Abstract
Ethnopharmacological relevance
Cocoa bean preparations were first used by the ancient Maya and Aztec civilizations of South America to treat a variety of medical ailments involving the cardiovascular, gastrointestinal, and nervous systems. Diets rich in foods containing abundant polyphenols, as found in cocoa, underlie the protective effects reported in chronic inflammatory diseases. Release of calcitonin gene-related peptide (CGRP) from trigeminal nerves promotes inflammation in peripheral tissues and nociception.
Aim of the study
To determine whether a methanol extract of Theobroma cacao L. (Sterculiaceae) beans enriched for polyphenols could inhibit CGRP expression, both an in vitro and an in vivo approach was taken.
Results
Treatment of rat trigeminal ganglia cultures with depolarizing stimuli caused a significant increase in CGRP release that was repressed by pretreatment with Theobroma cacao extract. Pretreatment with Theobroma cacao was also shown to block the KCl- and capsaicin-stimulated increases in intracellular calcium. Next, the effects of Theobroma cacao on CGRP levels were determined using an in vivo model of temporomandibular joint (TMJ) inflammation. Capsaicin injection into the TMJ capsule caused an ipsilateral decrease in CGRP levels. Theobroma cacao extract injected into the TMJ capsule 24 h prior to capsaicin treatment repressed the stimulatory effects of capsaicin.
Conclusions
Our results demonstrate that Theobroma cacao extract can repress stimulated CGRP release by a mechanism that likely involves blockage of calcium channel activity. Furthermore, our findings suggest that the beneficial effects of diets rich in cocoa may include suppression of sensory trigeminal nerve activation.
Keywords: Calcitonin gene-related peptide, Trigeminal, Inflammation, Temporomandibular joint, Polyphenols, Vanilloid receptor
1. Introduction
The trigeminal nerve is the primary sensory nerve of the face and head that connects the peripheral tissues and the central nervous system (O'Conner and Van der Kooy, 1986). Trigeminal nerves are responsible for the perception of touch, temperature, and nociception, and transmission of this information to the cell body in the trigeminal ganglion and trigeminal nucleus caudalis in the brain. Activation of trigeminal nerves, and the subsequent peripheral and central release of neuropeptides, is involved in the underlying pathology of chronic painful inflammatory conditions such as migraine (Buzzi, 2001; Pietrobon and Striessnig, 2003) and temporomandibular joint disorders (TMJD) (Kopp, 2001).
Calcitonin gene-related peptide (CGRP) is an abundant polypeptide widely expressed in both central and peripheral neurons, including trigeminal ganglion neurons. CGRP is known to promote inflammation and nociception (van Rossum et al., 1997). Elevated levels of CGRP have been reported to correlate with pain severity during migraine attacks (Buzzi and Moskowitz, 1992; Edvinsson and Goadsby, 1994) and TMJD (Holmlund et al., 1991; Appelgren et al., 1993). TMJD is a clinical term used to describe the number of related disorders affecting the jaw joint, masticatory muscle and associated structures (Schiffman et al., 1990). About 60–70% of the general population has at least one symptom of TMJD, defined as pain or limited joint opening (Dimitroulis, 1998). Currently, there are no FDA approved pharmacological treatments for TMJD.
Cocoa bean preparations were first used by the ancient Maya and Aztec civilizations of South America to treat a variety of medical ailments involving the cardiovascular, gastrointestinal, and nervous systems (Dillinger et al., 2000). In addition, cocoa was documented to be beneficial in the treatment of general and abdominal pain, toothache, and pain associated with joints (rheumatism). Thus, a therapeutic benefit of cocoa as a medical treatment appears to involve decreasing sensory nerve activation. Recently, phenolic phytochemicals have received growing scientific interest in an effort to explain some of the health benefits associated with fruit- and vegetable-rich diets. While polyphenols occur abundantly in teas, grapes, and berries, higher levels of total phenolics are reported in cocoa beans (Sanbongi et al., 1998; Lee et al., 2003). Polyphenols display a wide range of biochemical and pharmacological activities that strongly suggest a role in promoting health and preventing disease (Yoon and Baek, 2005). Recent efforts to understand the biological effects of polyphenols have primarily focused on modulation of immune and endothelial cell function (Middleton and Kandaswami, 1992; Stoclet et al., 2004; Williams et al., 2004). However, regulation of sensory neurons that mediate neurogenic inflammation and pain transmission by cocoa polyphenols has not been directly investigated. Thus, the goal of this study was to investigate the effect of a Theobroma cacao L. (Sterculiaceae) bean extract enriched for polyphenols on CGRP expression using trigeminal ganglia cultures and an in vivo model of TMJ inflammation.
2. Materials and methods
2.1. Preparation of Theobroma cacao bean extract
Organic raw Ecuadorian cacao beans, Theobroma cacao, were obtained from Nature's First Law (San Diego, CA) and stored at 4 °C. Beans (4 g), outer skin intact, were roasted for 10 min at 177 °C, finely crushed after cooling, homogenized 5 min in 30 ml of methanol at room temperature, and then mixed vigorously for 1 min. Following centrifugation at 3000 × g for 5 min, 10 ml of the supernatant was dispensed to a 100 mm Petri dish and concentrated by evaporation at room temperature to 1 ml. To remove remaining particulate matter, the concentrated liquid was centrifuged at 3000 × g for 5 min and the supernatant was transferred to a new tube. The concentrated methanol extract was stored at −20 °C and will be referred to as Theobroma cacao extract. The dry weight yield was 17 μg/g (w/w) of cocoa beans. The average total phenolic content of the extract (25.5 mg/ml; n = 3) expressed as gallic acid equivalents (GAEs) was determined using the Folin–Ciocalteu method (Singleton and Rossi, 1965; Lee et al., 2003). Briefly, 0.25 N Folin–Ciocalteu reagent was added to the diluted sample or gallic acid, the samples mixed, and incubated for 3 min at room temperature. Sodium carbonate (1 N) was added to each sample and after mixing the samples were incubated for 7 min. After addition of water, the samples were again mixed and then incubated for 2 h prior to measurement of absorbance at 760 nm.
2.2. Animals
Neonatal Sprague Dawley rats (Charles River Laboratories Inc., Wilmington, MA) were used in all in vitro experiments while adult female post-estrous Sprague Dawley rats were utilized for the in vivo studies. Animals were housed in clean plastic cages on a 12-h light/dark cycle with unrestricted access to food and water. All animal care and procedures were conducted in accordance with institutional and National Institutes of Health guidelines.
