Abstract
The long-term effects on marine fish populations of the recent increase worldwide in the incidence of coastal hypoxia are unknown. Here we show that chronic environmental exposure of Atlantic croaker (Micropogonias undulatus) to hypoxia in a Florida estuary caused marked suppression of ovarian and testicular growth which was accompanied by endocrine disruption. Laboratory hypoxia studies showed that the endocrine disruption was associated with impairment of reproductive neuroendocrine function and decreases in hypothalamic serotonin (5-HT) content and the activity of the 5-HT biosynthetic enzyme, tryptophan hydroxylase. Pharmacological restoration of hypothalamic 5-HT levels also restored neuroendocrine function, indicating that the stimulatory serotonergic neuroendocrine pathway is a major site of hypoxia-induced inhibition. Inhibition of tryptophan hydroxylase activity to downregulate reproductive activity could have evolved as an adaptive mechanism to survive periodic hypoxia, but in view of the recent increased incidence of coastal hypoxia could become maladaptive and potentially affect fish population abundance and threaten valuable fishery resources.
Keywords: endocrine disruption, reproduction, neuroendocrine mechanism, fish, hypoxia, estuary
1. Introduction
Seasonal hypoxia (dissolved oxygen, DO<2 mg l−1) occurs naturally in estuaries and is caused by vertical stratification due to the formation of haloclines and thermoclines and by seasonal temperature increases which drive high oxygen demand. However, the incidence of hypoxia in many coastal regions throughout the world such as the northern Gulf of Mexico and East China Sea has increased dramatically over the past 20 years as a result of eutrophication caused by several-fold increases in the inputs of nitrogen and other nutrients from anthropogenic sources (Rabalais et al. 2002; Wu 2002; Smith 2003) and now affects more than 104 km2 in these regions (Rabalais et al. 2002). This worldwide trend is expected to continue with further growth of intense agricultural practices and human populations in coastal regions, and also possibly with global warming (Justić et al. 2005). Consequently, there is growing concern over the long-term impacts of recurring seasonal hypoxia on the sustainability of coastal ecosystems and fishery resources (Pihl et al. 1991; Diaz & Rosenberg 1995; Wu 2002).
Reproduction is especially sensitive to interference by environmental stressors (Billard et al. 1981). Although hypoxia-induced reproductive impairment potentially could have important long-term consequences for population dynamics, currently there is practically no information on the effects of coastal hypoxia on reproduction in fishes and other marine organisms (Billard et al. 1981; Wu 2002). Seasonal reproduction in fishes, including gonadal crudescence and gametogenesis, is primarily controlled by hormones secreted by the hypothalamus–pituitary–gonad (HPG) axis. Reproductive impairment, due to interference of endocrine function by industrial and agricultural chemicals (endocrine-disrupting chemicals, EDCs) and other stressors, is frequently observed in fishes collected from contaminated and degraded environments (e.g. Jobling et al. 1998; Hashimoto et al. 2000). Atlantic croaker, Micropogonias undulatus, is an abundant inshore teleost species and is representative of a large number of early maturing, short-lived, iteroparous estuarine fish species in the Gulf of Mexico that migrate offshore to spawn (Gutherz 1976). Atlantic croaker can tolerate moderate hypoxia and do not show a strong avoidance response to hypoxic estuarine environments, unlike some other estuarine species (Bell & Eggleston 2005). Therefore, in the present study we investigated the effects of environmental and laboratory hypoxia exposure on gametogenesis and reproductive endocrine function in Atlantic croaker, a well-established estuarine teleost model of reproductive endocrinology (e.g. Hawkins et al. 2000; Khan & Thomas 2001; Thomas et al. 2006a,b), as well as the site of hypoxia interference on the HPG axis.
2. Material and methods
(a) Description of field study area
The Pensacola Bay system in the Florida panhandle is a shallow (mean depth 3 m), medium-sized (370 km2) drowned river estuary system and comprises several estuaries (figure 1), which receive freshwater inputs from three rivers. The water column displays a strong salinity-dependent density stratification, especially during periods of high river flow, because the diurnal tides have a low amplitude (less than 50 cm) and subtidal circulation and vertical diffusion are sluggish, resulting in little mixing (Hagy & Murrell 2007). Such conditions favour the development of seasonally persistent hypoxia which has been reported in this system regularly since the 1960s. Monthly water quality data for the Pensacola Bay system from 2002 to 2004, as well as earlier water surveys were used to calculate the extent of hypoxia and to construct salt and water balance models for determining the physical factors influencing hypoxia development (Hagy & Murrell 2007). Their study shows that the lower part of the system receives an adequate flow of oxygenated seawater through the pass from the Gulf of Mexico to prevent the development of hypoxia and that the oxygen level in the bottom water decreases and becomes hypoxic as it flows up the bays (Hagy & Murrell 2007). Once established, the extent and pattern of bottom-water hypoxia persists throughout the summer and consistently impacts a particular region of the bay system. Thus, the lower part of system (Pensacola Bay) is never hypoxic, whereas large areas of East Bay become hypoxic every summer.
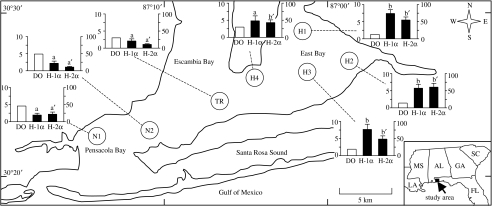
Figure 1.
Hypoxic sites (H1, H2, H3 and H4) in East Bay, normoxic sites (N1 and N2) in Pensacola Bay, Florida and transition site (TR) between the two bays where Atlantic croaker were collected in October and November 2003. Inset bar graphs: DO levels (mg l−1, clear bars) at the bottom of the water column (y-axis on left), and ovarian expression of HIF-1α (H-1α) and HIF-2α (H-2α) mRNAs (solid bars: relative units, y-axis on right) in croaker at the time of the October sampling (N=6). A nested ANOVA analysis indicates that HIF-1α and -2α mRNA levels in croaker from the normoxic sites are significantly different from those in fish from the hypoxic sites (p<0.001; results not shown). Individual site differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (a, b for HIF-1α and a′, b′ for HIF-2α, p<0.05).
