Abstract
The trade-off hypothesis of virulence evolution rests on the assumption that infection-induced mortality is a consequence of host exploitation by parasites. This hypothesis lies at the heart of many empirical and theoretical studies of virulence evolution, despite growing evidence that infection-induced mortality is very often a by-product of host immune responses. We extend the theoretical framework of the trade-off hypothesis to incorporate such immunopathology and explore how this detrimental aspect of host defence mechanisms affects the evolution of pathogen exploitation and hence infection-induced mortality. We argue that there are qualitatively different ways in which immunopathology can arise and suggest ways in which empirical studies can tease apart these effects. We show that immunopathology can cause infection-induced mortality to increase or decrease as a result of pathogen evolution, depending on how it covaries with pathogen exploitation strategies and with parasite killing by hosts. Immunopathology is thus an important determinant of whether public and animal health programmes will drive evolution in a clinically beneficial or detrimental direction. Immunopathology complicates our understanding of disease evolution, but can nevertheless be readily accounted for within the framework of the trade-off hypothesis.
Keywords: immunopathology, immune over-responsiveness, anti-disease vaccines, anti-sepsis therapy, ecological immunology, pathogenicity
1. Introduction
The best studied evolutionary explanation of why parasites harm their hosts is the virulence trade-off hypothesis (Anderson & May 1982; Ewald 1983; Frank 1996; Day 2003; Mackinnon et al. in press). This pathogen-centred theory asserts that virulence is a consequence of host exploitation by parasites. Pathogens that more aggressively exploit their hosts are assumed to produce more transmission forms per unit time and/or for longer before immune clearance. However, excessively exploitative pathogens risk killing their hosts and hence truncating their own infectious periods. Death through over-exploitation is thus the fitness cost said to be curbing excessively virulent pathogens. Yet, extensive biomedical data show that a very substantial proportion of infection-induced mortality is not due to transmission-enhancing exploitation of hosts by pathogens, but due to host immune responses against infection. For instance, among the infections of greatest concern to the World Health Organization are a set of immune-mediated diseases including tuberculosis, malaria, dengue fever and Chagas disease which, collectively, kill over 3 million people per year (Graham et al. 2005). Here, we ask how pathogen exploitation schedules should evolve given the reality of immune-induced host death.
Infectious agents can kill hosts via at least two conceptually distinct routes. If uncontrolled, parasites can directly kill hosts through excessive tissue damage. In the case of microparasites such as viruses, bacteria and protozoa, hosts are simply overwhelmed by parasite numbers. This is a major reason why immunodeficient animals die. This mechanism of host death is most often considered in current theory on virulence evolution: higher pathogen densities are assumed to be a consequence of greater host exploitation which, in the absence of host death, results in enhanced transmission. However, mortality rates often exceed that attributable to parasites alone. Immune effector mechanisms can cause serious damage to host tissue, and the damage can be lethal. The most extreme example is septic shock: the life-threatening symptoms of shock (multi-organ failure and low blood volume) stem from innate immune responses to bacteria, rather than direct effects of the bacteria themselves (Munford 2006). Likewise, lethal cases of prevalent tropical diseases such as malaria are frequently due to excessive immune effector activity rather than parasite density per se (Clark et al. 2004). Influenza induces much more immunological activity than is necessary to clear the virus, and it is this excess activity that does most of the damage to the lung (Hussell et al. 2001; Xu et al. 2004). Recent outbreaks of H5N1 avian flu, and possibly also the 1918 influenza pandemic, killed people owing to excessively exuberant inflammatory responses known as cytokine ‘storms’ triggered by high viral titres (de Jong et al. 2006; Kobasa et al. 2007).
From a biomedical perspective, the costs of defence due to excessive or misdirected immune effector mechanisms appear to be both large and ubiquitous. Experiments on laboratory animals have revealed that immune effectors such as antibodies, superoxides and collagen cause the death of mammalian hosts fighting a huge range of infections: from flaviviruses such as West Nile (King et al. 2007) or poxviruses such as smallpox (Stanford et al. 2007) through to metazoan parasites such as schistosomes (Hoffmann et al. 2002). Indeed, bacterial virulence seems to be mostly due to immune over-response rather than direct tissue damage by the replication of bacteria (Margolis & Levin in press). In immunology and clinical biomedicine, such immune-mediated disease is usually termed immunopathology. We here use this relatively concise term to mean damage to host tissue that is caused by the host's own immune effector mechanisms. We concern ourselves only with infection-related immunopathology, ignoring immunopathologies such as rheumatoid arthritis, which apparently occur independently of infection.
In the framework presented here, we consider infection-induced mortality to comprise two elements: mortality that is a direct consequence of parasite exploitation, and the mortality caused by host immune responses (and any interaction between the two). For simplicity, we refer to the second of these elements as immunopathology, but we note that the equations below are agnostic about the mechanistic basis of host-induced mortality. Thus, the mortality cost of defence we are discussing incorporates other costs not generally labelled as immunopathology in biomedical research, including those arising from resource reallocation and energetic constraints (Rolff & Siva-Jothy 2003). Our purpose is to determine how immunopathology alters the evolution of parasite-encoded virulence factors, and how this evolution then affects the total infection-induced mortality. Some previous models of virulence evolution have included aspects of immunopathology (e.g. Alizon & van Baalen 2005), but none has provided a systematic study of how immunopathology affects virulence evolution. Here, we use a simple model to do so and to illustrate that there are different ways in which immunopathology can act, each with its own consequences for virulence evolution.