2.3. Primary cultures of trigeminal neurons
Primary cultures of trigeminal ganglia were established based on our previously published protocols (Durham and Russo, 1999; Bowen et al., 2006). Briefly, cells from ganglia isolated from 3–5-day-old rats were resuspended in L15 medium containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), 50 mM glucose, 250 μM ascorbic acid, 8 μM glutathione, 2mM glutamine, and 10 ng/ml mouse 2.5 S nerve growth factor (Alomone Laboratories, Jerusalem, Israel). Penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (2.5 μg/ml, Sigma) were also added to the supplemented L15 media, which will be referred to as L15 complete medium. For secretion studies, dissociated cells from the equivalent of 24 ganglia were plated on 24-well poly-d-lysine (PDL)-coated tissue culture plates (BD Biosciences, Bedford, MA) and incubated at 37 °C at ambient CO2. For the calcium measurements, trigeminal cultures were enriched in neuronal cells (>90%) by density-gradient centrifugation as previously described (Bowen et al., 2006). Briefly, after dissociation, the cell pellet was resuspended in 3 ml plating medium containing 1 mg/ml bovine serum albumin (BSA). Cells were carefully layered onto 6 ml of plating medium containing 10 mg/ml BSA in a 15 ml conical tube and centrifuged at 100 × g for 3 min. The pellet was resuspended in L15 complete medium and plated at a density of 1.5–2 ganglia per well in a 24-well plate, which corresponds to 50,000–65,000 cells per well.
2.4. CGRP secretion assay
Trigeminal ganglion primary cultures maintained for 48 h in L15 complete medium were incubated in 250 μl per well of HEPES-buffered saline (HBS; 22.5 mM HEPES, 135 mM NaCl, 3.5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 3.3 mM glucose, and 0.1% BSA, pH 7.4). The amount of CGRP released into the HBS medium was determined using a CGRP-specific radioimmunoassay (Bachem/Peninsula Laboratories Inc., San Carlos, CA). In each experiment, the basal (unstimulated) amount of CGRP secreted into the medium in 1 h was determined and was used to normalize for differences between the wells.
The effects of 90 mM KCl or 2 μM 8-methyl-N-vanillyl-6-nonenamide (capsaicin; Sigma, St. Louis, MO) on CGRP secretion was determined by incubating the cells for 1 h in HBS and the amount of CGRP measured. For inhibition studies, cultures were pretreated for 30 min with 1.7, 8.5, 17, or 34 μg/g of extract dry weight, which corresponds to 10, 50, 100, or 200 μg/ml GAE of Theobroma cacao extract diluted in HBS. Following removal of the HBS medium, fresh HBS medium was added to the cultures with or without inhibitory compounds. Cultures were left unstimulated (control) or were stimulated with KCl or capsaicin for an additional 60 min and the amount of secreted CGRP measured. As another control, cultures were treated with equivalent amounts of the appropriate vehicle (dimethylsulfoxide (DMSO) for capsaicin or methanol for Theobroma cacao extract) for 60 min and the amount of secreted CGRP determined. To test for possible cytotoxic effects of the various treatments, neuronal viability was assessed in trigeminal cultures 24 h after treatment using the CellTiter 96 assay following manufacturer's instructions (Promega, Madison, WI). The absorbency at 490 nm was measured 2 h following addition of the CellTiter 96 Aqueous One Solution. Each experimental condition was performed in duplicate and repeated in at least three independent experiments. The data were reported as mean ± S.E.M. Statistical analysis was performed using the Mann–Whitney U-test. Results were considered significant when p was less than 0.05.
2.5. Immunocytochemistry
Dissociated trigeminal ganglion cells were plated directly on PDL (MW 30,000–70,000; Sigma)-coated 13-mm plastic coverslips equivalent to one ganglion per three coverslips in L15 complete medium. All cells were incubated for 48 h at 37 °C then rinsed briefly with phosphate buffered saline (PBS) and treated with 4% paraformaldehyde for 30 min at room temperature and with 0.2% Triton X-100 in PBS for an additional 15 min to fix and permeabilize the cells. Cultures were incubated overnight at 4 °C with chicken anti-CGRP antibodies (1:500 in PBS; Neuromics, Edina, MN) or rabbit anti-TRPV1 antibodies (1:1000 in PBS; Neuromics). Immunoreactive proteins were detected following incubation with Rhodamine Red X-conjugated donkey anti-chicken or FITC-conjugated donkey anti-rabbit IgG polyclonal antibodies (1:100 in PBS, Jackson Immuno-Research Laboratories, West Grove, PA) for 1 h at room temperature. Prior to viewing, cells were mounted using Vectashield mounting media (Vector Laboratories, Burlingame, CA) containing 4′,6 diamidino-2-phenylindole (DAPI) to identify nuclei. Cells were photographed with an Olympus DP70 camera mounted on an Olympus BX41 fluorescent microscope and image collection and analysis performed using Olympus MicroSuite Five image processing software (Center Valley, PA).
2.6. Intracellular calcium measurements
For intracellular calcium imaging, trigeminal ganglion cultures enriched for neurons were plated at a density equivalent to 1.5–2 ganglia per well on PDL-coated, plastic or glass coverslips. Cells were incubated with Fura-2AM and pleuronic acid (Invitrogen Inc., Eugene, OR) at final concentrations of 5 μM, using a similar protocol as previously described (Durham and Russo, 1999, 2000). Absorbance was determined at 340 nm and 380 nm for 1 s at 20 s intervals in a Victor 3V plate reader following manufacturer's instructions (Perkin-Elmer, Wellesley, MA). Basal levels were obtained for 5 min before treatment with 90 mM KCl, 2 μM capsaicin, or 17 μg/g of extract dry weight (100 μg/ml GAE Theobroma cacao extract). After addition of stimulatory agents or Theobroma cacao, intracellular calcium levels were determined for an additional 20 min. In some wells treated with Theobroma cacao extract, cultures were subsequently treated with 90 mM KCl or 2 μM capsaicin and intracellular calcium levels measured for another 5 min. Data are reported as the ratio of the 340–380 wavelength measurements and, thus, are the averages of all the cells in each well. Each experimental condition was repeated a minimum of six times.
2.7. In vivo studies
Female post-estrous rats (200–225 g) were anesthetized by intraperitoneal injection of ketamine (96 mg/kg) and xylazine (7.2 mg/kg) solution (0.2–0.3 ml; Sigma). Initially, the retrograde tracer True Blue (25 μl of 2 mg/ml in deiononized H2O; Biotium, Inc., Hayward, CA) was injected into each TMJ capsule. After 5–7 days, the animal was sacrificed and both trigeminal ganglion removed and immediately placed in Neg 50 mounting medium (Perkin-Elmer, Downers Grove, IL) at −25 °C for further analysis. For immunohistochemical studies, 50 μm sections were fixed, permeabilized, and incubated with anti-CGRP (1:500 in PBS; Neuromics) and/or TRPV1 antibodies (1:1000 in PBS; Neuromics) at 4 °C for 24 h. Immunoreactive proteins were visualized following incubation with fluorescently labeled secondary antibodies as described above. In some studies, multiple image analysis was performed to allow observation of the retrograde tracer True Blue and expression of CGRP and capsaicin receptor TRPV1 in the entire ganglion. For studies on CGRP levels in trigeminal ganglion, animals were either left untreated or both TMJ capsules were injected with capsaicin (25 μl of 10 μM solution) or vehicle (DMSO) 2 h prior to harvesting the ganglion. For the cocoa studies, Theobroma cacao extract (25 μl; 424 μg/g or 638 μg GAE) or vehicle (25 μl methanol) was injected into both capsules 24 h prior to injection of capsaicin. Following treatment, the animals were sacrificed and the posterior third of each ganglion, which corresponds to the mandibular or V3 branch, was removed, placed in HBS pH 7.4, homogenized, centrifuged, and the amount of CGRP in the supernatant determined by radioimmunoassay. The total amount of protein in each sample was determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA).