(b) Field sampling
Adult young-of-the-year (yr1) croaker were collected from four hypoxic sites (H1, H2, H3 and H4) in East Bay, two normoxic sites (N1 and N2) in adjoining Pensacola Bay and a transition site (TR) between the two bays during the period of gonadal development and gametogenesis in the autumn of 2003 (figure 1). Fish (length 15–18 cm) were caught (25–30 fish per site) between 08.00 and 12.00 on 6–9 October and 3–5 November, with 15 min bottom trawls. Tissue samples were collected and processed on board the vessel for subsequent analyses. DO measurements were taken at the time of collection with an oxygen meter (YSI 600 XLM Multi-Parameter Water Quality Monitor, YSI, Inc., Yellow Springs, OH; see electronic supplementary material, tables 1 and 2). DO levels at the sites or within 2–4 km of the sites were also monitored at one- to five-week intervals throughout the summer and autumn of 2003 (see electronic supplementary material, tables 3 and 4). From July to October, the bottom DO values recorded at the hypoxic sites remained between 0.2 and 2.0 mg l−1 (exception H4 in October, 3.2 mg l−1, moderately hypoxic, 2.0–3.5 mg l−1; see electronic supplementary material, tables 1–4), whereas a monitoring station near to N1 and N2 remained normoxic (DO>3.5 mg l−1) throughout this period with bottom DO values of 4.4–5.2 mg l−1 (site P07; Hagy & Murrell 2007).
(c) Laboratory hypoxia experiments
Adult yr1 croaker (length 14–15 cm) were collected locally and acclimated to laboratory conditions at the University of Texas Marine Science Institute for one month prior to experimentation and fed commercial pellets daily (Rangen, Angleton, TX, 5% body weight (BW) d−1). Croaker were exposed to hypoxic (1.72±0.01 mg l−1 DO), moderately hypoxic (2.70±0.01 mg l−1 DO) or normoxic conditions (5.33±0.02 mg l−1 DO) in 2000 l recirculating saltwater (salinity 32‰) tanks (22 fish of mixed sex per tank, three tanks per treatment) under controlled environmental conditions that mimicked the seasonal changes in temperature and photoperiod (photoperiod 11 L : 13 D, temperature 23°C) and were fed pellets (3% BW) daily for 10 weeks during the period of gonadal crudescence (October–December). Hypoxic conditions in the tanks were maintained by adjusting the aeration and water recirculation and DO was monitored two to three times daily (see electronic supplementary material, table 5) with a YSI 556 Multiprobe System. At the end of the exposure period, 43–45 fish (14–15 fish per tank) were captured between 09.00 and 12.00, humanely sacrificed and tissues collected for analysis.
(d) Luteinizing hormone response to gonadotrophin-releasing hormone analogue
The remaining fish (50; four to six per tank) in each DO treatment group received intraperitoneal (i.p.) injections of either gonadotrophin-releasing hormone analogue (GnRHa) (des-Gly10, [d-Ala6]-LHRH (1–9) ethylamide, Bachem, Torrance, CA, dose 50 ng g−1 BW) dissolved in saline (29 fish; 8–11 per treatment) or saline alone (21 fish; 7 per treatment), and blood was collected 1 hour later for the measurement of luteinizing hormone (LH) using a homologous radioimmunoassay (RIA; Copeland & Thomas 1989).
(e) Effects of 5-hydroxytryptophan treatment on hypothalamic levels of serotonin and GnRH mRNA
Eighty yr1 adult croaker of both sexes, equally divided among eight tanks, were exposed to hypoxic (1.7 mg l−1 DO) or normoxic (5.5 mg l−1 DO) conditions for two weeks as described above. The fish in half the tanks for each DO group were injected intraperitoneally with 5-hydroxytryptophan (5-HTP; 20 μg g−1 BW d−1; every other day for two weeks, totalling seven injections) and the remainder were injected under the same regimen with saline. Tissues were processed 24 hours after the last injection for determination of hypothalamic serotonin (5-HT) concentrations and GnRH mRNA in the preoptic–anterior hypothalamus (POAH). Ethical approval was obtained from the University of Texas at Austin IACUC for the field and laboratory studies with Atlantic croaker.
(f) Sex steroid and vitellogenin measurement
Plasma oestradiol-17β (E2), testosterone (T) and 11-ketotestosterone (11-KT) levels were measured by RIAs validated for croaker plasma (Singh & Thomas 1993). Plasma vitellogenin (Vg) concentrations were measured by sandwich ELISA using croaker Vg as standard and an antibody raised against Vg from a closely related species, spotted seatrout (Cynoscion nebulosus; Copeland & Thomas 1988).
(g) Quantification of hepatic oestrogen receptor α, hypothalamic GnRH and ovarian hypoxia-inducible factor-1α and -2α mRNA levels
Total RNA was extracted from hepatic, hypothalamic and ovarian tissues using TRI reagent (Sigma, St Louis, MO) and treated with DNase I (Zymo Research, Orange, CA). First-strand cDNA was synthesized using a reverse transcriptase kit (Amersham Biosciences, Piscataway, NJ) following the manufacturer's instructions. Gene-specific primers for oestrogen receptor alpha (ERα) sense: 5′-GTGAGCTTCAAATTGCACGA-3′ and antisense: 5′-TCTC- TCTGCCATGCACAAAC-3′, hypoxia-inducible factor (HIF)-1α sense: 5′-AGACCGAGGATGTGAAACCA-3′ and antisense: 5′-GCCCGAGTGAACAGTTTGAT-3′ and HIF-2α sense: 5′-GCCCGAGTGAACAGTTTGAT-3′ and antisense: 5′-CGAAGTCGAGGGAAATGATG-3′ were used for amplification of croaker ERα, HIF-1α and -2α mRNAs. The primers were designed from the nucleotide sequences of croaker ERα (Hawkins et al. 2000), HIF-1α and -2α (Rahman & Thomas 2007), respectively. The primers for 18S mRNA (sense: 5′-AGAAACGGCTACCACATCCA-3′ and antisense: 5′-TCCCGAGATCCAACTACGAG-3′) were designed from the croaker 18S rRNA sequence (GenBank accession no. AY866435) and used as an internal control. PCR was carried out in 25 μl buffer containing 10 μM of each primer, 12.5 μl PCR Master Mix (Promega, Madison, WI) and 1 ng of first-strand cDNA. PCR products (3 μl) were quantified using the Quant-iT DNA assay kit (Molecular Probes, Eugene, OR) on a Fluoro Star spectrophotometer (BMG Labtechnologies, Durham, NC) according to Rahman & Thomas (2007). Gene-specific primers for GnRH sense: 5′-GCACTGGTCGTATGGACTGA-3′ and antisense: 5′-TCTCCCGATCTGTGACTCC-3′ were used for the amplification of croaker GnRH I mRNA designed from the nucleotide sequence of sea bream-like GnRH (Mohamed et al. 2005). Croaker β-actin (primers, sense: 5′-GAGCGTGGCTACTCCTTCAC-3′ and antisense: 5′-CAGGACTCCATACCGAGGAA-3′) was used as an internal control. Real-time quantitative PCR was conducted to determine relative gene expression.
