Abstract
The processing and presentation of lipid antigens by antigen presenting cells (APC) is important for defense against infection, tumor immunosurveillance, and autoimmunity. CD1, a family of cell surface glycoproteins, is responsible for the binding and presentation of lipid antigens to receptors expressed on the surface of T lymphocytes. Among the several (glyco)lipids identified to cause T-cell stimulation in complex with CD1, α-galactosyl ceramide (α-Galcer), is one of the most well studied. A combination of structure-activity relationship (SAR), crystallographic studies, and discovery of new “natural” antigens has led to greater understanding of the structural requirements for optimal natural killer T-cell activation.
Introduction
The CD1 family of antigen presenting glycoproteins mediates T-cell responses through the presentation of self and foreign lipids, glycolipids, lipopeptides, or amphipathic small molecules to T-cell receptors (TCR)1-3. In humans, the various CD1 isoforms are categorized as group I (CD1a, b, c and e) and group II (CD1d) based on sequence similarity4. Through the binding and presentation of endogenous and exogenous lipid antigens to TCRs, the CD1 pathway is reminiscent of peptide presentation by major histocompatibility complex (MHC) class I and class II molecules5.
CD1 molecules are glycosylated heterodimers composed of a heavy chain polypeptide noncovalently associated with β2-microglobulin (β2m). Group I and II CD1 proteins are mainly expressed on cortical thymocytes, B-cell (CD1c) and antigen presenting cells (APC), such as dendritic cells (DC). The group II isoform, CD1d, is additionally expressed on macrophages, epithelial cells and hepatocytes1.
Crystal structures of human CD1a6, 7, hCD1b8, 9, hCD1d10 and mouse CD1d11-15. (mCD1d), some in complex with their respective antigens, have revealed how differences in the topology of their respective binding grooves enable them to have a degree of ligand specificity, while maintaining the ability to present a diverse set of antigenic lipids.
Wilson and co-workers solved the initial structure of mCD1d which revealed an overall fold similar to the MHC class I proteins. The α-chain folds into three domains (α1, α2, and α3) and is closely associated with β2m. The membrane distal α1, and α2, domains form the binding groove which is composed of an eight-stranded anti-parallel β-sheet floor traversed by two anti-parallel α-helices11.
In the antigen binding groove, two deep pockets, designated A′ and F′, are lined with hydrophobic residues that account for the affinity for long hydrophobic chains, such as lipid tails. Glycosylceramides are presented by CD1d in a specific manner, with the fatty acyl and sphingosine tails extending into the A′ and F′ pockets respectively. An extensive hydrogen bonding network tightly locks the sugar headgroup in place, which orients and stabilizes the glycolipid for presentation to the TCR10, 13. The recent elucidation of the T-cell receptor/α-Galcer/hCD1d complex has shed light upon the interactions found at the interface of the ternary complex16. It confirmed that the highly conserved α-chain of the TCR contributes a majority of the total buried surface area on binding of the TCR to hCD1d. Additionally, the high specificity displayed by the TCR for α-Galcer and closely related glycolipids was explained through the extensive hydrogen bonding formed with the 2′, 3′ and 4′ galactose hydroxyl groups and 3 hydroxyl of the sphingosine chain.
The crystal structure of hCD1b revealed how antigens with lipid chains of up to 80 carbons in length are accommodated. Whereas both hCD1a and hCD1d have only two antigen-binding pockets, A′ and F′, hCD1b has a total of four, that have been named A′, F′, C′ and T′. Interconnecting the A′, T′, and F′ pockets creates a continuous channel 70Å in length that can enclose the long hydrocarbon tails (up to C80) characteristic of hCD1b antigens. Although structurally conserved among the solved CD1 isoforms, the A′ pocket of CD1a is unlike that of hCD1b and mCD1d. It functions more like a ‘molecular ruler’ to selectively bind alkyl chains of distinct length since it abruptly ends at one terminus. This is in comparison to how the A′ pocket is part of a continuous channel as seen in the structures of hCD1b and mCD1d.
CD1 Antigens
A great assortment of lipids, glycolipids, lipopeptides and amphipathic small molecules have now been shown to bind to the CD1 isoforms, some of which are shown below.
These antigens are generally either of bacterial or self-origin. The ability to present a diverse set of structures arises from differences in the shape, connectivity and overall volume of the CD1 binding grooves17.
The Model Antigen: α-Galactosyl Ceramide
A great assortment of lipids, glycolipids, lipopeptides and amphipathic small molecules have now been shown to bind to the CD1 isoforms. The most well-studied is a CD1d-presented antigen, α-galactosyl ceramide (α-GalCer). It initially drew interest when extracts derived from the marine sponge, Agelas mauritianus, demonstrated antitumor properties in murine models. This potent activity was later traced to a family of α-linked glycosphingolipids, called agelasphins, from which α-GalCer was structurally optimized18-20. α-GalCer consists of a galactosyl moiety α1-linked to a ceramide, a long-chain amino alcohol, D-erythro-phytosphingosine, N-acylated with a 26 carbon fatty acid.
The use of α-GalCer has shown promising potential in the treatment of several diseases, including cancer21-23, malaria24, and hepatitis B25, while also helping to fend off certain bacterial infections26, 27. The molecule also functions in the suppression of autoimmune disorders28 and exhibits adjuvant activity29. Taniguchi and co-workers first identified α-GalCer as a ligand for the activation of CD1d-mediated natural killer T (NKT) cells, a subpopulation of T lymphocytes that express a semi-invariant TCR (Vα14-Jα18 in mice and Vα24-Jα18 in humans) and are reactive to α-GalCer30. NKT cells are able to modulate the immune system through rapid secretion of regulatory cytokines including, but not limited to, IFN-γ and IL-431. Stimulation of NKT cells is followed by downstream activation of other cells in the immune system, such as natural killer (NK) cells, DCs, macrophages, B cells, and conventional T cells32-36. Many of these cells, in turn, go on to secrete additional immune modulating cytokines creating an entire activation cascade and are responsible for the therapeutic effects of α-GalCer.