2. Theoretical development
Our approach supposes that there is a single trait of evolutionary interest in the parasite species, referred to as ‘exploitation level’. We use ‘exploitation’ in a very general sense, but practically, high exploiter pathogens might be those that secrete more tissue-degrading toxins, or more efficiently bind to host tissue or evade host responses. In many instances, exploitation level might be quantified as within-host parasite density. Whatever the underlying mechanism, our key assumption about exploitation is that it is positively associated with the transmission rate between hosts. The traditional trade-off hypothesis also posits that exploitation is positively associated with the mortality rate of the host (e.g. higher parasite density leads, directly, to higher host mortality). Otherwise, intermediate levels of exploitation are not expected to evolve. We will typically make this assumption here as well, but as will be seen, it is not always a requirement for the intermediate levels of infection-induced mortality to evolve.
Provided there is no co-infection or superinfection, we expect natural selection to maximize the parasite's R0. For a standard susceptible-infected (SI) epidemiological model (Anderson & May 1982), R0 has the form
| (2.1) |
Here, ϵ is the parasite exploitation strategy; β is transmission rate; μ is background host mortality rate; c is the recovery rate of infection via protective (parasite killing) immune responses; and γ is a constant that scales the effect of host exploitation on the infection-induced mortality (Frank 1996). In virtually all previous models, γϵ is what is taken as the definition of parasite virulence (Day 2002). The clearance rate, c, is the instantaneous rate at which infected individuals leave the infected class, and this is expected to be positively associated with a host's investment in an immunological response. For example, higher concentrations of antibodies are expected to be associated with higher rates of clearance. We note that most measures of exploitation and immune investment (and thus, the parameters ϵ, β, c) typically vary during the course of an infection, whereas expression (2.1) implicitly assumes that these are constant. In such cases, the epidemiological parameters in (2.1) should be interpreted as averages across infection age (appendix A). Finally, we suppose that transmission rate increases with exploitation with diminishing returns (mathematically, β′(ϵ)>0, β″(ϵ)<0).
Given expression (2.1) for parasite fitness, we can calculate the selection gradient on exploitation by differentiating (2.1) with respect to ϵ (Otto & Day 2007), giving
| (2.2) |
Expression (2.2) contains two terms, representing current and future reproduction by the parasite. The term dβ/dϵ represents the transmission benefit of increased exploitation, and γ is the survival cost of increased exploitation (i.e. a unit increase in exploitation increases the mortality rate by a factor γ). The latter term is weighted by β/(μ+c+γϵ), which we will refer to as the value of survival; it is the future reproduction that a parasite can expect if the current infection survives (conversely, it is the reproduction that is given up if the current infection does not survive). At the evolutionarily stable strategy (ESS) exploitation level, these costs and benefits exactly balance, making expression (2.2) zero (Frank 1996). From (2.2), it can also be seen that, in the absence of a direct mortality cost of exploitation (i.e. if γ=0), exploitation is expected to evolve upwards without bound (because dβ/dϵ>0).
With immunopathology, the mortality rate of the host is increased, which requires an additional term in the denominator of expression (2.1) for parasite fitness. Furthermore, immunopathology will often depend on pathogen exploitation, sometimes positively and sometimes negatively. High viral loads apparently trigger the cytokine storms that cause fatal H5N1 infections in humans (de Jong et al. 2006). Similarly, immunopathology will often be an unavoidable consequence of clearing pathogens (also referred to as friendly fire, collateral damage or bystander effects). For example, immune-mediated destruction of malaria-infected red blood cells can have a constant proportional side effect on uninfected red blood cells (Haydon et al. 2002). In such cases, better control of parasites will come at the cost of increased self-harm. On the other hand, hyperpathogenic strains of Marek's disease virus in poultry are massively immunosuppressive because they destroy lymphoid organs, substantially reducing the potential for immunopathology (Davison & Nair 2004). It is also possible that immunopathology is independent of both clearance and exploitation. Filariasis (elephantiasis) is an immune-mediated disease that is not associated with worm elimination or worm fecundity (Behnke et al. 1992; Sartono et al. 1997). Finally, it is also easy to envisage that, in a statistical sense, immunopathology could be associated with an interaction between rates of exploitation and clearance. Thus, our formulation allows immunopathology to depend on the parasite exploitation rate as well as host recovery rate in arbitrary ways.
We define the function f(ϵ, c) to be the additional mortality due to immunopathology and α(ϵ, c)≡γϵ+f(ϵ, c) to be the total infection-induced mortality, including both exploitation and immunopathology. This separation of sources of mortality into those directly related to host exploitation and those related to immunopathology is conceptually useful, but in practice it will typically not be possible to separate these two so clearly. However, this is of no consequence to the below results since all analyses can be conducted on the total infection-induced mortality.
