Abstract
The principal physical mechanism of sound generation is similar in songbirds and humans, despite large differences in their vocal organs. Whereas vocal fold dynamics in the human larynx are well characterized, the vibratory behaviour of the sound-generating labia in the songbird vocal organ, the syrinx, is unknown. We present the first high-speed video records of the intact syrinx during induced phonation. The syrinx of anaesthetized crows shows a vibration pattern of the labia similar to that of the human vocal fry register. Acoustic pulses result from short opening of the labia, and pulse generation alternates between the left and right sound sources. Spontaneously calling crows can also generate similar pulse characteristics with only one sound generator. Airflow recordings in zebra finches and starlings show that pulse tone sounds can be generated unilaterally, synchronously or by alternating between the two sides. Vocal fry-like dynamics therefore represent a common production mechanism for low-frequency sounds in songbirds. These results also illustrate that complex vibration patterns can emerge from the mechanical properties of the coupled sound generators in the syrinx. The use of vocal fry-like dynamics in the songbird syrinx extends the similarity to this unusual vocal register with mammalian sound production mechanisms.
Keywords: sound production, syrinx, high-speed video endoscopy, vocal fry, songbird
1. Introduction
In the human larynx, sound is generated by the vibrations of the vocal folds, which act as pneumatic valves. This principal phonatory mechanism is shared by the vocal organ of songbirds, the syrinx, despite large differences in the morphology, histology and biomechanics of the two structures. Unlike the human larynx (Whitehead et al. 1984; Seikel et al. 1997; Blomgren et al. 1998), the songbird syrinx contains two independently controlled sound sources, one in each bronchus near the tracheobronchial junction (Goller & Larsen 1997). Each sound generator contains a set of loose connective tissue masses, medial and lateral labia (King 1989; Goller & Larsen 2002) whose vibrations give rise to sound. Connective tissue (semilunar membrane), reinforced by a cartilaginous centre (pessulus), separates the two sound generators of the songbird syrinx and provides a potential tissue bridge for mechanical coupling (Goller & Larsen 1997). The two sound sources are typically tuned to different frequencies; the left side consistently contributes sounds with lower average frequency than does the right (Suthers 1997).
Information about the detailed vibratory behaviour of the human vocal folds comes from high-speed film material and indirect techniques, such as electroglottography. This research identified differences in vibration characteristics across the range of vocal frequencies. Based on the temporal characteristics of the vibratory behaviour, three main vocal registers have been identified: vocal fry (pulse tone register), modal register and falsetto, each covering a different range of frequencies (Seikel et al. 1997). The different vibration dynamics of these three main vocal registers appear to be correlated with different vocal fold configuration (Titze 1994), suggesting that they represent distinct mechanisms of sound production.
Comparable studies of the vibratory characteristics of the songbird labia are not available. Videographic observations of the syrinx in situ identified the labia as the primary sound-generating structures (‘labial hypothesis’; Goller & Larsen 1997, 2002) as opposed to the earlier model in which vibrations of the medial tympaniform membranes were seen as the primary sound source. However, the low frame rate (30 frames per second, fps) did not allow a detailed analysis of the vibratory characteristics of the labia for any vocalization. High-speed film material of the in situ syrinx does not exist and is limited for the preparations of the excised syrinx (Paulsen 1967; Fee et al. 1998; Fee 2002), leaving details about the vibratory behaviour of the labia during phonation unexplored.
Investigation of the physical and biomechanical mechanisms of sound generation is a key component in understanding the neural control of song production and song learning. In light of the wide frequency range of sounds in the songs of many species, the lack of detailed information on sound generation presents a major gap in our understanding of the complex vocal behaviour of songbirds. Specifically, it remains unexplained how relatively small birds can produce low-frequency sounds.
Here, we present an analysis of high-speed film material of the intact syrinx of anaesthetized hooded crows (Corvus corone cornix) and show vibration patterns similar to those observed during vocal fry vocalizations in the human larynx. This evidence in combination with physiological data on smaller songbird species shows that vocal fry-like vibratory patterns may be a general mechanism for generating low-frequency sounds.