Limitations of α-Galactosyl Ceramide
Activation of NKT cells occurs when a TCR recognizes the CD1d/α-GalCer bimolecular complex. The recognition event induces the rapid secretion of T helper 1 (Th1) and T helper 2 (Th2) cytokines IFN-γ and IL-4 respectively, by NKT cells. Secondary activation of other cell types include NK cells, B cells, CD8+ T cells, dendritic cells, and myeloid cells as well as the differentiation of CD4+ T cells into either Th1 or Th2 cells. This ability to influence both innate and adaptive immune responses puts NKT cells in the position to play a pivotal role in regulating immune responses in both host defense and autoimmune diseases37, 38.
However, the opposing Th1 and Th2 polarizing functions of α-GalCer limit its effectiveness as an immunomodulator. Th1 cytokines, such as IFN-γ, are stimuli which drive the development of naïve helper T cells towards Th1 type cell formation. In contrast, Th2 cytokines like IL-4 send pre-Th cells down the path of Th2 type cell formation39. Th1 cells participate in cell-mediated immunity (CMI) and are essential for controlling intracellular pathogens while Th2 cells participate in antibody-mediated immunity control of extracellular pathogens. The balance between Th1 and Th2 cytokines is carefully controlled and any disruption between the two can cause disease40. Therapeutic strategies could involve trying to restore Th1/Th2 balance through in vivo modulation of NKT cells. For instance, multiple sclerosis (MS) is characteristic of hyporesponsive Th2 cells and thus a Th1-like profile41, while many types of cancer have a predominant Th2 response42. In addition, upregulation of either pathway causes downregulation of the other through reciprocal inhibition43.
Th1 cytokines are thought to mediate the anti-tumor, antiviral and antibacterial effects of α-GalCer. Its effectiveness is limited though, because α-GalCer induced activation of NKT cells causes rapid secretion of both Th1 and Th2 cytokines, The production of IL-4 may mask or limit the beneficial effect if IFN-γ. In a Phase I study, α-GalCer was ineffective in the treatment of solid-tumors possibly because the therapeutic effects of IFN-γ was hindered by IL-4 giving no net benefit44. In animal models of various autoimmune diseases, NKT cell responses to glycolipid stimulation have also resulted in mixed outcomes.
Therapeutic Potential of α-Galactosyl Ceramide Analogs
α-GalCer, a vital tool in the field of NKT cell biology, has been utilized in studies for the treatment of many diseases. Its efficacy has been limited because of the reciprocal inhibition of Th1 and Th2 cytokines. Attempts to selectively control the rapid secretion of cytokines by NKT cells has led to the development of several α-GalCer analogs with different but interesting immunomodulatory properties. The mechanism by which these analogs elicit the dissimilar responses remains unclear.
With some exceptions such as asthma, certain autoimmune diseases are characteristic of hyporesponsiveness to Th2 and over activation of Th1 cells. Skewing of the cytokine release profile to Th2 would be beneficial for the treatment of these diseases, but any induction of IFN-γ may be harmful. A direct relationship has been shown relating the shortening of lipid tail lengths and biasing of the cytokine release profile towards a Th2 response45. OCH, a sphingosine and fatty acyl truncated analog of α-GalCer, protects mice against the development of experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis. This result was attributed to the biased production of IL-4 by OCH and the suppression of myelin antigen-specific Th1 responses46, 47. Another α-GalCer analog which exhibits skewing towards Th2 responses is C20:248. It is a variant that shortens the fatty acid from C26 to C20 and introduces two cis-double bonds at C11 and C14.
Conversely, another analog, α-C-GalCer, showed enhanced Th1 response and thus had 100-1000 fold improved activity against melanoma metastases and malaria compared with α-GalCer49, 50. Both are diseases where a Th1 response is beneficial. In this analog, the O-linkage between the sugar and ceramide is replaced with a C-linkage giving the glycosidic bond in vivo stability to enzymatic degradation. The improved activity of α-C-GalCer may also be attributed to changes in the electron density of the galactosyl moiety affected by α-anomeric atom.
Structural Optimization of α-Galactosyl Ceramide
Modification of the galactosyl moiety of α-GalCer
Since its discovery, α-GalCer has been the prototypical antigen for the study of NKT cell stimulation. The glycolipid itself is a modified analog of the natural class of compounds, agelasphins18. The unparalleled potency of α-GalCer has been the motivation for structure-activity relationship analysis and has led to many interesting observations about the specificity of the TCR/glycolipid/CD1d interaction. Initially, Taniguchi and coworkers examined several glycoside analogs of α-GalCer for their proliferative responses to murine NKT cells30. Of these analogs, α-linked glucosyl analog (α-GlcCer) showed slightly diminished activity compared to α-GalCer. On the other hand, α-linked mannosyl ceramide (α-ManCer) and β-GalCer were not at all active, suggesting the importance of the 2′-hydroxyl group of the galactosyl moiety and α-linkage to the anomeric carbon. Taniguchi and coworkers also tested several disaccharide analogs, Galα1-6Galα1-1′Cer, Galα1-2Galα1-1′Cer, Galα1-4Glcβ1-1′Cer, Galα1-6Glcα1-1′Cer, galactofuranoseβ1-3Galα1-1′Cer. The results illustrated the importance of the α-anomeric configuration of the inner sugar, although none of the diglycosylated compounds were more potent than the monoglycosylated α-GalCer. In a later study by Kronenberg and coworkers, an antigen processing by a lysosomal enzyme, α-galactosidase A, was shown to play a role in the generation of a monosaccharide epitope for the Galα1-2GalCer and the Galα1-3GalCer glycolipids but not Galα1-6GalCer51.