2.8. Statistical analysis
The data are reported as mean (pmol/mg total protein) ± S.E.M. Each experimental condition was repeated in at least three independent experiments. Statistical analysis was performed using the nonparametric Mann–Whitney U-test. Results were considered significant when p was less than 0.05. All statistical tests were preformed using Minitab Statistical Software, Release 14.
3. Results
3.1. Theobroma cacao extract represses stimulated CGRP secretion
Primary rat trigeminal ganglia cultures were utilized to investigate the effects of Theobroma cacao extract on CGRP secretion. Under our culture conditions, which are based on previously published procedures (Durham and Russo, 1999; Bellamy et al., 2006), >95% of the neurons express CGRP (Fig. 1A, 1C). The majority of the CGRP positive neurons (>90% cells) also expressed the TRPV1 receptor, which identifies capsaicin-responsive sensory neurons involved in pain transmission (Fig. 1B, D). As a control, no staining was observed in any trigeminal ganglion cells in the absence of primary antibodies (data not shown). These data demonstrate that under our culture conditions the majority of trigeminal neurons express both CGRP and TRPV1.
Fig. 1.
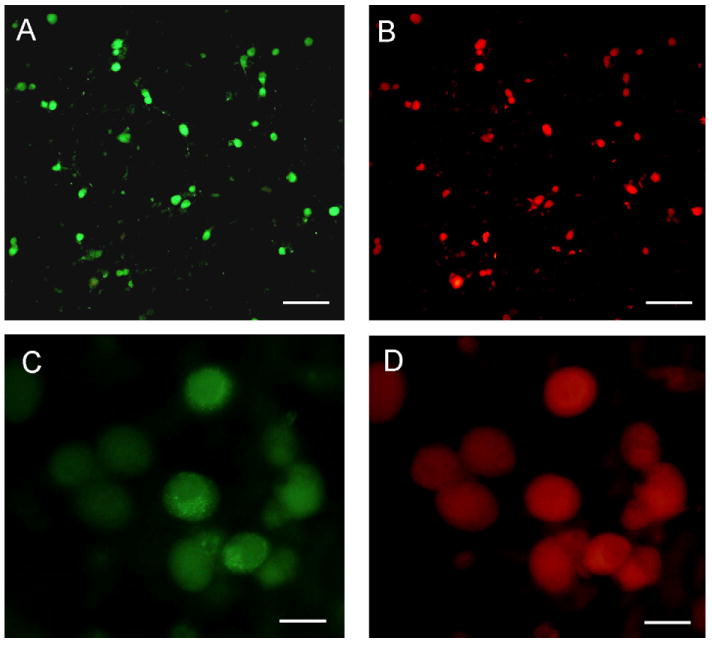
CGRP and TRPV1 are co-expressed in most cultured trigeminal ganglion neurons. (A and C) CGRP expression in d2 trigeminal ganglion neurons plated on poly-d-lysine-coated coverslips. (B and D) The same culture shown in panel A or C costained for TRPV1 expression. Magnification bar = 200 μm (A and B) or 40 μm (C and D).
To determine the effect of Theobroma cacao extract on stimulated release of CGRP from trigeminal neurons, cultures were pretreated for 30 min with Theobroma cacao extract prior to addition of KCl or capsaicin and the amount of CGRP secreted in 1 h measured. As seen in Fig. 2, stimulation with 90 mM KCl caused an almost 8-fold increase in CGRP release while treatment with 2 μM capsaicin caused a 5-fold increase. Pretreatment with increasing amounts of Theobroma cacao extract resulted in a significant reduction in the amount of CGRP secreted in response to KCl. Since not much difference was observed between the 8.5, 17, or 34 μg/g of Theobroma cacao extract dry weight (50, 100, and 200 μg/ml GAE), 17 μg/g or 100 μg/ml GAE was used in all subsequent experiments. At this concentration of Theobroma cacao extract, capsaicin stimulation of CGRP release was also greatly reduced to a level similar to the effect seen on KCl stimulation.
Fig. 2.
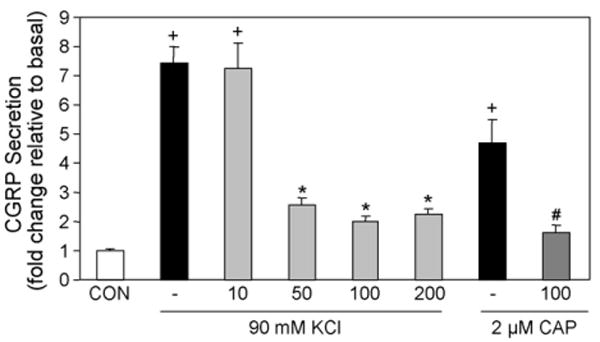
Theobroma cacao extract represses stimulated CGRP secretion. The relative amount of CGRP secreted from d2 trigeminal neurons in 1 h from untreated control cultures (CON), cultures treated with 90 mM KCl or 2 μM capsaicin (CAP), or cultures pretreated for 30 min with increasing amounts of Theobroma cacao extract. The final amount of Theobroma cacao extract (10, 50, 100, 200) is reported as μg/ml expressed as gallic acid equivalents or GAE, which corresponds to 1.7, 8.5, 17, or 34 μg/g of extract dry weight. The mean basal rate of CGRP release was 16.0 pg/h/well ± S.E.M. 0.685. The secretion rate for each condition (n > 6) was normalized to the basal rate for each well. + = p < 0.01 when compared to control, * = p < 0.001 when compared with KCl stimulated levels, # = p < 0.001 when compared to capsaicin-stimulated levels.
To determine whether Theobroma cacao extract could inhibit the basal amount of CGRP secreted, unstimulated cultures were incubated with the same amount of Theobroma cacao extract as described above. In contrast to the effect of Theobroma cacao on stimulated CGRP release, pretreatment with Theobroma cacao did not inhibit the amount of CGRP secreted under basal, unstimulated conditions (data not shown). In fact, at the higher concentrations (17 and 34 μg/g; 100 and 200 μg/ml GAE), Theobroma cacao extract slightly, yet significantly (p < 0.01), stimulated CGRP secretion (∼2-fold, n = 12). As a control, cultures were also treated for equal times with equivalent volumes of vehicle for Theobroma cacao extract (methanol) or capsaicin (DMSO). Secreted CGRP levels in the methanol or DMSO treated samples were not significantly different than those for the control samples (n = 10; p < 0.45 for both conditions). To establish that the inhibitory effect of Theobroma cacao was not due to cytotoxicity, neuronal viability was determined 24 h after treatments. Based on data from the CellTiter 96 assay (n = 3), no significant difference in neuronal viability was observed following treatment with Theobroma cacao extract or methanol when compared to untreated cultures. Taken together, these results demonstrate that Theobroma cacao extract can repress stimulated but not basal CGRP secretion, and the inhibitory effect is not due to cytotoxicity.
