Abstract
Animals exploiting their familiar food items often avoid spatio-temporal aggregation with others by avoiding scents, less rewarding areas or visual contacts, thereby minimizing competition or interference when resources are replenished slowly in patches. When animals are searching or assessing available food sources, however, they may benefit from reducing sampling costs by following others at food sites. Therefore, animals may adjust their responses to others depending on their familiarity with foraging situations. Here, we conducted field experiments to test whether nectar-collecting bumble bees make this adjustment. We allowed free-foraging bees to choose between two inflorescences, one occupied by a conspecific bee and another unoccupied. When bees were presented with flowers of a familiar type, they avoided occupied inflorescences. In contrast, bees visited an occupied inflorescence when the flower type was unfamiliar. To our knowledge, this is the first report suggesting that animals adjust their responses to feeding conspecifics depending on their familiarity with food sources. Such behavioural flexibilities should allow foragers to both explore and exploit their environments efficiently.
Keywords: Bombus diversus, context-dependent behaviour, foraging, inadvertent social information, local enhancement, plant–animal interactions
1. Introduction
Animals exploiting the same food source with other individuals of both the same and other species tend to compete and interfere with one another, thereby reducing their food consumption rate (Thomson et al. 1987; Sandlin 2000a). It follows from this that individuals would often avoid spatio-temporal aggregation or overlaps with other foragers in an area by responding to the presence of others (perceived by decreased reward levels, odours or encounters) in an antagonistic way. Many previous studies have suggested that animals indeed avoid one another while foraging, which sometimes leads to resource partitioning (Heinrich 1976b; Alanärä et al. 2001; Rodríguez-Gironés 2006) or ideal free distributions (reviewed by Sutherland 1996). Individuals arriving late at a patch or a habitat would especially tend to avoid occupied (and exploited) areas either when resources replenish slowly (Stout & Goulson 2002) or when competition acts in favour of experienced individuals through a systematic foraging (Possingham 1989) or when resident foragers defend their territories by aggression or exploitation (Paton & Carpenter 1984; Nagamitsu & Inoue 1997).
When animals are searching for novel food items or assessing profitability of available food sources in an unfamiliar habitat, however, they may benefit by following other feeding individuals, rather than avoiding them. Animals that can use a broad range of food items would initially have to find and learn its resources by gathering information through their own sampling efforts, as well as having to locate and sample other food sources to establish their new targets as food availability changes in an unpredictable way (Heinrich 1979; Thomson 1981). In these situations, individuals may effectively reduce the cost of exploration by using the presence of other foragers on a food source as an indirect cue of its location and profitability (Giraldeau & Beauchamp 1999; Valone & Templeton 2002; Danchin et al. 2004; Kawaguchi et al. 2006). Many vertebrates use such cues provided inadvertently by other individuals for the detection of unfamiliar food types or locations (reviewed by Danchin et al. 2004; Dall et al. 2005). Recent studies have revealed that even insects can use these social cues under similar conditions (Collins & Bell 1996; Raveret Richter & Tisch 1999; D'Adamo et al. 2000; Prokopy et al. 2000; Slaa et al. 2003; Leadbeater & Chittka 2005; Kawaguchi et al. 2006), although the possibility that an individual is attracted to an occupied resource simply because it is attracted to conspecifics themselves has been rarely excluded experimentally (but see Worden & Papaj 2005).
Rational responses for an animal towards resident individuals on food should largely depend on how familiar it is with the food it is exploiting, not solely on how intensely it is competing with others. If foragers are able to decide how to respond to others depending on their foraging contexts, therefore, they could greatly improve their lifetime foraging success by tracking changes in food availability more quickly and accurately. Such a conditional use of alternative behaviours would be advantageous to flower visitors exploiting nectar and pollen, whose abundance and quality vary considerably in time and space (Pleasants 1983; Waser 1983; Hunter & Price 1992). Indeed, many previous studies have found that flower visitors often avoid spatio-temporal aggregation with other foragers (Inouye 1978; Pimm et al. 1985; Thomson et al. 1987; Giurfa & Núñez 1992; Goulson et al. 1998; Sandlin 2000b; Gilbert et al. 2001; Ohashi & Yahara 2002; Makino & Sakai 2004, 2005), but other studies have reported that these animals were instead attracted to other foragers when sampling novel flowers (bat: Howell 1979; stingless bee: Slaa et al. 2003; Leadbeater & Chittka 2005; bumble bee: Kawaguchi et al. 2006). Despite these suggestive findings, however, no previous study has explicitly tested whether flower visitors adopt avoidance versus attraction in a conditional way depending on their foraging contexts in nature.
