Abstract
The subthalamic nucleus (STN) plays a pivotal role in normal and abnormal motor function. We used patch pipettes to study effects of 5-hydroxytryptamine (5HT) on synaptic currents evoked in STN neurons by focal electrical stimulation of rat brain slices. 5HT (10 μM) reduced glutamate-mediated excitatory postsynaptic currents (EPSCs) by 35 ± 4%. However, a much higher concentration of 5HT (100 μM) was required to inhibit GABA-mediated inhibitory postsynaptic currents (IPSCs) to a comparable extent. Concentration-response curves showed that the 5HT IC50 for inhibition of IPSCs (20.2 μM) was more than 5-fold greater than the IC50 for inhibition of EPSCs (3.4 μM). The 5HT-induced reductions in EPSCs and IPSCs were accompanied by increases in paired-pulse ratios, indicating that 5HT acts presynaptically to inhibit synaptic transmission. The 5HT1B receptor antagonist NAS-181 significantly antagonized 5HT-induced inhibitions of EPSCs and IPSCs. These studies show that 5HT inhibits synaptic transmission in the STN by activating presynaptic 5HT1B receptors.
Keywords: presynaptic inhibition, EPSC, IPSC, 5-hydroxytryptamine, serotonin, brain slice, patch clamp
The subthalamic nucleus (STN) is a basal ganglia structure comprised of glutamate-containing neurons that project to the two output nuclei of the basal ganglia: substantia nigra pars reticulata and globus pallidus interna (Albin et al., 1995). By way of its glutamatergic projections, the STN plays an important role in normal and pathological motor behaviors. For example, excessive burst firing of STN neurons is thought to contribute to the akinesia of Parkinson’s disease (Benedetti et al., 2004; Wichmann et al., 1994). The importance of the STN in the control of movement is also evidenced by the effective therapeutic effects of surgical lesions in an animal model of Parkinson’s disease (Bergman et al., 1990) and high frequency STN stimulation in Parkinson’s disease patients (Krack et al., 1998).
Among the neurotransmitter systems that regulate STN neuronal activity, the actions of 5-hydroxytryptamine (5HT) have yet to be fully investigated. The STN receives a dense 5HT-containing innervation that arises from the dorsal raphe nucleus (Canteras et al., 1990; Mori et al., 1985). Furthermore, the STN has been shown to express several 5HT receptor subtypes, including 5HT1A, 5HT1B, 5HT2C, and 5HT4 (Pompeiano et al., 1994; Wright et al., 1995; Bruinvels et al., 1993; Compan et al., 1996). Recently, our laboratory (Shen et al., 2007) and others (Stanford et al., 2005; Xiang et al., 2005) have reported electrophysiological effects of 5HT on membrane properties of STN neurons in slices of rat brain. However, no studies have been done thus far that examine presynaptic actions of 5HT that may regulate transmitter release in the STN. Therefore, the present study was undertaken to characterize physiological effects of 5HT on excitatory and inhibitory synaptic transmission in the rat STN.
EXPERIMENTAL PROCEDURES
Tissue preparation
Horizontal slices of midbrain and diencephalon (300 μm) were prepared from male Sprague-Dawley rats (120 - 180g; Harlan, Indianapolis, IN, USA) as described in detail previously (Shen and Johnson, 2000). Rats were euthanized under isoflurane anesthesia in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The brain was rapidly removed and slices were cut in cold physiological saline with a vibratome. A slice containing the STN was then placed on a supporting net and submerged in a continuously flowing solution (2 ml/min) of the following composition (in mM): NaCl, 126; KCl, 2.5; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 19; glucose, 11, gassed with 95% O2 and 5% CO2 (pH 7.4) at 36°C. Using a dissection microscope for visual guidance, the STN was located as grey matter approximately 2.7 mm lateral to the midline and 2 mm rostral to the center of the substantia nigra pars reticulata (Paxinos and Watson, 1986).