3.2. Theobroma cacao blocks KCl-mediated increase in intracellular calcium
Having shown that Theobroma cacao extract could inhibit CGRP release in response to KCl and capsaicin, calcium imaging was used to determine whether the inhibitory effect of Theobroma cacao on stimulated CGRP secretion involved inhibition of increased intracellular calcium levels. Basal calcium levels were measured in HBS medium prior to stimulation with 90 mM KCl or 2 μM capsaicin. Both KCl and capsaicin treatment alone caused a rapid increase in intracellular calcium above basal levels as shown in representative calcium plots (Fig. 3A and C). The mean and S.E.M. at the highest calcium level for KCl was 1.20 ± 0.02 while for capsaicin the level was 1.19 ± 0.02 relative units (n = 6). However, treatment with Theobroma cacao extract caused a prolonged decrease in basal intracellular calcium levels and prevented the typical calcium increase in response to KCl (Fig. 3B) or capsaicin as seen in representative calcium plots (Fig. 3D). The mean and S.E.M. following the addition of Theobroma cacao prior to KCl treatment was 0.68 ± 0.05, while after KCl treatment the mean level was 0.69 ± 0.06. Similarly, the mean and S.E.M. following the addition of Theobroma cacao prior to capsaicin treatment was 0.75 ± 0.03, while after capsaicin treatment was 0.75 ± 0.03. Based on these data, Theobroma cacao repression of KCl or capsaicin-stimulated CGRP release from trigeminal neurons is likely to involve inhibition of calcium channel activity and blocking the subsequent elevation of intracellular calcium levels.
Fig. 3.
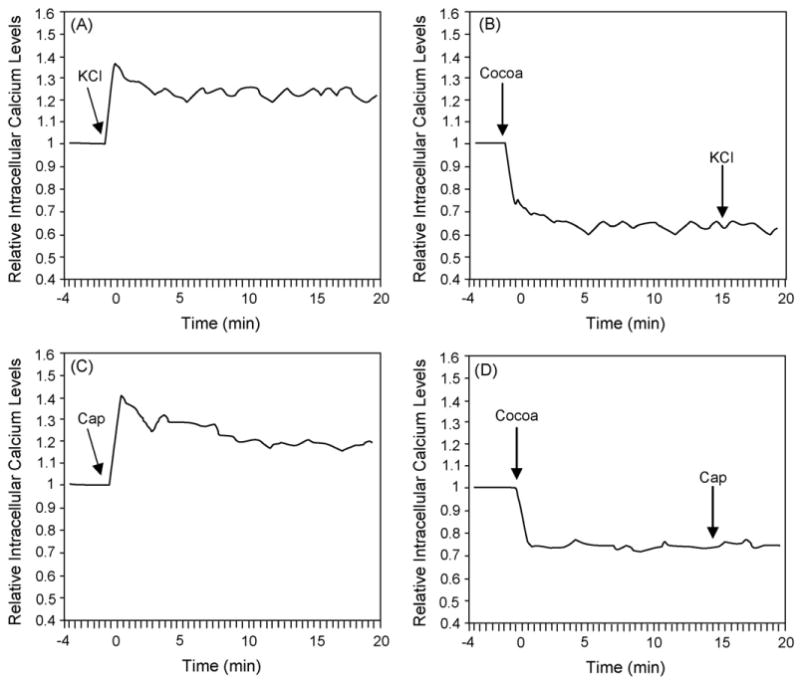
Theobroma cacao extract inhibits KCl and capsaicin-mediated increases in intracellular calcium levels. Relative intracellular calcium levels in d2 cultured trigeminal ganglion neurons in response to 90 mM KCl (A) or 2 μm capsaicin (C), or pretreated with Theobroma cacao extract (100 μg/ml GAE or 17 μg/g of extract dry weight) prior to addition of KCl (B) or capsaicin (D). The levels are the average of all the cells in each well. A representative graph from >6 independent experiments is shown for each experimental condition. All data are expressed relative to basal calcium levels, which were made equal to one.
3.3. Theobroma cacao repression of CGRP in an in vivo model of TMJ inflammation
To determine whether Theobroma cacao extract could inhibit CGRP expression in an in vivo model of TMJ inflammation, the effect of Theobroma cacao on capsaicin-stimulated CGRP levels in the trigeminal ganglion was investigated. In this model, capsaicin is injected into the TMJ capsule to activate nociceptive neurons that mediate peripheral inflammation and pain. Initially, the fluorescent dye, True Blue, was injected into the TMJ capsule to retrograde label neuronal cell bodies within the trigeminal ganglion that provide sensory innervation of the capsule. Multiple image alignment was used to obtain pictures of the entire ganglion including the ophthalmic (V1), maxillary (V2), and mandibular (V3) regions. After 5–7 days, the dye was readily observable in the cell body of neurons found in clusters or bands within the posterior lateral region of the ganglion, which corresponds to the mandibular or V3 branch (Fig. 4A). The number of labeled cells was greatest in the middle sections of the ganglion when compared to the most dorsal and ventral sections in which less than 10 cells were identified. The majority of neurons that provide sensory innervation of the TMJ capsule were found to express both CGRP (Fig. 4C) and TRPV1 (Fig. 4D).
Fig. 4.