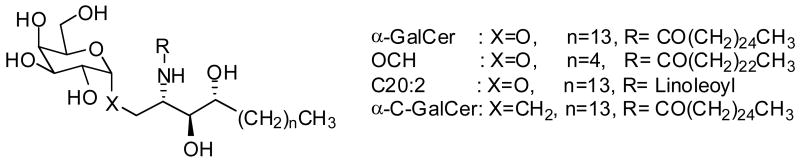
Modification of the 2′-hydroxyl of the galactose moiety has been extensively explored. Essentially, any modification of the 2′-hydroxyl to 2′-fluoro, 2′-deoxygalactosyl, or 2′-acetoamino-2′-deoxygalactosyl abolished any activation of murine NKT cell hybridomas52. Sulfation of the 3′-hydroxyl group resulted in a compound, 3-O-sulfo-α-GalCer, with almost comparable activity to the parent compound, α-GalCer, in activation assays of mice NKT hybridomas and a human NKT cell line53. The 4′- and 6′-positions have also proven to be amendable towards modification. The change from 4′-axial (gal) to equatorial (glu) only moderately affected activity30, while more severe changes to the 6′-position did not abolish activity.
Savage and coworkers substituted the 6′-hydroxy with an acetamide to yield a compound with comparable activity to α-GalCer and improved solubility in organic and aqueous solvents54. In addition, a variety of small fluorophores and biotin have been appended on the 6′-hydroxy and were well tolerated, making such compounds useful for the study of glycolipid trafficking and CD1d loading55. Oxidation of the 6′- position to a carboxylate, in the case of the Sphingomonas glycosphingolipids (GSLs) was accepted52, 56. In spite of all these studies, galactose still remains unrivaled as the prototypical head group to maximize NKT cell activation. Subsequent to a few of the aforementioned studies, the crystal structures of both human and murine CD1d in complex with α-GalCer10 and its truncated analog, PBS-2513, respectively, were determined. The crystallographic structures confirmed the earlier observation about the importance of the 2′-hydroxyl, which makes extensive interactions with CD1d. In the murine structure, the galactose headgroup makes an important hydrogen bond with Asp153, effectively helping to orient the sugar for presentation to the TCR. Even though the 3′-hydroxl also hydrogen bonds to Asp 153, it is more solvent accessible than the 2′-hydroxyl.
Modification of the phytosphingosine scaffold
More extensive studies have been done with the lipid portion of α-GalCer. The 2S, 3S, 4R orientation of the 2-amino-3, 4-diol are essential components of the phytosphingosine chain. The 3-hydroxyl group of the phytosphingosine chain is crucial for antitumor activity by the comparison of the 4-deoxy and 3,4-dideoxy analogs of α-GalCer18. Kronenberg and coworkers also proved that the lack of a 3-hydroxyl group on the phytosphingosine chain results in the complete loss of the binding between glycolipid-CD1d complex and TCR as observed by surface plasmon resonance57. Isosteric replacement of the 2-amino functionality with a 1,2,3-triazole created analogs with comparable stimulatory effects as α-GalCer and a skewing towards Th2 cytokine response58.
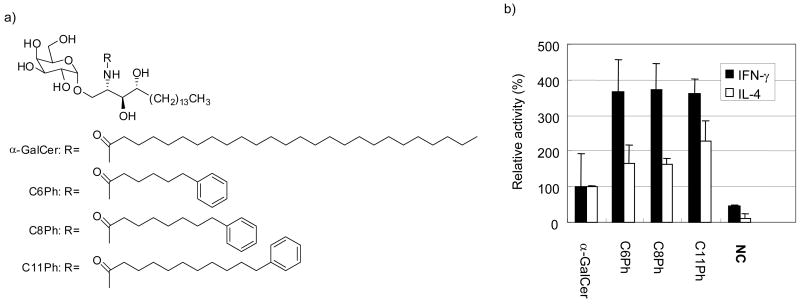
Comparison of the Sphingomonas glycolipid, GalA-GSL and α-GalCer gives insight into the role of the 4-hydroxyl group. The bacterial glycolipids possess a similar lipid structure as α-GalCer with major differences in the length of the fatty acyl chain, C14 versus C26 respectively, and the absence of the 4-hydroxyl group in the bacterial lipid structure. As expected, α-GalCer was the most potent of these compounds, and the existence of crystallographic structures for both GalA-GSL and α-GalCer in complex with CD1d offers a view into the role of the 4-hydroxyl59. Asp 80 of CD1d hydrogen bonds with the 3- and 4- diol causing a lateral shift of α-GalCer in the binding pocket. In the case of the bacterial glycolipid, only one hydroxyl group is available for hydrogen bonding with Asp 80 and thus sits lower in the binding groove, effecting how it is presented by CD1d. In another study, substitution of the phytosphingosine with a sphingosine tail, i.e., replacement of the 4-hydroxyl group with a double bond, also reduced activity60 in an α-sulfatide analog.