Electrophysiological recordings
Whole-cell recordings were made with pipettes containing (in mM): cesium gluconate, 130; MgCl2, 2; CaCl2, 1; EGTA, 11; HEPES, 10; ATP, 1.5; GTP, 0.3 (pH 7.3; osmolarity, 285 - 290). We used cesium rather than potassium gluconate in order to block 5HT-induced changes in postsynaptic conductance that might otherwise interfere with measuring synaptic currents (Shen et al., 2007). Membrane currents were recorded under voltage clamp (-70 mV) and amplified with an Axopatch-1D amplifier (Molecular Devices, Foster City, CA, USA). Data were acquired using a personal computer with a Digidata analog/digital interface and analyzed using pCLAMP 9.0 software (Molecular Devices). Holding currents were recorded continuously using a MacLab analog/digital interface, Chart software (AD Instruments, Castle Hill, Australia) and a Macintosh computer. Membrane potentials have been corrected for the liquid junction potential (10 mV).
Synaptic currents
Bipolar stimulation electrodes (tip separation 300 - 500 μm) were placed in the slice 300 μm rostral to the STN. Synaptic currents were evoked by a single rectangular pulse (0.1 ms duration) of constant current every 10 sec. During paired-pulse studies, pairs of synaptic currents were evoked with an interval of 50 msec. The amplitude of evoked synaptic currents was measured from recordings that represent the average of three responses. An inhibitory postsynaptic current (IPSC) mediated by GABAA receptors was isolated pharmacologically by recording in the presence of (±)-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) and 6-cyano-7-nitro-quinoxalone (CNQX, 10 μM) in order to block ionotropic glutamate receptors. An excitatory postsynaptic current (EPSC) mediated by ionotropic glutamate receptors was recorded in the presence of bicuculline (30 μM) or picrotoxin (100 μM) to block GABAA receptors. All synaptic currents were recorded with voltage clamped at -70 mV.
Drugs
All drugs were dissolved in aqueous stock solutions with the exception of CNQX, which was dissolved in dimethyl sulfoxide. Stock solutions of drugs were diluted at least 1:1000 to the desired concentration in superfusate immediately prior to their use. Dimethyl sulfoxide, diluted 1:1000 in artificial spinal fluid, had no effect on either holding current or synaptic currents. Approximately 30 sec were required for the drug solution to enter the recording chamber; this delay was due to passage of the perfusate through a heat exchanger. Tetrodotoxin (TTX), 5-hydroxytryptamine hydrochloride (5HT), bicuculline methiodide, AP5, and CNQX were obtained from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). SB216641, pindolol, and NAS-181 were obtained from Tocris Cookson Inc. (Ellisville, MO, USA).
Data analysis
Numerical data in the text and error bars in the figures are expressed as mean ± S.E.M.. Numbers of observations (“n” values) in the text indicate numbers of neurons, as opposed to numbers of brain slices or animals. Differences were tested for statistical significance with either paired or unpaired Student’s two-tailed t tests that were run using SigmaStat software (Jandel scientific, San Rafael, CA, USA) on a personal computer. A significant difference was accepted when P < 0.05. Prior to analyzing data for significance, a Kolmogorov-Smirnov test was performed to ensure that all data were normally distributed. The KaleidaGraph curve-fitting program (Synergy Software, Reading, PA, USA) was used to fit concentration-response data to the Hill-Langmuir equation, y = ax/(x + b), where y is the percentage effect, a is percentage of maximum effect, x is the drug concentration, and b is the concentration that produces 50% of maximum inhibition (IC50). Significant differences between concentration-response data were determined using 2-way ANOVA followed by Tukey multiple comparison tests.
RESULTS
5HT inhibits evoked synaptic currents
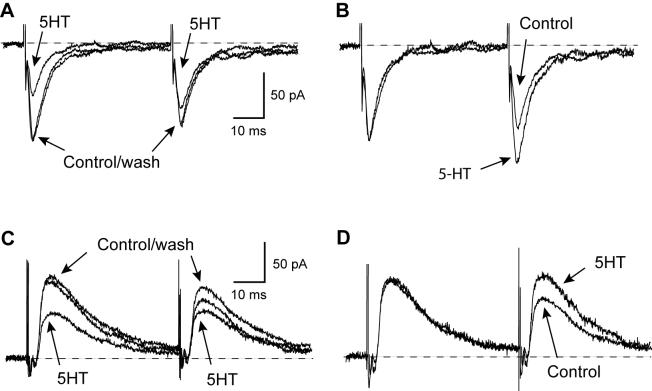
Bipolar stimulation electrodes placed in the rostral region of the slice were used to evoke synaptic currents at a holding potential of -70 mV. When recording in the presence of bicuculline (30 μM) or picrotoxin (100 μM), focal stimulation evoked an excitatory postsynaptic current (EPSC), as shown in Fig. 1A. This inward current was mediated by ionotropic glutamate receptors because it was completely blocked by the combination of AP5 (50 μM) and CNQX (10 μM; data not shown). Also seen in Fig. 1A, 5HT (10 μM) reversibly reduced the amplitude of the evoked EPSC. The summary graph in Fig. 1B shows that 10 μM 5HT reduced the amplitude of EPSCs by 35 ± 4% (n = 13). Effects of 5HT began within 1 min of perfusion, reached its peak within 5 min, and washed out completely within 10 - 15 min.
