Abstract
The pathogenic bacteria Bordetella parapertussis and Bordetella bronchiseptica express a lipopolysaccharide O antigen containing a polymer of 2,3-diacetamido-2,3-dideoxy-l-galacturonic acid. The O-antigen cluster contains three neighbouring genes that encode proteins belonging to the short-chain dehydrogenase/reductase (SDR) family, wbmF, wbmG and wbmH, and we aimed to elucidate their individual functions. Mutation and complementation implicate each gene in O-antigen expression but, as their putative sugar nucleotide substrates are not currently available, biochemical characterisation of WbmF, WbmG and WbmH is impractical at the present time. SDR family members catalyse a wide range of chemical reactions including oxidation, reduction and epimerisation. Because they typically share low sequence conservation, however, catalytic function cannot be predicted from sequence analysis alone. In this context, structural characterisation of the native proteins, co-crystals and small-molecule soaks enables differentiation of the functions of WbmF, WbmG and WbmH. These proteins exhibit typical SDR architecture and coordinate NAD. In the substrate-binding domain, all three enzymes bind uridyl nucleotides. WbmG contains a typical SDR catalytic TYK triad, which is required for oxidoreductase function, but the active site is devoid of additional acid–base functionality. Similarly, WbmH possesses a TYK triad, but an otherwise feature-poor active site. Consequently, 3,5-epimerase function can probably be ruled out for these enzymes. The WbmF active site contains conserved 3,5-epimerase features, namely, a positionally conserved cysteine (Cys133) and basic side chain (His90 or Asn213), but lacks the serine/threonine component of the SDR triad and therefore may not act as an oxidoreductase. The data suggest a pathway for synthesis of the O-antigen precursor UDP-2,3-diacetamido-2,3-dideoxy-l-galacturonic acid and illustrate the usefulness of structural data in predicting protein function.
Abbreviations used: dTDP, deoxythymidine diphosphate; l-GalNAc3NAcA, 2,3-diacetamido-2,3-dideoxy-l-galacturonic acid; GME, GDP-mannose 3,5-epimerase; GMER, GDP-4-keto-6-deoxymannose 3,5-epimerase/reductase; LPS, lipopolysaccharide; PDB, Protein Data Bank; BLAST, basic local alignment search tool; SDR, short-chain dehydrogenase/reductase; UDP-d-ManNAc3NAcA, UDP-2,3-diacetamido-2,3-dideoxy-d-mannuronic acid; UDP-l-GalNAc3NAcA, UDP-2,3-diacetamido-2,3-dideoxy-l-galacturonic acid; UMP, uridine monophosphate; MCS, multiple cloning site
Keywords: short-chain dehydrogenase/reductase, X-ray crystallography, Bordetella, lipopolysaccharide, O-antigen biosynthesis
Introduction
The acceleration of genome sequencing has created a challenge for modern biology, namely, interpretation of the vast and growing amount of genetic information in the databases. Search algorithms such as the basic local alignment search tool (BLAST)1 can efficiently find related genes, but only in a small minority of cases is the precise function of a novel enzyme equivalent to that of the highest scoring hit. In straightforward cases the output is useful to generate hypotheses for the novel enzyme's function, based on common catalytic chemistry, substrates or both, and these postulates are then amenable to direct biochemical investigation. To make full use of genome data it is important that where such standard bioinformatic analysis cannot predict precise roles and biochemical function assays are not possible, tools are developed to differentiate the functions of genes. A good example of such a challenging case is where gene products are annotated as short-chain dehydrogenase/reductases (SDRs). Members of this family share low sequence identity (typically 15–30%) and catalyse a wide range of chemical reactions, including oxidation, reduction, epimerisation, dehydration and decarboxylation (reviewed in Ref. 2).
Bordetella parapertussis and Bordetella bronchiseptica are pathogens of mammals. B. bronchiseptica is associated with respiratory tract infections in many animals including acute tracheobronchitis in dogs (kennel cough)3 and atrophic rhinitis in pigs,4 whereas B. parapertussis can cause whooping cough in humans.5 A separate lineage of B. parapertussis infects sheep.6
The major component of the outer leaflet of the outer membrane in Gram-negative bacteria is lipopolysaccharide (LPS). The complete structure of this macromolecule in B. bronchiseptica and B. parapertussis has been recently described.7 The lipid A domain of LPS, which consists of a diglucosamine backbone substituted with fatty acyl chains, forms the outer leaflet of the outer membranes of the bacteria. Lipid A is linked to a complex, branching oligosaccharide known as the core. Lipid A-core comprises a proportion of the LPS that is exposed on the cell surface and is known as band B. In B. bronchiseptica, lipid A-core can be substituted with a trisaccharide and this structure is known as band A. Synthesis of the band A trisaccharide requires functions encoded in the wlb gene cluster (previously named bpl).8
Both B. bronchiseptica and B. parapertussis also substitute their LPS with an O antigen. This O antigen contains 12 to 15 2,3-diacetamido-2,3-dideoxy-l-galactosaminuronic acid (l-GalNAc3NAcA) residues9 and is required for full virulence in animal and in vitro models of infection.10 O-antigen biosynthesis requires genes in the wbm cluster that is adjacent to the wlb genetic locus. The wbm locus contains three neighbouring SDR genes (wbmF, wbmG and wbmH), all of which have been annotated as nucleotide-sugar epimerases/dehydratases.
The structure of complete O antigen and the homology of wbm genes with genes of known function have led us to propose a pathway for biosynthesis of this polysaccharide and we are currently testing various steps in this scheme as part of an ongoing project to determine the functions of all 24 wbm genes. This report specifically concerns the roles of the SDR genes wbmF, wbmG and wbmH.
O-antigen residues are synthesised as sugar nucleotide precursors. The probable substrate for wbm locus-encoded biosynthesis of the nucleotide-activated l-GalNAc3NAcA is UDP-2,3-diacetamido-2,3-dideoxy-d-mannuronic acid (UDP-d-ManNAc3NAcA). This compound is related to the l-galacto configuration by inversion of the stereochemistry at the hexose 3 and 5 positions. In the bordetellae, UDP-d-ManNAc3NAcA is formed as a precursor for band A trisaccharide synthesis by 2-epimerisation of UDP-2,3-diacetamido-2,3-dideoxy-d-glucuronic acid, catalysed by WlbD.11 Because there are precedents for either a single12 or multiple13–16 SDR enzymes catalysing 3,5-epimerisation conversions of sugar nucleotides, we hypothesise that one or more of WbmF, WbmG and WbmH operate in this biosynthetic pathway to catalyse the conversion of UDP-d-ManNAc3NAcA to UDP-2,3-diacetamido-2,3-dideoxy-l-galacturonic acid (UDP-l-GalNAc3NAcA). Because of the low percentage conversion of the WlbD-catalysed reaction, UDP-d-ManNAc3NAcA is not currently available for biochemical studies and therefore this hypothesis cannot be directly tested at the present time.
In this report we demonstrate the involvement of wbmF, wbmG and wbmH in O-antigen expression by mutation of the genes in B. bronchiseptica. Because the putative substrate is unavailable, we elected to probe the catalytic functions of WbmF, WbmG and WbmH by characterising these proteins' three-dimensional structures. With soaking and co-crystallisation experiments, densities corresponding to cofactors and nucleotide portions of substrate analogues can be identified within the active sites. Biochemical and crystallographic studies have defined the structural basis for the catalytic chemistries of a range of other SDR enzymes (reviewed in Ref. 17). Our knowledge of these structure–function relationships enables us to interpret the structures of the wbm SDRs. We have analysed the potential for acid–base chemistry in the active sites to differentiate their potential roles in vivo. Based on the composition of O antigen, the genetic context of these wbm genes and information from the structural studies, we propose roles for these genes in O-antigen biosynthesis. The results demonstrate the usefulness and limitations of X-ray data in elucidating biochemical pathways where catalytic activity cannot be directly measured.