Here, we report on our field experiments which examine how nectar-collecting bumble bees (Bombus diversus) respond to conspecifics that are present at flowers of familiar and unfamiliar plant species (Abelia grandiflora and Kalanchoe blossfeldiana, respectively). Numerous studies have suggested that bumble bees learn to avoid spatio-temporal aggregation with other bees on flowers (Heinrich 1976a; Inouye 1978; Thomson et al. 1987; Dreisig 1995; Goulson et al. 1998; Ohashi & Yahara 2002; Stout & Goulson 2002; Makino & Sakai 2004, 2005; Saleh et al. 2006; but see Brian 1957; Leadbeater & Chittka 2005; Kawaguchi et al. 2006). Other studies have also reported that bumble bees occasionally sample alternate flowers while specializing on one or a few flower species, which may allow them to track variable resources and shift to other species whenever circumstances require (Heinrich 1976b, 1979; Thomson et al. 1997). Since sampling inevitably involves measurable time and energy costs, we would expect that following conspecifics would be beneficial for experienced bumble bees when sampling unfamiliar flowers (Kawaguchi et al. 2006). Using ‘interview bouquets’ technique (Thomson 1981; Thomson et al. 1982; Kearns & Thomson 2001), we tested whether free-foraging bees visited inflorescences with conspecifics when sampling flowers while they chose inflorescences without conspecifics when harvesting from familiar flowers.
2. Material and methods
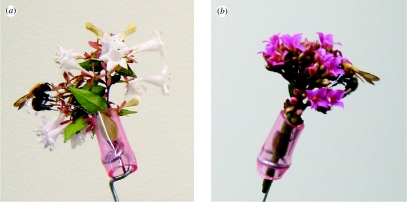
All experiments were conducted in a roadside artificial population of Abelia grandiflora Rehder (Caprifoliaceae) lining approximately 400 m along both sides of the Higashi Odori Road, Tsukuba, Ibaraki Prefecture, Japan (36°06′ N, 140°32′ E). A. grandiflora is a low tree species cultivated as garden or roadside plants. Each plant bears light pinkish-white flowers that are zygomorphic, perfect, tubular (approx. 15 mm in depth; figure 1a) and arranged in various angles and directions on numerous cymose inflorescences. Nectar accumulates in the basal part of the corolla tube. Flowering season extends from July to October and each flower lasts at least a few days. At our site, A. grandiflora was heavily visited by workers of Bombus diversus Smith. Other flower visitors included honeybees (Apis mellifera L. and Apis cerana japonica Rad.), skippers (Hesperiidae) and day-flying hawkmoths (Sphingidae). During our experiments, we found no other nearby plant population that attracts B. diversus as much as our Abelia population. B. diversus has a long proboscis (up to 14 mm) and prefers to visit tubular flower species. Workers visit Abelia mainly for nectar, but also accumulate corbicular pollen loads by bringing passively applied pollen to the hind legs.
Figure 1.
‘Occupied’ inflorescences used in experiments. (a) Abelia grandiflora (familiar flower species) and (b) Kalanchoe blossfeldiana (unfamiliar flower species). Each inflorescence is inserted into a plastic tube together with a thin wire to which a dead worker of B. diversus was attached.
To investigate whether responses of bumble bees to conspecifics vary with familiarity of the flower species that they are about to visit, we used Kalanchoe blossfeldiana Poelln (Crassulaceae) as the alternative unfamiliar flower species. K. blossfeldiana is cultivated as a garden plant. Each plant bears deep pink flowers arranged on several to ten umbels. Flowers are actinomorphic, perfect and upward-oriented. Each corolla consists of a slender tube (approx. 12 mm in depth) and a flat limb (figure 1b). Nectar accumulates in the narrow basal part of the corolla tube, and can be extracted by B. diversus with its long proboscis. Flowers of K. blossfeldiana thus differ greatly from those of A. grandiflora in colour, shape and arrangement. We bought potted Kalanchoe plants from a garden shop located approximately 9 km from our study site, and did not find this species within at least 1 km of our study site. We did not note any other concurrently blooming plants whose flowers look similar to K. blossfeldiana within the range.