With these specifications, expression (2.1) then becomes
| (2.3) |
We can again calculate the selection gradient on exploitation by differentiating (2.3) with respect to ϵ, treating c as being independent of ϵ, giving
| (2.4) |
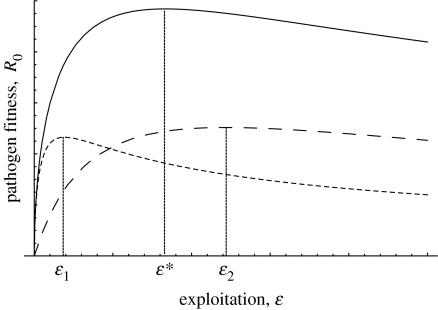
A comparison of expression (2.2) with expression (2.4) reveals that immunopathology has two effects on parasite evolution. First, it always decreases the value of survival because any infection that survives will no longer be as productive in the future (owing to the additional mortality; the f term in the denominator of (2.4)). This selects for increased exploitation. Second, if the extent of mortality caused by immunopathology depends on the level of parasite exploitation (i.e. ∂f/∂ϵ≠0), then the survival cost of exploitation will also change. For example, if mortality due to immunopathology increases with exploitation level, then the survival cost of exploitation will increase. The reason is simply that differences between strains in exploitation will then translate into larger differences in the probability that an infection will survive (i.e. γ in the absence of immunopathology versus γ+∂f/∂ϵ in the presence of immunopathology). This then selects for decreased exploitation. These considerations yield the following conclusions: if the extent of immunopathology is independent of exploitation or if it decreases with increasing exploitation, then immunopathology always causes the ESS level of exploitation to increase. If the extent of immunopathology is positively associated with the level of exploitation, however, then immunopathology causes a smaller increase in the ESS exploitation level and can even cause it to decrease (figure 1).
Figure 1.
Schematic of parasite fitness as a function of exploitation for three different scenarios: solid line, no immunopathology (ϵ* gives optimal exploitation); short dash, extent of immunopathology increases with increasing exploitation (ϵ1 gives optimal exploitation); and long dash, immunopathology is independent of exploitation (ϵ2 gives optimal exploitation). Either form of immunopathology results in an overall reduction in pathogen fitness, but the optimal value of exploitation can be shifted up or down depending on how immunopathology acts.
The above conclusions are phrased in terms of the ESS exploitation level, but the total infection-induced mortality rate is typically of more interest (and is more readily observable). This latter quantity is the summed effect of the exploitation level of the parasite, γϵ, as well as the mortality due to immunopathology itself, f (i.e. α(ϵ, c)=γϵ+f(ϵ, c)). Again, if immunopathology affects mortality rate independently of the level of parasite exploitation, or if it decreases with increasing exploitation, then immunopathology always causes the infection-induced mortality at the ESS level of exploitation, α(ϵ*, c), to be larger than when immunopathology is absent. The reason is simply that the ESS level of exploitation will be larger in this case, and the existence of immunopathology itself (i.e. f) increases the mortality rate α. If the mortality due to immunopathology increases with increasing exploitation, however, then the mortality directly due to exploitation, γϵ, will be affected less and might even decrease. At the same time, however, the existence of immunopathology itself will increase mortality. Therefore, the sum, γϵ+f(ϵ, c), might be larger, smaller or remain unchanged. Furthermore, since case mortality is monotonically related to α, the same qualitative conclusions hold if we quantify infection-induced mortality as the probability of an infection ending in death (conventionally called the case fatality rate in the biomedical literature; Day 2002).
To gain a more concrete appreciation for the above general results, it is helpful to specify a functional form for f(ϵ, c). A precise relationship between immunopathology and the rates of exploitation and clearance can sometimes be derived from assumptions about the mechanistic details of within-host parasite replication and the immune response (e.g. Krakauer & Nowak 1999; Wodarz & Krakauer 2000). The information required to do so is often not available, however, and therefore we take a more phenomenological approach.
Consider an experiment aimed at quantifying how infection-induced mortality changes in response to changes in clearance rates and/or exploitation (the latter perhaps being most readily measured as parasite density). The data might be analysed using a two-way ANOVA, with exploitation and clearance as predictor variables. The linear statistical model behind the analysis would have a constant term, a term for the main effects of each variable and a term for their interaction. Thus, the simplest complete expression for the mortality arising from immunopathology is
| (2.5) |
With this choice of f, the parameter ϕ0 specifies the degree of immunopathology that is independent of both recovery and exploitation, ϕ1 and ϕ2 determine its dependence on exploitation and recovery rate, respectively and ϕ3 determines the extent to which an interaction between exploitation and recovery affects immunopathology.