Results
Mutational analysis of wbmF, wbmG and wbmH
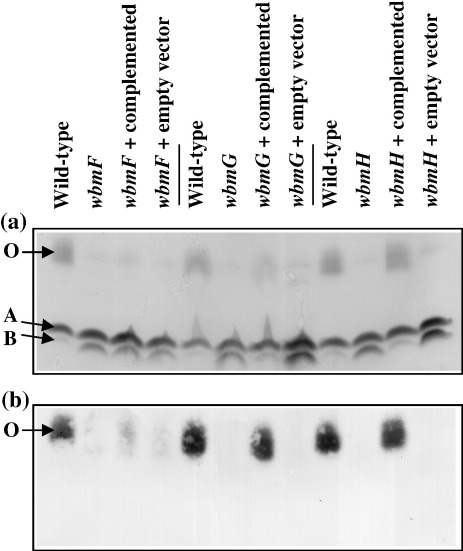
To test the hypothesis that wbmF, wbmG and wbmH are involved in O-chain biosynthesis, each gene was disrupted by insertion of a tetracycline-resistance cassette. Mutation of the chromosomal genes was confirmed by Southern hybridisation (data not shown). The effects of these mutations were assessed by silver-stained SDS-PAGE analysis and immunoblotting of mutant LPS (Fig. 1). Mutation of wbmG or wbmH results in apparent abrogation of O-antigen synthesis, as the LPS from these mutants lacks O band as detected by either silver stain or Western blot. O-antigen expression in the wbmF mutant, CNF0a, is dramatically reduced compared with the wild type, but this strain retains its ability to express a small amount of material with the electrophoretic mobility of O-band LPS and which binds the O-antigen-specific monoclonal antibody, D13B11.
Fig. 1.
(a) Silver stain analysis and (b) Western immunoblot of duplicate SDS-PAGE gels showing the LPS profiles of wild-type B. bronchiseptica (CN7635E) and CN7635E-derived mutants in wbmF (CNF0a), wbmG (CNG1a) and wbmH (CNH1d) and mutants carrying the complementation vectors for wbmF (pCompF), wbmG (pCompG) and wbmH (pCompH) or the empty vector (pCompEmpty). The positions of the B. bronchiseptica A-band (A) and B-band (B) species are indicated as well as the position of LPS that contains O antigen (O). The primary monoclonal antibody used in (b) that recognises O-band LPS was D13B11.35
Each of the mutations was complemented by expression of the cognate wild-type allele in trans (Fig. 1). Each coding sequence was cloned into the broad host range vector pBBR1MCS(kan) under the control of the flaA promoter to generate complementation vectors. Complementation of the wbmG and wbmH mutations restored O-antigen expression to near wild-type levels. O-antigen expression in the complemented wbmF mutant was increased relative to the wbmF mutant, but was still considerably less than in wild type. The reason for incomplete complementation of the wbmF mutation is unknown, but is not likely to be due to polar effects of the mutation because, due to the organisation of genes in the wbm locus, wbmF is at the 3′ end of an operon.18 Introduction of the empty complementation vector had no effect on O-antigen expression in any of the mutants.
The gene products WbmF, WbmG and WbmH are all in the extended SDR family
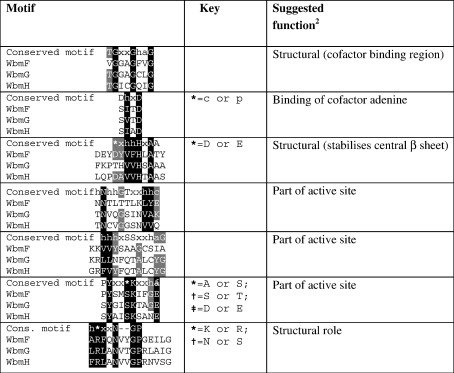
The SDR family is divided into the classical and extended subfamilies primarily on the basis of protein length and the sequence pattern of the glycine-rich cofactor-binding region close to the N terminus. According to both criteria, the products of wbmF, wbmG and wbmH, which are predicted to have 357, 310 and 313 amino acids, respectively, and all have the GxxGxxG motif near the N terminus, are extended SDRs2 (Fig. 2).
Fig. 2.
Conserved motifs in extended SDR enzymes. Sections from Wbm SDR protein sequences are aligned with the characteristic motifs of the extended SDR subfamily. Except for the alignments with Wbm sequences, the information in this table is from Ref. 2. Lowercase letters represent conserved biophysical properties of amino acid side chains: x, any residue; h, hydrophobic; c, charged; p, polar; a, aromatic. Conservation of motif features in Wbm proteins is indicated by shading.
WbmG and WbmH are closely related to each other, sharing 38% amino acid identity. WbmF, WbmG and WbmH are all homologous to Pfam01370 (NAD-dependent epimerase/dehydratase family). The most closely related, characterised homologues of these Wbm SDRs in the protein databases are deoxythymidine diphosphate (dTDP)-glucose 4,6-dehydratases from Escherichia coli and Streptomyces venezuelae with 27% or 28% sequence identity (Table 1).19 This level of sequence conservation between members of the SDR family does not imply conservation of either catalytic chemistry or substrate; rather this functional information can be better determined by examination of the residues at key positions in the active sites and binding pockets. Several conserved motifs within the extended SDR subfamily facilitate alignment of sections of the Wbm SDR sequences with homologues (Fig. 2) but many of the critical functional amino acids are located outside of these motifs, and with this low level of conservation, it is not possible to make full sequence alignments with confidence that all of these residues are correctly identified.
Table 1.
Closest homologues of WbmF, WbmG and WbmH in the protein databases
| Protein | Closest homologue | Sequence identity (%) | References |
|---|---|---|---|
| WbmF | dTDP-Glc 4,6-dehydratase, RffG, E. coli | 27 | 19 |
| WbmG | dTDP-Glc 4,6-dehydratase, DesIV, S. venezuelae | 28 | 23 |
| WbmH | dTDP-Glc 4,6-dehydratase, DesIV, S. venezuelae | 28 | 23 |
The sequence identity reported between each Bordetella protein and its closest homologue is based on the alignments produced by a third-iteration position-specific iterated BLAST.
Crystallisation of His6-WbmF, His6-WbmG and His6-WbmH
Thus far the data implicated each of these wbm SDRs in O-antigen synthesis, but did not indicate precise roles for each gene. Therefore, in order to elucidate their individual functions we characterised the gene products by X-ray crystallography. The overexpression, purification and crystallisation of these proteins are described elsewhere.20 The structures discussed in the present work are summarised in Table 2.
Table 2.