(h) Gonadosomatic index and gonadal histology
Gonadosomatic index (GSI) was calculated from the formula: gonad weight/(body weight−gonad weight)×100. Gonads were fixed in formalin, embedded in paraffin, sectioned at 7 μm and stained with haematoxylin–eosin. Oocytes were classified into peri-nucleolus, cortical alveoli, primary yolk, secondary yolk and tertiary yolk stages.
(i) Gamete production
The total number of vitellogenic oocytes (greater than 350 μm, fecundity) and their size distribution was estimated as described previously (Brown-Peterson et al. 1988). Sperm production was estimated by calculating the percentage of the total area of spermatogenic cells occupied by spermatozoa in each testicular section under a microscope using image analysis software (MetaVue, Universal Imaging, Downingtown, PA).
(j) Tryptophan hydroxylase activity
Tryptophan hydroxylase (TPH) activity was measured in homogenized hypothalamic tissues by radioenzymatic assay using [3H]-tryptophan as tracer and incubated under conditions validated for croaker (Khan & Thomas 2001).
(k) 5-HT and 5-hydroxyindoleacetic acid measurement
Fish were decapitated immediately after capture and brains rapidly excised (within 15–20 s) and frozen in liquid nitrogen. The hypothalamus was removed, homogenized, and 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were separated by reversed-phase HPLC and analysed by electrochemical detection (Waters 464 Electrochemical Detector and Breeze Software, Khan & Thomas 1997).
(l) Statistical analysis
For the field data, significant differences between the normoxic and hypoxic sites were examined by conducting a nested ANOVA (the single transition site, TR, was excluded from this analysis). To explore differences among the individual sampling sites, we performed Fisher's protected least-significant difference (PLSD) test for multiple comparisons. Laboratory data were analysed by one-way ANOVA, and where significant effects were found, this was followed by Fisher's PLSD test for multiple comparisons and Student's t-test for paired comparisons. Analyses were performed using Systat (Systat, San Jose, CA) and GraphPad Prism (GraphPad, San Diego, CA) computer software.
3. Results
(a) Field study on reproductive effects of hypoxia
Heavy rainfall and the resulting halocline resulted in the bottom 0.2–3.0 m of the water column throughout East Bay being hypoxic during the summer and first sampling period in the autumn (July–October), whereas West Pensacola Bay remained normoxic from April to November (Hagy & Murrell 2007) as assessed by periodic DO measurements and DO concentrations obtained at the time of sample collection (figure 1 and electronic supplementary material, tables 1–4). DO levels at all sites had increased at the time of the second collection period in November, but were still moderately hypoxic (DO<3.5 mg l−1) at three of the East Bay sites. Ovarian expression of HIF-1α and -2α mRNAs was significantly increased in fish collected from the hypoxic sites (H1–H4) in October (figure 1) and November (electronic supplementary material, figure 1), and reproductive and endocrine functions were impaired both in October and November (figures 2 and 3), which suggests that croaker are resident in these areas for extended periods.
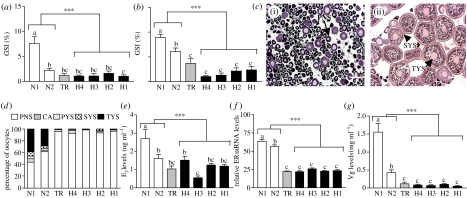
Figure 2.
Ovarian development and endocrine function in female croaker collected from hypoxic sites in (a,c–g) October and (b) November 2003. (a) GSI (ovarian growth) in October. (b) GSI in November. (c) Histological appearance of representative ovaries, (i) hypoxic site and (ii) normoxic site. (d) Percentage of oocytes at each development stage. (e) Plasma E2 levels. (f) Hepatic ER mRNA levels. (g) Plasma Vg levels. PNS, peri-nucleolus; CA, cortical alveoli; PYS, primary yolk; SYS, secondary yolk; TYS, tertiary yolk. Scale bar, 200 μm (N=9–22). Asterisks indicate significant differences between normoxic and hypoxic sites (nested ANOVA, ***p<0.001). Individual site differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05).
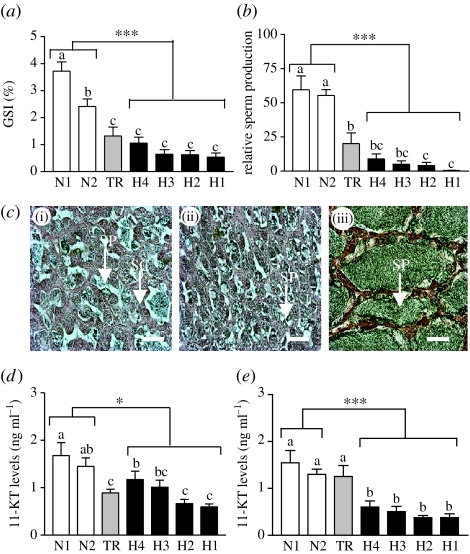
Figure 3.
Testicular development and endocrine function in male croaker collected from hypoxic sites in (a–d) October and (e) November 2003. (a) GSI (testicular growth). (b) Relative sperm production. (c) Histological appearance of representative testes, (i) (ii) hypoxic sites and (iii) normoxic site. (d,e) Plasma 11-KT levels. SP, spermatozoa; SC, spermatocytes. Scale bar, 200 μm (N=15–31). Asterisks indicate significant differences between normoxic and hypoxic sites (nested ANOVA, *p<0.05 and ***p<0.001). Individual site differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05).