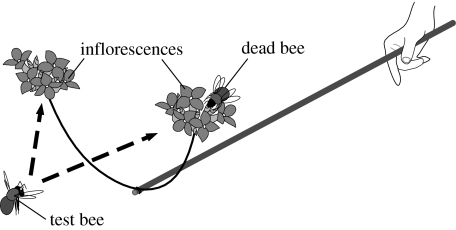
We used an ‘interview bouquet’ (Thomson 1981; Thomson et al. 1982; Kearns & Thomson 2001) to present free-foraging bees on Abelia with the two inflorescences, one of which was occupied by a conspecific and another was not (figure 2). We inserted each inflorescence into a short piece of plastic tube attached to each end of a 30 cm length of wire (1.5 mm diameter). The wire was bent in a crescent so that its ends faced upwards 20 cm apart from one another and was bound to one end of a 90 cm garden pole at the central part. To ensure that the flowers were filled with nectar and did not have odour left by recent insect visitors, we covered these inflorescences with 2 mm mesh bags for approximately 36 hours before we cut them from the plants for experiments. Free-flying workers of B. diversus were caught at random in other small populations of A. grandiflora within 3 km of our site. They were frozen to death at −20°C in clean plastic containers until we defrosted them approximately 30 min before experiments. In each trial, we attached a fresh dead bee to one of the two inflorescences with a piece of thin wire (0.28 mm in diameter) and we refer to it as an ‘occupied’ inflorescence. The other inflorescence without a dead bee we term ‘unoccupied’. Each inflorescence was randomly assigned to either the right or the left position. We trimmed the inflorescences to make occupied and unoccupied inflorescences the same display size (Abelia: 2–10 flowers, Kalanchoe: 3–15 flowers; figure 1).
Figure 2.
A schematic view of the experiment with ‘interview bouquet’ technique. The interview stick equipped with two inflorescence holders is extended towards a test bee.
During the peak forager activity in our Abelia population (06.00–09.00 hours), we continued to ‘interview’ arbitrarily selected foragers, half with the familiar inflorescences (Abelia) and half with the unfamiliar (Kalanchoe), in random order. In each trial, we extended the interview stick towards the test bee in such a way that the two inflorescences were equidistant from it, and observed which one it visited. We recorded the first inflorescence it landed on as ‘first landing’ and the first it probed as ‘first probing’. One trial would end when the test bee responded (approached, landed or probed) to either inflorescence at least once and then left. In total, 84 bees were tested on Abelia and 279 bees were tested on Kalanchoe. When a trial resulted in at least one landing or when more than 30 min had passed before any bee landed on the inflorescences, we replaced both inflorescences and the dead bee with new ones before the next trial. We also moved around between successive trials by walking 20–100 m or switching to the other side of the road (approx. 15 m wide) between successive trials. Since it normally took us only a few minutes to locate a new bee after the movement, we considered our experimental procedure would effectively minimize the possibility of testing with the same bees between successive trials. Daily experiments were repeated during the late flowering season of A. grandiflora in 2005 (17 September to 21 October).
For each experiment with A. grandiflora and K. blossfeldiana, we compared the frequency of bees' first visits to occupied and unoccupied inflorescences with a two-tailed binomial test. We also performed a two-tailed Fisher's exact test to compare bees' responses to occupied inflorescences between experiments with A. grandiflora and K. blossfeldiana.
3. Results
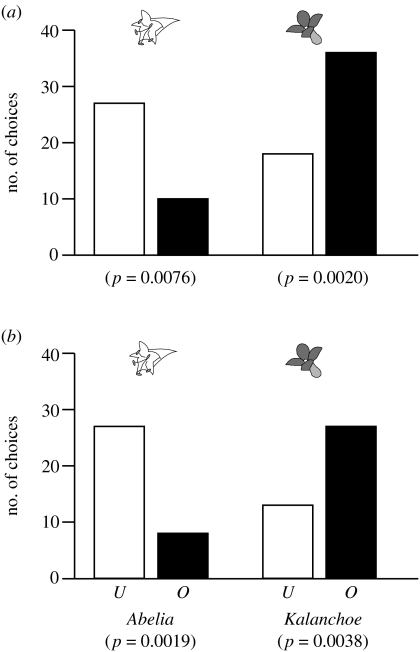
When bees were presented with familiar flower species (Abelia), significantly smaller portion of bees landed on or probed occupied inflorescences than unoccupied ones (two-tailed binomial test: p=0.0076 for first landing, p=0.0019 for first probing; figure 3). In contrast, bees landed on and probed occupied inflorescences significantly more often than unoccupied ones when they were presented with unfamiliar flower species (Kalanchoe; two-tailed binomial test: p=0.0020 for first landing, p=0.0038 for first probing; figure 3). For both first landing and first probing, we found that the observed trends in bees' responses towards occupied and unoccupied inflorescences significantly differed between Abelia and Kalanchoe (two-tailed Fisher's exact test: p=0.00027 for first landing, p=0.00017 for fist probing; figure 3); bees responded negatively to occupied inflorescences when they continued to visit familiar flower species, whereas they responded positively to occupied inflorescences when they sampled unfamiliar flower species.