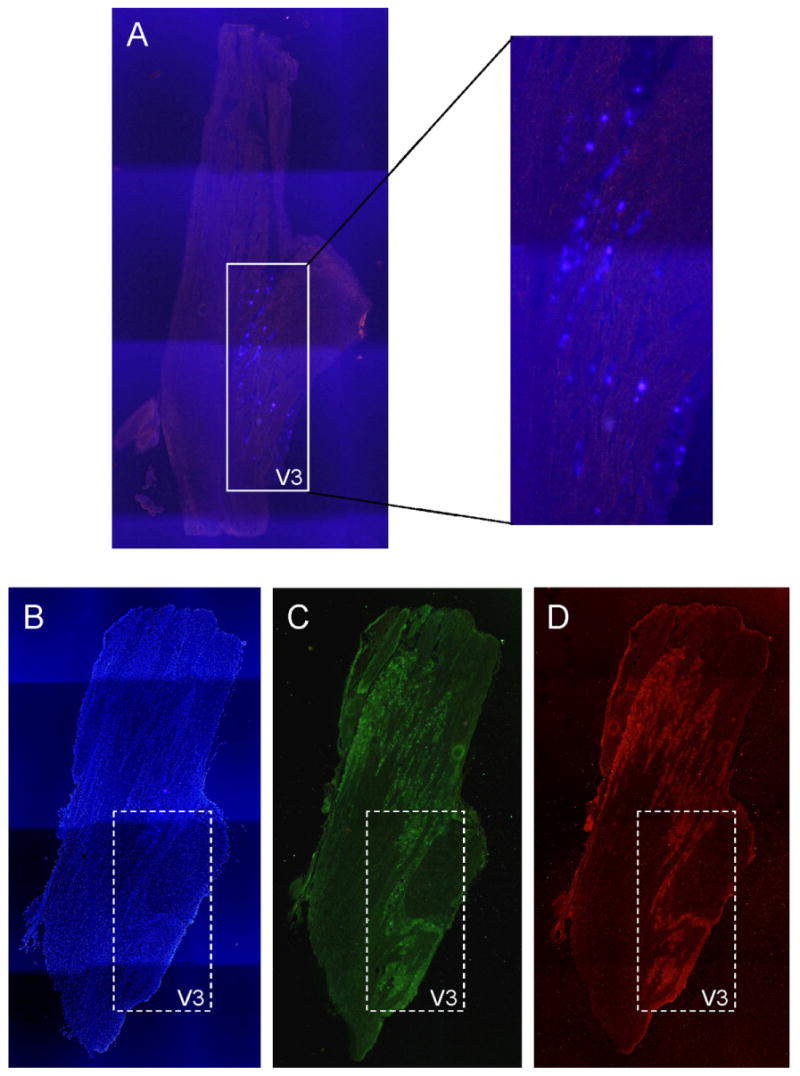
Expression of CGRP and TRPV1 in trigeminal ganglion neurons providing innervation of the TMJ capsule. (A) Cellular localization of True Blue in neuronal cells within the mandibular or V3 region of the trigeminal ganglion 5 days after injection of the dye into the TMJ capsule. The entire trigeminal ganglion is shown. Colocalization of CGRP (C) and TRPV1 (D) in neurons providing innervation to the TMJ capsule. The same section of trigeminal ganglion was stained with the nuclear dye DAPI to identify nuclei of both neuronal and glial cells (B).
Immunohistochemistry was used to study the effect of Theobroma cacao extract on CGRP-IR levels within the trigeminal ganglion of untreated animals or animals injected with capsaicin in the TMJ capsule. In untreated animals, CGRP was readily detected in the cell bodies of neuronal cells in the mandibular region of the ganglion (Fig. 5A). In contrast, the levels of CGRP within this region of the ganglion were greatly diminished after 2 h following capsaicin injection in the TMJ capsule (Fig. 5B). The decrease in immunoreactive CGRP is likely caused by the release of CGRP from the terminals and cell body of neurons in response to capsaicin stimulation, which has been previously reported to cause neuropeptide release (Jansen-Olesen et al., 1996; Garry et al., 2000). Significantly, the stimulatory effect of capsaicin on CGRP-IR levels in the ganglion was prevented in animals in which Theobroma cacao extract was injected into the TMJ capsule 24 h prior to capsaicin stimulation (Fig. 5D). In Theobroma cacao treated animals, the level of CGRP-IR within the ganglion was returned to levels observed in untreated, control animals. This in vivo finding is in agreement with our in vitro results in which Theobroma cacao repressed capsaicin stimulation of CGRP secretion from cultured trigeminal neurons (Fig. 2). In contrast, overnight treatment with Theobroma cacao did not appear to affect CGRP-IR levels in the mandibular region of the ganglion, since levels were comparable to those seen in untreated animals (Fig. 5C).
Fig. 5.
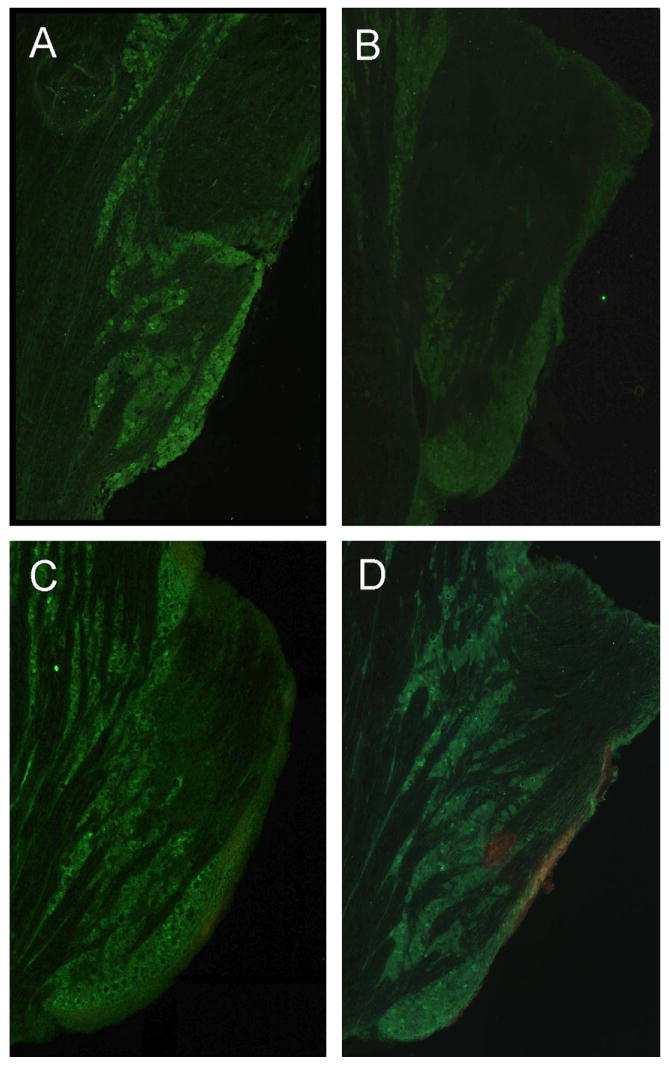
Theobroma cacao extract blocks effect of capsaicin on CGRP levels in vivo in trigeminal ganglion. CGRP-IR expression in the mandibular region of trigeminal ganglion sections (40 μm) obtained from control, untreated animals (A), animals injected with capsaicin for 2 h (B) or Theobroma cacao for 24 h (C), or animals injected with Theobroma cacao extract 24 h prior to capsaicin injection (D) is shown. Only the mandibular or V3 region of each ganglion obtained from multiple image alignment of the entire trigeminal ganglion is shown.
As a complementary approach to demonstrate that Theobroma cacao can repress the stimulatory effects of capsaicin-mediated trigeminal nerve activation, CGRP levels within the ganglion were determined by radioimmunoassay. The amount of CGRP is reported as pmol/mg of total protein. As seen in Fig. 6, capsaicin stimulation for 2 h caused a significant decrease in CGRP levels within the mandibular region of the ganglion when compared to control levels. This stimulatory effect was completely blocked by pretreatment with Theobroma cacao extract 24 h prior to capsaicin injection. Injection of DMSO (capsaicin vehicle) for 2 h or methanol (Theobroma cacao vehicle) for 24 h did not cause any significant change in CGRP levels compared to control values. These results, which are in agreement with our immunohistochemical findings, demonstrate that Theobroma cacao extract can repress capsaicin-stimulated CGRP release from trigeminal nerves that provide sensory innervation of the TMJ capsule.
Fig. 6.