Fig. 1.

5HT inhibits evoked glutamate EPSCs and GABAA IPSCs in STN neurons. (A) Current traces of EPSCs before, during and after application of 5HT (10 μM). (B) Summary graph showing the time course for the effect of 5HT (10 μM) on EPSCs. Each data point is the averaged response from 13 cells. (C) Current traces of IPSCs before, during and after application of 5HT (100 μM). (D) Summary graph showing the time course for the effect of 5HT (100 μM) on IPSCs. Each data point is the averaged response from 12 cells. In “B” and “D”, the amplitudes of synaptic currents were normalized for each cell based upon the mean amplitude of the first 10 synaptic currents recorded under control conditions.
When recording in the presence of AP5 (50 μM) and CNQX (10 μM), a single stimulus evoked an inhibitory postsynaptic current (IPSC), as shown in Fig. 1C. This outward current was mediated by GABAA receptors because it was completely abolished by perfusion with bicuculline (30 μM; data not shown). Also seen in Fig. 1C, 5HT (100 μM) reversibly reduced the amplitude of the evoked IPSC. However, at a concentration of 10 μM, 5HT only reduced IPSCs by 5 ± 3% (n = 12), which was significantly less than the effect of this 5HT concentration on EPSCs (P < 0.0001). In contrast, a concentration of 100 μM 5HT was required to reduce the IPSC to an extent that was comparable to the effect of 10 μM 5HT on EPSCs. Thus, 100 μM 5HT reduced the amplitude of IPSCs by 34 ± 3% (n = 19), as shown in Fig. 1D. Complete concentration-response curves for effects of 5HT on EPSCs and IPSCs are shown in Fig. 2. The 5HT IC50 for inhibition of IPSCs (20.2 μM) was more than 5-fold greater than the IC50 for inhibition of EPSCs (3.4 μM). These data show that 5HT is more potent for inhibiting EPSCs compared to IPSCs.
Fig. 2.
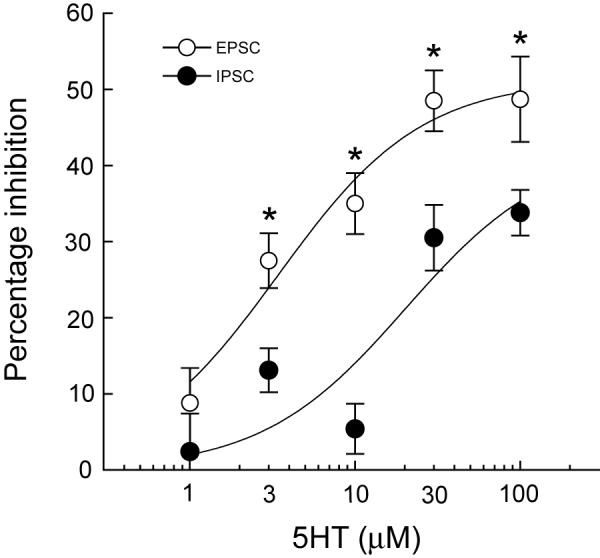
Concentration-response curves for effects of 5HT on EPSCs and IPSCs. 5HT is more potent for inhibiting EPSCs (IC50 = 3.4 μM) compared to inhibiting IPSCs (IC50 = 20.2 μM). Asterisks indicate significant differences based upon 2-way ANOVA and Tukey multiple comparison tests (P < 0.05). Each data point represents the mean ± SEM of 3 - 19 cells.