X-ray structures refinement data
| PDB ID |
2PZJ |
2Q1T |
2Q1U |
2Q1S |
2PZK |
2PZL |
2PZM |
2Q1W |
|---|---|---|---|---|---|---|---|---|
| Crystal |
WbmF-NAD+ |
WbmF-NAD+-UDP soak |
WbmF-NAD+-UDP co-crystal |
WbmF-NADH |
WbmG-GDP-Man soak |
WbmG-UDP-glucose soak |
WbmG-UDP co-crystal |
WbmH native |
| Ligands | NAD+ | NAD+, UDP | NAD+, UMP | NADH | NAD | NAD, UMP | NAD, UDP | NAD |
| Refinement | ||||||||
| Resolution (Å) | 40–1.90 | 30–1.75 | 50–1.70 | 50–1.50 | 30–2.10 | 30–2.40 | 30–2.00 | 80–2.20 |
| No. unique reflections | 27,275 | 33,895 | 81,311 | 57,271 | 38,661 | 27,549 | 51,158 | 49,975 |
| No. reflections used in refinement | 25,919 | 32,210a | 77,250a | 54,327 | 36,601a | 26,162 | 48,524a | 47,385 |
| Rwork/Rfree | 16.1/20.4 | 18.3/21.9 | 17.7/21.1 | 19.1/22.6 | 18.8/24.2 | 18.6/23.5 | 17.1/22.1 | 17.9/22.7 |
| No. atoms | 2774 | 2835 | 5791 | 2887 | 5048 | 4954 | 5359 | 7267 |
| Protein | 2538 | 2557 | 5154 | 2596 | 4643 | 4670 | 4750 | 6820 |
| Ligand/ion | 44 | 69 | 142 | 44 | 89 | 130 | 136 | 132 |
| Water | 192 | 209 | 495 | 247 | 316 | 154 | 473 | 415 |
| B-factors | ||||||||
| Protein | 30.5 | 41.3 | 19.7 | 44.9 | 34.3 | 34.6 | 27.2 | 29.1 |
| Ligand/ion | 21.2 | 35.6 | 27.8 | 27.4 | 27.5 | 27.8 | 39.7 | 24.4 |
| Water | 36.9 | 40.3 | 38.2 | 45.0 | 38.8 | 33.3 | 39.4 | 31.8 |
| RMSD | ||||||||
| Bond lengths (Å) | 0.014 | 0.015 | 0.015 | 0.014 | 0.014 | 0.015 | 0.017 | 0.016 |
| Bond angles (°) | 1.46 | 1.75 | 1.54 | 1.63 | 1.60 | 1.59 | 1.70 | 1.51 |
| Ramachandran plotb | ||||||||
| Favoured (%) | 98.1 | 98.2 | 98.9 | 98.8 | 98.4 | 97.2 | 97.9 | 97.3 |
| Allowed (%) | 1.5 | 1.5 | 1.1 | 0.9 | 1.1 | 2.1 | 1.6 | 2.4 |
| Outliers (%) | 0.3 | 0.3 | 0.0 | 0.3 | 0.5 | 0.7 | 0.5 | 0.3 |
Data collection statistics have been previously described.20R = ∑<Fobs − Fcalc>/∑<Fobs>.
The free set of reflections was included in the final round of refinement.
Ramachandran plot analysis was performed using RAMPAGE (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) for which expected values for well-refined, high-resolution structures are 98% favoured, 2% allowed.65
General architecture of His6-WbmF, His6-WbmG and His6-WbmH
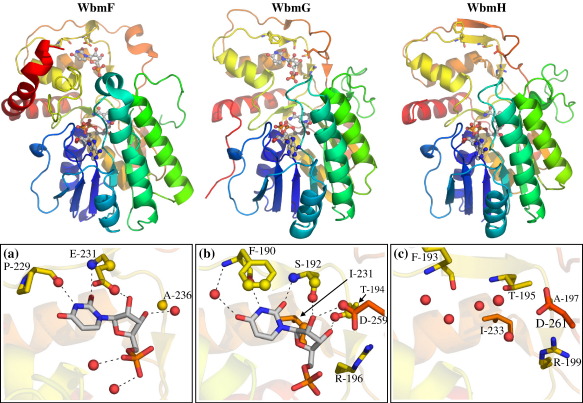
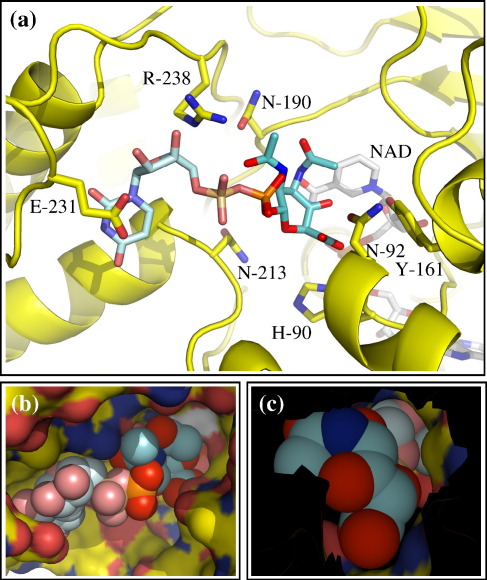
These proteins all exhibit the typical SDR family architecture (Fig. 3). The structures each comprise two domains. The first of these is the N-terminal Rossmann fold domain in which a central β sheet is flanked by two layers of α helices. The cofactor-binding motif GxxGxxG is located at the C-terminal edge of this β sheet, which has seven parallel β strands running in the order 3, 2, 1, 4, 5, 6, 7. The second domain is largely made up of C-terminal sequence and contains all of the residues involved in binding of the nucleotide portion of the substrate. The catalytic sites in SDR enzymes are located at the interface of these two domains where, in sugar-nucleotide-modifying enzymes, the substrate hexose is brought into proximity with the cofactor nicotinamide ring. The main architectural differences between these three proteins lie in their C-terminal regions (Fig. 3). The C terminus of His6-WbmG consists of a loop that stretches out from the C-terminal domain to interact with the Rossmann domain. In His6-WbmH, this loop is not visible in the density, although this difference may not reflect any distinction between the real structures of the two proteins in solution. In WbmF, the last 30 residues form a large bent helix that covers two faces of the C-terminal domain. There is also an insertion of 14 amino acids in WbmF relative to the other structures, including all residues from Gly198 to Arg212. This extra loop extends over the nicotinamide end of the cofactor-binding pocket.
Fig. 3.
Nucleotide binding in WbmF, WbmG and WbmH. Proteins are oriented with the Rossmann domains in the lower part of each structure. Cartoons are used to represent protein backbone; bound nucleotides are shown as ball-and-stick and key protein side chains as sticks. The boxed panels show details of UMP-binding pockets in (a) His6-WbmF, UDP co-crystal, (b) His6-WbmG crystal soaked with UDP-glucose and (c) His6-WbmH. In (a) and (b), UMP is shown as sticks, and spheres represent atoms within 3.5 Å of the bound nucleotide. Probable hydrogen-bonding interactions are shown as dashed lines. In (c) spheres represent ordered water molecules in this binding pocket. Carbon atoms are coloured rainbow for protein, white for bound nucleotides; oxygen atoms are coloured red; nitrogen is blue and phosphorus orange.
SDRs typically form dimers or higher oligomers. The crystal packing of these proteins suggests that WbmF, WbmG and WbmH have the conserved four-helix bundle usually found at the dimer interface. Analytical gel filtration chromatography indicated a hydrodynamic radius for each of these proteins consistent with either a monomer or dimer (data not shown). We conclude, therefore, that these proteins are present in solution predominantly in dimer form. In the WbmG and WbmH structures, the dimer interaction buries 1316 Å2 (5.7% of total protein surface) and 1562 Å2 (7.2%) of surface per dimer, respectively. In the WbmF structure the interface region is extended to include a short antiparallel β sheet composed of the residues Ile151 to Ser153 from each monomer as well as the loops that connect these strands to the bundle (Leu154 to Pro160); 2131 Å2 is buried in the WbmF dimer interface, which is equivalent to 8.6% of the total protein surface. In all three cases, the majority of the residues buried at these interfaces are hydrophobic or aromatic.