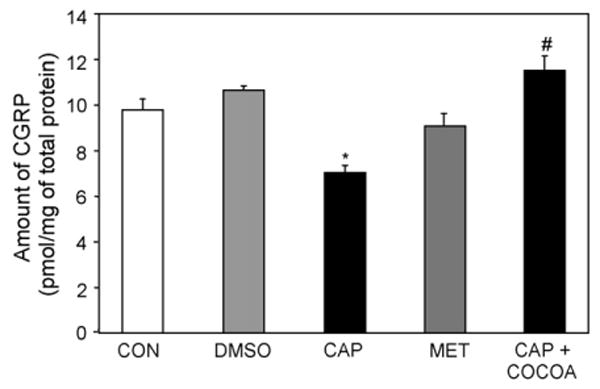
Theobroma cacao extract represses stimulatory effect of capsaicin on CGRP levels in trigeminal ganglion. The amount of CGRP was determined by radioimmunoassay in trigeminal ganglion obtained from control, untreated animals (CON, n = 13) or animals treated for 2 h with DMSO (n = 4) or capsaicin (CAP, n = 7). CGRP levels were also measured in ganglion obtained from animals 24 h after injection of methanol (MET, n = 4) or from animals initially injected with Theobroma cacao for 24 h and then with capsaicin 2 h prior to harvesting (n = 4). * p < 0.005 when compared to control, # p < 0.001 when compared to capsaicin-stimulated levels.
4. Discussion
Based on epidemiological studies, populations that consume foods and drinks rich in polyphenols have a lower incidence of chronic inflammatory diseases such as arthritis, atherosclerosis, and chronic heart failure (Stoclet et al., 2004; Goggs et al., 2005; Buijsse et al., 2006; McCullough et al., 2006). The beneficial effects of polyphenols in cardiovascular disease have been shown to involve inhibition of key enzymes and transcription factors that are known to promote inflammation (Bode and Zigang, 2003; Yoon and Baek, 2005). Much of this research has focused on the antioxidant and radical scavenging capacity of polyphenols in immune and endothelial cells to suppress inflammation (Sanbongi et al., 1998; Stoclet et al., 1999; Kempuraj et al., 2005). Furthermore, many of the health benefits attributed to dietary polyphenols are due to their ability to repress inflammatory responses by inhibiting the production and release of cytokines and other inflammatory mediators from immune cells (Chen et al., 2002; Veronique et al., 2004; Wheeler et al., 2004). However, the effect of polyphenols on sensory neuron function and excitability is not well understood. Towards this end, we investigated whether biologically active compounds isolated from Theobroma cacao beans, which contain higher levels of polyphenols than red wine or green tea, could inhibit CGRP expression in cultured trigeminal ganglia neurons and in vivo model of TMJ inflammation.
In this study, we showed that the stimulatory effect of KCl and capsaicin on CGRP secretion from trigeminal ganglion neurons was significantly repressed by pretreatment with Theobroma cacao extract enriched for polyphenols. The concentration of polyphenols in our Theobroma cacao extract, which was found to suppress neuronal activity and was not toxic to neuronal cells, was similar to concentrations reported to regulate gene expression in non-neuronal cells (Noe et al., 2004; Ramiro et al., 2005). While KCl is known to activate neurons via chemical depolarization, capsaicin selectively excites nociceptive C and Aδ fibers to cause neuropeptide release (Caterina et al., 1997). Based on our calcium imaging studies, we speculate that Theobroma cacao repression of trigeminal neuron activity involves blockage of the normal physiological increase in intracellular calcium levels in response to KCl and capsaicin. It is well established that the cellular effects of KCl and capsaicin can be mediated by activation of calcium channels (Bell, 1993; Kopanitsa et al., 1995; Brosenitsch et al., 1998; Mohri et al., 2003). Our finding is in agreement with a recent study that reported an inhibitory effect of the flavonols, a class of plant polyphenols, on stimulated interleukin secretion which involved suppression of intracellular calcium levels in cultured mast cells (Kempuraj et al., 2005). However, our data provide the first evidence, to our knowledge, of Theobroma cacao extract blocking neuropeptide release from sensory neurons via a mechanism that is likely to involve inhibition of calcium channel activity.
Previously, we had reported that the anti-migraine drug sumatriptan could inhibit stimulated but not basal release of CGRP from trigeminal neurons (Durham and Russo, 1999). Similarly, in this study, we found that Theobroma cacao did not inhibit basal CGRP secretion but rather, only repressed stimulated CGRP release. Interestingly, while both sumatriptan and Theobroma cacao extract were shown to regulate CGRP secretion from cultured trigeminal ganglion neurons in a similar manner, the mechanism of repression appears to be quite distinct. Sumatriptan is a member of the triptan class of serotonergic drugs which are commonly used to abort the pain associated with migraine headache (Dodick and Martin, 2004). Relief of migraine headache with sumatriptan has been shown to correlate with a return of CGRP to normal levels (Buzzi et al., 1991; Goadsby and Edvinsson, 1991, 1993). Sumatriptan repression of KCl-stimulated CGRP release involves a sustained elevation of intracellular calcium and activation of specific phosphatases (Durham and Russo, 1999). In contrast, Theobroma cacao extract caused a prolonged decrease in intracellular calcium levels, a cellular event not seen with sumatriptan. Furthermore, pretreatment with Theobroma cacao prevented the normal rapid transient increase in intracellular calcium in response to KCl and capsaicin. Thus, it appears that Theobroma cacao extract represses CGRP secretion from trigeminal neurons by a different mechanism than reported for the migraine abortive drug sumatriptan.
CGRP is believed to play an important role in the genesis and maintenance of inflammation and pain associated with both migraine and TMJ pathology (Lassen et al., 2002; Juhasz et al., 2003; Lobbezoo et al., 2004). In this study, we used an in vivo model of TMJ inflammation to investigate the effect of Theobroma cacao extract on trigeminal nerve activation and CGRP release. Initially, we used a retrograde tracer to identify neurons that provide sensory innervation to the TMJ capsule within the posterior lateral region of the trigeminal ganglion. Neuronal cell bodies were arranged in discrete bands within the mandibular region of the ganglion as reported in a previous study (Takeda et al., 2005). We found that the majority of the neurons providing innervation of the TMJ capsule co-expressed CGRP and the TRPV1 receptor. Similarly, others (Ichikawa et al., 2004; Loi et al., 2006) have reported TRPV1 and CGRP co-expression in nerves providing innervation of cells lining the synovium of the TMJ capsule. TRPV1 receptors, which are activated by capsaicin, heat, and protons, are abundantly expressed by unmyelinated C-fibers involved in pain transmission (Caterina et al., 1997). In addition, activation of TRPV1 receptors has been shown to cause CGRP release from sensory neurons (Carleson et al., 1997; Spears et al., 1998; Gibbs et al., 2006). Peripheral release of CGRP promotes inflammation within the joint (Spears et al., 2005), while its release centrally in the trigeminal nucleus caudalis is correlated with increased pain perceptions and can lead to central sensitization and allodynia (Hutchins et al., 2000; Jenkins et al., 2004). In this study, injection of capsaicin into the TMJ capsule was shown to cause a decrease in CGRP levels in trigeminal neurons within the mandibular or V3 region of the ganglion. This decrease was likely due to release of CGRP from nerve terminals as well as from the cell body of the neurons. Our capsaicin findings are in agreement with other studies in which CGRP levels in the trigeminal ganglion of capsaicin treated animals were found to be lower than levels in control animals (Spears et al., 1998).
