Abstract
The importance of neutral dynamics is contentiously debated in the ecological literature. This debate focuses on neutral theory's assumption of fitness equivalency among individuals, which conflicts with stabilizing fitness that promotes coexistence through niche differentiation. I take advantage of competition–colonization trade-offs between species of aquatic micro-organisms (protozoans and rotifers) to show that equalizing and stabilizing mechanisms can operate simultaneously. Competition trials between species with similar colonization abilities were less likely to result in competitive exclusion than for species further apart. While the stabilizing mechanism (colonization differences) facilitates coexistence at large spatial scales, species with similar colonization abilities also exhibited local coexistence probably due to fitness similarities allowing weak stabilizing mechanisms to operate. These results suggest that neutral- and niche-based mechanisms of coexistence can simultaneously operate at differing temporal and spatial scales, and such a spatially explicit view of coexistence may be one way to reconcile niche and neutral dynamics.
Keywords: competition–colonization trade-off, microcosm, niche versus neutral dynamics, spatial scale, species coexistence
1. Introduction
The idea that ecological communities are regulated by neutral processes (Bell 2001; Hubbell 2001) has had a profound effect on ecology, but this remains contentious (Gaston & Chown 2005). Data-driven studies generally refute some aspect of neutral patterns or processes (McGill 2003; Gilbert & Lechowicz 2004; Turnbull et al. 2005; Wootton 2005), denying that neutral dynamics can produce observable ecological patterns. Similarly, completely niche-based explanations have failed to adequately explain extant community patterns (Chave 2004; Holyoak & Loreau 2006). Consequently, a number of studies have attempted to reconcile neutral and niche dynamics (Chave 2004; Tilman 2004; Gravel et al. 2006; Holyoak & Loreau 2006; Adler et al. 2007). Chesson (2000) best anticipated the divergence and reconciliation between neutral and niche dynamics by explicitly viewing coexistence mechanisms as either equalizing or stabilizing (Chave 2004; Adler et al. 2007). Stabilizing coexistence describes species differences that result in reduced niche overlap, thus minimizing the impact of fitness inequalities on competitive interactions. Equalizing mechanisms promote similarities in species responses to environmental conditions (i.e. fitness equivalency) and reduce the rate of competitive exclusion as well as allow coexistence from weak stabilizing mechanisms. Often invoked as the fundamental assumption for neutral dynamics, equivalency does not mean that species are the same in all respects, rather that equalizing mechanisms diminish fitness inequalities.
Although Chesson (2000) viewed stabilizing and equalizing mechanisms as small-scale resource competition, here I view stabilizing and equalizing as contributions to coexistence in more general terms. Here I consider stabilizing mechanisms to be synonymous with any ecological difference that allows two species to stably coexist together at some definable spatial scale. Similarly, I define equalizing mechanisms as those that produce equivalent fitness responses to environmental conditions. Thus, equalizing coexistence is a product of environmental constraints and is observed at some finite spatial or temporal scale. It is important to note that in using Chesson's schema, I am explicitly viewing the outcome of neutral-type dynamics at the population level (i.e. persistence time) and not considering the more appropriate view of neutrality as stochasticity at the individual level (see Volkov et al. (2005) for a treatment on this difference).
With this more general definition, we can examine coexistence mechanisms at different spatial scales and specifically ask how dispersal and colonization play a role in our understanding of coexistence (McPeek & Holt 1992; Tilman 1994; Holt & McPeek 1996; Tilman & Kareiva 1997; Kinzig et al. 1999; Amarasekare 2003; Mouquet & Loreau 2003; Kneitel & Chase 2004; Holyoak et al. 2005). A number of coexistence models explicitly consider species as having a trade-off between their competitive and colonizing abilities (Levins & Culver 1971; Horn & Macarthur 1972; Tilman 1994; Pacala & Rees 1998; Yu & Wilson 2001; Yu et al. 2001; Levine & Rees 2002; Mouquet & Loreau 2003; Mouquet et al. 2006). I will argue in this paper that whether we view coexistence in a competition–colonization trade-off as stabilizing or equalizing probably depends on the scale of observation.