To take this example further, suppose that β(ϵ)=ϵn, where 0<n<1 is a parameter affecting the transmission benefits of increasing exploitation (n<1 implies that the relationship is saturating). The ESS level of exploitation is then
| (2.6) |
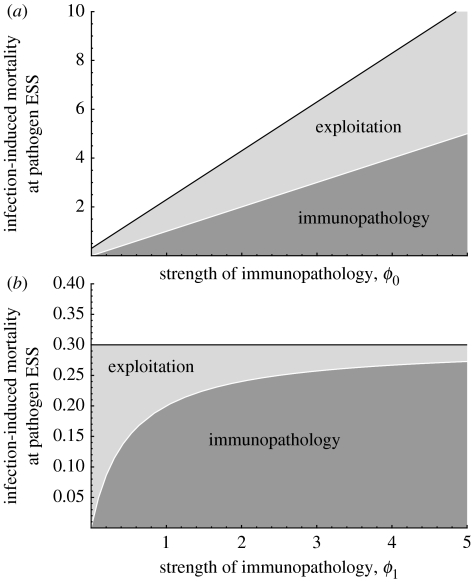
The effect of immunopathology on the ESS level of exploitation can be seen by comparing the full expression (2.6) with the case where we set the ϕi in (2.6) to zero. For example, if ϕ1=ϕ3=0, then immunopathology is independent of the level of exploitation. In this case, equation (2.6) illustrates the general prediction that such immunopathology causes the ESS level of exploitation to be larger (figure 2a). On the other hand, if immunopathology increases with exploitation, then the ESS exploitation can be smaller than that occurring in the absence of immunopathology. In particular, the ESS will be smaller than in the absence of immunopathology whenever (figure 2b).
Figure 2.
Infection-induced mortality at the pathogen ESS exploitation, α(ϵ*, c), plotted against increasing strength of immunopathology (measured as ϕ0 in (a) and ϕ1 in (b)). Results based on equations (2.6) and (2.7). (a) All immunopathology is independent of exploitation. The infection-induced mortality at the pathogen ESS increases (solid black line), and the amount of this mortality due to immunopathology at the ESS increases (dark grey), as does that due to exploitation (light grey). Parameter values: n=1/2, μ=1/(80×365), ϕ1=ϕ2=ϕ3=0, γ=1/2, c=3/10. (b) All immunopathology is positively associated with exploitation. The infection-induced mortality at the pathogen ESS remains constant at 0.3 (solid black line), but the amount of this mortality due to immunopathology increases (dark grey), while that due to exploitation decreases (light grey). Parameter values: n=1/2, μ=1/(80×365), ϕ0=ϕ2=ϕ3=0, γ=1/2, c=3/10.
The total infection-induced mortality at the ESS is also readily calculated for this model (provided that ϵ* is finite)
| (2.7) |
Equation (2.7) can be compared with the classical result, in which there is no immunopathology. In this case, we can see that the infection-induced mortality at the ESS level of exploitation is always larger than the classical result provided that there is some immunopathology that is independent of exploitation (i.e. if ϕ0>0 and/or if ϕ2>0; figure 2a). On the other hand, if all immunopathology is positively associated with exploitation (ϕ0=ϕ2=0 but ϕ1>0 and/or ϕ3>0), then, in this example, the mortality rate at the ESS is identical to that which occurs in the absence of immunopathology. This second prediction is somewhat counter-intuitive and occurs owing to the contrasting effects that immunopathology has on infection-induced mortality. If immunopathology acts solely in response to exploitation, then the ESS level of exploitation will be smaller (as seen in equation (2.6)). At the same time, the additional mortality caused by the immunopathology itself counteracts this effect. In this specific example, these two opposing factors happen to exactly cancel; the total infection-induced mortality remains unchanged, but the part of this that is directly due to exploitation is decreased (at evolutionary equilibrium) and this decrease is exactly compensated for by the extra mortality arising from immunopathology (figure 2b). In other cases, the two opposing forces need not exactly cancel, so that infection-induced mortality at the new pathogen ESS can be smaller or larger than it would be in the absence of immunopathology.
Equation (2.6) also clearly illustrates that, in the presence of immunopathology, intermediate levels of exploitation can evolve even if exploitation does not directly cause host mortality (i.e. if γ=0). There is evidence that the mortality experienced by mice infected with lymphocytic choriomeningitis virus (LCMV) is entirely due to host defence mechanisms. LCMV is a non-cytopathic virus and thus, in the absence of a strong, antigen-specific immune response, the virus can persist in mice without causing detrimental effects (Moskophidis et al. 1995; Moskophidis & Zinkernagel 1996). The occurrence of a CD8+T cell response can clear the virus by lysing infected cells, but this tends also to be detrimental to the host owing to collateral damage to the meninges. In the context of equation (2.6), this might be described by setting γ=ϕ0=ϕ1=0. Thus, so long as the mouse population is immunocompetent, exploitation and thus transmission (and infection-induced mortality) will evolve to intermediate values. For a population of immunodeficient hosts, however, exploitation and thus transmission would evolve to very high values.
Finally, it is useful to consider how the presence of immunopathology alters the effect of host-mediated parasite killing on parasite evolution. Previous analyses of the evolution of virulence in the absence of immunopathology have demonstrated that ESS exploitation and thus virulence increases as host recovery rate increases (van Baalen 1998; Day & Burns 2003). A comparison of expressions (2.2) and (2.4), however, reveals that immunopathology has the potential to qualitatively alter this prediction. An increase in recovery rate reduces the value of survival by decreasing future reproductive output. This selects for increased exploitation. At the same time, if the dependence of immunopathology on exploitation is affected by recovery (i.e. a c×ϵ interaction, where ∂f/∂ϵ in expression (2.4) increases with recovery rate), then the survival cost of increased exploitation is also increased. This generates selection for decreased exploitation. The net outcome then depends on the relative strength of these two factors.