2. Material and methods
(a) Surgery
Three hooded crows were captured in fields near Odense, Denmark and spontaneous calls were recorded in an open field with an omni-directional half-inch microphone (G.R.A.S. type 40AF with preamplifier type 26AH and power amplifier type 12AA) and stored on tape (TEAC type RD-135T DAT data recorder). These same individuals were then used for the filming of the syrinx. Prior to surgery, crows were anaesthetized by intramuscular injections of a ketamine–rompun mixture. The trachea was exposed through a small incision 3 cm below the glottis and part of a tracheal cartilage was removed for insertion of the angioscope. One of the thoracic air sacs was cannulated by inserting the needle of a winged infusion set (VENOFIX 0.65 mm), which was connected to a calibrated custom-built pressure transducer (with a SensorTechnics HCX pressure sensor), the output of which was recorded on one channel of a data recorder (TEAC, type RD-135T DAT; 22.05 kHz sampling rate). A second channel of the data recorder received the output from a precision sound level meter (Brüel & Kjær, type 2235 with a half-inch type 4176 microphone) placed approximately 10 cm from the beak of the subject bird.
(b) High-speed filming
The flexible angiofibrescope (Olympus AF, type 28C; 2.8 mm outer diameter, 2–50 mm depth of field, 75° field of view) was connected to a 300 W Xenon light source (Olympus, type CLV-A) and via a TV adapter (Olympus AF, type MD-869) to a high-speed digital video camera (Redlake Imaging Corporation, Hi-G Camera Head type 9400-0016, Low-light Camera Option). An additional optic fibre (Newport, type F-SV 10; 150 μm outer diameter, 3 μm core diameter) was inserted into the water channel of the angioscope and attached to fibre coupling optics allowing us to focus laser light (20 mW; He–Ne; λ=633) through the fibre onto the syrinx. High-speed video sequences of 8.19 s duration and frame rates of 125–2000 fps were collected simultaneously with pressure and acoustic data while sound production was induced by gently pushing on the thorax of the bird. The high-speed video files were gamma corrected (gamma value around 2, Corel Photo Paint v. 8.0) to enhance the relative luminance on the video monitor for optimal detection of details by the human eye. The sound channel was used to synchronize video recordings with pressure data.
(c) Bronchial plugging
Spontaneous calls of four additional captive hooded crows were recorded in a large anechoic sound booth over several days. Crows were then anaesthetized with a ketamine/diazepam mixture, and the syrinx was accessed through incisions into the skin and interclavicular air sac membrane. To prevent airflow through one of the sound generators, we injected dental impression medium (Reprosil, type 1, low viscosity) into one of the bronchi below the syrinx and secured this plug to the bronchial endothelium with tissue adhesive. After closing the air sac membrane and skin with surgical suture and tissue adhesive, crows were allowed to recover. On the following 4 days, we recorded spontaneous calls. Then crows were sacrificed and the bronchial plug was inspected. In all cases, the bronchus was still firmly plugged at that time.
(d) Airflow recordings
A detailed description of bilateral airflow and air sac pressure recording techniques was given previously (Suthers et al. 1994). Briefly, birds were anaesthetized with chloropent and a flexible cannula was inserted below the last rib into a thoracic air sac. This cannula was connected to a small pressure transducer (Fujikura FPM-02PG), which was attached on the back to a thoracic belt. Flow probes were built from microbead thermistors (Thermometrics BB05JA202) and fine lead wires (Phoenix wire). The thermistor bead was inserted into the bronchial lumen, and the probe was secured to the bronchus with tissue adhesive. The wires were routed out of the interclavicular air sac and subcutaneously to the backpack from where stronger wire was used to connect the probe to signal conditioning equipment (Hector Engineering). In this feedback system, airflow is proportional to the voltage required to drive the supplied current and maintain the thermistor bead at approximately 60°C. After recovery from surgery, air sac pressure and bilateral airflow were recorded together with sound (Audiotechnica AT8356) during spontaneously generated song. Data from three European starlings (Sturnus vulgaris) and three zebra finches (Taeniopygia guttata) were used for this study.