Spatially implicit competition models (e.g. Hastings 1980; Caswell & Cohen 1991; Tilman 1994; Pacala & Rees 1998) show that in an environment where local disturbances (i.e. density-independent mortality) cause small-scale extinctions, a good colonizer/poor competitor and a poor colonizer/good competitor can stably coexist at larger spatial scales. Because there is a trade-off, neither strategy could replace the other in a moderately disturbed system and the relative occupancy of competitors depends upon disturbance frequency. However, in these models, local coexistence between these two strategies is impossible because the dominant competitor always replaces the better colonizer within a patch. The presence of any trade-off is often cited as evidence against the role of neutral dynamics in structuring communities (Turnbull et al. 2005; Ellis et al. 2006). Yet several recent publications suggest that even though relatively few strategies along a niche gradient can coexist, within any single niche strategy, multiple functionally similar or equivalent species can coexist, mimicking neutral-type dynamics (Hubbell 2005; Gravel et al. 2006; Holt 2006; Scheffer & van Nes 2006). Recently, Fukami et al. (2007) showed that adaptive radiation in Pseudomonas bacteria resulted in both the filling of empty niches and the evolution of ecological equivalents coexisting within niches. Thus, the presence of trade-offs may not necessarily refute neutral dynamics (Hubbell 2005). If we view the stabilizing mechanism (colonization ability) as part of a strict trade-off, then two species that have similar colonization abilities will also have similar competitive abilities within local patches. In the absence of any other local niche partitioning, these two, similarly competing species, should have similar fitness responses to local environmental conditions (Chesson 2000), meaning that either competitive exclusion takes many generations to occur or weak stabilizing mechanisms promote coexistence. I use data from aquatic microcosm experiments to test whether the risk of competitive exclusion decreases and time to local extinction increases as species become more similar.
2. A simple model
With a competition–colonization trade-off, species can stably coexist at larger spatial scales despite competitive differences. However, within local patches, such coexistence is not possible if we assume that there is not any spatial subsidy effect enhancing one species birth rates over another (Mouquet et al. 2006). Furthermore, many competition–colonization models assume instantaneous competitive exclusion, but in considering a gradient from niche to neutral dynamics, the relative time for competitive exclusion is fundamentally important. Incorporating succession requires the addition of local niche dynamics to trade-off models (Pacala & Rees 1998). Here I assume that there is a strict trade-off between colonization and competitive ability. I am explicitly considering the dynamics of unicellular micro-organisms of a single trophic level inhabiting homogeneous, spatially discrete patches (e.g. Cadotte 2006, 2007). Given this simple system, the competition–colonization trade-off can be defined by two parameters: the intrinsic rate of increase for species i, ri, and the strength of interspecific competition (bij, the effect of species j on i). Here I assume that intraspecific effects, bii, are constant. The population size of species i at time t is given by
| (2.1) |
where Ni,t−1 is the population size at time t−1 and ϵ is the normally distributed stochasticity with a mean of 0 and standard deviation of 1.
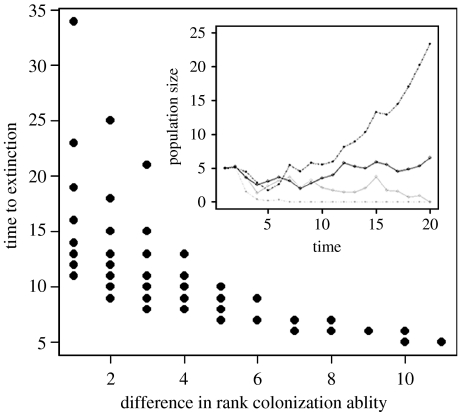
If there is a trade-off, then as ri increases, its effect on the other species, bji, must decrease. As the difference increases, the disparity between competitive effects also increases. Thus, within patches, increasing D means that the inferior competitor goes extinct faster (figure 1). Species with identical r's (and thus b's) will persist indefinitely, but even species with small D may persist for many generations if the magnitude of the difference in b's is less than demographic stochasticity (ϵ in equation (2.1)).