Figure 3.
Number of (a) first landings and (b) first probings exhibited by bumble bees (open bars, unoccupied inflorescences (U); filled bars, occupied inflorescences (O)) when bees were presented with Abelia (familiar flower species, left two bars), and when they were presented with Kalanchoe (unfamiliar flower species, right two bars). The p-values in parentheses are obtained from two-tailed binomial tests.
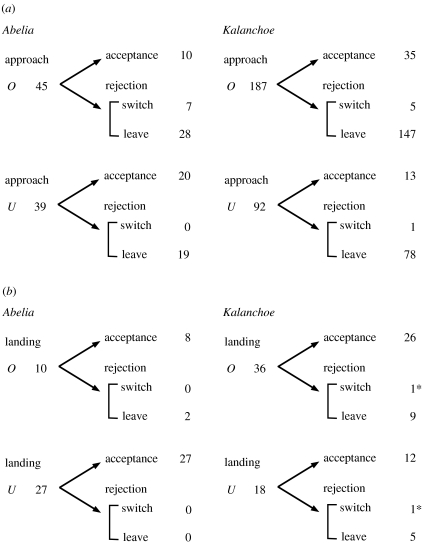
At the beginning of our data collection, we noted that a substantial number of bees approached and hovered in front of the test inflorescences prior to landing, and they sometimes flew away without touching either of the inflorescences even after several approaches. The data on landing and probing therefore did not include all the decision-makings made at approaching distances (1–5 cm off the inflorescences). So we additionally recorded the first inflorescence a bee approached as ‘first approach’ (figure 4a). When bees were presented with familiar species (Abelia), they showed no significant difference between occupied and unoccupied inflorescences in the frequency of first approaches (two-tailed binomial test: O : U=45 : 39, p=0.59). When bees were presented with unfamiliar species (Kalanchoe), on the other hand, they approached occupied inflorescences significantly more often than unoccupied ones (two-tailed binomial test: O : U=187 : 92, p<0.0001).
Figure 4.
Flow diagrams of bees' behavioural sequences (a) from first approach to first landing and (b) from first landing to first probing. Arrows and values indicate transitions from one behaviour to another and the number of observed transitions, respectively. *The two bees had landed on the same inflorescence that they first approached, but they switched to the other inflorescence while probing.
If bees approached one inflorescence and landed on it subsequently, the visit was recorded as an ‘acceptance’, but if they did not, the visit was recorded as a ‘rejection’. Then we tested whether the proportion of rejection of inflorescence by bees varies depending on which option bees approached first. Out of 45 first approaches to occupied inflorescence of Abelia, 77.8% ended with rejection, while 48.7% of 39 first approaches to unoccupied inflorescences ended with rejection. Approaching bees had significant tendency to reject occupied inflorescences more frequently than unoccupied ones (two-tailed Fisher's exact test: p=0.0068). When bees were presented with Kalanchoe, 81.3% of 187 first approaches to occupied inflorescence ended with rejection, while 85.9% of 92 first approaches to unoccupied inflorescence ended with rejection. The proportion of first approaches that ended with rejection did not differ between occupied and unoccupied inflorescences (two-tailed Fisher's exact test: p=0.40).
When bees rejected one inflorescence after the first approach, they sometimes landed on the other option (figure 4a). We tested whether the proportion of switch to the other option to acceptance of the first inflorescence varies depending on which option they approached first. Out of 17 first approaches to occupied inflorescence of Abelia, 41.2% switched to unoccupied option when landing. On the other hand, none of 20 first approaches to unoccupied inflorescence ended with landing on occupied option. Thus, approaching bees had a significant tendency to switch from occupied to unoccupied more frequently than from unoccupied to occupied when landing on Abelia inflorescences (two-tailed Fisher's exact test: p=0.0019). Out of 40 first approaches to occupied inflorescence of Kalanchoe, 12.5% switched to unoccupied option after the rejection, while 7.1% of 14 first approaches to unoccupied inflorescence ended with landing on occupied option. Thus, approaching bees did not switch to the alternative inflorescence so frequently when landing on Kalanchoe inflorescences, and the proportion of switches did not vary depending on which option they approached first (two-tailed Fisher's exact test: p=1).
We also observed that bees sometimes flew away without probing either inflorescence after the first landing (figure 4b). We tested whether the proportion of rejection of inflorescence by bees varies depending on which option bees landed on first. Out of 10 first landings on occupied inflorescence of Abelia, 20.0% ended with rejection, while none of 27 first landings on unoccupied inflorescence ended with rejection. Regarding tests with Kalanchoe, 27.8% of 36 first landings on occupied inflorescence ended with rejection, while 33.3% of 18 first landings on unoccupied inflorescence ended with rejection. In either case, the proportion of first landings that ended with rejection did not differ between occupied and unoccupied flowers (two-tailed Fisher's exact test: p=0.068 for Abelia; p=0.76 for Kalanchoe).