Two other lipid scaffolds, in addition to the ceramide base, have also been observed in natural and synthetic NKT cell antigens. Serine-based lipids have been utilized as a ceramide mimic with success in other applications. However, galactosyl serine-type ceramide analogs were not as potent as the ceramide based glycolipid61. This may indicate that a proton donor is preferred at least in the 3- position of the lipid moiety. Glycerol-type scaffolds, found in known CD1d-presented antigens phosphatidylinositol mannoside (PIM)62, phosphatidylcholine (PC)14, phosphatidyl ethanolamine (PE)63, and the Borellia glycolipid, α-galactosyl 1,2-diacyl sn-glycerol, are also functional frameworks64. Interestingly, small variations in the acyl tail length and degree of unsaturation had great influence upon antigenic potency though overall, the potency of Borellia glycolipids were weaker than that of α-GalCer65. These compounds lack the corresponding phytosphingosine 2-amide, 3- and 4-hydroxyl group which were observed to make important hydrogen bonding interactions with CD1d in crystallographic structures. Overall, glycolipids containing ceramide-based frameworks are generally more potent in the activation of NKT cells than glycerol-based compounds.
Modification of lipid chains
Crystal structures of various glycolipids in complex with mouse and human CD1d confirmed that the lipid chains were accommodated in the binding grooved created by the α1 and α2 domains10-14. Hydrophobic interactions between the lipid tails and residues lining the binding groove of CD1d are the principal contributing factors of binding energy and therefore, a number of SAR studies modifying lipid chains have been reported resulting in interesting changes to the cytokine release profile of NKT cells. Savage and coworkers showed that truncation of the fatty acyl or the phytosphingosine chains has shown to bias cytokine secretion toward a Th2 response.45 Similarly, OCH selectively induces Th2 cytokines from NKT cells and suppressed autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and diabetes in NOD mice.46 Also, introduction of unsaturation into the fatty acyl chain of α-GalCer, C20:1 cis/trans and C20:2 analogs seem to bias toward a Th2 response.48 Although the origins of the Th2 bias are somewhat unclear, a possibility introduced to partially explain the differences relates to the relative stability of the glycolipids in complex with CD1d.66 A direct relationship has been shown correlating the shortening of the length of the lipid tails and the biasing of the cytokine release profile towards a Th2 response45 Miyake and coworkers demonstrated that IFN-γ, a Th1-type cytokine, production by NKT cells requires longer TCR stimulation than IL-4 production. Thus, glycolipids with shorter lipid tails have reduced ability to form stable complexes with CD1d. This altered stability of the CD1d/glycolipid complex directly influences the stability of the TCR/glycolipids/CD1d complex, which may be a factor in the cytokine profile produced.
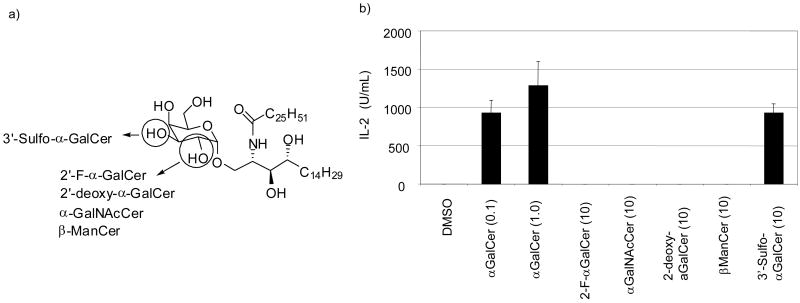
Conversely, we have found that introduction of terminal aromatic groups into the fatty acyl tail of α-GalCer enhances stability of the glycolipid/CD1d complex and biases the profile towards a Th1 response.67 A majority of the binding energy between the acyl tails and CD1d is mainly due to non-specific hydrophobic interactions. Therefore, through inclusion of one or multiple aromatic groups in either acyl chain, additional favorable interactions could be introduced via ring stacking or other more specific contacts. The vital amino alcohol stereocenters of the sphingosine would be kept and therefore the orientation of the sugar should be held intact or only subtly affected. α-GalCer analogs bearing a 6-phenylhexanoyl (C6Ph), 8-phenyloctanoyl (C8Ph), or 11-phenylundecanoyl group (C11Ph) as the fatty acyl chain demonstrated more potent overall cytokine production and biased NKT cell activation toward Th1 type response as measured by IFN-γ cytokine production.
In vivo studies of the aromatic ring containing α-GalCer analogs also supported Th1-type biased NKT cell activation68. Glycolipids that induced more Th1-biased cytokines in vitro exhibited greater anticancer activities in mice bearing breast or lung cancers.
Modeling of selected glycolipids in the hCD1d hydrophobic groove predicted binding of the aromatic analogs to be in a similar fashion as observed in the crystal structure of α-GalCer and CD1d. The phytosphingosine and fatty acyl tail extended into the F′ and A′ pockets respectively, and there was not a notable shift of the galactose headgroup. The installation of a terminal aromatic group at the end the fatty acyl tail seemed to allow for additional specific interactions with the aromatic side chains lining the CD1d pocket.
A competitive binding assay system using isoelectric focusing (IEF) electrophoresis was conducted as a qualitative method to relate binding affinities of glycolipids to CD1d.69 In our hands, C6Ph, C8Ph and C11Ph demonstrated more potent inhibition of GT1b-hCD1d binding than α-GalCer and a less potent analog bearing an isonicotinoyl group as fatty acyl chain (4-Py). Therefore we suspect that that introduction of more specific interactions between glycolipid and CD1d greatly enhances the overall production of both Th1 and Th2 type cytokines and also skews the balance towards a Th1 type response. C6Ph, C8Ph and C11Ph represent the first examples of NKT cell agonists which are more potent than α-GalCer and also exhibit a stronger Th1 cytokine response, possibly due to enhanced binding to CD1d. However, the origin of the enhanced potency and Th1 selectivity remains to be fully addressed. These new synthetic glycolipid compounds with altered binding to CD1d yields novel therapeutic compounds and extends the library of glycolipids available for the study of NKT cell biology.