Figure 1.
The time to observe a local extinction for pairwise species combinations from equation (2.1). Here 12 species are modelled with a strict competition–colonization trade-off. The best colonizer had ri=1.325 and was ranked 1, and subsequent species r's were decreased by 0.025, with the 12th ranked species having ri=1.05. The 12th ranked species was also the best competitor with bi12=0.65 and lower ranked species had lower bij's by 0.05, with the best colonizer having bi1=0.1. The inset shows two example simulations: one simulation is between two species with similar abilities (solid lines) and the other is for two species with very different abilities (dashed lines). For the similar species: solid black line, species rank 5; solid grey line, species rank 4. For the different species: dashed black line, species rank 11; dashed grey line, species rank 3.
3. A test using micro-organisms
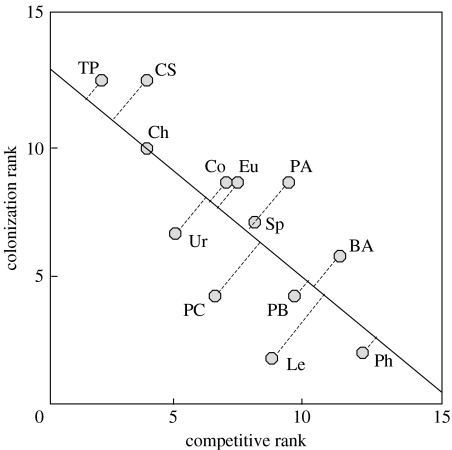
Here I use data from the competition–colonization experiment of Cadotte et al. (2006). Using an artificial system of aquatic micro-organisms (protozoans and rotifers; see figure 2 for species list), Cadotte et al. (2006) revealed that species exhibited competition–colonization trade-offs, where the best competitors were generally poor colonizers and the best colonizers were typically poor competitors (figure 2). Colonization was measured as the relative time for species to colonize every patch in a discrete five-patch system, whereas competition was measured as the extinction probability in pairwise combinations with every other species (see Cadotte et al. (2006) for detailed methods). The patches in the colonization experiment were 125 ml Nalgene bottles filled with 100 ml of bacterialized nutrient solution, and with 4.76 mm threaded holes having nylon tube fittings (Cadotte et al. 2006). Patches were linked serially, connected with 12.5 cm of clear Nalgene 4.76 mm PVC tubing. For the competition experiment, the two species were added to 50 ml of bacterialized solution in 250 ml glass jars. For both experiments, the presence of species was assessed with weekly 5 ml samples (and replaced with 5 ml of sterile nutrient solution). All experiments were replicated three times.
Figure 2.
The relationship between competitive and colonization abilities, showing a competition–colonization trade-off (adapted from Cadotte et al. (2006)). Dashed lines show species locations along regression line. Species with similar colonization abilities are assumed to have similar fitnesses given laboratory conditions and resource availability. BA, Blepharisma americanum; Ch, Chilomonas sp.; Co, Coleps sp.; CS, Colpidium striatum; Eu, Euplotes sp.; Le, Lepadella sp.; PA, Paramecium aurelia; PB, P. bursaria; PC, P. caudatum; Ph, Philodina sp.; Sp, Spirostomum sp.; TP, Tetrahymena pyriformis; Ur, Uronema sp.