3. Discussion
Several authors have pointed out that the fact that host responses cause much disease is a complication for parasite-centric trade-off models of virulence evolution (e.g. Lipsitch & Moxon 1997; Ebert & Bull 2003, in press; Graham et al. 2005; Margolis & Levin in press). Here, we have shown formally that this is indeed so, but that the trade-off framework can be expanded to accommodate this reality.
Immunopathology has two broad classes of effect on infection-induced mortality when parasites are allowed to evolve. First, if the degree of mortality induced by immunopathology is independent of the level of exploitation by the parasite, then there will be higher host mortality at the ESS than there would be in the absence of immune-mediated disease. Second, if immunopathology increases with parasite exploitation, then immunopathology will have a smaller effect on the infection-induced mortality rates at the ESS and might even lead to pathogen evolution which lowers overall infection-induced mortality.
From this, we make several predictions. All else being equal, we expect the highest infection-induced mortality in host–pathogen systems where there is a lot of immunopathology that is relatively independent of the exploitation. This is because immune self-harm undermines the survival benefits of restrained exploitation and this sort of immunopathology will occur whatever the parasite does. In contrast, overall mortality rates should be lower in systems in which immune-mediated disease rises as parasite exploitation increases. Here, evolution will favour pathogen strains better able to avoid inducing immunopathology.
The same logic also generates related predictions about the relative contributions of direct pathogen damage and immunopathology to disease outcome. A large component of infection-induced mortality will be due to direct damage by the pathogen in systems where immunopathology is strong and relatively independent of what the pathogen does. Again this is because, all else being equal, exploitation-induced mortality is expected to increase in the presence of such immunopathology (figure 2a). In contrast, in systems where immunopathology is a consequence of pathogen exploitation, the contribution to the overall mortality of direct pathogen damage will be lower relative to that due to immune self-harm. Selection will favour less exploitative pathogens where exploitation-related mortality is further inflated by exploitation-related immunopathology (figure 2b).
Modern laboratory immunology provides a tool kit to experimentally disentangle parasite-derived mortality and immunologically derived mortality, especially in rodent models of infectious disease. For example, reagents are commercially available, which can enhance immune responses against microparasites such as malaria (Li et al. 2003). In effect, these reagents remove the regulatory control of immune effector mechanisms. In the case of malaria, use of such reagents increased the proportion of malaria virulence that, prior to parasite clearance, was due to immunopathology rather than parasite density (G. Long, B. Chan, J. Allen, A. Read, A. Graham 2007, unpublished work). In terms of equation (2.5), this might represent a manipulation of clearance rate and thus would indicate that ϕ2>0 and/or ϕ3>0 for this pathogen (i.e. higher clearance rates result in greater disease severity). An opposing treatment (also commercially available) that depletes immune effectors but has no effect on parasite densities reduced malaria virulence, further indicating that ϕ2>0 and/or ϕ3>0 in this system (Long et al. 2006). One could therefore use such treatments to determine if parasite exploitation evolves in a way predicted by the theory. Experimental evolution in such systems would be difficult but, by analogy with other studies (e.g. Mackinnon & Read 2003), it should be possible to compare the fitness of high- and low-virulence parasite strains, and deduce which would be favoured by selection in different immune environments.
Various clinical and public health interventions can also affect infection-related immunopathology. How would we expect infected-induced mortality to evolve in response to these? Previous studies have focused on the evolutionary consequences of vaccination and have shown that predictions about pathogen evolution depend on whether vaccination affects transmission rate, parasite-induced mortality or clearance rate (Gandon et al. 2001; Mackinnon et al. in press). In the presence of immunopathology, vaccines can have even more varied and subtle effects that will influence pathogen evolution and hence infection-induced mortality. With malaria, for example, much pathogenesis is due to a proinflammatory cytokine cascade triggered by parasite molecules such as glycosylphosphatidylinositol (GPI). These responses are believed to be first-line defences necessary to control otherwise lethal pathogen replication during acute primary infections. However, the responses themselves cause substantial collateral damage to the host (Clark et al. 2004). Antibody-mediated control of parasites takes longer to develop but is associated with considerably less immune-mediated disease. Many candidate malaria vaccines are aimed at priming protective antibody response, which should achieve parasite control without proinflammatory immunopathology. Moreover, some candidate vaccines are aimed at directly reducing immune-mediated disease by eliciting anti-GPI antibodies to deliberately remove these potent immunostimulatory parasite molecules (Schofield & Grau 2005; Riley et al. 2006). What will be the consequences of these sorts of vaccines on pathogen evolution?
Space limitations preclude a full analysis of the evolutionary consequences of different kinds of vaccines here, but a few predictions can be made using the results already obtained. In the absence of immunopathology, vaccines that increase clearance rate are predicted to lead to the evolution of higher levels of exploitation (Gandon et al. 2001). With immunopathology, however, the outcome will depend on the extent to which there is an interaction between recovery rate and exploitation in determining immunopathology. If the effect of exploitation on immunopathology is independent of the rate of parasite clearance, then earlier predictions remain valid. In the presence of a positive interaction between exploitation and recovery, the predicted effect can be reversed (clearance-enhancing vaccines lead to the evolution of lower exploitation).