Finally, we tested whether the proportion of switch to the other option to acceptance of the inflorescence varies depending on which option they landed on first. When bees first landed on Abelia inflorescence, they never switched to the alternative inflorescence after the rejection (figure 4b). When bees landed on Kalanchoe inflorescence, they occasionally switched to the other option: 1 out of 27 landings on occupied inflorescence and 1 out of 13 landings on unoccupied inflorescence ended with probing of the other option. We detected no significant difference in these trends (two-tailed Fisher's exact test: p=1). We did not find any bee that switched inflorescences more than once during its first approach to first probing.
4. Discussion
Our experiments have clearly demonstrated that bumble bees responded negatively to inflorescences with conspecifics when they foraged from flowers of the familiar species (Abelia), but they responded positively to inflorescences with conspecifics when the flowers were of an unfamiliar species (Kalanchoe; figure 3). Many previous studies have suggested that flower visitors reduce overlaps of their foraging areas at various spatial scales by avoiding either scent marks left on flowers or flowers with decreased reward levels or other foragers in sight (Inouye 1978; Pimm et al. 1985; Wetherwax 1986; Thomson et al. 1987; Giurfa & Núñez 1992; Goulson et al. 1998; Sandlin 2000b; Gilbert et al. 2001; Makino & Sakai 2004, 2005; Saleh et al. 2006). Conversely, several authors have found that these animals in novel food environments are attracted to other foragers on flowers or the same flower types fed by others (bat: Howell 1979; stingless bee: Slaa et al. 2003; Leadbeater & Chittka 2005; Worden & Papaj 2005; bumble bee: Kawaguchi et al. 2006). Our data reconcile these seemingly discordant findings by suggesting that bumble bees adopt these alternative behaviours depending on their foraging contexts.
Contrasting responses of bumble bees to conspecifics on flowers can be understood by comparing costs and benefits resulting from each behaviour in different foraging contexts. In general, it will be costly for animals to exploit the same food types or locations with other foragers in terms of increased inter- or intraspecific competition (Thomson et al. 1987; Dreisig 1995; Sandlin 2000b; Rodríguez-Gironés 2006). When searching for novel flowers to sample, however, it will benefit naive foragers to follow or copy others in terms of a reduction of the time consumed for food finding and subsequent decision-makings (Kawaguchi et al. 2006). Moreover, novel foods found by following others may have higher energetic value or lower risk of predation (Dukas & Morse 2003). Such benefits will be highest when an animal is entirely unfamiliar with food sources within a habitat, but will rapidly decrease as it accumulates knowledge about the food environment. One can expect, therefore, that animals alter their responses towards other foragers from positive to negative as they gain experience at a food site. Consistent with this prediction, Slaa et al. (2003) found that newly recruited stingless bees preferred to visit flowers occupied by other conspecifics over unoccupied ones, but this tendency gradually shifted to avoidance of others over consecutive foraging trips to the feeders. Leadbeater & Chittka (2005) also found that naive bumble bees were attracted to flowers occupied by conspecifics, but the preference for the presence of others decreased to chance levels once they found nectar in these flowers. In our experiments, the benefits of following conspecifics would have outweighed the costs of competition when bees sampled unfamiliar Kalanchoe flowers, but the benefits would have disappeared on familiar Abelia flowers. It should be noted that our test bees were attracted to conspecifics when sampling Kalanchoe flowers, even when they had already learned to avoid conspecifics while visiting Abelia flowers. This suggests that these animals are able to change their responses to others in either direction throughout their foraging career.
We note that the bees appeared to respond to conspecifics at different points in behavioural sequence before landing on familiar and unfamiliar inflorescences. In our tests with familiar species (Abelia), bees approached occupied and unoccupied inflorescences at similar frequencies. These bees rejected the occupied inflorescences more often than the unoccupied ones. Moreover, they often ended with landing on the unoccupied inflorescence when they first approached the occupied one, but they never switched to the other option when they first approached the unoccupied inflorescence. On the other hand, when bees were presented with unfamiliar species (Kalanchoe), bees approached occupied inflorescences significantly more often than unoccupied ones. In other words, while the avoidance of resident bees on Abelia occurred after bees approached these inflorescences, the attraction towards resident bees on Kalanchoe showed up just when bees were about to inspect the inflorescences. These data could reflect the bees' tendency to selectively pay attention to more familiar objects (reviewed by Chittka et al. 1999). Alternatively, bees may be able to detect familiar objects more quickly than unfamiliar ones. Since we observed opposite trends on familiar and unfamiliar flower species, the reaction towards conspecifics is unlikely to be solely an adaptively neutral by-product of bees' cognitive processes. At the same time, however, we believe that more research is needed to clarify how perceptual components (e.g. visual or olfactory similarity between familiar and unfamiliar flowers, etc.) affected the point at which bees' decisions were made.