Here I assume that the outcomes of species competition in Cadotte et al. (2006) result from fitness inequalities. I also assume that species inhabit a stable environment, are limited by a single resource and have colonization abilities that reflect maximal population growth rates (Warren et al. 2006). Competitive interactions are estimated in an extremely conservative manner: whether one of the two populations goes extinct, ostensibly due to competitive exclusion. Colonization ability was ranked by time to colonize all patches. Rank was calculated as the mean rank from 10 000 random draws of the individual replicates (see Cadotte et al. 2006). Since I observed exclusions over an eight-week interval, with weekly samplings, the data are said to be right-censored. Right-censored data are common in ‘time to’ experiments where observations end at some arbitrary time and therefore represent a biased sampling where parameter estimation does not conform to widely used parametric estimations (Hosmer & Lemeshow 1999). Which species went extinct is not important here because as long as there was extinction, then these two species are said to exhibit fitness inequalities. Therefore, to estimate the probability of coexistence, I used the Kaplan–Meier product-limit estimator (Hosmer & Lemeshow 1999), which calculates the probability that a given population will persist beyond time t. The maximum-likelihood estimate of this probability is given by
| (3.1) |
where ni is the number of surviving populations and di is the number of deaths at time ti. I used a parametric regression fitting the probability of coexistence to a Weibull distribution against the absolute difference in colonization rank, and evaluated the model using a likelihood ratio test (presented as Χ2-value) comparing this model with a model containing only an intercept. Survival analysis was performed using the Survival Package, v. 2.31 with R v. 2.4.1 maintained by Thomas Lumley (www.r-project.org).
4. Results
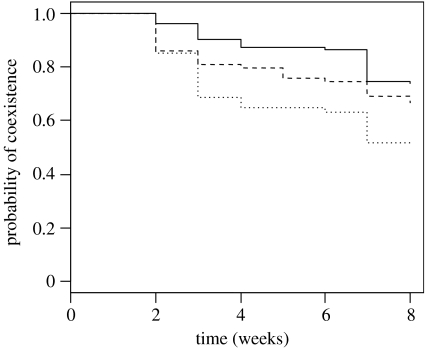
The regression analysis reveals that the probability of successful coexistence between any two species is negatively related to the difference in their colonization rank (Χ12=7.56, p=0.006; coefficient±s.e.=−0.07892±0.0289). To best illustrate this relationship, I grouped the colonization differences into three classes: (i) difference in colonization rank<3.00, (ii) difference≥3.00 and ≤6.00, and (iii) difference>6.00. The probability of coexistence as a function of time is shown in figure 3, and the relationship between the probability of coexistence and the colonization difference classes is very similar to that for the continuous model above (Χ12=8.75, p=0.003; coefficient±s.e.=−0.2814±0.0976).
Figure 3.
The Kaplan–Meier product-limit estimator of the probability of coexistence over time. The three lines refer to species classified by differences in colonization rank (D). Small (solid line), D<3; medium (dashed line), 3≤D≤6; large (dotted line), D>6. For clarity, 95% CIs were removed.
5. Implications: how niche and neutral processes can coexist
One of the earliest axioms of ecology was that two species occupying the same niche results in competitive exclusion of the inferior competitor (Grinnell 1904, 1917; Gause 1934). However, this ‘competitive exclusion principle’ was quickly cast into doubt as examples of coexistence in ecologically similar species surfaced (Ross 1957; Udvardy 1959; den Boer 1986). Since then, the idea that species coexist due to their similarities rather than their differences has repeatedly surfaced, primarily by ecologists studying tropical forests (e.g. Webb 2000; Hubbell 2006) and freshwater algae (Hutchinson 1967; Lewis 1977; McCormick 1996). The debate, whether coexistence results from ecological differences or similarities, is almost as old as the science of ecology. Hubbell (2006) rightly noted that experimental evidence of equivalency was lacking. I would argue that this is largely due to the fact that experimenters have not been explicitly looking for equalizing mechanisms, especially in concert with niche processes.
To look for niche versus neutral (i.e. stabilizing versus equalizing) processes, Adler et al. (2007) recently suggested quantifying intra- versus interspecific effects on vital rates, or measuring frequency-dependent population growth. Here I look at the outcome of negative interspecific effects on vital rates by enumerating population extinctions. The results reveal that the more similar two species are in their colonization ability, the more likely that they persist together for long periods, ostensibly due to fitness equivalency. Fitness similarities allow for coexistence from weak stabilizing mechanisms.