Paired-pulse studies
In order to examine site of action, we measured effects of 5HT on EPSCs and IPSCs that were evoked by pairs of stimuli separated by an interval of 50 msec. Under control conditions, the amplitude of the second EPSC was always somewhat smaller than that of the first, yielding a ratio of the second EPSC divided by the first of 0.95 ± 0.05 (n = 15). However, 5HT (10 μM) caused a significant increase in the EPSC paired-pulse ratio, to 1.12 ± 0.06 (P < 0.001, paired t test) in the same neurons (Figs. 3A and B). We found a similar result when examining effects of 5HT on paired IPSCs, as seen in Figs. 3C and D. Under control conditions, the IPSC paired-pulse ratio was 0.72 ± 0.02 (n = 12). However, 5HT (100 μM) significantly increased the IPSC paired-pulse ratio to 0.85 ± 0.03 (P = 0.0001, paired t test) in the same neurons. Because a change in the paired-pulse ratio is consistent with a presynaptic mechanism (Dunwiddie and Haas, 1985; Davies and Collingridge, 1996), these results suggest that 5HT inhibits EPSCs and IPSCs by acting presynaptically to inhibit transmitter release.
Fig. 3.
5HT increases the paired-pulse ratio of EPSCs and IPSCs. (A) Superimposed EPSCs evoked by pairs of stimuli in the absence (control and wash) and presence of 5HT (10 μM). (B) Current traces are the same as those in “A” except that the amplitude of the first EPSC recorded in 5HT has been normalized to match the amplitude of the first control EPSC. (C) Superimposed IPSCs evoked by pairs of stimuli in the absence (control and wash) and presence of 5HT (100 μM). (D) Current traces are the same as those in “C” except that the amplitude of the first IPSC recorded in 5HT has been normalized to match the amplitude of the first control IPSC.
5HT receptor pharmacology
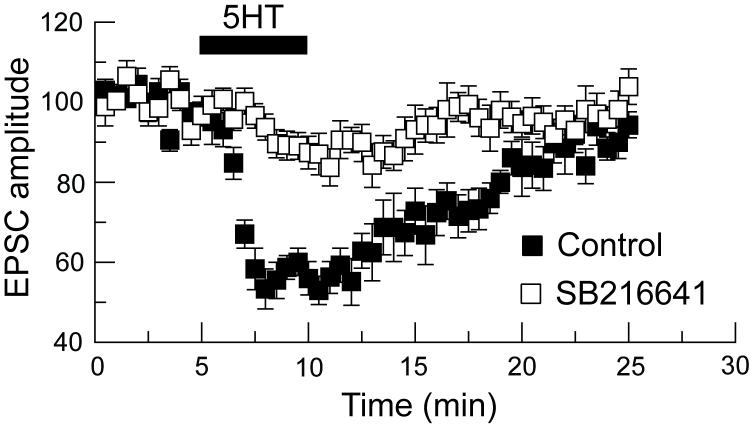
Having found that 5HT was more potent for inhibiting EPSCs compared IPSCs, we proceeded to identify the 5HT receptor subtype that mediate these actions. We first tested the effect of pindolol, a mixed antagonist at 5HT1A/1B receptors, on evoked EPSCs. In the presence of pindolol (10 μM), 5HT at 10 μM reduced the amplitude of EPSCs by 14 ± 2 % (n = 8), which was significantly smaller than the magnitude of 5HT-induced inhibition of EPSCs recorded under control conditions (P < 0.0001). Figure 4 shows the effect of SB216641, a selective 5HT1B receptor antagonist (Price et al., 1997). In the presence of SB21641 (10 μM), 5HT (30 μM) reduced EPSCs by only 13 ± 4%, which was significantly less than the 44 ± 4% reduction in EPSC amplitude produced by 5HT alone in the same eight neurons (P = 0.0001). In the presence of NAS-181 (10 μM), another selective 5HT1B antagonist (Stenfors et al., 2000), 5HT (3 μM) only reduced EPSCs by 1 ± 1% (n = 6). Furthermore, this concentration of NAS-181 completely blocked the ability of 5HT (30 μM) to inhibit IPSCs, and in fact resulted in a small 5HT-dependent increase (9 ± 4%) in the amplitude of IPSCs (n = 8). These data suggest that 5HT1B receptors mediate the ability of 5HT to inhibit both IPSCs and EPSCs in STN neurons.