3. Results
We filmed the crow syrinx through an angioscope at frame rates up to 2000 fps while inducing sound production by gently pushing on the thorax of the bird. At the threshold of 1.1 kPa sound production was initiated (defined as first sound pulse exceeding noise by 10 dB). Induced sounds consistently displayed characteristic acoustic features. Short pulses, 0.5–0.8 ms in duration, occurred at approximately 3 ms intervals, generating the fundamental frequency of the sound near 300 Hz (figure 1a,b). High-speed video recordings (1000–2000 fps) show that each acoustic pulse is accompanied by an opening of the labia (figure 2a–g; movie S1 in the electronic supplementary material). Each labial opening is visible only on one or two frames, suggesting that its duration is approximately 1.5 ms. The amplitude of the labial aperture is small compared with labial abduction during quiet expiration. In between the rapid opening events, the medial edges of the medial and lateral labia are in contact, which most likely causes complete closure of both bronchial valves to airflow. During the main part of the induced calls, labial opening alternates between the two sound generators (figure 2a–e). Occasional simultaneous bilateral opening was observed only at the on- and offset of sounds. The opening events and acoustic pulses occur in clusters of two or three (figure 2h,i), separated by longer closed periods of 3–7 ms. The inter-cluster interval corresponds to a duration that is consistent with a skipped pulse±observed jitter. In the case of two openings per cluster, each side contributes one opening event, with the left side leading (figure 2h). The labia on both sides therefore oscillate at the same frequency of approximately 150 Hz. In all cases of three-pulse clusters, it is the right side that opens for the first and third pulse, while the left side opens for the second pulse (figure 2i). Here the right labia therefore oscillate at twice the frequency of the left labia. The duration of acoustic pulses was independent of the subsyringeal pressure (linear regression for crow 1: r=−0.1, p=0.66 (n=19), pitch range: 326–568 Hz; crow 2: r=−0.01, p=0.97 (n=20), pitch range: 323–565 Hz; crow 3: r=−0.35, p=0.09 (n=20), pitch range: 297–505 Hz), but the inter-pulse interval decreased with increasing pressure (linear regression for crow 1: r=−0.54, p<0.0001 (n=20); crow 2: r=−0.38 (n=20), p=0.1; crow 3: r=−0.8, p<0.0001 (n=20)).
Figure 1.
((a), Red line) push-induced sounds occurred during elevated air sac pressure and are characterized by pulse rates near 300 Hz. (b) Pulses are clustered into triplets, expanded segment from (a). (c) Basic acoustic structure similar to that of natural crow calls, expanded in (d). The induced calls were less resonant than natural ones (compare (b) and (d)) evident as a reduced energy level of the tracheal resonance approximately 1.6 kHz in the power spectrum of induced calls ((e) black line) in comparison with ((e) red line), natural calls (see text). Plugging one bronchus to airflow, thus preventing phonation on that side, does not alter the basic pulse structure of the call: (f) before plugging and (g) after plugging. However, after plugging the pulses were more rounded (compare (f) and (g)) evident as a reduced high-frequency energy content of ((h) blue line), the power spectrum in comparison with ((h) red line), before plugging.
Figure 2.
High-speed imaging (at 1000 fps) reveals an alternating opening of the left and right side of the syrinx. (a)–(e) Frames (frame number in white) from a sequence during a push-induced vocalization illustrating (a) full bilateral opening prior to sound production, (b) full bilateral closure, (c) left opening, (d) right opening and (e) bilateral opening during phonation. (f) Schematic explaining the view in frames (a–e). OFL, optical fibre layer; LLS, laser light spot; LLl, LLr, left and right lateral labium; MLl, MLr, left and right medial labium; D, dorsal; V, ventral; SLM, semilunar membrane. (g) Segment of sound with superimposed opening events of the syringeal sides. (h)–(i) Two push-induced sounds illustrating a (h) doublet and (i) triplet pattern of pulse formation. Grey peaks indicate r.m.s. values of the sound track. The blue trace indicates the cross correlation values for comparisons of pulse shape, indicating that the shape of pulses is very constant. The shape of one sound pulse randomly chosen in the middle of the call was correlated with the shape of all the other pulses in the trace.