The Search for “Natural” NKT antigens
Due to the unusual origin and structure of α-GalCer, it has mainly been thought of as a surrogate ligand for CD1d-mediated NKT cell activation37. Although glycosphingolipids are commonly found within mammalian cellular membranes, they typically contain a β- and not an α-anomeric linkage between the sugar and ceramide. The α-anomeric configuration between the sugar and ceramide is essential for activity since β-GalCer is not able to cause NKT cell activation30. However, the endogenous antigen, isoglobotrihexosylceramide (iGb3) has the ability to stimulate NKT cells despite the β-linkage between the sugar and ceramide70. The requisite α-linkage is found at the terminal sugar of the iGb3 trisaccharide. There also has been no biochemical detection of α-linked glycosphingolipids in mammalian cells. It is also doubtful that mice and human T-cell populations are selected for the recognition of marine sponge antigens. The physiological significance of α-GalCer in mice and humans remained unclear, as it was unknown why an α-galactosyl ceramide of marine origin was such a potent NKT cell agonist. This issue has raised many questions as to the nature of the physiological ligand for CD1d-restricted NKT cells and has led to the investigation of mammalian, bacterial, and plant species as sources of natural ligands for NKT cells. In addition, the identification of biologically relevant antigens may offer some insight to understanding how structure influences the cytokine release profile.
α-GalCer's unique structural features provided clues in the search for more physiologically relevant antigens. Sphingomonas bacteria, commonly found in soil and sea water, are highly abundant in the environment71. They are Gram-negative, LPS-negative bacteria in which the outer LPS membrane has been replaced with glycosphingolipids (GSL)72-74. The composition of the GSL layer has been characterized in detail and shown to comprise of α-glucuronosylceramides. Three independent studies have reported that these glycosphingolipids (GSLs) of the bacterial cell wall were shown to be broadly recognized by both mice and human NKT cells. Although structurally similar to α-GalCer, the Sphingomonas GSLs are unique because they contain glucuronic or galacturonic acids α-linked to a ceramide base. Differences in the length of the N-fatty acyl tail and the absence of a sphingosine 4-OH also make Sphingomonas GSLs distinct from α-GalCer.
Glycolipids from more virulent strains of bacteria also promise to be sources of CD1d presented antigens. Lyme disease is caused by the tick-borne spirochete Borrelia burgdorferi and is transmitted to humans through the bites of infected ticks. A serious infectious disease affecting over 15,000 people a year, it has become the most common vector-borne disease in the United States75, 76. CD1d has been implicated to play a role in the initial host resistance to B. burgdorferi infection. CD1d-deficient (CD1d -/-) mice were shown to have an impaired defense against infection by B. burgdorferi, making the bacterium's glycolipids attractive compounds for further study as possible natural CD1d antigens77, 78. Two major classes of Borrelia burgdorferi glycolipids (BbGL), which comprise approximately 36% of the total lipid mass, were structurally characterized as cholesteryl 6-O-acyl-β-D-galactopyranoside (B. burgdorferi glycolipid 1, BbGL-I) and 1,2-di-O-acyl-3-O-α-D-galactopyranosyl-sn-glycerol (BbGL-II)79. BbGL-II was of special interest not only because of its α-configuration but also because its lipid moiety closely resembles phosphatidyl choline or phosphatidyl ethanolamine, two glycolipids found to bind CD1d63, 80. Although galactose was the only saccharide detected, a variety of major, C16:0 and C18:1, and minor, C14:0, C18:0, and C18:2, fatty acids were found during NMR analysis suggesting that BbGL-I and –II were acylated with a mixture of fatty acids. Additionally, as in the case of the Sphingomonas bacteria, there has been no evidence for the presences of LPS in the Borrelia species81, making these glycolipids possible alternative antigens. The discovery of such bacterial antigens suggests that they may serve as triggers for an innate-type immune response providing protection against bacteria that lack cell-wall ligands such as LPS and cannot be detected by the Toll-like receptors (TLRs).
Concluding Remarks
Over the past decade, it was shown that NKT cells play critical roles in both innate and adaptive immunity. Various natural/synthetic glycolipids which bind to CD1d were made known to activate NKT cells via CD1d mediated antigen presentation. Some of these glycolipids seemed to possess a more biased cytokine profile for either Th1 cytokines or Th2 cytokines than that of α-GalCer, the first glycolipid reported to activate NKT cells. Whether this skewing of the profile was directly correlated to the binding affinity the glycolipids to CD1d or a combination of other factors has not been definitively determined. A comprehensive study comparing side-by-side the disassociation constants of the now numerous α-GalCer analogs to their respective Th1/Th2 cytokine ratios could help interrelate these two factors.
Moreover, the natural role of NKT cells and how they regulate Th1/Th2 balance has not been clearly established. More information is needed relating the structure of CD1d-presented glycolipids to NKT cell activation and regulation of immune responses downstream. The systematic modification of α-GalCer to alter its trafficking and/or physical properties may lead to new therapeutically useful ligands. Identification of additional natural NKT cell antigens would give insight into the biological role NKT cells play in immune regulation and offer new scaffolds upon which to design better agonists. Ultimately, the advent of glycolipid-based immunomodulation is dependent upon more fully understanding NKT cell biology.
Figure 1. CD1 complexes.
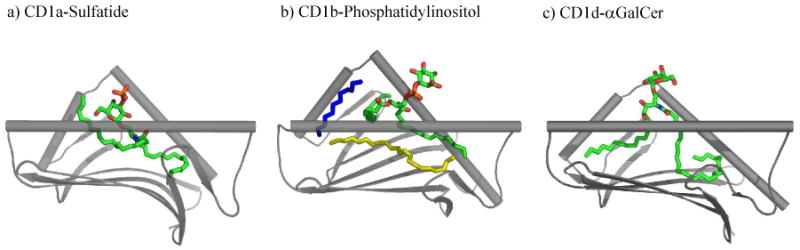
Crystal structures of CD1a, CD1b and CD1d bound to their respective ligands. The α3 and β2m subunit domains have been omitted for clarity.