These results also suggest that whether apparent coexistence is due to strong stabilizing or equalizing mechanisms depends upon the spatial and temporal scales at which observations are being made. From Chesson's (2000) framework, equalizing coexistence is ultimately unstable since fitness equivalency results in intrinsic rates of population increase that equal zero. Therefore, a population affected by a density-independent mortality event will not be able to recover when in the presence of a competitor with equivalent fitness. For inferior competitors, equalizing coexistence is trumped by the inevitable eventual immigration of the superior competitors (Cadotte 2006).
However, stabilizing coexistence from a competition–colonization trade-off occurs over large spatial and temporal scales. For superior competitors, periodic disturbances or environmental changes eventually eliminate local populations. Species distributed along a competition–colonization trade-off have differing strategies where coexistence depends on chance events happening at larger spatial and temporal scales than within patch dynamics alone (Amarasekare 2003; Kneitel & Chase 2004). In the absence of disturbance, dominant competitors will eventually exclude the inferior ones even at large spatial scales (Cadotte 2007), and in the microcosm system, space appears necessary for coexistence, contrary to Adler & Mosquera (2000).
Recently, Turnbull et al. (2005) concluded that the presence of a trade-off necessarily negates the possibility that neutral processes structure communities. They examined the potential for coexistence among grassland pioneer species and nicely showed that an ‘establishment/colonization’ trade-off best explained coexistence patterns (Turnbull et al. 2005). This trade-off describes the fact that species with larger seeds (i.e. poorer dispersers) can tolerate a greater range of environmental hazards while small-seeded species are more susceptible to hazards, but they have a better chance of dispersing to optimal microsites (Turnbull et al. 2005). The current results are completely compatible with Turnbull et al. (2005), in that in a strict stabilizing trade-off, only species with differing strategies should coexist. However, given the fact that Turnbull et al. (2005) were able to group species according to seed mass in their analyses, the question becomes: do species with similar seed sizes coexist, and if so, is this coexistence best explained by similar fitnesses as opposed to strong stabilizing mechanisms? Other plant community studies have shown that while several stabilizing traits appear to allow for coexistence, species composition within any trait appears haphazard and historically contingent (Fukami et al. 2005; Ejrnaes et al. 2006)
With a competition–colonization trade-off, coexistence is often thought of as the product of non-equilibrium, spatially dependent processes (Connell 1978; Huston 1979), where these chance events present different niche opportunities for species. Connell's (1978) intermediate disturbance hypothesis viewed local richness as dependent on the time since disturbance, where soon after a disturbance colonizing species establish populations. A result of the logic in this hypothesis is that if immigration ceased, then succession would be arrested and species richness would cease to change. The mechanism that allows coexistence within any successional stage was attributed to microhabitat niche partitioning (Connell 1978). The current results support the notion that species show equivalency and thus neutral processes probably dominate within successional stages, while niche processes drive patterns among successional stages (Denslow 1980; Ellner & Fussmann 2003; Cadotte 2007).
Acknowledgments
I am indebted to J. A. Drake, R. D. Holt, J. M. Levine, N. Sanders and D. Simberloff for their input and advice, and also to B. J. Cardinale, I. Carroll, T. Fukami, J. M. Levine, R. Shulman and two anonymous reviewers for their helpful comments on previous versions of this manuscript. Any errors or logical flaws are completely my own. Invaluable laboratory help was provided by Sam Jantz, Monica Keele and Donny Mai. Financial support was generously provided by the Department of Ecology and Evolutionary Biology, the Yate's Fellowship, Science Alliance, and Scholarly Activity and Research Incentive Funds, all at the University of Tennessee, and by the University of California at Santa Barbara.