Results obtained here support our initial hypothesis that flower visitors can change their responses to feeding conspecifics according to familiarity of the flower species they are about to visit. Such flexible behaviour would enhance an animal's ability to keep track of changing resources through time quickly and accurately. When the flower species on which a flower visitor is focusing in an area provide the highest net profits, it should keep foraging on the same species while avoiding others on flowers to minimize local competition. As a species' flowering period comes to an end or the number of competitors increases, however, the animal must locate and sample other flowers of concurrently blooming species to establish its new targets (Heinrich 1979; Thomson 1981). Some flower visitors are also known to occasionally sample alternate flowers even when their target species are not declining, which may allow them to track variable resources (Heinrich 1976a; Thomson et al. 1997). Since sampling or individual exploration inevitably involves measurable time and energy costs (Kawaguchi et al. 2006), following other conspecifics in such situations will provide animals with an efficient shortcut to currently rewarding flowers. If flower visitors have to move between familiar and unfamiliar flowers more often than once during their lifetime, therefore, a conditional use of these alternative behaviours would be highly advantageous. Further experiments will be needed with various flower visitors as well as a range of their familiar and unfamiliar flower species to clarify how widespread is such behaviour in nature. Rapid tracking of resources may also benefit a wide range of other animals that can learn to use multiple food items each of which has shorter lifespan than themselves. Animals foraging on plant products, such as fruits, seeds, and foliage (reviewed by Hunter & Price 1992); carrion (Heinrich 1989); and many prey organisms (reviewed by White 1978) will fall into this category. Moreover, recent studies have suggested that animals participating in habitat selection, mate choice and predation avoidance also use the presence of other conspecifics as cues for ‘resource’ status (Chittka & Leadbeater 2005; Dall et al. 2005). We hope that our findings will encourage future studies to explore the possibility that animals exploiting such various resources could adopt a conditional use of behaviours in response to cues provided inadvertently from others.
Acknowledgments
We are grateful to three anonymous reviewers for critical reading of the manuscript; J.E. Cresswell for his careful proofreading; and K. Fujii and many other colleagues for their helpful suggestions. This work was supported in part by grants from the Ministry of Education, Japan (17405004) to Y.T. and from the Development Project of Prevention and Control for Ecological Risks of Commercial Bumblebee (Chief: K. Goka, National Institute of Environmental Studies) to Y.T. The experiments described here complied fully with all current laws in Japan.
References
- Alanärä A, Burns M.D, Metcalfe N.B. Intraspecific resource partitioning in brown trout: the temporal distribution of foraging is determined by social rank. J. Anim. Ecol. 2001;70:980–986. doi:10.1046/j.0021-8790.2001.00550.x [Google Scholar]
- Brian A.D. Differences in the flowers visited by four species of bumble-bees and their causes. J. Anim. Ecol. 1957;26:71–98. doi:10.2307/1782 [Google Scholar]
- Chittka L, Leadbeater E. Social learning: public information in insects. Curr. Biol. 2005;15:R869–R871. doi: 10.1016/j.cub.2005.10.018. doi:10.1016/j.cub.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Chittka L, Thomson J.D, Waser N.M. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86:361–377. doi:10.1007/s001140050636 [Google Scholar]
- Collins R.D, Bell W.J. Enhancement of resource finding efficiency by visual stimuli in Musca domestica (Diptera: Muscidae) J. Kansas Entomol. Soc. 1996;69:204–207. [Google Scholar]
- D'Adamo P, Corley J, Sackmann P, Lozada M. Local enhancement in the wasp Vespula germanica—are visual cues all that matter? Insect. Soc. 2000;47:289–291. doi:10.1007/PL00001717 [Google Scholar]