Gonadal growth in croaker collected from the hypoxic sites in East Bay was impaired compared with that of individuals collected from the normoxic sites in Pensacola Bay. It is unlikely that chemical contaminants contributed significantly to this reproductive impairment because their environmental levels are relatively low in East Bay and lower than those in Pensacola Bay where normal gonadal growth was observed (Karouna-Renier et al. 2007). The GSI in October was greatest in females collected from normoxic site N1 (GSI 7.5%), which is furthest from the hypoxic zones in East Bay (figure 2a). By November, the GSIs of females from both normoxic sites were above 6%, significantly greater than those from the hypoxic sites (GSI<2.8%), whereas fish from the TR had an intermediate GSI (3.5%; figure 2b). The marked seasonal increase in ovarian GSI is primarily due to enlargement of the oocytes as a result of sequestering large amounts of Vg. Gametogenesis was impaired in females at the hypoxic sites (figure 2c(i)) and in October large vitellogenic (tertiary yolk) oocytes capable of forming mature fertilizable eggs were only identified in ovaries from fish at the normoxic sites (figure 2c(ii),d). By November, a greater proportion of the oocytes in ovaries collected from the TR (30%) had also reached the tertiary yolk stage, whereas fewer than 10% of the oocytes in ovaries from any of the hypoxic sites had reached this stage (electronic supplementary material, figure 2a). The fecundities (number of tertiary yolk stage oocytes; greater than 350 μm in diameter) of females from the hypoxic sites (10–40×103) in November were markedly lower than those from the normoxic sites (8–25×104; electronic supplementary material, figure 2b). It is unlikely that substantial further oocyte development occurred at any of the sites after the November sampling date because shortly afterwards croaker migrate to their spawning grounds offshore. Vg is produced in the liver under the influence of oestrogen, which acts through the oestrogen receptor, ERα. Plasma levels of E2 (figure 2e) and T (electronic supplementary material, figure 2c) were significantly lower in fish collected from the hypoxic sites in October in comparison with the plasma levels in fish from the normoxic sites. Similar differences between hypoxic and normoxic sites in oestrogen signalling were apparent when hepatic ERα mRNA and plasma Vg levels, which integrate oestrogen signalling over longer time periods, were measured. The relative levels of ERα mRNA were threefold higher in fish collected from the normoxic sites in October than in those at the hypoxic sites (figure 2f). Similarly, circulating levels of Vg were several-fold higher in fish collected from the normoxic sites in October (figure 2g) and November (electronic supplementary material, figure 2d).
Testicular growth was significantly lower in males collected at the hypoxic sites in October (GSI<1.2%) compared with that observed in fish at the normoxic sites (GSI 2.5–3.5%; figure 3a) and did not change during the three-week period between the October and November sampling times (electronic supplementary material, figure 2e). Sperm production in males from the hypoxic sites in both months was negligible in comparison with that of fish from the normoxic sites (figure 3b; electronic supplementary material, figure 2f). Gametogenesis was impaired in males collected from the hypoxic sites, all stages of spermatogenesis were present and only a small proportion of the spermatocytes had progressed to the spermatozoa stage (figure 3c(i)(ii)). In contrast, testes obtained at the normoxic sites contained very large lumens full of mature spermatozoa with few earlier spermatogenic stages present (figure 3c(iii)). The concentrations of 11-KT, the major androgen regulating spermatogenesis in teleosts, and T were significantly lower in plasma of fish collected from the hypoxic sites in comparison with those at the normoxic sites in October (figure 3d; electronic supplementary material, figure 2g) and November (figure 3e; electronic supplementary material, figure 2h).
(b) Laboratory study on reproductive effects of hypoxia
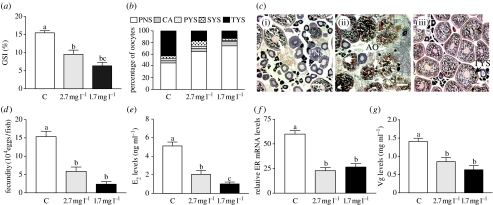
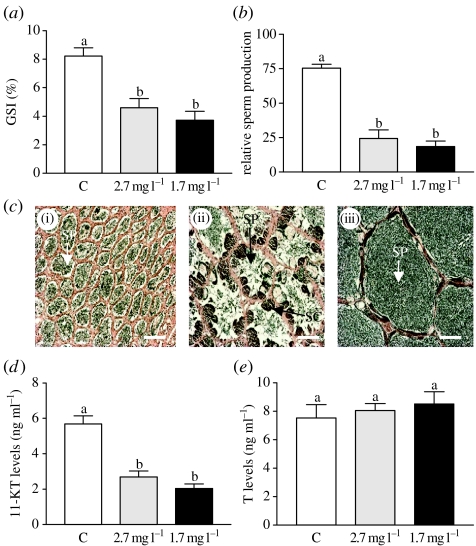
Low DO treatments (1.7 and 2.7 mg l−1 DO) caused a dramatic decrease in gonadal growth, gametogenesis and reproductive endocrine function in both males and females (figures 4 and 5). The patterns and extent of gonadal and endocrine disturbances after laboratory exposure to hypoxia were almost identical to those observed in the field study. At the end of the period of gonadal growth when the GSI in females held under normoxic conditions had reached 15%, the GSIs had only reached 6.8 and 3.9% in fish exposed to 2.7 and 1.7 mg l−1 DO, respectively (p<0.001, N=21–23; figure 4a). These declines in gonadal growth were accompanied by parallel decreases in gametogenesis and reproductive endocrine function. As observed in the field studies, there was a dramatic reduction in the development and growth of vitellogenic oocytes compared with controls (tertiary yolk stage; figure 4b,c(i)–(iii)). As a result, fecundity was reduced to 30 and 10% of that under normoxic conditions after exposure to 2.7 and 1.7 mg l−1 DO, respectively (figure 4d). The oestrogen signalling pathway regulating Vg production was impaired in females exposed to low DO as shown by lower plasma E2 levels, hepatic ERα mRNA levels and circulating Vg concentrations compared with controls (figure 4e–g). Similarly in males, gonadal crudescence was significantly impaired (figure 5a). There was a marked decrease in spermatogenesis and the production of fully formed sperm compared with controls in the low DO groups (figure 5b,c (i)–(iii)), which was associated with decreased 11-KT signalling regulating gametogenesis (figure 5d), whereas T levels were unaltered (figure 5e).