References
- Adler F.R, Mosquera J. Is space necessary? Interference competition and limits to biodiversity. Ecology. 2000;81:3226–3232. [Google Scholar]
- Adler P.B, HilleRisLambers J, Levine J.M. A niche for neutrality. Ecol. Lett. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. doi:10.1111/j.1461-0248.2006.00996.x [DOI] [PubMed] [Google Scholar]
- Amarasekare P. Competitive coexistence in spatially structured environments: a synthesis. Ecol. Lett. 2003;6:1109–1122. doi:10.1046/j.1461-0248.2003.00530.x [Google Scholar]
- Bell G. Neutral macroecology. Science. 2001;293:2143–2148. doi: 10.1126/science.293.5539.2413. doi:10.1126/science.293.5539.2413 [DOI] [PubMed] [Google Scholar]
- Cadotte M.W. Metacommunity influences on community richness at multiple spatial scales: a microcosm experiment. Ecology. 2006;87:1008–1016. doi: 10.1890/0012-9658(2006)87[1008:miocra]2.0.co;2. doi:10.1890/0012-9658(2006)87[1008:MIOCRA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cadotte M.W. Competition–colonization tradeoffs and disturbance effects at multiple scales. Ecology. 2007;88:823–829. doi: 10.1890/06-1117. doi:10.1890/06-1117 [DOI] [PubMed] [Google Scholar]
- Cadotte M.W, Mai D.V, Jantz S, Collins M.D, Keele M, Drake J.A. On testing the competition–colonization tradeoff in a multispecies assemblage. Am. Nat. 2006;168:704–709. doi: 10.1086/508296. doi:10.1086/508296 [DOI] [PubMed] [Google Scholar]
- Caswell H, Cohen J.E. Disturbance, interspecific interaction and diversity in metapopulations. Biol. J. Linn. Soc. 1991;42:193–218. [Google Scholar]
- Chave J. Neutral theory and community ecology. Ecol. Lett. 2004;7:241–253. doi:10.1111/j.1461-0248.2003.00566.x [Google Scholar]
- Chesson P. Mechanism of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. doi:10.1146/annurev.ecolsys.31.1.343 [Google Scholar]
- Connell J.H. Diversity in tropical forests and coral reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. doi:10.1126/science.199.4335.1302 [DOI] [PubMed] [Google Scholar]
- den Boer P.J. The present status of the competitive exclusion principle. Trends Ecol. Evol. 1986;1:25–28. doi: 10.1016/0169-5347(86)90064-9. doi:10.1016/0169-5347(86)90064-9 [DOI] [PubMed] [Google Scholar]
- Denslow J.S. Patterns of plant species diversity during succession under different disturbance regimes. Oecologia. 1980;46:18–21. doi: 10.1007/BF00346960. doi:10.1007/BF00346960 [DOI] [PubMed] [Google Scholar]
- Ejrnaes R, Bruun H.H, Graae B.J. Community assembly in experimental grasslands: suitable environment or timely arrival? Ecology. 2006;87:1225–1233. doi: 10.1890/0012-9658(2006)87[1225:caiegs]2.0.co;2. doi:10.1890/0012-9658(2006)87[1225:CAIEGS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ellis A.M, Lounibos L.P, Holyoak M. Evaluating the long-term metacommunity dynamics of tree hole mosquitoes. Ecology. 2006;87:2582–2590. doi: 10.1890/0012-9658(2006)87[2582:etlmdo]2.0.co;2. doi:10.1890/0012-9658(2006)87[2582:ETLMDO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner S.P, Fussmann G. Effects of successional dynamics on metapopulation persistence. Ecology. 2003;84:882–889. doi:10.1890/0012-9658(2003)084[0882:EOSDOM]2.0.CO;2 [Google Scholar]
- Fukami T, Bezemer T.M, Mortimer S.R, van der Putten W.H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 2005;8:1283–1290. doi:10.1111/j.1461-0248.2005.00829.x [Google Scholar]
- Fukami T, Beaumont H.J.E, Zhang X.-X, Rainey P.B. Immigration history controls diversification in experimental adaptive radiation. Nature. 2007;446:436–439. doi: 10.1038/nature05629. doi:10.1038/nature05629 [DOI] [PubMed] [Google Scholar]
- Gaston K.J, Chown S.L. Neutrality and the niche. Funct. Ecol. 2005;19:1–6. doi:10.1111/j.0269-8463.2005.00948.x [Google Scholar]