Next we wanted to assess whether the observed syringeal dynamics during induced sounds might also be employed during spontaneously generated calls. We compared the acoustic structure of induced calls (n=3 individuals) with that of spontaneous calls recorded in the field (recording distance approximately 1 m; n=4 individuals). Natural calls have very similar acoustic characteristics to those observed in the induced calls (compare figure 1a,b and c,d). Short acoustic pulses are repeated at 3–4 ms intervals, thus spanning the range of the fundamental frequency observed in induced sounds. In general, pulses are followed by damped oscillations (figure 1f). These oscillations are presumably resonances of the trachea constricted at one end by the syrinx and at the other by the glottis (the oscillation period equals approximately the sound wave travel time of two times this tracheal length of approximately 9 cm). The induced calls were less resonant than the natural ones (compare figure 1b and d), which presumably is due to the lack of active adjustments of upper vocal tract structures in the anaesthetized bird and disruption of resonances by the relative large incision made in the trachea for filming. This is evident in the lower energy (about 4 dB) around the tracheal resonance peak near 1.6 kHz in the power spectrum of induced relative-to-natural calls (figure 1e).
In four crows, we plugged one bronchus to eliminate one of the two sound sources and recorded calls before and after this treatment. We hypothesized that the elimination of one sound source would result in a significant decrease in fundamental frequency. However, plugging of the right (n=2) or left bronchus (n=2) did not drastically change the basic acoustic structure of calls. Fundamental frequency of calls shifted by less than 20 Hz in three birds and by 61 Hz in the fourth. The frequency shift was inconsistent, in that in each group one bird showed an increase and the other a decrease. The pulse-like acoustic structure remained intact. Subtle differences in calls generated after plugging were observed. The calls contained more rounded pulses (compare figure 1f and g), which is evident in the reduced energy level at higher frequencies (>2.5 kHz) in the power spectrum of induced relative-to-natural calls (figure 1h). It is unclear whether this acoustic change was caused by mechanical effects of the plug or by an elimination of dynamic interactions between vibrations in the two sound generators.
The high-speed filming and bronchial plug experiments show that pulse tone-like sounds can be generated either by alternation between the two sound generators, by simultaneous vibration or by each sound generator alone. However, owing to the artificial nature of these experiments, the question remains whether this range of possibilities is also used in natural vocalizations of songbirds. To investigate how natural sounds are generated, we simultaneously recorded airflow through both sides of the syrinx, subsyringeal air sac pressure and acoustic output in spontaneously singing birds.
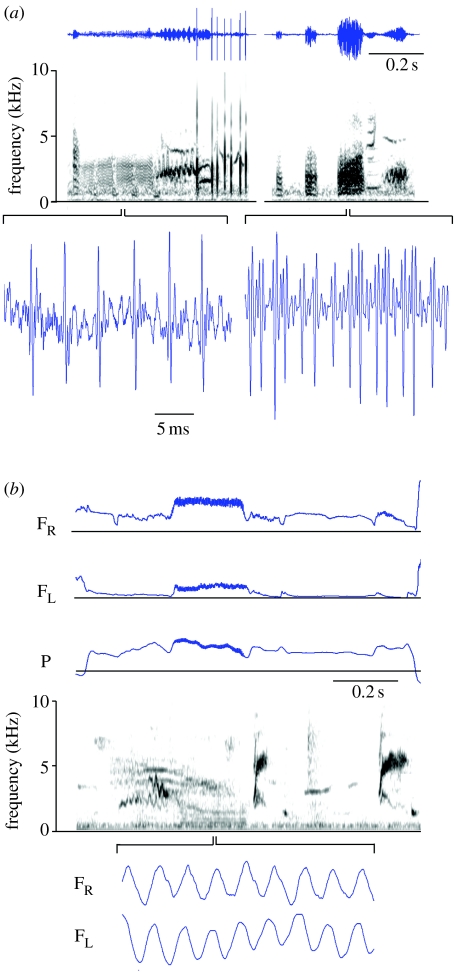
We identified pulse tone-like acoustic characteristics in low-frequency syllables (fundamental frequency between 300 and 800 Hz) of the European starling (figure 3a) and the zebra finch (figure 4). In starlings, the low-frequency sounds are always produced with contributions from both sides of the syrinx as indicated by bilateral airflow (figure 3b). This is in contrast to sounds of higher frequency (>1 kHz), which are generated by only one side, although each side can generate an independent tone simultaneously (as has also been observed in other species; for review see Suthers (1997); Suthers et al. (1999); Suthers & Zollinger (2004)). In this particular example of a low-frequency sound, the peaks in left and right airflow are offset by approximately 1 ms (figure 3b). Under the experimental conditions in the laboratory, starlings did not sing these low-frequency sounds frequently and only at very low amplitude. Therefore, it was not possible to record many examples and obtain a good signal-to-noise ratio in the acoustic recordings. However, free-living starlings sing a number of different low-frequency syllables as part of their warble song, and their acoustic pulse structure suggests that the two sides contribute either synchronously (figure 3a, left panel) or by alternating opening (figure 3a, right panel; see for comparison figure 2h). The airflow data from zebra finches show that all these postulated interactions between the left and right sound generators during pulse tone sounds do occur (figure 4). In the time span of a few vibrations, the left–right coordination can switch from a very short offset between flow peaks to an alternating pattern, changing the acoustic structure from one pulse in the former to two pulses in the latter. Unilateral pulse tone generation, although infrequently present in zebra finch syllables, was also observed (figure 4).