Figure 2. The mouse CD1d structure.
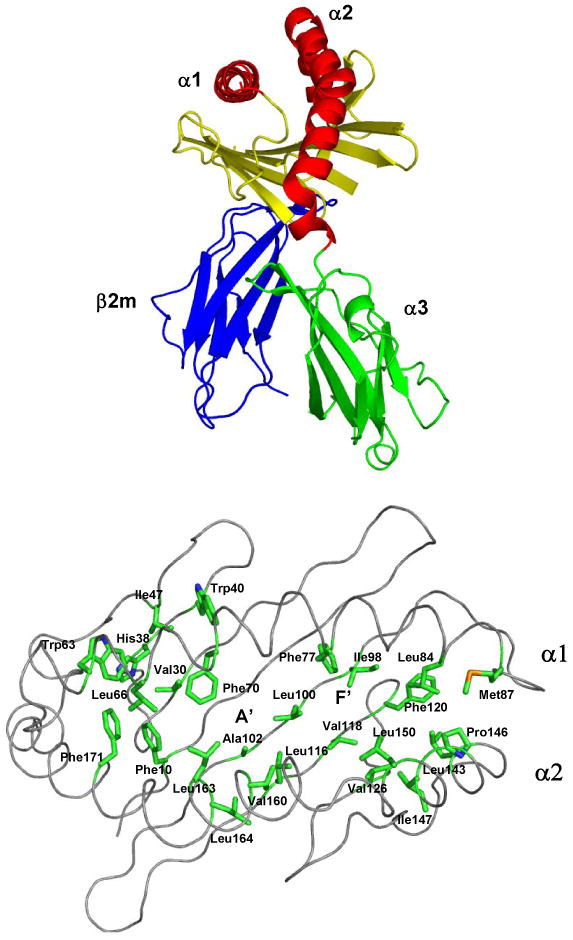
A) A ribbon diagram of the mCD1d structure is shown. Each domain is labeled. The anti-parallel helices (red) and β-sheet (yellow) of the α1 and α2 domains form a binding groove that accommodates the long hydrocarbon tails of (glyco)lipid antigens. B) mCD1d binding pocket is lined with hydrophobic residues. A view looking down into the binding groove. The side chains (green) of hydrophobic residues are labeled along with the location of the A′ and F′ pockets.
Figure 3. Selected CD1 antigens.
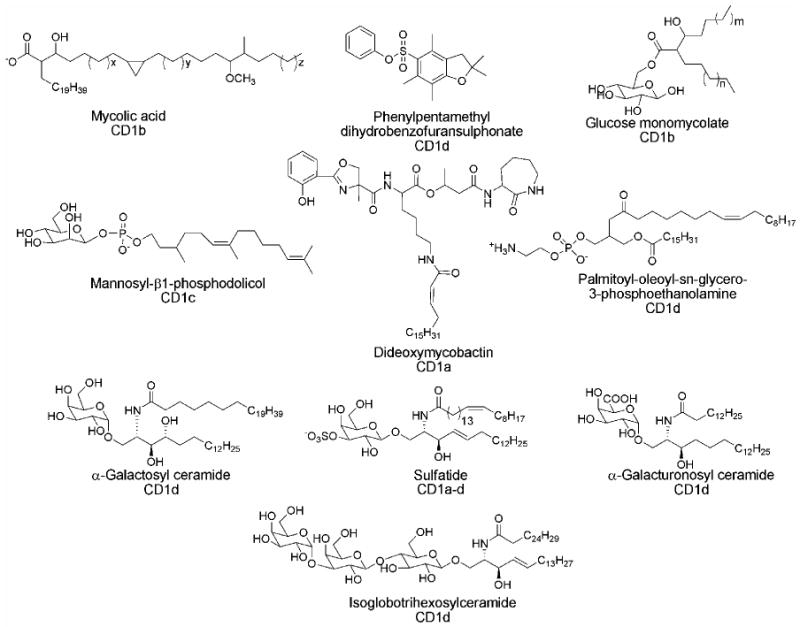
The majority of antigenic ligands identified are bound to CD1 through hydrophobic interactions allowing for presentation of a polar head group to an incoming TCR.
Figure 4. Structures of agelasphines and α-GalCer.
α-galactosyl ceramide is a chemically optimized variant of the numerous agelasphins found naturally.
Figure 5. α-GalCer analogs.
Numerous analogs, including changes to the anomeric oxygen, lipid tail length, and degree of unsaturation have been examined.
Figure 6. Modification of galactose head group.
a) Structures of galactose head group analogs of α-GalCer. b) IL-2 secretion by murine 1.2 hybridoma when stimulated by indicated glycolipids (μg/well) loaded on CD1d-coated plates. CD1d molecules (10 μg/mL in PBS) were coated in a 96-well plate by incubation for 1h at 37°C. IL-2 release was measured after 16h of culture in a sandwich ELISA.
Figure 8. Modification of fatty acyl chain of α-GalCer.
a) Structure of aromatic fatty acyl chain analogs. b) IFN-γ and IL-4 secretion by human NKT cell line when stimulated by the 10 ng/mL of indicated glycolipids. IFN-γ and IL-4 release was measured after 16 h of culture. Results are expressed as relative activities as mean of duplicate assays ± SD. Representative data from one of three experiments are shown.
Acknowledgments
The authors thank Dr. Yuki Kinjo for preparing figure 7b and 7c.
