the dorsal or ventral portions of pollinators (e.g. Brown & Kodric-Brown 1979; Nilsson et al. 1987; Tschapka et al. 2006); even if this divergence does represent a response to competition, it would be difficult to verify that such a pattern is not simply due to chance.
Acknowledgments
This research was approved by the Ministry of the Environment of Ecuador (permit no. 018-IC-FLO-DBAP) and the Animal Care and Use Committee of the University of Miami (protcol no. 05-037).
We are grateful to J. Vizuete, A. Caiza, D. Proaño and A. Jarrín for their help with fieldwork, and to M. Tschapka for advice on working with captive bats. T. Fleming, S. Armbruster, W. Searcy and C. Horvitz and four anonymous reviewers provided their valuable comments on drafts of the manuscript. We thank the many people who allowed us to work in their reserves, especially H. Greeney of Yanayacu and R. Parsons of Bellavista. Funding was provided by a Graduate Research Fellowship from the National Science Foundation and a Student Grant from Bat Conservation International.
Supplementary Material
R code to create and analyse null communities
Data file to be used with Null_Model.R containing data from table 1
The local exsertion length for Burmeistera polulations in 18 different cloud forest sites
References
- Akaike H. A new look at the statistical model identification. IEEE Trans. Automat. Control. 1974;AC-19:719–723. [Google Scholar]
- Armbruster W.S. Reproductive interactions between sympatric Dalechampia species: are natural assemblages “random” or organized? Ecology. 1986;67:522–533. doi:10.2307/1938595 [Google Scholar]
- Armbruster W.S, Herzig A.L. Partitioning and sharing of pollinators by four sympatric species of Dalechampia (Euphorbiaceae) in Panama. Ann. Miss. Bot. Garden. 1984;71:1–16. doi:10.2307/2399053 [Google Scholar]
- Armbruster W.S, Edwards M.E, Debevec E.M. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. doi:10.2307/1939537 [Google Scholar]
- Armbruster W.S, Pelabon C, Hansen T.F, Mulder C.P.H. Floral integration, modularity, and accuracy: distinguishing complex adaptations from genetic constraints. In: Pigliucci M, Preston K.A, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford University Press; Oxford, UK: 2004. pp. 23–49. [Google Scholar]
- Arnold M.L, Hodges S.A. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 1995;10:67–71. doi: 10.1016/S0169-5347(00)88979-X. doi:10.1016/S0169-5347(00)88979-X [DOI] [PubMed] [Google Scholar]
- Ashton P.S, Givnish T.J, Appanah S. Staggered flowering in the Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. Am. Nat. 1988;132:44. doi:10.1086/284837 [Google Scholar]
- Brown B.J, Mitchell R.J. Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia. 2001;129:43–49. doi: 10.1007/s004420100700. doi:10.1007/s004420100700 [DOI] [PubMed] [Google Scholar]
- Brown J.H, Kodric-Brown A. Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology. 1979;60:1022–1035. doi:10.2307/1936870 [Google Scholar]
- Brown W.L, Wilson E.O. Character displacement. Syst. Zool. 1956;5:49–64. doi:10.2307/2411924 [Google Scholar]
- Campbell D.R, Motten A.F. The mechanism of competition for pollination between two forest herbs. Ecology. 1985;66:554–563. doi:10.2307/1940404 [Google Scholar]
- Caruso C.M, Alfaro M. Interspecific pollen transfer as a mechanism of competition: effect of Castilleja linariaefolia pollen on seed set of Ipomopsis aggregata. Can. J. Bot. 2000;78:600–606. doi:10.1139/cjb-78-5-600 [Google Scholar]
- Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecol. Lett. 2005;8:875–894. doi:10.1111/j.1461-0248.2005.00791.x [Google Scholar]
- Dressler R.L. Pollination by euglossine bees. Evolution. 1968;22:202–210. doi: 10.1111/j.1558-5646.1968.tb03463.x. doi:10.2307/2406664 [DOI] [PubMed] [Google Scholar]
- Erbar C, Leins P. Portioned pollen release and the syndromes of secondary pollen presentation in the Campanulales–Asterales-complex. Flora. 1995;190:323–338. [Google Scholar]
- Feinsinger P, Tiebout H.M., III Competition among plants sharing hummingbird pollinators: laboratory experiments on a mechanism. Ecology. 1991;72:1946–1952. doi:10.2307/1941549 [Google Scholar]
- Feinsinger P, Tiebout H.M, III, Young B.E. Do tropical bird-pollinated plants exhibit density-dependent interactions? Field experiments. Ecology. 1991;72:1953–1963. doi:10.2307/1941550 [Google Scholar]
- Hansen T.F, Armbruster W.S, Antonsen L. Comparative analysis of character displacement and spatial adaptations as illustrated by the evolution of Dalechampia blossoms. Am. Nat. 2000;156:S17–S34. doi: 10.1086/303413. doi:10.1086/303413 [DOI] [PubMed] [Google Scholar]
- Howell D.J. Time sharing and body partitioning in bat–plant pollination systems. Nature. 1977;270:509–510. doi:10.1038/270509a0 [Google Scholar]
- Kay K.M. Reproductive isolation between two closely related hummingbird-pollinated Neotropical ginger. Evolution. 2006;60:538–552. [PubMed] [Google Scholar]
- Kearns C.A, Inouye D.W. University Press of Colorado; Niwot, CO: 1993. Techniques for pollination biologists. [Google Scholar]
- Kephart S, Theiss K. Pollinator-mediated isolation in sympatric milkweeds (Asclepias): do floral morphology and insect behavior influence species boundaries? New Phytol. 2004;161:265–277. doi:10.1046/j.1469-8137.2003.00956.x [Google Scholar]