Cofactor binding
NAD can be modelled into the electron density in all three proteins. His6-WbmG and His6-WbmH copurify with this compound from the E. coli expression host, but in order to stabilise the protein in solution His6-WbmF required the addition of exogenous cofactor,20 and this is the most likely source of this molecule in these His6-WbmF structures. The preference in each case for NAD cofactor as opposed to NADP is indicated by the close interaction (2.7 Å hydrogen bonds) of an aspartate side chain with the 2′-hydroxyl of the adenylic ribose. This side chain would disfavour binding of NADP by both steric and electrostatic repulsion of the NADP 2′-phosphate. In some SDR structures [e.g., GDP-4-keto-6-deoxymannose 3,5-epimerase/reductase (GMER)21 and RmlD22] the bound cofactor has direct access to solvent, indicating a pathway via which spent NAD/NADP can be replaced from solution. In these structures, the bound cofactor is buried within the core of the protein; in the case of the His6-WbmF structure, the 14-amino-acid insertion supplies an additional occluding loop, further blocking release of cofactor in this conformation.
Binding of substrate
Soaking and co-crystallisation experiments with His6-WbmF and His6-WbmG revealed the binding mode for the nucleotide portion of the sugar nucleotide substrates of these enzymes (Fig. 3). The X-ray data from a His6-WbmG crystal soaked with UDP-glucose revealed extra density that could be modelled as far as the α-phosphate. The location of this molecule is consistent with the nucleotide-binding site for SDR homologue structures and with the hydrogen-bonding chemistry expected to bind uridine. No density was visible corresponding to the hexose portion of the soaked compound nor the second phosphate, suggesting that either this portion of the molecule is disordered within the crystal, or the compound was partially hydrolysed and the smaller uridine monophosphate (UMP) preferentially soaked into the substrate-binding pocket. In either case, the data indicate that the glucose-1-phosphate moiety of UDP-glucose is not strongly bound in a single conformation by WbmG. A similar soaking experiment but with GDP-mannose did not indicate any binding of this sugar nucleotide (data not shown), indicating a possible preference for the uracil base. The hydrogen bonding between the WbmG pocket and UMP suggests a basis for this preference (Fig. 3b). Although we were unable to obtain any complex of WbmH with a substrate analogue bound, the nucleotide-binding domain of WbmH is conserved in sequence and structure with WbmG, implying identical specificity and a common binding mode for the nucleoside portion of their substrates (Fig. 3c). UDP density to the α-phosphate is also visible in the WbmF/NAD/UDP ternary complex. The UMP density in this structure and the chemistry of its interaction with the protein suggests that this protein, like WbmG and WbmH, recognises a UDP-sugar substrate.
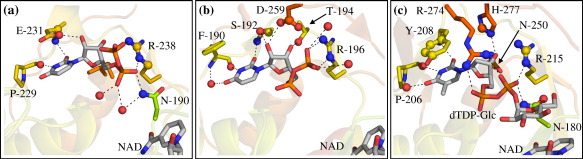
Both α and β nucleotide phosphates could be modelled in the data from a UDP soak into a WbmF-NAD+ co-crystal and co-crystallisation of His6-WbmG with UDP. In both structures the β-phosphate is coordinated by a conserved arginine (Fig. 4a and b), although the precise location of this phosphate is different in the two structures and both are inconsistent with the β-phosphate positions of bound substrate in the structure of a D128N, E129Q mutant of dTDP-glucose 4,6-dehydratase, DesIV, from S. venezuelae (Fig. 4c).23 In this DesIV structure [Protein Data Bank (PDB) ID 1R6D] the position of the substrate is consistent with the necessary overlap of molecular orbitals required for hydride transfer from the glucose C-4 to the cofactor. It is likely, therefore, that the β-phosphate positions in WbmF and WbmG structures do not represent the positions of equivalent phosphates when the native substrates for these enzymes are bound in the active site.
Fig. 4.
The UDP-binding pockets are shown for (a) His6-WbmF, soaked with UDP and (b) the His6-WbmG, UDP co-crystal. Spheres represent atoms within 3.5 Å of the bound UDP; NAD indicates the nicotinamide ring of the NAD cofactor. (c) dTDP-glucose bound in the active site of a D128N, E129Q mutant of dTDP-glucose 4,6-dehydratase DesIV from S. venezuelae (PDB ID 1R6D).23 The substrate-binding pockets are all shown from the same angle to enable comparison of the relative positions of the UDP diphosphates in (a) and (b) with the phosphates in dTDP-glucose in (c).
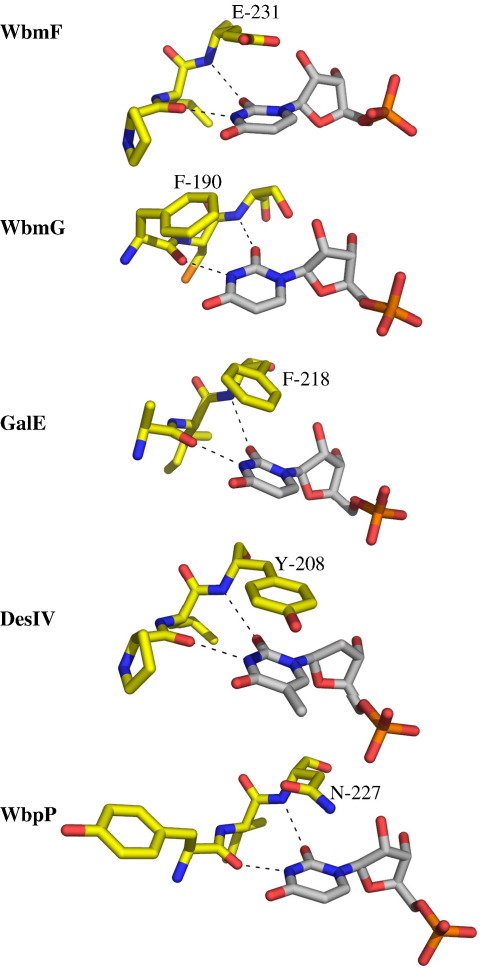
One common feature of substrate binding in SDR enzymes is pi stacking of the nucleobase with an amino acid side chain. In WbmF, this interaction involves Glu231 (Fig. 3a). Unusually, in WbmG the pi-stacking residue (Phe190) is the one that hydrogen bonds with the uracil N-3 (Fig. 3b). In WbmF, E. coli UDP-galactose 4-epimerase (GalE),24 S. venezuelae DesIV,23 and Pseudomonas aeruginosa WbpP,25 the hydrophobic contact is provided by the residue in the n + 2 position, whose backbone amide hydrogen bonds with the base's O2 group (Fig. 5). This unusual feature of the substrate binding in WbmG could not have been predicted from sequence alignments alone.
Fig. 5.
Pi-stacking interactions in binding of uracil or thymine. Amino acids that directly interact with the substrate nucleobase are shown for B. bronchiseptica WbmF and WbmG, E. coli GalE, S. venezuelae DesIV and P. aeruginosa WbpP with carbon atoms coloured yellow. UMP or dTDP from each structure is shown with carbon atoms in white. Amino acid side chains that have pi-stacking interactions with the base are labelled, and hydrogen-bonding interactions with the peptide backbone are indicated as dashed lines. Images for GalE, DesIV and WbpP were prepared using PDB files 1LRL,241R6D23 and 1SB8,25 respectively.