- Dall S.R.X, Giraldeau L.-A, Olsson O, McNamara J.M, Stephens D.W. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. doi:10.1016/j.tree.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Danchin E´, Giraldeau L.-A, Valone T.J, Wagner R.H. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. doi:10.1126/science.1098254 [DOI] [PubMed] [Google Scholar]
- Dreisig H. Ideal free distributions of nectar foraging bumblebees. Oikos. 1995;72:161–172. doi:10.2307/3546218 [Google Scholar]
- Dukas R, Morse D.H. Crab spiders affect flower visitation by bees. Oikos. 2003;101:157–163. doi:10.1034/j.1600-0706.2003.12143.x [Google Scholar]
- Gilbert F, Azmeh S, Barnard C, Behnke J, Collins S.A, Hurst J. Individually recognizable scent marks on flowers made by a solitary bee. Anim. Behav. 2001;61:217–229. doi: 10.1006/anbe.2000.1542. doi:10.1006/anbe.2000.1542 [DOI] [PubMed] [Google Scholar]
- Giraldeau L.-A, Beauchamp G. Food exploitation: searching for the optimal joining policy. Trends Evol. Ecol. 1999;14:102–106. doi: 10.1016/s0169-5347(98)01542-0. doi:10.1016/S0169-5347(98)01542-0 [DOI] [PubMed] [Google Scholar]
- Giurfa M, Núñez J.A. Honeybees mark with scent and reject recently visited flowers. Oecologia. 1992;89:113–117. doi: 10.1007/BF00319022. doi:10.1007/BF00319022 [DOI] [PubMed] [Google Scholar]
- Goulson D, Hawson S.A, Stout J.C. Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim. Behav. 1998;55:199–206. doi: 10.1006/anbe.1997.0570. doi:10.1006/anbe.1997.0570 [DOI] [PubMed] [Google Scholar]
- Heinrich B. The foraging specializations of individual bumblebees. Ecol. Monogr. 1976a;46:105–128. doi:10.2307/1942246 [Google Scholar]
- Heinrich B. Resource partitioning among some eusocial insects: bumblebees. Ecology. 1976b;57:874–889. doi:10.2307/1941054 [Google Scholar]
- Heinrich B. “Majoring” and “minoring” by foraging bumblebees, Bombus vagans: an experimental analysis. Ecology. 1979;60:245–255. doi:10.2307/1937652 [Google Scholar]
- Heinrich B. Summit Books; New York, NY: 1989. Ravens in winter. [Google Scholar]
- Howell D.J. Flock foraging in nectar-feeding bats: advantages to the bats and to the host plants. Am. Nat. 1979;114:23–49. doi:10.1086/283452 [Google Scholar]
- Hunter M.D, Price P.W. Natural variability in plants and animals. In: Hunter M.D, Ohgushi T, Price P.W, editors. Effects of resource distribution on animal-plant interactions. Academic Press; San Diego, CA: 1992. pp. 1–12. [Google Scholar]
- Inouye D.W. Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology. 1978;59:672–678. doi:10.2307/1938769 [Google Scholar]
- Kawaguchi L.G, Ohashi K, Toquenaga Y. Do bumble bees save time when choosing novel flowers by following conspecifics? Funct. Ecol. 2006;20:239–244. doi:10.1111/j.1365-2435.2006.01086.x [Google Scholar]
- Kearns C.A, Thomson J.D. University Press of Colorado; Boulder, CO: 2001. The natural history of bumblebees: a sourcebook for investigations. [Google Scholar]
- Leadbeater E, Chittka L. A new mode of information transfer in foraging bumblebees? Curr. Biol. 2005;15:R447–R448. doi: 10.1016/j.cub.2005.06.011. doi:10.1016/j.cub.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Makino T.T, Sakai S. Findings on spatial foraging patterns of bumblebees (Bombus ignitus) from a bee-tracking experiment in a net cage. Behav. Ecol. Sociobiol. 2004;56:155–163. doi:10.1007/s00265-004-0773-x [Google Scholar]
- Makino T.T, Sakai S. Does interaction between bumblebees (Bombus ignitus) reduce their foraging area?: bee-removal experiments in a net cage. Behav. Ecol. Sociobiol. 2005;57:617–622. doi:10.1007/s00265-004-0877-3 [Google Scholar]
- Nagamitsu T, Inoue T. Aggressive foraging of social bees as a mechanism of floral resource partitioning in an Asian tropical rainforest. Oecologia. 1997;110:432–439. doi: 10.1007/s004420050178. doi:10.1007/s004420050178 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Yahara T. Visit larger displays but probe proportionally fewer flowers: counterintuitive behaviour of nectar-collecting bumble bees achieves an ideal free distribution. Funct. Ecol. 2002;16:492–503. doi:10.1046/j.1365-2435.2002.00644.x [Google Scholar]