Another possibility is that a vaccine simply reduces the degree of immunopathology experienced by the host. The proposed anti-GPI malaria vaccines discussed above are intended to act in this way (Schofield & Grau 2005; Riley et al. 2006). If immunopathology is independent of exploitation, then reducing immune-mediated disease via vaccination will lead to an evolutionary reduction in exploitation and thus infection-induced mortality (as measured in an unvaccinated host). This is because the vaccines are, in effect, reducing mortality over which the parasite has no control, increasing the fitness gains to be had by exploiting the host more prudently. If immunopathology increases strongly with exploitation, then a reduction in immunopathology via vaccination will lead to an evolutionary increase in exploitation and thus higher mortality rates among the unvaccinated. Anti-GPI vaccines will probably do this because concentrations of GPI molecules, which come from parasite membranes, almost certainly increase with parasite density and replication rate. If so, the widespread use of anti-GPI vaccines will allow more exploitative pathogen strains to spread because these strains can accrue the fitness benefits of exploitation at reduced cost (Gandon et al. 2001; Mackinnon et al. in press). More generally, however, vaccination might affect the various components of infection-induced mortality differently. For example, one can readily imagine vaccines whose effects arise from a modulation of the values of γ, ϕ0, ϕ1, ϕ2 and ϕ3 in different ways. The evolutionary consequences of these vaccines would require a more in-depth analysis than is possible here.
Other medical interventions that modulate immunopathology might also have similar effects on pathogen evolution, if their use is to become sufficiently widespread. Statins, for instance, beneficially modulate the inflammatory cascades that trigger severe sepsis and shock, and their administration has been suggested as a potential prevention and treatment strategy (Terblanche et al. 2006; other analogous possibilities are reviewed by Margolis & Levin (in press)). The efficacy of such measures in terms of symptom alleviation is relatively straightforward to determine through standard biomedical protocols. The longer-term consequences of their use for pathogen evolution will require an interplay between the experimental dissections of the sort discussed above and models of the sort we have presented here. By analogy with the vaccine discussion above, they could favour the evolution of more or less pathogenic parasites, depending on the details.
The framework we have developed here could be extended in several directions. First, although the general results presented in equations (2.3) and (2.4) allow for any dependence of immunopathology on clearance and exploitation, most of our considerations have assumed that immunopathology either increases or decreases monotonically with these parameters. Theoretical studies of within-host pathogen replication and host immune responses suggest that the highest levels of immunopathology might sometimes occur at the intermediate values of these parameters (Krakauer & Nowak 1999). In such cases, it seems plausible that a greater variety of evolutionary outcomes would be possible.
Second, we have supposed here that the sole impact of immunopathology on pathogen fitness is through increased host mortality. In many cases, immunopathology also impacts on pathogen transmission while the host is alive. For instance, disease-causing cytokines elicited by malaria parasites transiently reduce infectiousness to mosquitoes (Karunaweera et al. 1992). Immunopathology can also enhance transmission. In tuberculosis, for instance, immunopathological necrosis of the lung enhances transmission, and damage of host tissue by immune effectors is also associated with increased transmission in schistosomiasis, dengue and leishmaniasis (reviewed by Graham et al. (2005)). Similarly, immunopathology causes considerable morbidity, and Ewald (1994) has argued that morbidity enhances transmission of many vector-borne diseases by reducing anti-vector behaviour in the host. The evolution of exploitation strategies when immunopathology directly affects transmission rate (β) could be analysed using the framework adopted here, as could the situation where host death is required for transmission.
Third, we have focused attention on the evolution of a single pathogen trait (exploitation level), but there are many other aspects of a pathogen's life cycle that might evolve jointly with this trait. For example, the extent to which immunopathology occurs (as quantified by the ϕ parameters in our model) is probably determined, in part, by the tissue in which the pathogen replicates. Replication in some tissues might elicit higher levels of immunopathology than others, if it is more difficult (e.g. in terms of killing efficiency per antibody) for the host to mount a pathogen-specific attack in those tissues. Thus, the tissue tropism of a pathogen might evolve as a means to hide from host defence mechanisms. However, the extent to which this occurs will depend on the associated costs of such changes, including any reduced transmission potential that results, as well as any increased likelihood of eliciting immunopathology (which can be detrimental to both the host and parasite) in tissues that are acutely sensitive to inflammatory damage. These are issues that warrant a more detailed theoretical examination than is possible in the context of the current paper.
Finally, it seems probable that in many cases, the degree of infection-induced mortality, as well as immunopathology, will often be the outcome of a coevolutionary dynamic between hosts and parasites (van Baalen 1998; Day & Burns 2003). Here, we have considered only the evolution of the parasite, in part to be comparable with previous models of parasite virulence evolution, and because for fast-evolving pathogens, the evolution of hosts (particularly large vertebrates) can be safely ignored. However, a variety of coevolutionary scenarios can be envisaged, including the evolution of immunomodulatory manipulations by the pathogen, which might sometimes be mutually beneficial for both parasite and host. Host evolution is also probable, with the optimal clearance rate dependent on how immunopathology scales with protective defence and what the pathogen is doing. An analysis of the impact of immunopathology on the evolution of host defence and host–parasite coevolution will be presented in a future article.