- Gause G.F. Hafner Publishing Company; New York, NY: 1934. The struggle for existence. [Google Scholar]
- Gilbert B, Lechowicz M.J. Neutrality, niches, and dispersal ina temporate forest understory. Proc. Natl Acad. Sci. USA. 2004;101:7651–7656. doi: 10.1073/pnas.0400814101. doi:10.1073/pnas.0400814101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel D, Canham C.D, Beaudet M, Messier C. Reconciling niche and neutrality: the continuum hypothesis. Ecol. Lett. 2006;9:399–409. doi: 10.1111/j.1461-0248.2006.00884.x. doi:10.1111/j.1461-0248.2006.00884.x [DOI] [PubMed] [Google Scholar]
- Grinnell J. The origin and distribution of the chestnut-backed chickadee. Auk. 1904;21:364–382. [Google Scholar]
- Grinnell J. The niche relationships of the California thrasher. Auk. 1917;34:427–433. [Google Scholar]
- Hastings A. Disturbance, coexistence, history, and competition for space. Theor. Popul. Biol. 1980;18:363–373. doi:10.1016/0040-5809(80)90059-3 [Google Scholar]
- Holt R.D. Emergent neutrality. Trends Ecol. Evol. 2006;21:531–533. doi: 10.1016/j.tree.2006.08.003. doi:10.1016/j.tree.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Holt R.D, McPeek M.A. Chaotic population dynamics favors the evolution of dispersal. Am. Nat. 1996;148:709–718. doi:10.1086/285949 [Google Scholar]
- Holyoak M, Loreau M. Reconciling empirical ecology with neutral community models. Ecology. 2006;87:1370–1377. doi: 10.1890/0012-9658(2006)87[1370:reewnc]2.0.co;2. doi:10.1890/0012-9658(2006)87[1370:REEWNC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Holyoak M, Leibold M.A, Holt R.D, editors. Metacommunities: spatial dynamics and ecological communities. University of Chicago Press; Chicago, IL: 2005. [Google Scholar]
- Horn H.S, Macarthur R.H. Competition among fugitive species in a Harlequin environment. Ecology. 1972;53:749–752. doi:10.2307/1934797 [Google Scholar]
- Hosmer D.W, Lemeshow S. Wiley; New York, NY: 1999. Applied survival analysis. [Google Scholar]
- Hubbell, S. P. 2001 The unified neutral theory of biodiversity and biogeography Monographs in population biology. Princeton, NJ: Princeton University Press.
- Hubbell S.P. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 2005;19:166–172. doi:10.1111/j.0269-8463.2005.00965.x [Google Scholar]
- Hubbell S.P. Neutral theory and the evolution of ecological equivalence. Ecology. 2006;87:1387–1398. doi: 10.1890/0012-9658(2006)87[1387:ntateo]2.0.co;2. doi:10.1890/0012-9658(2006)87[1387:NTATEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huston M. A general hypothesis of species diversity. Am. Nat. 1979;113:81–101. doi:10.1086/283366 [Google Scholar]
- Hutchinson G.E. Wiley; New York, NY: 1967. A treatise on limnology. [Google Scholar]
- Kinzig A.P, Levin S.A, Dushoff J, Pacala S. Limiting similarity, species packing, and system stability for hierarchical competition–colonization models. Am. Nat. 1999;153:371–383. doi: 10.1086/303182. doi:10.1086/303182 [DOI] [PubMed] [Google Scholar]
- Kneitel J.M, Chase J.M. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 2004;7:69–80. doi:10.1046/j.1461-0248.2003.00551.x [Google Scholar]
- Levine J.M, Rees M. Coexistence and relative abundance in annual plant assemblages: the roles of competition and colonization. Am. Nat. 2002;160:452–467. doi: 10.1086/342073. doi:10.1086/342073 [DOI] [PubMed] [Google Scholar]
- Levins R, Culver D. Regional coexistence of species and competition between rare species. Proc. Natl Acad. Sci. USA. 1971;68:1246–1248. doi: 10.1073/pnas.68.6.1246. doi:10.1073/pnas.68.6.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W.M. Net growth rate through time as an indicator of ecological similarity. Ecology. 1977;58:149–157. doi:10.2307/1935116 [Google Scholar]