Figure 3.
In starlings, low-frequency sounds are an important part of the vocal repertoire during warble song. (a) Two segments of warble song (shown as oscillogram and spectrographically on top) contain several different low-frequency sounds, whose pulse structure is shown in an expanded view (bottom). (b) Starlings generate the low-frequency sounds while air flows through both sides of the syrinx, whereas other syllables are generated with unilateral airflow (right-side airflow in the example). The segment of warble song shows one expiratory pressure pulse, left and right airflow and a spectrographic representation of the sound (P (horizontal line), ambient pressure. FL and FR (horizontal lines), zero airflow). Bilateral airflow and sound (S, oscillogram) during the low-frequency sound (expanded segment on the bottom) indicate that both syringeal sides open for each sound pulse with minimal temporal delay between the two sides (<1 ms). Airflow modulation indicates syringeal opening events. The temporal resolution of the flow probes is limited and reflects a low-pass filtered view of the actual changes in airflow. Accordingly, zero airflow is not always observed because the thermistor does not have the frequency response to capture these rapid changes in airflow.
Figure 4.
(a) Two characteristic zebra finch syllables shown spectrographically and with the concurrent airflow and air sac pressure patterns. (b) Two expanded segments illustrating flow modulations on the left and right side of the syrinx whose phase relationship changes from (i) short delay (grey bar no. 1) to out-of-phase oscillation (grey bar no. 2). (ii) Right, unilateral sound generation (grey bar no. 3) switching to bilateral, out-of-phase sound generation (grey bar no. 4). Time resolution of flow measurements is limited as described in figure 3. Abbreviations are the same as given in figure legend 3b.
4. Discussion
We provide evidence for pulse register phonation in the songbird syrinx, which can be generated by all possible interactions of the two sound sources, but typically involves the use of both sound generators and always gives rise to sounds with a rich harmonic structure. Since sounds were induced in deeply anaesthetized crows, the short labial opening events and the alternation of opening between the left and right sound generators must reflect passive mechanical dynamics of the vocal organ. The alternation pattern indicates coupling between the two sound sources, which might include mechanical coupling presumably via the semilunar membrane and/or direct acoustic coupling. Muscular control of the observed vibration pattern is highly unlikely. Even if the thoracic compression induces a reflex-like neural response, the syringeal muscles would not be fast enough to account for rapid opening events of 1.5 ms duration (Elemans et al. 2004; Rome 2006). We therefore conclude that the observed vibratory pattern and resulting complex acoustic structure can arise from passive properties of the syrinx in a state where syringeal muscles are relaxed. This interpretation is confirmed by electromyogram recordings from the syringeal muscles of zebra finches (Vicario 1991). Low-frequency sounds are accompanied by very low activation of ventral and dorsal syringeal muscles. Furthermore, in a preparation of the excised zebra finch syrinx, in which all neural connections were severed, sound pressure recordings showed pulse-like characteristics for low-frequency harmonic stacks. When adductive muscle activity was simulated by pushing the lateral labium into the bronchial lumen, the oscillations became more sinusoidal (Fee et al. 1998).