Figure 7. Modification of phytosphingosine chain.
a) Structures of GalGSL, GalAGSL, serine analogs and BbGL-II compounds. b) IL-2 secretion by murine 1.2 hybridoma when stimulated by Sphingomonas GSLs (ng/well) loaded on CD1d-coated plates. CD1d molecules (10 μg/mL in PBS) were coated in a 96-well plate by incubation for 1h at 37°C. IL-2 release was measured after 16h of culture in a sandwich ELISA. c) IL-2 secretion by murine hybridoma 2C12 when stimulated by BbGL-II analogs and GalAGSL (ng/well) loaded on CD1d-coated plates. Release of IL-2 was measured by ELISA of the supernatant after 16h of culture. BbGL-IIa: (R1=Palmitoyl, R2=Oleyl); b: (R1=Palmitoyl, R2=Linoleyl); c: (R1=Oleyl, R2=Palmitoyl); d: (R1=Oleyl, R2= Linoleyl); e: (R1=Linoleyl, R2= Palmitoyl); f: (R1= Linoleyl, R2=Oleyl); g: (R1=Palmitoyl, R2=Stearyl); h: (R1=Palmitoyl, R2= Palmitoyl).
Biography
Douglass Wu received his Bachelor's degree in chemistry from Cornell University in 2001. He then joined the laboratory of Dr. Chi-Huey Wong at The Scripps Research Institute, La Jolla, California working on the synthesis of glycolipids for CD1d-mediated NKT-cell activation. Doug received his Ph.D. in 2006 and is currently with Optimer Pharmaceuticals, San Diego, California as a Senior Research Scientist.
Masakazu Fujio received his B.S. in 1993 followed by M.S. in 1995 from Kyushu University, Fukuoka, Japan. Then he joined Yoshitomi Pharmaceutical Industries in 1995. In 2002, he received his Ph.D. in Pharmaceutical Sciences from Kyushu University, Fukuoka, Japan. He spent two years as a visiting investigator at The Scripps Research Institute with Dr. Chi-Huey Wong from 2004 to 2006. Currently he is a medicinal chemist at Mitsubishi Pharmaceutical Corporation, Yokohama, Japan.
Chi-Huey Wong received his B.S. and M.S. degrees from National Taiwan University, and Ph.D. in Chemistry (with George M. Whitesides) from Massachusetts Institute of Technology in 1982. He then moved with Professor Whitesides to Harvard University as a postdoctoral fellow for another year. He started his independent career as Assistant Professor of Chemistry at Texas A&M University in 1983, became Associate Professor in 1986 and Professor in 1987. He was Professor and Ernest W. Hahn Chair in Chemistry at the Scripps Research Institute (1989-2006) and Director of the Genomics Research Center at Academia Sinica, Taipei (2003-2006). Since October 2006, he has been Professor of Chemistry at The Scripps Research Institute and President of Academia Sinica.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22(1):817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17(1):297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem Soc Rev. 2007;35(9):771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]
- 4.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19(2):285–92. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 5.Porcelli S. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 6.Zajonc DM, Elsliger MA, Teyton L, Wilson IA. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat Immunol. 2003;4(8):808–815. doi: 10.1038/ni948. [DOI] [PubMed] [Google Scholar]
- 7.Zajonc DM, Crispin MDM, Bowden TA, Young DC, Cheng TY, Hu JD, Costello CE, Rudd PM, Dwek RA, Miller MJ, Brenner MB, Moody DB, Wilson IA. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22(2):209–19. doi: 10.1016/j.immuni.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Batuwangala T, Shepherd D, Gadola SD, Gibson KJC, Zaccai NR, Fersht AR, Besra GS, Cerundolo V, Jones EY. The crystal structure of human CD1b with a bound bacterial glycolipid. J Immunol. 2004;172(4):2382–2388. doi: 10.4049/jimmunol.172.4.2382. [DOI] [PubMed] [Google Scholar]
- 9.Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol. 2002;3(8):721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- 10.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6(8):819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 11.Zeng ZH, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277(5324):339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 12.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202(11):1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zajonc DM, Cantu C, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6(8):810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giabbai B, Sidobre S, Crispin MDM, Sanchez-Ruiz Y, Bachi A, Kronenberg M, Wilson IA, Degano M. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: a molecular basis for NKT cell activation. J Immunol. 2005;175(2):977–984. doi: 10.4049/jimmunol.175.2.977. [DOI] [PubMed] [Google Scholar]
- 15.Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural Characterization of Mycobacterial Phosphatidylinositol Mannoside Binding to Mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- 16.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 17.De Libero G, Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol. 2005;5:485–496. doi: 10.1038/nri1631. [DOI] [PubMed] [Google Scholar]
- 18.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38(12):2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 19.Natori T, Koezuka Y, Higa T. Agelasphins, novel α-galactosylceramides from the marine sponge Agelas mauritianus. Tetrahedron Lett. 1993;34(35):5591–5592. [Google Scholar]
- 20.Natori T, Morita M, Akimoto K, Koezuka Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritianus. Tetrahedron. 1994;50(9):2771–2784. [Google Scholar]
- 21.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998;95(10):5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, Durrant S, Juji T. Human invariant Vα24+ natural killer T cells activated by α-galactosylceramide (KRN7000) have cytotoxic anti tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99(2):229–234. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195(5):617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97(10):5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skold M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun. 2003;71(10):5447–5455. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skold M, Behar SM. The role of group 1 and group 2 CD1-restricted T cells in microbial immunity. Microbes Infect. 2005;7(3):544–551. doi: 10.1016/j.micinf.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Kaer LV. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5(1):31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 29.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, Schmidt RR, Lunt SJ, Williams KJ, Stratford IJ, Harris AL, Cerundolo V. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Paul W. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN- gamma upon activation by anti-CD3 or CD1. J Immunol. 1997;159(5):2240–2249. [PubMed] [Google Scholar]
- 32.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163(9):4647–4650. [PubMed] [Google Scholar]