Having demonstrated that Theobroma cacao extract could inhibit KCl- and capsaicin-stimulated CGRP secretion from cultured trigeminal neurons, we wanted to determine whether Theobroma cacao could block capsaicin-stimulated CGRP release from trigeminal ganglion neurons in vivo. Injection of Theobroma cacao extract 24 h prior to capsaicin administration was shown to effectively block changes in CGRP levels within the ganglion when compared to untreated control animals. Thus, importantly, our in vivo findings agree with our in vitro studies. In another in vivo study, a cocoa extract was shown to significantly decrease NO release from macrophages as well as down-regulate mRNA expression of inflammatory cytokines and chemokines (Ramiro et al., 2005). It will be of interest to determine whether Theobroma cacao extract enriched in polyphenols will decrease CGRP gene expression. Repression of CGRP expression may be clinically important given the role of CGRP in inflammatory and neuropathic pain (van Rossum et al., 1997; Ma and Quirion, 2006). For example, CGRP has been reported to play an important role in the initiation and maintenance of capsaicin-induced secondary allodynia and hyperalgesia and, thus, in the development of central sensitization in response to peripheral inflammation (Sun et al., 2003). In addition, results from in vivo studies have demonstrated that CGRP contributes to nociceptive behavior associated with inflammation (Han et al., 2005; Ambalavanar et al., 2006). Based on our findings, it is likely that repression of stimulated CGRP expression by biologically active compounds isolated from Theobroma cacao would be beneficial in treating TMJ disorders, for which there are no FDA approved pharmaceutical treatments.
In summary, results from this study have shown that CGRP secretion from stimulated trigeminal neurons can be directly inhibited by a methanol extract of Theobroma cacao beans. Significantly, it was shown that Theobroma cacao extract could block CGRP release from cultured trigeminal neurons as well as in an in vivo model of TMJ inflammation. Based on our calcium imaging data, the inhibitory effect of Theobroma cacao extract on trigeminal neurons likely involves blockage of calcium channel activity. Thus, our findings demonstrate that Theobroma cacao extract contains biologically active compounds that repress trigeminal nerve activation and, hence, release of CGRP, which is known to contribute to inflammation and pain within the TMJ. Furthermore, our results provide evidence that the medical benefits attributed to the use of cocoa in ancient Mayan and Aztec cultures for the treatment of tooth and joint ailments may have involved repression of sensory nerve activation.
Acknowledgments
The authors would like to thank Ryan Cady for his technical assistance.
Footnotes
This work was supported by NIH grants DE015385 and DE017805.
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit:
References
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143:875–884. doi: 10.1016/j.neuroscience.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Relation between intra-articular temperature of the arthritic temporomandibular joint and presence of calcitonin gene-related peptide in the joint fluid. A clinical study. Acta Odontologica Scandinavica. 1993;51:285–291. doi: 10.3109/00016359309040579. [DOI] [PubMed] [Google Scholar]
- Bell J. Selective blockade of spinal reflexes by omega-conotoxin in the isolated spinal cord of the neonatal rat. Neuroscience. 1993;52:711–716. doi: 10.1016/0306-4522(93)90419-g. [DOI] [PubMed] [Google Scholar]
- Bellamy J, Bowen E, Russo A, Durham P. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. The European Journal of Neuroscience. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode A, Zigang D. Signal transduction pathways: targets for green and black tea polyphenols. Journal of Biochemistry and Molecular Biology. 2003;36:66–77. [PubMed] [Google Scholar]
- Bowen E, Schmidt T, Firm C, Russo A, Durham P. Tumor necrosis factor-α stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. Journal of Neurochemistry. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch T, Salgado-Commissariat D, Kunze D, Katz D. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. The Journal of Neuroscience. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijsse B, Feskens E, Kok F, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Archives of Internal Medicine. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- Buzzi M. Trigeminal pain pathway: Peripheral and central activation as experimental models of migraine. Functional Neurology. 2001;16:77–81. [PubMed] [Google Scholar]
- Buzzi M, Carter W, Shimizu T, Heath H, III, Moskowitz M. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior saggital sinus during electrical stimulation of the trigeminal ganglia. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- Buzzi M, Moskowitz M. The trigemino-vascular system and migraine. Pathologie-Biologie (Paris) 1992;40:313–317. [PubMed] [Google Scholar]
- Carleson J, Kogner P, Bileviciute I, Theodorsson E, Appelgren A, Appelgren B, Kopp S, Yousef N, Lundeberg T. Effects of capsaicin in temporomandibular joint arthritis in rats. Archives of Oral Biology. 1997;42:869–876. doi: 10.1016/s0003-9969(97)00005-8. [DOI] [PubMed] [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen P, Wheeler D, Malhotra V, Odoms K, Denenberg A, Wong H. Green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26:233–241. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillinger T, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti L. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. The Journal of Nutrition. 2000;130:2057S–2072S. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- Dimitroulis G. Temporomandibular disorders: a clinical update. British Medical Journal. 1998;317:190–194. doi: 10.1136/bmj.317.7152.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick D, Martin V. Triptans and CNS side-effects: pharmacokinetic and metabolic mechanisms. Cephalalgia. 2004;24:417–424. doi: 10.1111/j.1468-2982.2004.00694.x. [DOI] [PubMed] [Google Scholar]
- Durham P, Russo A. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. The Journal of Neuroscience. 1999;19:3423–3429. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham P, Russo A. Differential regulation of mitogen-activated protein kinase-responsive genes by the duration of a calcium signal. Molecular Endocrinology. 2000;14:1570–1582. doi: 10.1210/mend.14.10.0529. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Goadsby P. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]
- Garry M, Walton L, Davis M. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide by not by cyclic GMP. Brain Research. 2000;861:208–219. doi: 10.1016/s0006-8993(99)02448-8. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Flores C, Hargreaves K. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain. 2006;124:167–174. doi: 10.1016/j.pain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Goadsby P, Edvinsson L. Sumatriptan reverses the changes in calcitonin gene-related peptide seen in the headache phase of migraine. Cephalalgia. 1991;11:3–4. [Google Scholar]
- Goadsby P, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Annals of Neurology. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goggs R, Vaughan-Thomas A, Clegg P, Carter S, Innes J, Mobasheri A, Shakibaei M, Schwab W, Bondy C. Nutraceutical therapies for degenerative joint diseases: a critical review. Critical Reviews in Food Science and Nutrition. 2005;45:145–164. doi: 10.1080/10408690590956341. [DOI] [PubMed] [Google Scholar]