Both the acoustic and the videographic evidence strongly indicate that the vibratory behaviour of the syringeal labia during induced sounds parallels that of the human vocal folds during the lowest vocal register, vocal fry. Human vocal fry occurs during speech and overtone singing (e.g. Fuks et al. 1998; Lindestad et al. 2001; Sakakibara et al. 2001) and is produced with thick vocal folds and flaccid vocal fold margins at low subglottal pressure (Whitehead et al. 1984; Seikel et al. 1997; Blomgren et al. 1998). Vocal fold opening events are short and are followed by long closed intervals. Pulse rate in human vocal fry ranges from 10 to 90 Hz, and frequency changes are thought to be dependent mainly on subglottal pressure (Hollien et al. 1969; Allen & Hollien 1973).
All these characteristics were shared by the observed labial vibration pattern in the crow syrinx during the induced sounds. Crows were deeply anaesthetized, and the labia were therefore in a flaccid state, because syringeal muscles were probably not activated. This vibratory mode of the labia was the only one induced by artificial pressurization at low air sac pressures (1.1 kPa is equivalent to 11.2 cm H2O, which is at the low end of typical phonatory pressure in songbirds). The alternation between the two sound generators resulted in very long closed phases on each side. Bronchial plugging showed that unilaterally produced calls are similar to those produced with both sides intact. Clearly, each sound source of the crow syrinx by itself can generate this call, and the similarity in acoustic structure suggests that a pulse tone mechanism also characterizes the vibratory pattern of a single syringeal sound source. Although passive compression of the thorax and plugging of a bronchus may artificially modify sound production, the airflow data in zebra finches and starlings confirm that the two syringeal sides can give rise to pulse tones with different phase relationships between the left and right labia. In the zebra finch, left and right vibrations can switch in the course of a few vibratory cycles from unilateral to simultaneous or out-of-phase vibrations, which results in acoustic changes, such as doubling of the pulse rate in the case of 180° phase relationship. These interactions suggest that either coupling between the sides may change passively, perhaps as air sac pressure changes slightly, or direct neural control may affect coupling. In either case, control of acoustic changes requires only simple changes in the neural control mechanism.
The observed alternating phonation pattern of the two syringeal sides differs from the typical and well-described use of the two sound generators for most song syllables, where sounds are generated unilaterally. When the left and right sides phonate simultaneously, they typically produce independent tones (Suthers 1997; Suthers et al. 1999; Suthers & Zollinger 2004). The observation that both sound sources, despite their different tuning (Suthers 1997; Suthers et al. 1999) and, especially in the case of the right side, otherwise much higher frequency range, can generate the same low frequency is consistent with data on human vocal fry (Chen et al. 2002). Fundamental frequency in humans is not different between males and females during pulse tone production, whereas for modal sounds there is a clear frequency difference. Furthermore, the observation that the principal vibratory characteristics are present in induced sounds and after denervation of the vocal organ in zebra finches (Simpson & Vicario 1990) indicates that this complex vibratory behaviour occurs without the need for sophisticated neural control. In summary, the physical properties of the syrinx alone can give rise to highly complex sounds.
It is unclear as to what extent nonlinear dynamics of the two syringeal sound sources play a role in pulse tone production. In the high-speed films, we observed that the pulse rate on the left side of the crow syrinx sometimes decreased by a factor of 2 as subsyringeal air sac pressure fell below approximately 2 kPa. This rapid step-like change suggests a period-doubling event. However, through the interaction of the two sides this event did not become manifested in the acoustic output. Perhaps, interactions of the two sound sources provide fairly linear acoustic parameters despite the highly nonlinear characteristics of each sound generator.