Potential catalytic chemistry
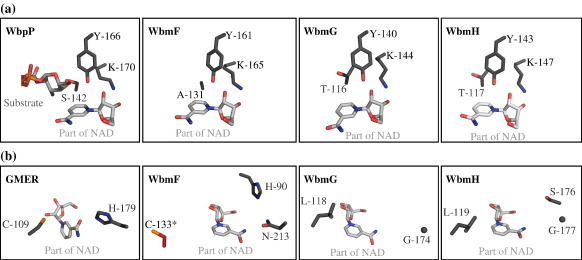
SDR enzymes have a conserved catalytic triad that is involved in their oxidoreductase activity and that is classically composed of a spatially conserved serine, tyrosine and lysine (SYK) triad, for example, Ser142, Tyr166 and Lys170 in WbpP. The first member of this triad is sometimes found as a threonine (TYK),23 and the tyrosine can be replaced by a methionine (SMK).26 The SDR triad is conserved in WbmG and WbmH as TYK but in WbmF Ala131 superimposes onto the Ser/Thr position (Fig. 6a).
Fig. 6.
Comparison of active-site residues in WbmF, WbmG and WbmH compared with WbpP25 and GMER. (a) The SDR catalytic triad is conserved in WbmG and WbmH but not in WbmF where the residue normally found as serine or threonine superimposes onto the position of Ala131. (b) GMER is shown as an example of an SDR that catalyses 3,5-epimerisation of its substrate.21 The GMER epimerase catalytic residues Cys109 and His179 superimpose onto hydrophobic residues in WbmG and WbmH. WbmF has side chains in these positions (Cys133 and Asn213) that may be capable of acid–base chemistry. *Cys133 is not resolved in the WbmF crystal; its position here is inferred from the neighbouring residue (Gly132), which is visible in the electron density.
In the context of O-antigen synthesis in Bordetella we are interested in the potential of the wbm SDR enzymes to catalyse secondary reactions of their substrates beyond the oxidoreductase reactions common to all SDRs. Of particular significance is the identification of a potential 3,5-epimerase. Despite the lack of a structure in which a full sugar nucleotide substrate analogue is bound, the cavity into which the substrate hexose binds is easily identifiable by comparison with homologue structures and because it is necessarily interposed between the UMP-binding site and the cofactor nicotinamide ring. The active sites of WbmG and WbmH are devoid of amino acid side chains capable of acid–base chemistry except for their respective TYK triads and, in WbmH, Ser176. SDR enzymes that catalyse 3,5-epimerisation of their substrates have a spatially conserved catalytic cysteine and a basic side chain. In GMER these residues are Cys109 and His179;21 in GDP-mannose 3,5-epimerase (GME) they are Cys145 and Lys217.12 In WbmG and WbmH these amino acids superimpose onto hydrophobic side chains, but in WbmF this catalytic cysteine is conserved (Cys133) and there are two candidates for the basic side chain, Asn213 and His90 (Fig. 6b). Cys133 is disordered in all of our WbmF crystals, indicating that an element of induced fit may be required to bring all of the active-site components into place. His90 has a very unusual backbone conformation, with a cis peptide bond to the following residue. This is strongly suggestive of a functional role for this residue, since such conformations are rarely observed except where they are required to place a key side chain in the active site.
Modelling of the putative substrate into the WbmF active site
The analysis of the potential catalytic chemistry of WbmF suggested that its role in O-antigen biosynthesis may be to catalyse the 3,5-epimerisation required in the overall conversion of UDP-d-ManNAc3NAcA to UDP-l-GalNAc3NAcA. The 4-keto derivative of UDP-d-ManNAc3NAcA is therefore a likely substrate or reaction intermediate for WbmF. This compound contains a bulkier sugar than any of the substrates of the characterised sugar-nucleotide-modifying SDRs. An important test of our proposed pathway is that the enzymes must be able to accommodate these unusually large substrates. We modelled 4-keto UDP-d-ManNAc3NAcA into the active site of WbmF (Fig. 7). This showed that it is feasible for the substrate to occupy the active site of WbmF in a manner that is consistent with the experimentally determined binding site for UMP, the proximity of the most likely catalytic site, and good geometry.
Fig. 7.
A model of the proposed substrate in the binding site of WbmF. (a) Overview of modelled interaction of WbmF with the 4-keto derivative of UDP-d-ManNAc3NAcA. Protein is shown as cartoon, with key (labelled) side chains, NAD and sugar nucleotide in sticks. The elements of the UDP-sugar found in the experimental structure have paler colours to highlight the modelled sections. (b) and (c) Close-up of the modelled sugar ring (shown as spheres) demonstrates that there are no clashes between the proposed ligand and the protein surface. The two views show opposite sides of the sugar: (c) shows the surface with the imaged slab cut at the level of the first sugar atom, with NAD surface also removed for clarity. Carbon atoms are yellow for protein, white for the NAD and cyan for 4-keto UDP-d-ManNAc3NAcA; oxygen is red, nitrogen is blue, phosphorus is orange and sulfur is purple.
Discussion
The abrogation or reduction of O-antigen expression as a result of wbmF, wbmG or wbmH mutations implicates all three genes in the biosynthesis of this molecule, and is consistent with the hypothesis that these three adjacent SDR genes catalyse the UDP-d-ManNAc3NAcA to UDP-l-GalNAc3NAcA conversion, which is probably required for O-antigen synthesis.
Structural analysis of these three wbm SDR gene products also enables us to distinguish between them in terms of potential catalytic chemistry. Both WbmG and WbmH have the conserved SDR catalytic triad (TYK in these cases), which suggests that they should be competent to function as oxidoreductases. In both of these enzymes, the pocket that surrounds the active site is hydrophobic and featureless compared with other SDR enzymes; the lack of side chains capable of acid–base chemistry indicates that the functions of these enzymes are likely to be limited to oxidation and/or reduction and probably rules out specific 3,5-epimerase activity. The hydroxyl of Ser176 in WbmH may be capable of facilitating proton exchange; however, an amino acid pair capable of acid–base chemistry is required for 3,5-epimerase function.
Some SDRs that catalyse both oxidation and reduction bind cofactor irreversibly and recycle it within the active site.27 In other SDRs, this binding is reversible and the cofactor-binding site is situated at the bottom of a solvent-accessible groove (e.g., in E. coli GMER, PDB ID 1BWS).28 No such groove is visible in the WbmF, WbmG and WbmH structures, where the cofactor-binding sites are almost completely occluded. However, we do not take this observation as being diagnostic of irreversible cofactor binding in these proteins for the following reasons. Firstly, exogenous cofactor is required for WbmF to be stable in solution with respect to precipitation.20 Presumably either cofactor that copurifies with WbmF can be released, resulting in unfolding and aggregation of the protein, or apo-WbmF must bind cofactor supplied from the medium in order to fold correctly. Either case would suggest that NAD can be exchanged between WbmF and solvent. Secondly, human GalE reversibly binds cofactor,29 and yet does not have an open binding site like that of GMER. Finally, a conversion of configuration from d-manno to l-galacto can be catalysed by a single tight NAD+-binding enzyme such as GME,12 but we cannot envisage a pathway in which two or three such enzymes contribute to this transformation. Instead, we suggest that the protein loops that cover the NAD-binding site may be flexible and move to allow release and replacement of spent cofactor.