- 34.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165(8):4305–4311. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 35.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30(4):985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99(4):1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa Y, Godfrey DI, Smyth MJ. α-Galactosylceramide: potential immunomodulatory activity and future application. Cur Med Chem. 2004;11:241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 38.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82(3):315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 40.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18(6):263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 41.Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24(3):511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 42.Muhammad Ali Tahir S, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-γ production by Iinvariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 43.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3(3):211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 44.Giaccone G, Punt CJA, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BME, Scheper RJ, van der Vliet HJJ, van den Eertwegh AJM, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A Phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- 45.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, Teyton L, Bendelac A, Savage PB. Effects of lipidchain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126(42):13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu KOA, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of {alpha}-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102(9):3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang G, Schmieg J, Tsuji M, Franck RW. The C-glycoside analogue of the immunostimulant α-galactosylceramide (KRN7000): synthesis and striking enhancement of activity. Angew Chem Int Ed Engl. 2004;43(29):3818–3822. doi: 10.1002/anie.200454215. [DOI] [PubMed] [Google Scholar]
- 50.Schmieg J, Yang G, Franck RW, Tsuji M. uperior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198(11):1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291(5504):664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 52.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1531–1536. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing GW, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong CH. Synthesis and human NKT cell stimulating properties of 3-O-sulfo-α/β-galactosylceramides. Bioorg Med Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, III, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312(12):34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Zhou XT, Forestier C, Goff RD, Li C, Teyton L, Bendelac A, Savage PB. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6″-amino-6″-deoxy-galactosylceramides. Org Lett. 2002;4(8):1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 56.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 57.Sidobre S, Hammond KJL, Bénazet-Sidobre L, Maltsev SD, Richardson SK, Ndonye RM, Howell AR, Sakai T, Besra GS, Porcelli SA, Kronenberg M. The T cell antigen receptor expressed by Vα14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc Natl Acad Sci USA. 2004;101(33):12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee T, Cho M, Ko SY, Youn HJ, Baek DJ, Cho WJ, Kang CY, Kim S. Synthesis and Evaluation of 1,2,3-Triazole Containing Analogues of the Immunostimulant α-GalCer. J Med Chem. 2007;50(3):585–589. doi: 10.1021/jm061243q. [DOI] [PubMed] [Google Scholar]
- 59.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franchini L, Matto P, Ronchetti F, Panza L, Barbieri L, Costantino V, Mangoni A, Cavallari M, Mori L, De Libero G. Synthesis and evaluation of human T cell stimulating activity of an alpha-sulfatide analogue. Bioorg Med Chem. 2007;15(16):5529–2236. doi: 10.1016/j.bmc.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 61.Fan GT, Pan YS, Lu KC, Cheng YP, Lin WC, Lin S, Lin CH, Wong CH, Fang JM, Lin CC. Synthesis of α-galactosyl ceramide and the related glycolipids for evaluation of their activities on mouse splenocytes. Tetrahedron. 2005;61(7):1855–1862. [Google Scholar]
- 62.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SHE, Schaible UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rauch J, Gumperz J, Robinson C, Skold M, Roy C, Young DC, Lafleur M, Moody DB, Brenner MB, Costello CE, Behar SM. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278(48):47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuji M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63(16):1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 66.Berkers CR, Ovaa H. Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends Pharmacol Sci. 2005;26(5):252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-Based Discovery of Glycolipids for CD1d-Mediated NKT Cell Activation: Tuning the Adjuvant versus Immunosuppression Activity. J Am Chem Soc. 2006;128(28):9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 68.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104(25):10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantu C, III, Benlagha K, Savage PB, Bendelac A, Teyton L. The Paradox of Immune Molecular Recognition of α-Galactosylceramide: Low Affinity, Low Specificity for CD1d, High Affinity for αβ TCRs. J Immunol. 2003;170:4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 70.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal Glycosphingolipid Recognition by NKT Cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 71.Neef A, Witzenberger R, Kampfer P. Detection of sphingomonads and in situ identification in activated sludge using 16S rRNA-targeted oligonucleotide probes. J Ind Microbiol Biotechnol. 1999;23:261–267. doi: 10.1038/sj.jim.2900768. [DOI] [PubMed] [Google Scholar]
- 72.Kawahara K, Moll H, Knirel YA, Seydel U, Zahringer U. Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem. 2000;267(6):1837–1846. doi: 10.1046/j.1432-1327.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- 73.Kawahara K, Seydel U, Matsuura M, Danbara H, Rietschel ET, Zahringer U. Chemical structure of glycosphingolipids isolated from Sphingomonas paucimobilis. FEBS Lett. 1991;292(12):107–110. doi: 10.1016/0014-5793(91)80845-t. [DOI] [PubMed] [Google Scholar]
- 74.Kawasaki S, Moriguchi R, Sekiya K, Nakai T, Ono E, Kume K, Kawahara K. The cell envelope structure of the lipopolysaccharide-lacking gram-negative bacterium Sphingomonas paucimobilis. J Bacteriol. 1994;176(2):284–290. doi: 10.1128/jb.176.2.284-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steere AC. Lyme Disease. N Engl J Med. 2001;345(2):115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 76.Orlosk iK, Hayes E, Campbell G, Dennis D. Surveillance for Lyme disease -- United States, 1992-1998. Mor Mortal Wkly Rep CDC Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 77.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting Edge: CD1d Deficiency Impairs Murine Host Defense Against the Spirochete, Borrelia burgdorferi. J Immunol. 2000;165(9):4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 78.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174(9):5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100(13):7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, Postle AD, De Libero G, Porcelli SA, Spinozzi F. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005;202(2):295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takayama K, Rothenberg R, Barbour A. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55(9):2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]