The use of a pulse tone phonatory mechanism explains how small songbirds (e.g. zebra finch mass is 12–13 g) can generate low-frequency sounds, and suggests that this additional mechanism can function to extend the frequency range of a species' vocal repertoire. This discovery that vocal fry-like vibration characteristics occur in the uniquely avian syrinx, together with its proposed wide-spread use in mammals (Riede & Zuberbühler 2003; Döllinger et al. 2005), suggests that the pulse tone vibratory mode is a more important component of acoustic communication in non-human animals than it is in humans. The presence of two sound generators in songbirds and the more diverse patterns of pulse tone generation provide a system that allows detailed exploration of the biomechanics of this vocal register. The diversity of birdsong suggests that the transition between vocal fry-like and modal vibrations is more gradual than is evident from the human example. These findings add to the surprising parallels in physical sound-generating mechanisms between the human larynx and songbird syrinx and illustrate further the usefulness of the avian system as a model for detailed experimental investigation of sound production mechanisms.
Acknowledgments
We would like to express our gratitude to Claus Næsbye Larsen for making the artwork for figure 2, to Horst-Günter Rubahn for letting us borrow his laser set-up, to Peter Bollen for providing us with laboratory space and microscopes at the Biomedical Laboratory, to Jason Whittington for field recordings and to gamekeeper Benny Larsen for advice on catching crows. The study was performed with permissions and counselling from the Danish Ringing Centre, The Danish Forest and Nature Agency, the Danish Animal Experiments Inspectorate and the Institutional Animal Care and Use Committee at the University of Utah. The research was funded by grants from the Danish Science Research Council (SNF 23155-4) to O.N.L. and the National Institutes of Health (R01 DC 06876 and R01 DC 04390 to F.G. and NRSA 05722 to B.G.C.).
Supplementary Material
Representative film segement showing simulatenous and alternate opening of the two syrigneal valves during a push-induced sound in the hooded crow
References
- Allen E, Hollien H. A laminagraphic study of pulse (vocal fry) phonation. Folia Phoniatr. 1973;25:241–250. doi: 10.1159/000263709. [DOI] [PubMed] [Google Scholar]
- Blomgren M, Chen Y, Ng M.L, Gilbert H.R. Acoustic, aerodynamic, physiologic, and perceptual properties of modal and vocal fry registers. J. Acoust. Soc. Am. 1998;103:2649–2658. doi: 10.1121/1.422785. doi:10.1121/1.422785 [DOI] [PubMed] [Google Scholar]
- Chen Y, Robb M.P, Gilbert H.R. Electroglottographic evaluation of gender and vowel effects during modal and vocal fry phonation. J. Speech Lang. Hear. Res. 2002;45:821–829. doi: 10.1044/1092-4388(2002/066). doi:10.1044/1092-4388(2002/066) [DOI] [PubMed] [Google Scholar]
- Döllinger M, Berry D.A, Berke G.S. Medial surface dynamics of an in vivo canine vocal fold during phonation. J. Acoust. Soc. Am. 2005;117:3174–3183. doi: 10.1121/1.1871772. doi:10.1121/1.1871772 [DOI] [PubMed] [Google Scholar]
- Elemans C.P.H, Spierts I.L.Y, Müller U.K, van Leeuwen J.L, Goller F. Superfast muscles control dove's trill. Nature. 2004;431:146. doi: 10.1038/431146a. doi:10.1038/431146a [DOI] [PubMed] [Google Scholar]
- Fee M.S. Measurement of the linear and nonlinear mechanical properties of the oscine syrinx: implications for function. J. Comp. Physiol. A. 2002;188:829–839. doi: 10.1007/s00359-002-0349-z. doi:10.1007/s00359-002-0349-z [DOI] [PubMed] [Google Scholar]
- Fee M.S, Shraiman B, Pesaran B, Mitra P.P. The role of nonlinear dynamics of the syrinx in the vocalizations of a songbird. Nature. 1998;395:67–71. doi: 10.1038/25725. doi:10.1038/25725 [DOI] [PubMed] [Google Scholar]
- Fuks L, Hammarberg B, Sundberg J. A self-sustained vocal-ventricular phonation mode: acoustical, aerodynamic and glottographic evidences. KTH TMH-QPSR. 1998;3:49–59. [Google Scholar]
- Goller F, Larsen O.N. A new mechanism of sound generation in songbirds. Proc. Natl Acad. Sci. USA. 1997;94:14 787–14 791. doi: 10.1073/pnas.94.26.14787. doi:10.1073/pnas.94.26.14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen O.N. New perspectives on mechanisms of sound generation in songbirds. J. Comp. Physiol. A. 2002;188:841–850. doi: 10.1007/s00359-002-0350-6. doi:10.1007/s00359-002-0350-6 [DOI] [PubMed] [Google Scholar]
- Hollien H, Damste H, Murry T. Vocal fold length during vocal fry phonation. Folia Phoniatr. 1969;21:257–265. doi: 10.1159/000263256. [DOI] [PubMed] [Google Scholar]
- King, A. S. 1989 Functional anatomy of the syrinx. In Form and function in birds, vol. 4 (eds A. S. King & J. McLelland), pp. 105–192. London, UK; San Diego, CA: Academic Press.