Acknowledgments
We thank three anonymous reviewers for their comments that improved the presentation of these ideas. This work was begun when T.D. was on sabbatical in Edinburgh supported by a BBSRC International Fellowship, and finished when A.F.R. and A.L.G. were at the Wissenshaftskolleg zu Berlin. Our empirical work is funded by the Wellcome Trust and the BBSRC. A.L.G. is supported by a BBSRC Fellowship (BB/D01977X/1).
Appendix A. When parameters depend on infection age
One epidemiological model that gives rise to expression (2.1) for R0 is
| (A1) |
where S and I are the number of susceptible and infected hosts. Model (A 1) implicitly assumes that clearance rate and exploitation (and thus transmission, β, and exploitation-induced mortality, γϵ) are constant during an infection. A simple extension of (A 1) that allows for infection age is (Day 2001)
| (A2) |
with boundary condition . Now, let us define as the total number of infected individuals at time t. Also, for any function of infection age, x(a), let us define its average at time t as . We can then integrate across infection age in (A 2) to obtain
| (A3) |
Over time, we expect the age distribution of infections, I(a, t)/IT, to reach a steady state, making constant. Thus, model (A 3) can be seen as analogous to model (A 1), except where the epidemiological parameters are averaged over the distribution of infection ages.
References
- Alizon S, van Baalen M. Emergence of a convex trade-off between transmission and virulence. Am. Nat. 2005;165:E155–E167. doi: 10.1086/430053. doi:10.1086/430053 [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Behnke J.M, Barnard C.J, Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int. J. Parasitol. 1992;22:861–907. doi: 10.1016/0020-7519(92)90046-n. doi:10.1016/0020-7519(92)90046-N [DOI] [PubMed] [Google Scholar]
- Clark I.A, Alleva L.M, Mills A.C, Cowden W.B. Pathogenesis of malaria and clinically similar conditions. Clin. Microbiol. Rev. 2004;17:509–539. doi: 10.1128/CMR.17.3.509-539.2004. doi:10.1128/CMR.17.3.509-539.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison F, Nair V, editors. Marek's disease: an evolving problem. Elsevier; Oxford, UK: 2004. [Google Scholar]
- Day T. Parasite transmission modes and the evolution of virulence. Evolution. 2001;55:2389–2400. doi: 10.1111/j.0014-3820.2001.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Day T. On the evolution of virulence and the relationship between various measures of mortality. Proc. R. Soc. B. 2002;269:1317–1323. doi: 10.1098/rspb.2002.2021. doi:10.1098/rspb.2002.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. Virulence evolution and the timing of disease life-history events. Trends Ecol. Evol. 2003;18:113–118. doi:10.1016/S0169-5347(02)00049-6 [Google Scholar]
- Day T, Burns J.G. A consideration of patterns of virulence arising from host–parasite coevolution. Evolution. 2003;57:671–676. doi: 10.1111/j.0014-3820.2003.tb01558.x. [DOI] [PubMed] [Google Scholar]
- de Jong M.D, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. doi:10.1038/nm1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Bull J.J. Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol. 2003;11:15–20. doi: 10.1016/s0966-842x(02)00003-3. doi:10.1016/S0966-842X(02)00003-3 [DOI] [PubMed] [Google Scholar]
- Ebert, D. & Bull, J. J. In press. The evolution and expression of virulence. In Evolution in health and disease Oxford, UK: Oxford University Press.
- Ewald P.W. Host–parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 1983;14:465–485. doi:10.1146/annurev.es.14.110183.002341 [Google Scholar]
- Ewald P.W. Oxford University Press; Oxford, UK: 1994. Evolution of infectious disease. [Google Scholar]
- Frank S.A. Models of parasite virulence. Q. Rev. Biol. 1996;71:37–78. doi: 10.1086/419267. doi:10.1086/419267 [DOI] [PubMed] [Google Scholar]
- Gandon S, Mackinnon M.J, Nee S, Read A.F. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. doi:10.1038/414751a [DOI] [PubMed] [Google Scholar]
- Graham A.L, Allen J.E, Read A.F. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 2005;36:373–397. doi:10.1146/annurev.ecolsys.36.102003.152622 [Google Scholar]
- Haydon D.T, Matthews L, Timms R, Colegrave N. Top-down or bottom-up regulation of intra-host blood-stage malaria: do malaria parasites most resemble the dynamics of prey or predator? Proc. R. Soc. B. 2002;270:289–298. doi: 10.1098/rspb.2002.2203. doi:10.1098/rspb.2002.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K.F, Wynn T.A, Dunne D.W. Cytokine-mediated host responses during schistosome infections: walking the fine line between immunological control and immunopathology. Adv. Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. doi:10.1016/S0065-308X(02)52014-5 [DOI] [PubMed] [Google Scholar]