WbmF lacks the serine/threonine of the SDR catalytic triad. The conservation of the classical SDR catalytic triad is not absolute amongst members of the family. For example, in the very unusual SDR enzyme, biliverdin IXβ reductase (which catalyses the reduction of a range of substrates including some flavins and non-α-isomers of biliverdin), a histidine occupies the usual place of the catalytic lysine and tyrosine residues.30 The shapes and electron delocalisation exhibited by biliverdin IXβ reductase substrates imply that compared with the putative substrates of the Wbm SDR enzymes, they will have rather special requirements for binding geometry and reaction intermediate stabilisation during catalysis. In those SDRs that oxidise or reduce sugar nuclotides at the hexose C-4 position, however, the catalytic triad serine/threonine residue is important because it modifies the pKa of the C-4 hydroxyl by forming a short hydrogen bond.23 Mutation of the triad threonine (Thr134) to alanine in E. coli dTDP-Glc 4,6-dehydratase results in a 200-fold drop in kcat,31 whereas mutation of Ser124 to alanine in E. coli GalE reduces epimerase activity (kcat) almost 3000-fold.32 The incomplete WbmF catalytic triad raises doubts about the competence of this enzyme as a sugar nucleotide oxidoreductase. It is possible that the function of the missing side chain is provided by another active-site residue; this implies a different substrate-binding geometry and further studies will be required to investigate this possibility. Conversely, if a standard substrate-binding orientation is assumed, the active site of WbmF resembles that of 3,5-epimerases and our structures of this protein identify Cys133 and either Asn213 or His90 as potential mediators of proton exchange in the epimerisation reaction—one group abstracts a proton from the sugar while the other donates a proton to the opposite face.
By modelling the compound 4-keto UDP-d-ManNAc3NAcA into the WbmF structure we have demonstrated that there is space within this active site to accommodate this bulky sugar nucleotide. Other solutions produced in the modelling process demonstrated alternative plausible substrate positions (data not shown). For this reason, we do not suggest that the solution presented here (Fig. 7) represents the true active conformation. While we did not attempt similar modelling experiments with WbmG and WbmH, visual inspection of these structures indicates that they also have spacious active sites compared with other SDRs and can therefore accommodate diacetamido uronic acids in their hexose-binding pockets.
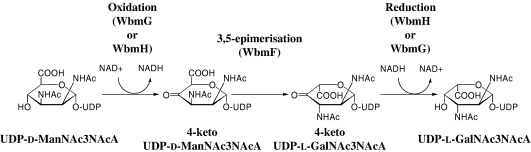
We therefore propose the following biosynthetic pathway (Fig. 8) for the 3,5-epimerisation of UDP-d-ManNAc3NAcA: One of the putative oxidoreductases, WbmG or WbmH, catalyses the initial oxidation at C-4, and the other oxidoreductase is responsible for the final reduction step. Introduction of the keto group lowers the pKa of the C-3 and C-5 hydrogen atoms, allowing WbmF to catalyse proton exchange effecting 3,5-epimerisation. This scheme is the most consistent with all of the available data. The function suggested for WbmF is very unusual in that we suggest that this enzyme does not catalyse oxidation or reduction of its substrate. If this is the case, WbmF would be the first such member of the SDR family, and this would imply that the cofactor in WbmF has a purely structural role, without participating in the catalytic cycle. It has been suggested that the transcriptional regulation protein NmrA from Aspergillus nidulans is a NAD-binding SDR family member that is not an enzyme at all. NmrA appears to function in control of nitrogen metabolism through physical interaction with the GATA family transcription factor AreA, possibly by controlling the rate of entry of AreA into the nucleus,33 and the structural equivalents of the SDR triad tyrosine and serine/threonine in NmrA are Met127 and Met113, respectively.34 Since the NmrA structure was reported, however, several SDR enzymes with SMK triads have been reported (e.g., WbpM from P. aeruginosa),26 so that the nonconservation of the SDR catalytic triad in NmrA should not rule out an enzyme function as absolutely as first appeared.34 An alternative possibility to the biosynthetic pathway we have outlined (Fig. 8) is that WbmF does in fact catalyse either oxidation or reduction as well as the 3,5-epimerisation, in which case the third SDR may participate in O-antigen expression by performing a function in the synthesis of the complicated sugar residue that adorns the nonreducing terminus of the O-chain.35 The nonredox role we propose for WbmF is consistent, however, with the lack of the conserved SDR triad in this enzyme, and if this protein operates purely as a 3,5-epimerase this may also suggest a mechanism by which the wbmF mutant is able to express O antigen, albeit in greatly reduced amounts. The product of the initial oxidation reaction is activated for proton exchange α- to the keto group and it may be that racemisation at these positions occurs at a biologically significant rate without an absolute requirement for enzyme catalysis.
Fig. 8.
Proposed pathway for the synthesis of UDP-l-GalNAc3NAcA catalysed by WbmF, WbmG and WbmH.
Recent structural studies of the 3,5-epimerase in the dTDP-rhamnose pathway, RmlC,36 suggest that the rate-limiting step in its reaction involves a transition state in which the anomeric linkage is in the disfavoured equatorial position (which is also adopted by the reaction product), and that in order to avoid steric clashes and satisfy the requirement for axial proton abstraction, the reaction mechanism requires a twist-boat conformation such as that proposed in the GME reaction.12 Binding of substrate in a C-1 equatorial conformation may be part of the way that RmlC reduces kinetic barriers to this reaction. For the same reasons, the product of the proposed WbmF-catalysed 3,5-epimerisation will have the nucleotide attached to the l-GalNAcNAcA in the equatorial position in order to avoid 1,3,5-triaxial clashes. This transformation therefore involves considerable kinetic and thermodynamic barriers and will be slow without enzyme catalysis. However, if sufficient starting material is generated by the upstream oxidase, and the 3,5-epimerisation product is efficiently and selectively removed by the downstream reductase, the flux through this bottleneck may be sufficient to allow O-chain synthesis. Furthermore, the slower 3,5-epimerisation rate resulting from mutation of the wbmF gene may make relatively little impact on O-antigen expression levels if this conversion does not usually comprise the rate-limiting step for O-antigen synthesis. A similar effect may operate in the dTDP-l-noviose pathway in Streptomyces spheroides where observation of a naturally occurring l-rhamnoside analogue of novobiocin37 shows that 3,5-epimerisation of the dTDP-noviose precursor (dTDP-6-deoxy-d-xylo-4-hexulose) does occur despite the fact that in vitro, the 3,5-epimerisation catalysed by NovW has a 2000-fold lower kcat than RmlC38 (discussed in Ref. 39).
In conclusion, X-ray crystallography of WbmF, WbmG and WbmH enabled definition of these enzymes' NAD-cofactor preference and the substrate nucleotide specificity. We have been able to distinguish the likely function of WbmF from WbmG and WbmH, but cannot differentiate the roles of WbmG and WbmH. In order to achieve this, these proteins must be characterised in vitro. This will require chemical synthesis of UDP-d-ManNAc3NAcA and is beyond the scope of the present study. Subject to future experimental verification of predictions we have made, our data illustrate the usefulness of structural studies for investigating such challenging problems. While they also show why this methodology cannot universally supplant direct in vitro characterisation, this kind of structural analysis will prove essential for researchers to make full use of the gene databases.
Materials and Methods
Bacterial strains, plasmids and culture conditions
Bacterial strains used in this study are described in Table 3. Bordetella was grown on Bordet–Gengou agar (Difco) supplemented with 10% defibrinated horse blood (TCS Cellworks Ltd). E. coli was cultured in Luria–Bertani (LB) broth or on LB agar. All strains were incubated at 37 °C and ampicillin (100 μg ml− 1), kanamycin (50 μg ml− 1), tetracycline (10 μg ml− 1 for E. coli, 5 μg ml− 1 for B. bronchiseptica) or streptomycin (200 μg ml− 1) were added where required. Suicide plasmids were based on the host-restricted pEX100T backbone40 and broad host range shuttle vectors were based on a Kmr derivative of pBBR1MCS.41 For preparation of LPS, B. bronchiseptica was grown in medium supplemented with 50 mM MgSO4, as this maximises O-antigen expression in this strain.