Fig. 4.
5HT-induced inhibition of EPSCs is mediated by 5HT1B receptors. The reduction EPSC amplitude by 5HT (30 μM) is antagonized by SB216641 (10 μM), a selective 5HT1B receptor antagonist. EPSC amplitudes are expressed as a percentage of control. Data points represent the paired responses of 8 cells.
None of the 5HT1B antagonists affected EPSC or IPSC amplitude when applied alone. Furthermore, the 5HT uptake inhibitor fluoxetine (10 μM) failed to alter the amplitude of EPSCs (n = 4) and IPSCs (n = 2). These results suggest that levels of endogenous 5HT are too low to exert a tonic effect on synaptic transmission in the brain slice preparation.
DISCUSSION
The main finding of our study is that 5HT reduces the amplitude of both glutamatergic EPSCs and GABAergic IPSCs in the STN. 5HT-induced inhibition of synaptic currents is associated with a significant increase in the paired-pulse ratios of evoked EPSCs and IPSCs, suggesting that 5HT acts presynaptically to suppress both glutamate and GABA release. However, 5HT is more potent for reducing EPSCs compared with IPSCs. This inhibitory effect is mediated via activation of 5HT1B receptors because selective 5HT1B antagonists blocked 5HT-induced inhibition of EPSCs and IPSCs. Taken together, our results suggest that 5HT causes presynaptic inhibition of transmission by activating 5HT1B receptors.
Although 5HT1B receptors are widely expressed in the brain, this receptor subtype and its human correlate, the 5HT1D receptor, are expressed most densely in globus pallidus, substantia nigra, striatum, and deep cerebellar nuclei (Varnäs et al., 2001; Sari et al., 1999). However, significant amounts of 5HT1B receptor are also expressed in the STN (Bruinvels et al., 1993). Mismatches between mRNA and binding sites reveal that 5HT1B and 5HT1D receptors are expressed predominantly on nerve terminals (Boschert et al., 1994; Maroteaux et al., 1992). Consistent with its presynaptic location, 5HT1B receptor activation has been shown to cause presynaptic inhibition of glutamate-mediated EPSCs in many brain regions such as the hypoglossal nucleus (Singer et al., 1996) and nucleus accumbens (Muramatsu et al., 1998), and to inhibit GABA-mediated transmission in structures such as the dorsolateral septum (Matsuoka et al., 2004) and substantia nigra (Johnson et al., 1992; Stanford and Lacey, 1996). Results of the present study clearly show that 5HT1B receptor activation also causes presynaptic inhibition of transmission in the STN.
One may note that concentrations of 5HT used in the present series of experiments are relatively high compared to what would likely be present during synaptic transmission in vivo. Relatively high concentrations of 5HT may be needed in brain slice experiments due to problems with diffusion into the brain slice, the influence of endogenous 5HT metabolism, or active 5HT reuptake. When taking these factors into consideration, it is interesting to speculate on the basis for our finding that 5HT is much more potent for inhibiting EPSCs compared to IPSCs. It is possible that differential potencies of 5HT may be due to differences in 5HT metabolism or reuptake at glutamate and GABA synapses. Alternatively, 5HT1B receptors located on GABA nerve terminals may be less tightly coupled to second messenger systems than are receptors at glutamate nerve terminals. Future studies are needed to evaluate these possible explanations for the differential potency of 5HT on excitatory and inhibitory synaptic transmission.
Although our data show that 5HT is more potent for reducing glutamate-mediated neurotransmission compared to inhibitory transmission, it is difficult to know if this in vitro result would have functional significance in vivo. According to a widely used model of basal ganglia function, a reduction in excitatory glutamate input to the STN would be expected to improve symptoms of Parkinson’s disease (DeLong, 1990). Indeed, injection of 5HT1B agonists systemically (Oberlander et al., 1987) or into the STN (Martinez-Price and Geyer, 2002) has been reported to increase locomotion in rats. However, 5HT1B receptor stimulation has also been reported to reduce levodopa-induced dyskinesia in a rat model of Parkinson’s disease (Carta et al., 2007), which is not what one would predict based upon inhibition of excitatory input to the STN. Moreover, 5HT1B agonists have also been reported to interfere with the benefit of levodopa in a marmoset model of Parkinson’s disease (Jackson et al., 2004). Thus, it appears that further studies will be needed to clarify the functional consequence of 5HT1B receptor stimulation on motor function.