- McCormick P.V. Resource competition and species coexistence in freshwater benthic algal assemblages. In: Stevenson R.J, Bothwell M.L, Lowe R.L, editors. Algal ecology. Academic Press; New York, NY: 1996. pp. 229–252. [Google Scholar]
- McGill B.J. A test of the unified neutral theory of biodiversity. Nature. 2003;422:881–888. doi: 10.1038/nature01583. doi:10.1038/nature01583 [DOI] [PubMed] [Google Scholar]
- McPeek M.A, Holt R.D. The evolution of dispersal in spatially and temporally varying environments. Am. Nat. 1992;6:1010. doi:10.1086/285453 [Google Scholar]
- Mouquet N, Loreau M. Community patterns in source–sink metacommunities. Am. Nat. 2003;162:544–557. doi: 10.1086/378857. doi:10.1086/378857 [DOI] [PubMed] [Google Scholar]
- Mouquet N, Miller T.E, Daufresne T, Kneitel J.M. Consequences of varying regional heterogeneity in source–sink metacommunities. Oikos. 2006;113:481–488. doi:10.1111/j.2006.0030-1299.14582.x [Google Scholar]
- Pacala R.W, Rees M. Models suggesting field experiments to test two hypotheses explaining successional diversity. Am. Nat. 1998;152:729–737. doi: 10.1086/286203. doi:10.1086/286203 [DOI] [PubMed] [Google Scholar]
- Ross H.H. Principles of natural coexistence indicated by leafhopper populations. Evolution. 1957;11:113–129. doi:10.2307/2406045 [Google Scholar]
- Scheffer M, van Nes E.H. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA. 2006;103:6230–6235. doi: 10.1073/pnas.0508024103. doi:10.1073/pnas.0508024103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. doi:10.2307/1939377 [Google Scholar]
- Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl Acad. Sci. USA. 2004;101:10 854–10 861. doi: 10.1073/pnas.0403458101. doi:10.1073/pnas.0403458101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman, D. & Kareiva, P. (eds) 1997 The role of space in population dynamics and interspecific interactions Monographs in population biology. Princeton, NJ: Princeton University Press.
- Turnbull L.A, Manley L, Rees M. Niches, rather than neutrality, structure a grassland pioneer guild. Proc. R. Soc. B. 2005;272:1357–1364. doi: 10.1098/rspb.2005.3084. doi:10.1098/rspb.2005.3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy M.F.D. Notes on the ecological concepts of habitat, biotope and niche. Ecology. 1959;40:725–728. doi:10.2307/1929830 [Google Scholar]
- Volkov I, Banavar J.R, He F, Hubbell S.P, Maritan A. Density dependence explains tree species abundance and diversity in tropical forests. Nature. 2005;438:658–661. doi: 10.1038/nature04030. doi:10.1038/nature04030 [DOI] [PubMed] [Google Scholar]
- Warren P.H, Law R, Weatherby A.J. Invasion biology as a community process: messages from microcosms. In: Cadotte M.W, McMahon S.M, Fukami T, editors. Conceptual ecology and invasions biology: reciprocal approaches to nature. Springer; Dordrecht, The Netherlands: 2006. pp. 343–367. [Google Scholar]
- Webb C.O. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 2000;156:145–155. doi: 10.1086/303378. doi:10.1086/303378 [DOI] [PubMed] [Google Scholar]
- Wootton J.T. Field parameterization and experimental test of the neutral theory of biodiversity. Nature. 2005;433:309–312. doi: 10.1038/nature03211. doi:10.1038/nature03211 [DOI] [PubMed] [Google Scholar]
- Yu D.W, Wilson H.B. The competition–colonization trade-off is dead; long live the competition–colonization trade-off. Am. Nat. 2001;158:49–63. doi: 10.1086/320865. doi:10.1086/320865 [DOI] [PubMed] [Google Scholar]
- Yu D.W, Wilson H.B, Pierce N.E. An empirical model of species coexistence in a spatially structured environment. Ecology. 2001;82:1761–1771. [Google Scholar]