- Hussell T, Pennycook A, Openshaw P.J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. doi:10.1002/1521-4141(200109)31:9<2566::AID-IMMU2566>3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- Karunaweera N.D, Carter R, Grau G.E, Kwiatkowski D, Del Giudice G, Mendis K.N. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin. Exp. Immunol. 1992;88:499–505. doi: 10.1111/j.1365-2249.1992.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.J.C, Getts D.R, Getts M.T, Rana S, Shrestha B, Kesson A.M. Immunopathology of flavivirus infections. Immunol. Cell Biol. 2007;85:33–42. doi: 10.1038/sj.icb.7100012. doi:10.1038/sj.icb.7100012 [DOI] [PubMed] [Google Scholar]
- Kobasa D, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. doi:10.1038/nature05495 [DOI] [PubMed] [Google Scholar]
- Krakauer D.C, Nowak M. T-cell induced pathogenesis in HIV: bystander effects and latent infection. Proc. R. Soc. B. 1999;266:1069–1075. doi: 10.1098/rspb.1999.0745. doi:10.1098/rspb.1999.0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Sanni L.A, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in IL-10-deficient mice are ameliorated by anti-TNF-alpha and exacerbated by anti-TGF-beta antibodies. Infect. Immun. 2003;71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. doi:10.1128/IAI.71.9.4850-4856.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Moxon E.R. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. doi:10.1016/S0966-842X(97)81772-6 [DOI] [PubMed] [Google Scholar]
- Long G.H, Chan B.H.K, Allen J.E, Read A.F, Graham A.L. Parasite genotype does not alter the effects of TNF-α on the virulence of murine malaria. Parasitology. 2006;133:673–684. doi: 10.1017/S003118200600117X. doi:10.1017/S003118200600117X [DOI] [PubMed] [Google Scholar]
- Mackinnon M.J, Read A.F. The effects of host immunity on virulence–transmissibility relationships in the rodent malaria parasite Plasmodium chabaudi. Parasitology. 2003;126:103–112. doi: 10.1017/s003118200200272x. doi:10.1017/S003118200200272X [DOI] [PubMed] [Google Scholar]
- Mackinnon, M. J., Gandon, S. & Read, A. F. In press. Virulence evolution in response to vaccination: the case of malaria. Vaccine [DOI] [PMC free article] [PubMed]
- Margolis, E. & Levin, B. R. In press. The evolution of bacteria–host interactions: virulence and the immune over-response. In Introduction to the evolutionary biology of bacterial and fungal pathogens (eds J. A. Gutirrez & F. Baquero).
- Moskophidis D, Zinkernagel R.M. Immunobiology of cytotoxic T-cell resistant virus variants: studies on lymphocytic choriomeningitis virus (LCMV) Virology. 1996;7:3–11. doi: 10.1128/jvi.69.4.2187-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D, Battegay M, Vandenbroek M, Laine E, Hoffmannrohrer U, Zinkernagel R.M. Role of virus and host variables in virus persistence or immunopathological disease caused by a noncytolytic virus. J. Gen. Virol. 1995;76:381–391. doi: 10.1099/0022-1317-76-2-381. [DOI] [PubMed] [Google Scholar]
- Munford R.S. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu. Rev. Pathol. Mech. Dis. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. doi:10.1146/annurev.pathol.1.110304.100200 [DOI] [PubMed] [Google Scholar]
- Otto S.P, Day T. Princeton University Press; Princeton, NJ: 2007. A biologist's guide to mathematical modeling in ecology and evolution. [Google Scholar]
- Riley E.M, Wahl S, Perkins D.J, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. doi:10.1111/j.1365-3024.2006.00775.x [DOI] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. doi:10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- Sartono E, Kruize Y.C, Kurniawan A, Maizels R.M, Yazdanbakhsh M. Depression of antigen-specific IL-5 and IFN-γ responses in human lymphatic filariasis as a function of clinical status and age. J. Infect. Dis. 1997;175:1276–1280. doi: 10.1086/593701. [DOI] [PubMed] [Google Scholar]
- Schofield L, Grau G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005;5:727–735. doi: 10.1038/nri1686. doi:10.1038/nri1686 [DOI] [PubMed] [Google Scholar]
- Stanford M.M, McFadden G, Karupiah G, Chaudhri G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 2007;85:93–102. doi: 10.1038/sj.icb.7100033. doi:10.1038/sj.icb.7100033 [DOI] [PubMed] [Google Scholar]
- Terblanche M, Almog Y, Rosenson R, Smith T, Hackam D. Statins: panacea for sepsis? Lancet Infect. Dis. 2006;6:242–248. doi: 10.1016/S1473-3099(06)70439-X. doi:10.1016/S1473-3099(06)70439-X [DOI] [PubMed] [Google Scholar]
- van Baalen M. Coevolution of recovery ability and virulence. Proc. R. Soc. B. 1998;265:317–325. doi: 10.1098/rspb.1998.0298. doi:10.1098/rspb.1998.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz D, Krakauer D.C. Defining CTL-induced pathology: implications for HIV. Virology. 2000;274:94–104. doi: 10.1006/viro.2000.0399. doi:10.1006/viro.2000.0399 [DOI] [PubMed] [Google Scholar]
- Xu L, Yoon H, Zhao M.Q, Liu J, Ramana C.V, Enelow R.I. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-α by antiviral CD8+ T cells. J. Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]