- Kingston T, Jones G, Zubaid A, Kunz T.H. Resource partitioning in rhinolophoid bats revisited. Oecologia. 2000;124:332–342. doi: 10.1007/PL00008866. doi:10.1007/PL00008866 [DOI] [PubMed] [Google Scholar]
- Lammers T.G. Royal Botanical Gardens; Kew, UK: 2007. World checklist and bibliography of Campanulaceae. [Google Scholar]
- Losos J.B. A phylogenetic analysis of character displacement in Caribbean Anolis lizards. Evolution. 1990;44:558–569. doi: 10.1111/j.1558-5646.1990.tb05938.x. doi:10.2307/2409435 [DOI] [PubMed] [Google Scholar]
- Miyake T, Inoue K. Character displacement in style length between pollinator-sharing Clerodendrum trichotomum and C. izuinsulare (Verbenaceae) Plant Syst. Evol. 2003;243:31–38. doi:10.1007/s00606-003-0059-1 [Google Scholar]
- Muchhala N. Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora. Oecologia. 2003;134:373–380. doi: 10.1007/s00442-002-1132-0. [DOI] [PubMed] [Google Scholar]
- Muchhala N. Nectar bat stows huge tongue in its rib cage. Nature. 2006a;444:701–702. doi: 10.1038/444701a. doi:10.1038/444701a [DOI] [PubMed] [Google Scholar]
- Muchhala N. The pollination biology of Burmeistera (Campanulaceae): specialization and syndromes. Am. J. Bot. 2006b;93:1081–1089. doi: 10.3732/ajb.93.8.1081. [DOI] [PubMed] [Google Scholar]
- Muchhala, N. In press. Functional significance of interspecific variation in Burmeistera floral morphology: evidence from nectar bat captures. Biotropica
- Muchhala N, Jarrin-V P. Flower visitation by bats in cloud forests of western Ecuador. Biotropica. 2002;34:387–395. [Google Scholar]
- Murcia C, Feinsinger P. Interspecific pollen loss by hummingbirds visiting flower mixtures: effects of floral architecture. Ecology. 1996;77:550–560. doi:10.2307/2265629 [Google Scholar]
- Murray K.G, Feinsinger P, Busby W.H, Linhart Y.B, Beach J.H, Kinsman S. Evaluation of character displacement among plants in two tropical pollination guilds. Ecology. 1987;68:1283–1293. doi:10.2307/1939213 [Google Scholar]
- Nilsson L.A, Jonsson L, Ralison L, Randrianjohany E. Angraecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. Biotropica. 1987;19:310–318. doi:10.2307/2388628 [Google Scholar]
- Pleasants J.M. Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology. 1980;61:1446–1459. doi:10.2307/1939053 [Google Scholar]
- R Development Core Team (RDCT) R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing.http://www.R-project.org [Google Scholar]
- Rathcke B.J. Competition and facilitation among plants for pollination. In: Real L, editor. Pollination biology. Academic Press; New York, NY: 1983. pp. 305–329. [Google Scholar]
- Sargent R.D, Otto S.P. The role of local species abundance in the evolution of pollinator attraction in flowering plants. Am. Nat. 2006;167:67–80. doi: 10.1086/498433. doi:10.1086/498433 [DOI] [PubMed] [Google Scholar]
- Schluter D. Ecological character displacement in adaptive radiation. Am. Nat. 2000;156:S4–S16. doi:10.1086/303412 [Google Scholar]
- Simberloff D, Boecklen W. Santa Rosalia reconsidered: size ratios and competition. Evolution. 1981;35:1206–1228. doi: 10.1111/j.1558-5646.1981.tb04990.x. doi:10.2307/2408133 [DOI] [PubMed] [Google Scholar]
- Stone G.N, Willmer P, Rowe J.A. Partitioning of pollinators during flowering in an African Acacia community. Ecology. 1998;79:2808–2827. [Google Scholar]
- Stone L, Dayan T, Simberloff D. On desert rodents, favored states, and unresolved issues: scaling up and down regional assemblages and local communities. Am. Nat. 2000;156:322–328. doi: 10.1086/303384. doi:10.1086/303384 [DOI] [PubMed] [Google Scholar]
- Strong D.R, Jr, Szyska L.A, Simberloff D.S. Tests of community-wide character displacement against null hypotheses. Evolution. 1979;33:897–913. doi: 10.1111/j.1558-5646.1979.tb04743.x. doi:10.2307/2407653 [DOI] [PubMed] [Google Scholar]
- Tschapka M, Dressler S, von Helversen O. Bat visits to Marcgravia pittieri and notes on the inflorescence diversity within the genus Marcgravia (Marcgraviaceae) Flora. 2006;201:383–388. [Google Scholar]
- Vamosi J.C, Knight T.M, Steets J.A, Mazer S.J, Burd M, Ashman T.-L. Pollination decays in biodiversity hotspots. Proc. Natl Acad. Sci. USA. 2006;103:956–961. doi: 10.1073/pnas.0507165103. doi:10.1073/pnas.0507165103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser N.M. Competition for pollination and floral character differences among sympatric plant species: a review of the evidence. In: Jones C.E, Little R.J, editors. Handbook of experimental pollination biology. Van Nostrand Reinhold; New York, NY: 1983. pp. 277–293. [Google Scholar]
- Wolf P.G, Campbell D.R, Waser N.M, Sipes S.D, Toler T.R, Archibald J.K. Tests of pre- and postpollination barriers to hybridization between sympatric species of Ipomopsis (Polemoniaceae) Am. J. Bot. 2001;88:213–219. doi:10.2307/2657012 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R code to create and analyse null communities
Data file to be used with Null_Model.R containing data from table 1
The local exsertion length for Burmeistera polulations in 18 different cloud forest sites