Table 3.
Bacterial strains
| Strain | Description or genotype | Reference |
|---|---|---|
| E. coli SM10λpir | thi thr leu tonA lacY supE recA∷RP4-2-Tc∷Mu kanrλpir | 44 |
| E. coli CC118 | araD139, Δ(ara, leu)7697, ΔlacX74, phoAΔ20, galE, galK, thi, rpsE, rpoB, argEam, recA1 | 46 |
| E. coli S17-1 pNJ5000 | RP4 Res−, tra+, Tcr, pri, PstIC− | 44,47 |
| B. bronchiseptica CN7635E | Wild type, Smr | 51 |
| B. bronchiseptica CNF0a | wbmF∷Tcr, Smr | This study |
| B. bronchiseptica CNG1a | wbmG∷Tcr, Smr | This study |
| B. bronchiseptica CNH1d | wbmH∷Tcr, Smr | This study |
DNA methods
Standard methods were used for DNA manipulations. Oligonucleotides were supplied by Sigma-Genosys. PCR was performed with template from boiled bacteria42 and Taq DNA polymerase (Promega) or KOD Hot Start DNA polymerase (Novagen).
Construction of wbmF, wbmG and wbmH mutants
The multiple cloning site (MCS) from pBluescript II SK+ (Stratagene) was excised using SacI and KpnI, blunt-ended and ligated into SmaI-cut pEX100T, to generate the MCS-containing suicide vector pEXMCS. The wbmF allelic exchange construct was prepared as follows. The wbmF gene in the plasmid Bb540g06 (a clone generated in the B. bronchiseptica RB50 genome sequencing project)43 was disrupted by insertion of a blunt-ended tetracycline-resistance (Tc) cassette into the internal NsiI site. The mutant allele was excised using Acc65I and XbaI, blunt ended, and then cloned into EcoRV-digested pEXMCS. For wbmG, the gene was amplified using primers 5′-ATATCTAGACATATGCGTATTCTGATCACCG-3′ (XbaI site underlined) and 5′-ATAAAGCTTTGATTACTGGCAACTCTTC-3′, and the PCR product was topoisomerase cloned into pCR2.1-TOPO using a TOPO-cloning kit (Invitrogen). wbmG was excised using XbaI and cloned into the XbaI site in pEXMCS. The wbmG gene was then disrupted by cloning a blunt-ended Tc cassette into the internal Acc65I site. The wbmH mutant allele was obtained by in vitro transposon-mediated mutagenesis of the wbm locus-containing cosmid, BbLPS118 (accession number AJ007747) using an EZ-Tn5™ <Tet-1> insertion kit (Epicentre). The <Tet-1> transposon, plus flanking wbmH DNA, was cut out by partial digestion with AluI and ligated into SmaI-cut pEX100T. Allelic exchange constructs were electroporated into E. coli SM10λpir and transferred to B. bronchiseptica by conjugation with E. coli SM10λpir as donor.44 Loss of the plasmid-encoded sacB gene in allelic exchange mutagenesis of B. bronchiseptica was selected for by growth on LB agar with reduced salt concentration and supplemented with 10% (w/v) sucrose.45
Complementation of mutations
The B. bronchiseptica flaA promoter was amplified using primers 5′-GCTCTAGATAGGCGCATGCCATGGCC-3′ (XbaI site underlined) and 5′-AAGGATCCCATATGGAGGCTCCCAAGAGAGAA-3′ (BamHI and NdeI sites underlined), and cloned into the XbaI and BamHI sites in pBBR1MCS-kan to generate the vector pCompEmpty. wbmF was amplified using primers 5′-AAAAAAACATATGTTGCCAGTAATCATGAATGC-3′ (NdeI site underlined) and 5′-AAAAAGCTTAGATCTAGTCCGCCGTCTTATTTG-3′ (HindIII site underlined) and cloned into pCompEmpty using NdeI and HindIII to generate the wbmF complementation vector pCompF. wbmG was PCR amplified using primers 5′-AAAAAAACATATGCGTATTCTGATCACC-3′ and 5′-AAAAAGCTTAGATCTGCAACTCTTCAGGTCTTG-3′ and cloned into pCompEmpty using NdeI and HindIII, generating pCompG. wbmH was amplified using 5′-GAGAATTCCATATGAAGAAAGTATTTATTACGG-3′ and 5′-AAAAAGCTTAGATCTTTGTCGATGACCAGGATT-3′ and cloned into pCompEmpty using NdeI and HindIII, generating pCompH. Shuttle vectors were moved into B. bronchiseptica by conjugation with E. coli CC118 as donor46 and trans-acting transfer functions provided by E. coli S17-1 pNJ5000 as helper.44,47
SDS-PAGE, silver stain and immunoblot analysis of LPS
LPS was obtained from B. bronchiseptica using a modification of the method of Hitchcock and Brown48 as has been described.49 SDS-PAGE of LPS was performed using Novex precast 16% tricine gels (Invitrogen). LPS was oxidised in-gel with periodic acid50 and visualised with the Silver Stain Plus kit (BioRad). Western blotting was performed as previously reported51 using the monoclonal antibody D13B11 that specifically binds the O-antigen-containing LPS of B. bronchiseptica strain CN7635E.35
X-ray crystallography
Protein production, purification, crystallisation and data collection were performed as described.20 Data were processed using DENZO and SCALEPACK (version 1.97).52 Data were truncated and converted to structure factors using TRUNCATE53 from the CCP4 Suite.54 Molecular replacement was carried out using PHASER55 as described.20 For the initial WbmG structure, phases were determined experimentally from selenomethionine-labelled protein: peak, inflection point and high-energy remote data were used to determine initial phases using the PHENIX package.56 Model building was carried out using COOT,57 and refinement was performed with REFMAC version 5.0.58 Initial models were improved using ARP/wARP version 6.1 where the data quality permitted.59 The CCP4i interface60 was used where appropriate. TLS parameters were assigned (where used) using the TLSMD server.61 CNS62 was used to perform simulated annealing. Structures were validated using COOT, PROCHECK,63 WHATCHECK,64 and RAMPAGE.65 Table 2 provides a summary of the crystallographic statistics. Protein structures were analysed using Pymol,66 COOT,57 and CCP4MG.67 Surface area calculations were performed using the Richards' Rolling Probe method.68 More extensive details of the crystallographic methods are provided in the Supplementary Data.
Modelling of putative substrate into WbmF structure
Coordinates and parameters for the 4-keto derivative of UDP-2,3-dideoxy-2,3-diacetamido-d-mannuronic acid were prepared using the CCP4 monomer library sketcher with refinement in REFMAC 5.2.58 An initial structure was prepared by modelling the proposed substrate into the structure of WbmF co-crystallised with UDP in COOT.57 The observed density was used to place the atoms present in UMP, and to place sugar atoms in positions occupied by water or glycerol in the observed structure. Different orientations were used in the two molecules in the asymmetric unit to increase the sampling of conformational space. The structure of WbmF co-crystallised with UDP, with 4-keto UDP-d-ManNAc3NAcA placed in, was truncated to include only protein, NAD and 4-keto UDP-d-ManNAc3NAcA atoms. This was used as an input to MODELLER version 9.2 to determine an optimised structure for the complex with the sugar nucleotide.69 After modelling, the ligand coordinates were regularised in COOT to correct minor distortions. Ten modelling trials were performed with different seeds, and the best was selected by eye on the basis of geometry, agreement with the determined structures and lack of bad interactions.