Figure 4.
Effects of laboratory hypoxia exposure on ovarian development and endocrine function in female croaker. (a) GSI (ovarian growth). (b) Oocyte development. (c) Histological appearance of representative ovaries, (i) (ii) hypoxia exposure and (iii) normoxic conditions. (d) Fecundity. (e) Plasma E2. (f) Hepatic ER mRNA. (g) Plasma Vg. See figure 2 for key to abbreviations. Scale bar, 200 μm (N=20–23). Significant differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05). Preliminary results presented at Pollution Responses in Marine Organisms Symposium (Thomas et al. 2006b).
Figure 5.
Effects of laboratory hypoxia exposure on testicular development and endocrine function in male croaker. (a) GSI (testicular growth). (b) Relative sperm production. (c) Histological appearance of representative testes, (i) (ii) hypoxia exposure and (iii) normoxic conditions. (d) Plasma 11-KT. (e) Plasma T. See figure 3 for key to abbreviations. Scale bar, 200 μm (N=8–24). Significant differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05).
(c) Neuroendocrine mechanism of reproductive impairment
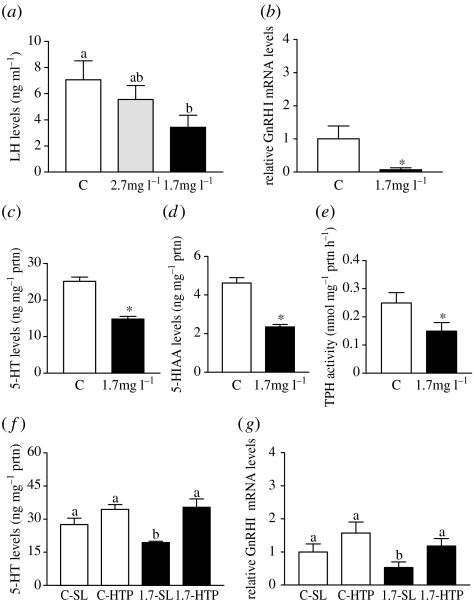
Possible impairment of the reproductive neuroendocrine system was investigated to determine whether it could account for the impairment of gonadal functions observed in males and females. There were no significant differences between male and female croaker for any of the neuroendocrine responses (electronic supplementary material, table 6), so the results of both sexes were combined (figure 6). LH secretion in response to GnRHa injection was significantly attenuated in croaker exposed to low DO (figure 6a). Decreased LH secretion in response to GnRHa in croaker is related to a decline in GnRH receptor concentrations on the gonadotrophs, which in turn is associated with decreases in GnRH synthesis and secretion (Khan & Thomas 2001). Consistent with this mechanism of decreased pituitary responsiveness, the expression of GnRH mRNA was significantly decreased in the POAH of croaker exposed to 1.7 mg l−1 DO (figure 6b). An important stimulatory role for 5-HT in the control of LH secretion and the presence of serotonergic neurons in close association with GnRH ones in the POAH have been identified in croaker (Khan & Thomas 1997). Exposure to hypoxia caused a decrease in hypothalamic 5-HT concentrations, suggesting an impairment of the serotonergic system (figure 6c). Tissue 5-HT levels are influenced by the rate-limiting enzyme in 5-HT synthesis, TPH, and degradation, via monoamine oxidase, both of which require molecular oxygen and could potentially be influenced by low DO. Tissue concentrations of the metabolite of 5-HT, 5-HIAA, demonstrated a similar decline to that of 5-HT, while the ratio of 5-HIAA/5-HT was unaffected, suggesting that the rate of degradation was not altered and that 5-HT synthesis via TPH was decreased (figure 6d). A follow-up experiment demonstrated that TPH activity in the POAH was significantly decreased after exposure to 1.7 mg l−1 DO (figure 6e). If TPH is the primary site of impairment of the reproductive neuroendocrine system during hypoxia, injection with 5-HTP, an immediate precursor of 5-HT which bypasses the biosynthetic step catalyzed by TPH, should restore hypothalamic 5-HT concentrations as well as GnRH neuronal function. As observed in the earlier experiment, exposure to hypoxia caused a significant decrease in 5-HT content. Injection with 5-HTP increased 5-HT levels in both control and hypoxia-exposed fish (figure 6f). These changes in 5-HT content were accompanied by increases in GnRH mRNA expression in the POAH to normal levels in the hypoxia-exposed fish (figure 6g).
Figure 6.
Effects of hypoxia on neuroendocrine functions in croaker. Results from both sexes were combined because they were not significantly different. Mean±s.e.m. values and N for each sex in the experimental groups are shown in electronic supplementary material, table 6. (a) Plasma LH in response to GnRHa injection (N=8–11). LH values (N=7) for all saline-injected groups were below the detection limit of the assay and are not included in the figure. (b) GnRH mRNA expression in POAH (N=7). (c) 5-HT and (d) 5-HIAA levels in hypothalamus (N=19). (e) Hypothalamic TPH activity (N=6–7). (f,g) Effects of 5-HTP injection on (f) 5-HT (N=10) and (g) GnRH I mRNA levels in POAH (N=7–11). (a,f,g) Significant differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05). (b–e) Asterisk indicates significant difference from controls (Student's t-test, p<0.05).
4. Discussion
These extensive field observations with croaker provide the first clear evidence, to our knowledge, of widespread reproductive impairment and endocrine disruption in a teleost species chronically exposed to hypoxia in its natural environment. Gonadal development was impaired in croaker collected from four hypoxic sites in East Bay, Florida in 2003, but was normal in fish collected from adjoining normoxic sites in Pensacola Bay. The extent of the reproductive disturbance was remarkable in that gametogenesis and sex steroid signalling were dramatically inhibited in both males and females collected throughout a 42 km2 estuarine region. By comparison, the reproductive and endocrine disturbances reported in fish collected from heavily industrialized estuaries contaminated with endocrine-disrupting chemicals and other industrial pollutants are typically less pronounced, with only partial impairment of gametogenesis affecting a subset of the population of one of the sexes (Allen et al. 1999; Hashimoto et al. 2000). The specificity of the reproductive responses in croaker to hypoxia was confirmed in the controlled laboratory studies, where the patterns of gametogenic and endocrine changes were remarkably similar to those observed in the field samples. In contrast, indicators of several other physiological processes in croaker such as growth were unaltered at the end of the reproductive season in November by environmental exposure to hypoxia (results not shown). Previously, we obtained preliminary evidence that several indices of reproductive endocrine function and gametogenesis were impaired in croaker collected from sites that had experienced periodic seasonal hypoxia in Mobile Bay, Alabama (Thomas et al. 2006b). Reproductive and endocrine dysfunction has also been observed in a freshwater species, carp, after exposure to very low DO (less than 1 mg l−1; Wu et al. 2003). Taken together, these results suggest that reproduction in fishes is especially sensitive to disturbance by hypoxia and that reproductive functions are preferentially curtailed in croaker when their estuarine environment becomes hypoxic.