Acknowledgements
This study was supported by USPHS grant NS38175 and by the Portland Veterans Affairs Parkinson’s Disease Research, Education, and Clinical Center.
Abbreviations
- AP5
(±)-2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitro-quinoxalone
- EPSC
excitatory postsynaptic current
- GABA
γ-aminobutyric acid
- 5HT
5-hydroxytryptamine
- IC50
inhibitory concentration 50%
- IPSC
inhibitory postsynaptic current
- STN
subthalamic nucleus
- TTX
tetrodotoxin
Footnotes
Section Editor: Dr. Yoland Smith (neuropharmacology)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albin RL, Young AB, Penney JB., Jr The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nature Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neurosci. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA. Afferent connections of the subthalamic nucleus: a combined regrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1990;513:43–59. doi: 10.1016/0006-8993(90)91087-w. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A. Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci. 1996;8:2591–2598. doi: 10.1111/j.1460-9568.1996.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. J Physiol (Lond) 1996;496:451–470. doi: 10.1113/jphysiol.1996.sp021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trend Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: Evidence for a presynaptic site of action. J Physiol (Lond) 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ, Al-Barghouthy G, Pearce RKB, Smith L, Hagan JJ, Jenner P. Effect of 5-HT1B/D receptor agonist and antagonist administration on motor function in haloperidol and MPTP-treated common marmosets. Pharmacol Biochem Behav. 2004;79:391–400. doi: 10.1016/j.pbb.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Mercuri NB, North RA. 5-Hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons, via presynaptic inhibition. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Benazzouz A, Pollak P, Limousin P, Piallat B, Hoffmann D, Xie J, Benabid A-L. Treatment of tremor in Parkinson’s disease by subthalamic nucleus stimulation. Movement Disord. 1998;13:907–914. doi: 10.1002/mds.870130608. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5-HT1B serotonin receptor: Cloning, functional expression and localization in motor control centers. Proc Natl Acad Sci (USA) 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Price DL, Geyer MA. Subthalamic 5-HT1A and 5-HT1B receptor modulation of RU 24969-induced behavioral profile in rats. Pharmacol Biochem Behav. 2002;71:569–580. doi: 10.1016/s0091-3057(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci Res. 2004;48:229–238. doi: 10.1016/j.neures.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mori S, Takino T, Yamada H, Sano Y. Immunohistochemical demonstration of serotonin nerve fibers in the subthalamic nucleus of the rat, cat and monkey. Neurosci Lett. 1985;62:305–309. doi: 10.1016/0304-3940(85)90566-x. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MDS, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Oberlander C, Demassey Y, Verdu A, Van de Velde D, Bardelay C. Tolerance to the serotonin 5-HT1 agonist RU 24969 and effects on dopaminergic behaviour. Eur J Pharmacol. 1987;139:205–214. doi: 10.1016/0014-2999(87)90253-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. SB-216641 and BRL-15572 - compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel M-C, Brisorgueil M-J, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neurosci. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol (Lond) 2000;525:331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-Z, Kozell LB, Johnson SW. Multiple conductances are modulated by 5-hydroxytryptamine receptor subtypes in rat subthalamic nucleus neurons. Neurosci. 2007;148:996–1003. doi: 10.1016/j.neuroscience.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT2C, 5-HT4 and 5-HT1A receptors. Neuropharmacol. 2005;49:1228–1234. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors C, Yu H, Ross SB. Enhanced 5-HT metabolism and synthesis rate by the new selective r5-HT1B receptor antagonist, NAS-181 in the rat brain. Neuropharmacol. 2000;39:553–560. doi: 10.1016/s0028-3908(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT1B and 5-HT1D receptors in the post mortem human brain using [3-H]GR 125743. Brain Res. 2001;915:47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin-1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Wang L, Kitai ST. Modulation of spontaneous firing in rat subthalamic neurons by 5-HT receptor subtypes. J Neurophysiol. 2005;93:1145–1157. doi: 10.1152/jn.00561.2004. [DOI] [PubMed] [Google Scholar]