- Lindestad P.-Å, So¨dersten M, Merker B, Granqvist S. Voice source characteristics in Mongolian “throat singing” studied with high-speed imaging technique, acoustic spectra and inverse filtering. J. Voice. 2001;15:78–85. doi: 10.1016/S0892-1997(01)00008-X. doi:10.1016/S0892-1997(01)00008-X [DOI] [PubMed] [Google Scholar]
- Paulsen K. Akademsiche Verlagsgesellschaft; Frankfurt, Germany: 1967. Das Prinzip der Stimmbildung in der Wirbeltierrehe und beim Menschen. [Google Scholar]
- Riede T, Zuberbühler K. Pulse register phonation in Diana monkey alarm calls. J. Acoust. Soc. Am. 2003;113:2919–2926. doi: 10.1121/1.1567278. doi:10.1121/1.1567278 [DOI] [PubMed] [Google Scholar]
- Rome L.C. Design and function of superfast muscles: new insights into the physiology of skeletal muscle. Annu. Rev. Physiol. 2006;68:193–221. doi: 10.1146/annurev.physiol.68.040104.105418. doi:10.1146/annurev.physiol.68.040104.105418 [DOI] [PubMed] [Google Scholar]
- Sakakibara, K. I., Konishi, T., Kondo, K., Murano, E. Z., Kumada, M., Imagawa, H., Niimi, S. 2001 Vocal fold and false vocal fold vibrations and synthesis of khöömei. In Proc. Int. Computer Music Conference 2001, pp. 135–138. San Francisco, CA: Interational Computer Music Association.
- Seikel J.A, King D.W, Drumright D.G. Singular Publishing Group, Inc; San Diego, CA; London, UK: 1997. Anatomy and physiology for speech and language. [Google Scholar]
- Simpson H.B, Vicario D.S. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J. Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers R.A. Peripheral control and lateralization of birdsong. J. Neurobiol. 1997;33:632–652. doi:10.1002/(SICI)1097-4695(19971105)33:5<632::AID-NEU10>3.0.CO;2-B [PubMed] [Google Scholar]
- Suthers R.A, Zollinger S.A. Producing song. The vocal apparatus. Ann. NY Acad. Sci. 2004;1016:109–129. doi: 10.1196/annals.1298.041. doi:10.1196/annals.1298.041 [DOI] [PubMed] [Google Scholar]
- Suthers R.A, Goller F, Hartley R.S. Motor dynamics of song production by mimic thrushes. J. Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. doi:10.1002/neu.480250803 [DOI] [PubMed] [Google Scholar]
- Suthers R.A, Goller F, Pytte C. The neuromuscular control of birdsong. Phil. Trans. R. Soc. B. 1999;354:927–939. doi: 10.1098/rstb.1999.0444. doi:10.1098/rstb.1999.0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I.R. Prentice Hall; Englewood Cliffs, NJ: 1994. Principles of voice production. [Google Scholar]
- Vicario D.S. Contributions of syringeal muscles to respiration and vocalization in the zebra finch. J. Neurobiol. 1991;22:63–73. doi: 10.1002/neu.480220107. doi:10.1002/neu.480220107 [DOI] [PubMed] [Google Scholar]
- Whitehead R.L, Metz D.E, Whitehead B.H. Vibratory patterns of the vocal folds during pulse register phonation. J. Acoust. Soc. Am. 1984;75:1293–1297. doi: 10.1121/1.390737. doi:10.1121/1.390737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative film segement showing simulatenous and alternate opening of the two syrigneal valves during a push-induced sound in the hooded crow