The laboratory studies with 5-HTP demonstrate that hypoxia impairs reproductive functions in croaker through decreasing the activity of the stimulatory 5-HT component of the reproductive neuroendocrine system. Hypoxia also downregulates the brain serotonergic system in mammals which is associated with a general decrease in neural function (Brown et al. 1975; Hedner et al. 1977). Many other environmental stressors have been shown to inhibit the endocrine system and reproduction in fishes (Billard et al. 1981). However, the decrease in POAH 5-HT levels in croaker does not appear to be a general non-specific stress response because other environmental and social stressors have been shown to exert opposite effects, elevating neural 5-HT levels in fishes and mammals (Fuller 1990; Winberg & Nilsson 1993) which results in activation of neuroendocrine pathways mediating the corticosteroid stress response (Höglund et al. 2002). We have confirmed that hypothalamic 5-HT content is also elevated in croaker exposed to a typical laboratory stressor, capture by netting and a 1 min exposure to air once a day for 5 days. Hypothalamic 5-HT concentrations were significantly increased 60 min after the last combined stressor treatment (22.9±1.1 ng mg−1 protein) compared with controls (18.6±1.2 ng mg−1 protein; N=6, p<0.05). These results suggest that the decline in hypothalamic 5-HT content through decreased TPH activity is a specific response to hypoxia stress. However, the mechanism by which TPH activity is downregulated is unknown and could potentially involve signalling molecules altered during hypoxia such as HIF and gonadal steroids.
The occurrence of a specific response induced by low DO involving downregulation of TPH activity to inhibit the HPG axis suggests that inhibition of this neuroendocrine pathway and curtailment of reproduction are important for adaptation of croaker to periodic hypoxia. Hypoxia-tolerant fish species initially respond to low DO by increasing oxygen delivery (Hochachka & Somero 2002). A second strategy is to conserve energy expenditure through metabolic suppression (Wu 2002), the main benefit of which is to slow down biological time, enabling the organism to survive until environmental conditions again become favourable (Hochachka & Somero 2002). Hypoxia is a natural seasonal phenomenon in estuaries, so it is not surprising that many estuarine teleost species, such as croaker, have adapted to periodic hypoxia (Bell & Eggleston 2005), presumably through the development of mechanisms to conserve energy. Fishes invest enormous amounts of energy in reproduction and their life-history strategies involve balancing the amount of energy invested in reproduction with that used for growth and metabolism (Williams 1966). Thus, a major portion of the total available energy in estuarine fishes such as croaker is diverted to ovarian growth (approx. 20% of BW) during the reproductive season, a period when seasonal hypoxia can also occur. Therefore, we propose that inhibition of gonadal growth during chronic exposure to hypoxia would be a highly effective strategy to dramatically reduce energy and oxygen requirements in estuarine fishes, enabling them to survive until favourable normoxic conditions return. In support of this idea, hypoxia-induced decreases in reproductive function, including impairment of gonadal growth, have been observed in several freshwater species (Wu et al. 2003; Shang et al. 2006) and decreases in egg production are often observed when energy from food is limited (Leggett & Carscadden 1978). Croaker and many other fishes that experience highly variable environmental conditions during the reproductive season are iteroparous (repeat spawners), which increases their fitness by not putting all their energy into a single spawn (Leggett & Carscadden 1978), especially when food is limited (Wootton 1973). Some fish species do not spawn annually in nutrient-poor environments, particularly if the energy demands of reproduction would result in increased mortality, as a strategy to increase lifetime offspring production (Bull & Shine 1979; Trippel & Harvey 1989). Our results suggest that a similar curtailment of reproduction occurs in croaker when their environment becomes hypoxic for an extended period, as observed in East Bay in 2003 when there was total failure of reproduction in both males and females, suggesting that they did not contribute significantly to the spawning population in that year but delayed reproduction until a subsequent year when environmental conditions were favourable.
Seasonal hypoxia in estuaries has become more common and widespread in the past 20 years, affecting 35–60% of the total estuarine area along Gulf of Mexico and Atlantic coasts (Engle et al. 1999). Our field results provide the first clear evidence that HIF-1α and -2α mRNAs are upregulated in fishes during chronic environmental exposure to hypoxia and support the conclusion of our laboratory studies that they are potential molecular biomarkers of persistent hypoxia exposure (Rahman & Thomas 2007). Croaker that experience normoxic estuarine conditions and complete gonadal crudescence could still experience hypoxia-induced reproductive failure when they migrate into the Gulf of Mexico to complete gamete maturation and spawn. Recent laboratory studies show that these final stages of the croaker reproductive cycle are also impaired by hypoxia (P. Thomas & M. S. Rahman 2004, unpublished observations). The hypoxic ‘dead zone’ off the mouth of the Mississippi River has doubled in size over the past 25 years and now extends along the entire eastern Louisiana coast to East Texas, an area of 16–20 000 km2 (Rabalais et al. 2002). Although the long-term effects of these increases in estuarine and coastal hypoxia on marine ecosystems are unknown, our results suggest that hypoxia-induced inhibition of reproduction could have serious consequences for the maintenance of croaker populations and other coastal marine finfish resources.
Acknowledgments
We are grateful to Dr Richard Snyder (West Florida State University) and Dr James Hagy (US EPA Gulf Breeze Laboratory) for kindly providing the bottom DO data for the summer to autumn 2003 in electronic supplementary material, tables 3 and 4. We also thank Dr Ken Dunton, Dr Paul Montagna and Dr Alfredo Ojanguren for their advice with statistical analyses. The assistance of Susan Lawson with the laboratory experiments is greatly appreciated. This work was supported by US Environmental Protection Agency's Science to Achieve Results (STAR) Estuaries and Great Lakes (EaGLe) program through funding to the Consortium for Ecoindicator Research for the Gulf of Mexico (CEER-GOM) grant no. R-82945801 (to P.T.).