- Han J, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behaviour. The Journal of Neuroscience. 2005;25:10717–10728. doi: 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund A, Ekblom A, Hansson P, Lind J, Lundenberg T, Theodorsson E. Concentration of neuropeptide substance – P, Neurokinin A, calcitonin gene related peptide, neuropeptide Y and vasoactive intestinal polypeptide in the synovial fluid of the – human temporomandibular joint. International Journal of Oral and Maxillofacial Surgery. 1991;20:228–231. doi: 10.1016/s0901-5027(05)80181-x. [DOI] [PubMed] [Google Scholar]
- Hutchins B, Spears R, Hinton R, Harper R. Calcitonin gene-related peptide and substance P immunoreactivity in rat trigeminal ganglia and brainstem following adjuvant induced inflammation of the temporomandibular joint. Archives of Oral Biology. 2000;45:335–345. doi: 10.1016/s0003-9969(99)00129-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin H, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Research. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- Jenkins D, Langmead C, Parsons A, Strijbos P. Regulation of calcitonin gene-related peptide release from rat trigeminal nucleus caudalis slices in vitro. Neuroscience Letters. 2004;366:241–244. doi: 10.1016/j.neulet.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Modos E, Olajos S, Jakab B, Nemeth J, Szolcsanyi J, Vitrai J, Bagdy G. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo C, Theoharides T. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. British Journal of Pharmacology. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanitsa M, Panchenko V, Magura E, Lishko P, Krishtal O. Capsaicin blocks Ca2+ channels in isolated rat trigeminal and hippocampal neurons. Neuroreport. 1995;6:2338–2340. doi: 10.1097/00001756-199511270-00016. [DOI] [PubMed] [Google Scholar]
- Kopp S. Neuroendocrine, immune, and local responses related to temporomandibular disorders. Journal of Orofacial Pain. 2001;15:9–28. [PubMed] [Google Scholar]
- Lassen L, Haderslev P, Jacobsen V, Iversen H, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Kim Y, Lee H, Lee C. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. Journal of Agricultural and Food Chemistry. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- Lobbezoo F, Drangsholt M, Peck C, Sato H, Kopp S, Svensson P. Topical review: new insights into the pathology and diagnosis of disorders of the temporomandibular joint. Journal of Orofacial Pain. 2004;18:181–191. [PubMed] [Google Scholar]
- Loi H, Kido M, Zhang J, Yamaza T, Nakata S, Nakasima A, Tanaka T. Capsaicin receptor expression in the rat temporomandibular joint. Cell and Tissue Research. 2006;325:47–54. doi: 10.1007/s00441-006-0183-7. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. Increased calcitonin gene-related peptide in neuroma and invading macrophages is involved in the up-regulation of interleukin-6 and thermal hyperalgesia in a rat model of mononeuropathy. Journal of Neurochemistry. 2006;98:180–192. doi: 10.1111/j.1471-4159.2006.03856.x. [DOI] [PubMed] [Google Scholar]
- McCullough M, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz H, Coletti C, Campos H, Hollenberg N. Hypertension, the Kuna, and the epidemiology of flavanols. Journal of Cardiovascular Pharmacology. 2006;47:S103–S109. doi: 10.1097/00005344-200606001-00003. discussion 119–121. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C. Effects of flavonoids on immune function and inflammatory cell functions. Biochemical Pharmacology. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- Mohri Y, Katsura M, Shuto K, Tsujimura A, Ishii R, Ohkuma S. L-type high voltage gated calcium channels cause an increase in diazepam binding inhibitor mRNA expression after sustained exposure to ethanol in mouse cerebral cortical neurons. Brain Research. 2003;113:52–56. doi: 10.1016/s0169-328x(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Noe V, Penuelas S, Lamuela-Raventos R, Permanyer J, Cuidad C, Izquierdo-Pulido M. Epicatechin and a cocoa polyphenolic extract modulate gene expression in human Caco-2 cells. The Journal of Nutrition. 2004;134:2509–2516. doi: 10.1093/jn/134.10.2509. [DOI] [PubMed] [Google Scholar]
- O'Conner T, Van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. The Journal of Neuroscience. 1986;6:2200–2207. doi: 10.1523/JNEUROSCI.06-08-02200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D, Striessnig J. Neurobiology of migraine. Nature Reviews. Neuroscience. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- Ramiro E, Franch A, Castellote C, Perez-Cano F, Permanyer J, Izquierdo-Pulido M, Castell M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. Journal of Agricultural and Food Chemistry. 2005;53:8506–8511. doi: 10.1021/jf0511042. [DOI] [PubMed] [Google Scholar]
- Sanbongi C, Osakabe N, Natsume M, Takizawa T, Gomi S, Osawa T. Antioxidative polyphenols isolated from Theobroma cacao. Journal of Agricultural and Food Chemistry. 1998;46:454–457. doi: 10.1021/jf970575o. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Fricton J, Haley D, Shapiro B. The prevalence and treatment needs of subjects with temporomandibular disorders. Journal of the American Dental Association. 1990;120:295–303. doi: 10.14219/jada.archive.1990.0059. [DOI] [PubMed] [Google Scholar]
- Singleton V, Rossi J. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Spears R, Dees L, Sapozhnikov M, Bellinger L, Hutchins B. Temporal changes in inflammatory mediator concentrations in an adjuvant model of temporomandibular joint inflamation. Journal of Orofacial Pain. 2005;19:34–40. [PubMed] [Google Scholar]
- Spears R, Hutchins B, Hinton R. Capsaicin application to the temporomandibular joint alters calcitonin gene-related peptide levels in the trigeminal ganglion of the rat. Journal of Orofacial Pain. 1998;12:108–115. [PubMed] [Google Scholar]
- Stoclet J, Chataigneau T, Ndiaye M, Oak M, El Bedoui J, Chataigneau M, Schini-Kerth V. Vascular protection by dietary polyphenols. European Journal of Pharmacology. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Stoclet J, Kleschyov A, Andriambeloson E, Diebolt M, Andriantsitohaina R. Endothelial no release caused by red wine polyphenols. Journal of Physiology and Pharmacology: an Official Journal of the Polish Physiological Society. 1999;50:535–540. [PubMed] [Google Scholar]
- Sun R, Lawand N, Willis W. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Shima Y, Ohta H, Matsumoto S. Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. Journal of Neurophysiology. 2005;93:2723–2738. doi: 10.1152/jn.00631.2004. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch U, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neuroscience and Biobehavioral Reviews. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Veronique N, Penuelas S, Lamuela-Raventos R, Permanyer J, Ciudad C, Izquierdo-Pulido M. Epicatechin and a cocoa polyphenolic extract modulate gene expression in human caco-2 cells. The Journal of Nutrition. 2004;134:2509–2516. doi: 10.1093/jn/134.10.2509. [DOI] [PubMed] [Google Scholar]
- Wheeler s, Catravas J, Odoms K, Denenberg A, Malhotra V, Wong H. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1B-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. The Journal of Nutrition. 2004;134:1039–1044. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- Williams R, Spencer J, Rice-Evans C. Flavonoids: antioxidants or signaling molecules? Free Radical Biology & Medicine. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yoon J, Baek S. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Medical Journal. 2005;46:585–596. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]