- Paton D.C, Carpenter F.L. Peripheral foraging by territorial Rufous hummingbirds: defense by exploitation. Ecology. 1984;65:1808–1819. doi:10.2307/1937777 [Google Scholar]
- Pimm S.L, Rosenzweig M.L, Mitchell W. Competition and food selection: field tests of a theory. Ecology. 1985;66:798–807. doi:10.2307/1940541 [Google Scholar]
- Pleasants J.M. Structure of plant and pollinator communities. In: Jones C.E, Little R.J, editors. Handbook of experimental pollination biology. Scientific and Academic Editions; New York, NY: 1983. pp. 375–391. [Google Scholar]
- Possingham H.P. The distribution and abundance of resources encountered by a forager. Am. Nat. 1989;133:42–60. doi:10.1086/284900 [Google Scholar]
- Prokopy R.J, Miller N.W, Duan J.J, Vargas R.I. Local enhancement of arrivals of Ceratitis capitata females on fruit mimics. Entomol. Exp. Appl. 2000;97:211–217. doi:10.1023/A:1004064005423 [Google Scholar]
- Raveret Richter M, Tisch V.L. Resource choice of social wasps: influence of presence, size and species of resident wasps. Insect. Soc. 1999;46:131–136. doi:10.1007/s000400050123 [Google Scholar]
- Rodríguez-Gironés M.A. Resource partitioning among flower visitors: extensions of Possingham's model. Evol. Ecol. Res. 2006;8:765–783. [Google Scholar]
- Saleh N, Ohashi K, Thomson J.D, Chittka L. Facultative use of the repellent scent mark in foraging bumblebees: complex versus simple flowers. Anim. Behav. 2006;71:847–854. doi:10.1016/j.anbehav.2005.06.014 [Google Scholar]
- Sandlin E.A. Cue use affects resource subdivision among three coexisting hummingbird species. Behav. Ecol. 2000a;11:550–559. doi:10.1093/beheco/11.5.550 [Google Scholar]
- Sandlin E.A. Foraging information affects the nature of competitive interactions. Oikos. 2000b;91:18–28. doi:10.1034/j.1600-0706.2000.910102.x [Google Scholar]
- Slaa E.J, Wassenberg J, Biesmeijer J.C. The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 2003;28:369–379. doi:10.1046/j.1365-2311.2003.00512.x [Google Scholar]
- Stout J.C, Goulson D. The influence of nectar secretion rates on the responses of bumblebees (Bombus spp.) to previously visited flowers. Behav. Ecol. Sociobiol. 2002;52:239–246. doi:10.1007/s00265-002-0510-2 [Google Scholar]
- Sutherland W.J. Oxford University Press; Oxford, UK: 1996. From individual behaviour to population ecology. [Google Scholar]
- Thomson J.D. Field measures of flower constancy in bumblebees. Am. Midl. Nat. 1981;105:377–380. doi:10.2307/2424756 [Google Scholar]
- Thomson J.D, Maddison W.P, Plowright R.C. Behavior of bumble bee pollinators of Aralia hispida Vent. (Araliaceae) Oecologia. 1982;54:326–336. doi: 10.1007/BF00380001. doi:10.1007/BF00380001 [DOI] [PubMed] [Google Scholar]
- Thomson J.D, Peterson S.C, Harder L.D. Response of traplining bumble bees to competition experiments: shifts in feeding location and efficiency. Oecologia. 1987;71:295–300. doi: 10.1007/BF00377298. doi:10.1007/BF00377298 [DOI] [PubMed] [Google Scholar]
- Thomson J.D, Slatkin M, Thomson B.A. Trapline foraging by bumble bees. II. Definition and detection from sequence data. Behav. Ecol. 1997;8:199–210. doi:10.1093/beheco/8.2.199 [Google Scholar]
- Valone T.J, Templeton J.J. Public information for the assessment of quality: a widespread social phenomenon. Phil. Trans. R. Soc. B. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. doi:10.1098/rstb.2002.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser N.M. The adaptive nature of floral traits: ideas and evidence. In: Real L, editor. Pollination biology. Academic Press; Orlando, FL: 1983. pp. 241–285. [Google Scholar]
- Wetherwax P.B. Why do honeybees reject certain flowers? Oecologia. 1986;69:567–570. doi: 10.1007/BF00410364. doi:10.1007/BF00410364 [DOI] [PubMed] [Google Scholar]
- White T.C.R. The importance of a relative shortage of food in animal ecology. Oecologia. 1978;33:71–86. doi: 10.1007/BF00376997. doi:10.1007/BF00376997 [DOI] [PubMed] [Google Scholar]
- Worden B.D, Papaj D.R. Flower choice copying in bumblebees. Biol. Lett. 2005;1:504–507. doi: 10.1098/rsbl.2005.0368. doi:10.1098/rsbl.2005.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]