This research has not been subjected to EPA's peer and policy review and does not reflect the views of the agency and no official endorsement should be inferred.
Supplementary Material
Electronic supplementary material, figure 1. Relative ovarian expression of hypoxia-inducible factor (HIF)-1α and HIF-2α mRNAs in croaker at the time of the November sampling. N=6. A nested ANOVA analysis indicates HIF-1α and HIF-2α mRNA levels in croaker from the normoxic sites are significantly different from those in fish from the hypoxic sites (***p<0.001). Individual site differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05)
Electronic supplementary material, figure 2. Inhibition of gonadal development and disruption of endocrine functions in croaker collected from hypoxic sites in October (c,g) and November (a,b,d–f,h) 2003
Dissolved oxygen levels at field sites and in experimental tanks
Neuroendocrine responses in male and female croaker in laboratory hypoxia experiment
References
- Allen Y, Scott A.P, Matthiessen P, Haworth S, Thain J.E, Feist S. Survey of estrogenic activity in United Kingdom estuarine and coastal waters and its effects on gonadal development of the flounder Platichthys flesus. Environ. Toxicol. Chem. 1999;18:1791–1800. doi:10.1897/1551-5028(1999)018<1791:SOEAIU>2.3.CO;2 [Google Scholar]
- Bell G.W, Eggleston D.B. Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Mar. Biol. 2005;146:761–770. doi:10.1007/s00227-005-1614-9 [Google Scholar]
- Billard R, Bry C, Gillet C. Stress, environment and reproduction in teleost fish. In: Pickering A.D, editor. Stress and fish. Academic Press; London, UK: 1981. pp. 185–208. [Google Scholar]
- Brown R.M, Kehr W, Carlsson A. Functional and biochemical aspects of catecholamine metabolism in brain under hypoxia. Brain Res. 1975;85:491–509. doi: 10.1016/0006-8993(75)90822-7. doi:10.1016/0006-8993(75)90822-7 [DOI] [PubMed] [Google Scholar]
- Brown-Peterson N, Thomas P, Arnold C.R. Reproductive biology of the spotted seatrout, Cynoscion nebulosus, in south Texas. Fish. Bull. 1988;86:373–388. [Google Scholar]
- Bull J.J, Shine R. Iteroparous animals that skip opportunities for reproduction. Am. Nat. 1979;114:296–303. doi:10.1086/283476 [Google Scholar]
- Copeland P.A, Thomas P. The measurement of plasma vitellogenin levels in a marine teleost, the spotted seatrout (Cynoscion nebulosus) by homologous radioimmunoassay. Comp. Biochem. Physiol. B. 1988;91:17–23. doi: 10.1016/0305-0491(88)90108-3. doi:10.1016/0305-0491(88)90108-3 [DOI] [PubMed] [Google Scholar]
- Copeland P.A, Thomas P. Purification of maturational gonadotropin from Atlantic croaker (Micropogonias undulatus) and development of a homologous radioimmunoassay. Gen. Comp. Endocrinol. 1989;73:425–441. doi: 10.1016/0016-6480(89)90200-1. doi:10.1016/0016-6480(89)90200-1 [DOI] [PubMed] [Google Scholar]
- Diaz R.J, Rosenberg R. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 1995;33:245–303. [Google Scholar]
- Engle V.D, Summers J.K, Macauley J.M. Dissolved oxygen conditions in northern Gulf of Mexico estuaries. Environ. Monit. Assess. 1999;57:1–20. doi: 10.1007/BF00545979. doi:10.1023/A:1005980410752 [DOI] [PubMed] [Google Scholar]
- Fuller R.W. Serotonin receptors and neuroendocrine responses. Neuropsychopharmacology. 1990;3:495–502. [PubMed] [Google Scholar]
- Gutherz E.J. The northern Gulf of Mexico ground fish fishery, including a brief life history of Atlantic croaker (Micropogonias undulatus) Proc. Gulf Coast Fish. Inst. 1976;29:87–101. [Google Scholar]
- Hagy J.D, Murrell M.C. Susceptibility of a northern Gulf of Mexico estuary to hypoxia: an analysis using box models. Estuar. Coast. Shelf Sci. 2007;74:239–253. doi:10.1016/j.ecss.2007.04.013 [Google Scholar]
- Hashimoto S, Bessho H, Hara A, Nakamura M, Iguchi T, Fujita K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectes yokohamae) from Tokyo Bay, Japan. Mar. Environ. Res. 2000;49:37–53. doi: 10.1016/s0141-1136(99)00047-1. doi:10.1016/S0141-1136(99)00047-1 [DOI] [PubMed] [Google Scholar]
- Hawkins M.B, Thornton J.W, Crews D, Skipper J.K, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc. Natl Acad. Sci. USA. 2000;97:10 751–10 756. doi: 10.1073/pnas.97.20.10751. doi:10.1073/pnas.97.20.10751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner T, Lundborg P, Engel J. Effect of hypoxia on monoamine synthesis in brains of developing rats. Biol. Neonate. 1977;31:122–126. doi: 10.1159/000240952. [DOI] [PubMed] [Google Scholar]
- Hochachka P.W, Somero G.N. Oxford University Press; New York, NY: 2002. Biochemical adaptation: mechanisms and process in physiological adaptation evolution. [Google Scholar]
- Höglund E, Balm P.H.M, Winberg S. Stimulatory and inhibitory effects of 5-HT1A receptors on adrenocorticotropic hormone and cortisol secretion in a teleost fish, the Arctic charr (Salvelinus alpinus) Neurosci. Lett. 2002;324:193–196. doi: 10.1016/s0304-3940(02)00200-8. doi:10.1016/S0304-3940(02)00200-8 [DOI] [PubMed] [Google Scholar]
- Jobling S, Tyler C.R, Brighty G, Sumpter J.P. Widespread sexual disruption in fish. Environ. Sci. Technol. 1998;32:2498–2506. doi:10.1021/es9710870 [Google Scholar]
- Justić D, Rabalais N.N, Turner R.E. Coupling between climate variability and coastal eutrophication: evidence and outlook for the northern Gulf of Mexico. J. Sea Res. 2005;54:25–35. doi:10.1016/j.seares.2005.02.008 [Google Scholar]
- Karouna-Renier N.K, Snyder R.A, Allison J.G, Wagner M.G, Rao K.R. Accumulation of organic and inorganic contaminants in shellfish collected in estuarine waters near Pensacola, Florida: contamination profiles and risks to human consumers. Environ. Pollut. 2007;145:474–488. doi: 10.1016/j.envpol.2006.04.035. doi:10.1016/j.envpol.2006.04.035 [DOI] [PubMed] [Google Scholar]
- Khan I.A, Thomas P. Aroclor 1254-induced alterations in hypothalamic monoamine metabolism in the Atlantic croaker (Micropogonias undulatus): correlation with pituitary gonadotropin release. Neurotoxicology. 1997;18:553–560. [PubMed] [Google Scholar]
- Khan I.A, Thomas P. Disruption of neuroendocrine control of luteinizing hormone secretion by Aroclor 1254 involves inhibition of hypothalamic tryptophan hydroxylase activity. Biol. Reprod. 2001;64:955–964. doi: 10.1095/biolreprod64.3.955. doi:10.1095/biolreprod64.3.955 [DOI] [PubMed] [Google Scholar]
- Leggett W.C, Carscadden J.E. Latitudinal variation in reproductive characteristics of American shad (Alosa sapidissima): evidence for population specific life history strategies in fish. J. Fish. Res. Board Can. 1978;35:1469–1478. [Google Scholar]
- Mohamed J.S, Thomas P, Khan I.A. Isolation, cloning and expression of three prepro-GnRH mRNAs in Atlantic croaker brain and pituitary. J. Comp. Neurol. 2005;488:384–395. doi: 10.1002/cne.20596. doi:10.1002/cne.20596 [DOI] [PubMed] [Google Scholar]
- Pihl L, Baden S.P, Diaz R.J. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar. Biol. 1991;108:349–360. doi:10.1007/BF01313644 [Google Scholar]
- Rabalais N.N, Turner R.E, Scavia D. Beyond science into policy: Gulf of Mexico hypoxia and the Mississippi river. Bioscience. 2002;52:129–142. doi:10.1641/0006-3568(2002)052[0129:BSIPGO]2.0.CO;2 [Google Scholar]
- Rahman M.S, Thomas P. Molecular cloning, characterization and expression of two hypoxia-inducible transcription factors (HIF-1α and HIF-2α) in a hypoxia-tolerant marine teleost, Atlantic croaker (Micropogonias undulatus) Gene. 2007;396:273–282. doi: 10.1016/j.gene.2007.03.009. doi:10.1016/j.gene.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Shang E.H.H, Yu R.M.K, Wu R.S.S. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio) Environ. Sci. Technol. 2006;40:3118–3122. doi: 10.1021/es0522579. doi:10.1021/es0522579 [DOI] [PubMed] [Google Scholar]
- Singh H, Thomas P. Mechanism of stimulatory action of growth hormone on ovarian steroidogenesis in spotted seatrout, Cynoscion nebulosus. Gen. Comp. Endocrinol. 1993;89:341–353. doi: 10.1006/gcen.1993.1042. doi:10.1006/gcen.1993.1042 [DOI] [PubMed] [Google Scholar]
- Smith V. Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ. Sci. Pollut. Res. 2003;10:126–139. doi: 10.1065/espr2002.12.142. doi:10.1065/espr2002.12.142 [DOI] [PubMed] [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006a;71:310–316. doi: 10.1016/j.steroids.2005.09.015. doi:10.1016/j.steroids.2005.09.015 [DOI] [PubMed] [Google Scholar]
- Thomas P, Rahman M.S, Kummer J.A, Lawson S. Reproductive endocrine dysfunction in Atlantic croaker exposed to hypoxia. Mar. Environ. Res. 2006b;62:S249–S252. doi: 10.1016/j.marenvres.2006.04.031. doi:10.1016/j.marenvres.2006.04.031 [DOI] [PubMed] [Google Scholar]
- Trippel E.A, Harvey H.H. Missing opportunities to reproduce: an energy dependent or fecundity gaining strategy in white sucker (Catostomus commersoni)? Can J. Zool. 1989;67:2180–2188. [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection: a critique of some current evolutionary thought. [Google Scholar]
- Winberg S, Nilsson G.E. Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions, with particular reference to fish. Comp. Biochem. Physiol. C. 1993;106:597–614. doi:10.1016/0742-8413(93)90216-8 [Google Scholar]
- Wootton R.J. The effect of size of food ration on egg production in the female three-spined stickleback, Gasterosteus aculeatus L. J. Fish. Biol. 1973;5:89–96. doi:10.1111/j.1095-8649.1973.tb04433.x [Google Scholar]
- Wu R.S.S. Hypoxia: from molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002;45:35–45. doi: 10.1016/s0025-326x(02)00061-9. doi:10.1016/S0025-326X(02)00061-9 [DOI] [PubMed] [Google Scholar]
- Wu R.S.S, Zhou B.S, Randall D.J, Woo N.Y.S, Lam P.K.S. Aquatic hypoxia is an endocrine disruptor and impairs fish reproduction. Environ. Sci. Technol. 2003;37:1137–1141. doi: 10.1021/es0258327. doi:10.1021/es0258327 [DOI] [PubMed] [Google Scholar]
Notice of correction
Figure 4 is now presented in the correct form.
30 August 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material, figure 1. Relative ovarian expression of hypoxia-inducible factor (HIF)-1α and HIF-2α mRNAs in croaker at the time of the November sampling. N=6. A nested ANOVA analysis indicates HIF-1α and HIF-2α mRNA levels in croaker from the normoxic sites are significantly different from those in fish from the hypoxic sites (***p<0.001). Individual site differences identified with a multiple range test, Fisher's PLSD, are indicated with different letters (p<0.05)
Electronic supplementary material, figure 2. Inhibition of gonadal development and disruption of endocrine functions in croaker collected from hypoxic sites in October (c,g) and November (a,b,d–f,h) 2003
Dissolved oxygen levels at field sites and in experimental tanks
Neuroendocrine responses in male and female croaker in laboratory hypoxia experiment