Protein Data Bank accession codes
X-ray amplitudes, phases and the derived atomic coordinates have been deposited with the PDB under the accession codes 2PZJ, 2Q1T, 2Q1U, 2Q1S, 2PZK, 2PZL, 2PZM and 2Q1W.
Acknowledgements
This work was supported by Wellcome Trust Programme Grant 054588 to D.J.M. J.D.K. was the recipient of a Wellcome Trust prize studentship (065482). N.J.H. was funded by Wellcome Trust Programme Grant 064597 to T.L.B. R.A.F. holds a UK Engineering and Physical Sciences Research Council grant (GR/S82046/01).
Edited by I. Wilson
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2007.09.055
Supplementary data
References
- 1.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson B., Kallberg Y., Oppermann U., Jornvall H. Coenzyme-based functional assignments of short-chain dehydrogenases/reductases (SDRs) Chem. Biol. Interact. 2003;143–144:271–278. doi: 10.1016/s0009-2797(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 3.Burns E.H., Jr, Norman J.M., Hatcher M.D., Bemis D.A. Fimbriae and determination of host species specificity of Bordetella bronchiseptica. J. Clin. Microbiol. 1993;31:1838–1844. doi: 10.1128/jcm.31.7.1838-1844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magyar T., Chanter N., Lax A.J., Rutter J.M., Hall G.A. The pathogenesis of turbinate atrophy in pigs caused by Bordetella bronchiseptica. Vet. Microbiol. 1988;18:135–146. doi: 10.1016/0378-1135(88)90059-4. [DOI] [PubMed] [Google Scholar]
- 5.Cherry J.D. Historical review of pertussis and the classical vaccine. J. Infect. Dis. 1996;174:S259–S263. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 6.Porter J.F., Connor K., Donachie W. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology. 1994;140:255–261. doi: 10.1099/13500872-140-2-255. [DOI] [PubMed] [Google Scholar]
- 7.Preston A., Petersen B.O., Duus J.O., Kubler-Kielb J., Ben-Menachem G., Li J., Vinogradov E. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J. Biol. Chem. 2006;281:18135–18144. doi: 10.1074/jbc.M513904200. [DOI] [PubMed] [Google Scholar]
- 8.Preston A., Thomas R., Maskell D.J. Mutational analysis of the Bordetella pertussis wlb LPS biosynthesis locus. Microb. Pathog. 2002;33:91–95. doi: 10.1006/mpat.2002.0511. [DOI] [PubMed] [Google Scholar]
- 9.Di Fabio J.L., Caroff M., Karibian D., Richards J.C., Perry M.B. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol. Lett. 1992;76:275–281. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 10.Burns V.C., Pishko E.J., Preston A., Maskell D.J., Harvill E.T. Role of Bordetella O antigen in respiratory tract infection. Infect. Immun. 2003;71:86–94. doi: 10.1128/IAI.71.1.86-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westman E.L., McNally D.J., Rejzek M., Miller W.L., Kannathasan V.S., Preston A. Identification and biochemical characterization of two novel UDP-2,3-diacetamido-2,3-dideoxy-alpha-d-glucuronic acid 2-epimerases from respiratory pathogens. Biochem. J. 2007;405:123–130. doi: 10.1042/BJ20070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major L.L., Wolucka B.A., Naismith J.H. Structure and function of GDP-mannose-3′,5′-epimerase: an enzyme which performs three chemical reactions at the same active site. J. Am. Chem. Soc. 2005;127:18309–18320. doi: 10.1021/ja056490i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsburg V. Formation of guanosine diphosphate l-fucose from guanosine diphosphate d-mannose. J. Biol. Chem. 1960;235:2196–2201. [PubMed] [Google Scholar]
- 14.Ginsburg V. Studies on the biosynthesis of guanosine diphosphate l-fucose. J. Biol. Chem. 1961;236:2389–2393. [PubMed] [Google Scholar]
- 15.Giraud M.F., Naismith J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000;10:687–696. doi: 10.1016/s0959-440x(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 16.Tonetti M., Sturla L., Bisso A., Zanardi D., Benatti U., De Flora A. The metabolism of 6-deoxyhexoses in bacterial and animal cells. Biochimie. 1998;80:923–931. doi: 10.1016/s0300-9084(00)88889-6. [DOI] [PubMed] [Google Scholar]
- 17.Field R.A., Naismith J.H. Structural and mechanistic basis of bacterial sugar nucleotide-modifying enzymes. Biochemistry. 2003;42:7637–7647. doi: 10.1021/bi0345079. [DOI] [PubMed] [Google Scholar]
- 18.Preston A., Allen A.G., Cadisch J., Thomas R., Stevens K., Churcher C.M. Genetic basis for lipopolysaccharide O-antigen biosynthesis in bordetellae. Infect. Immun. 1999;67:3763–3767. doi: 10.1128/iai.67.8.3763-3767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegeman A.D., Gross J.W., Frey P.A. Probing catalysis by Escherichia coli dTDP-glucose-4,6-dehydratase: identification and preliminary characterization of functional amino acid residues at the active site. Biochemistry. 2001;40:6598–6610. doi: 10.1021/bi010441a. [DOI] [PubMed] [Google Scholar]
- 20.Harmer N.J., King J.D., Palmer C.M., Preston A., Maskell D.J., Blundell T.L. Cloning, expression, purification and preliminary crystallographic analysis of the short-chain dehydrogenase enzymes WbmF, WbmG and WbmH from Bordetella bronchiseptica. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2007;63:711–715. doi: 10.1107/S174430910703477X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosano C., Bisso A., Izzo G., Tonetti M., Sturla L., De Flora A., Bolognesi M. Probing the catalytic mechanism of GDP-4-keto-6-deoxy-d-mannose epimerase/reductase by kinetic and crystallographic characterization of site-specific mutants. J. Mol. Biol. 2000;303:77–91. doi: 10.1006/jmbi.2000.4106. [DOI] [PubMed] [Google Scholar]
- 22.Blankenfeldt W., Kerr I.D., Giraud M.F., McMiken H.J., Leonard G., Whitfield C. Variation on a theme of SDR. dTDP-6-deoxy-l-lyxo-4-hexulose reductase (RmlD) shows a new Mg2+-dependent dimerization mode. Structure. 2002;10:773–786. doi: 10.1016/s0969-2126(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 23.Allard S.T., Cleland W.W., Holden H.M. High resolution X-ray structure of dTDP-glucose 4,6-dehydratase from Streptomyces venezuelae. J. Biol. Chem. 2004;279:2211–2220. doi: 10.1074/jbc.M310134200. [DOI] [PubMed] [Google Scholar]
- 24.Thoden J.B., Henderson J.M., Fridovich-Keil J.L., Holden H.M. Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase. Teaching an old dog new tricks. J. Biol. Chem. 2002;277:27528–27534. doi: 10.1074/jbc.M204413200. [DOI] [PubMed] [Google Scholar]
- 25.Ishiyama N., Creuzenet C., Lam J.S., Berghuis A.M. Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa: substrate specificity in UDP-hexose 4-epimerases. J. Biol. Chem. 2004;279:22635–22642. doi: 10.1074/jbc.M401642200. [DOI] [PubMed] [Google Scholar]
- 26.Creuzenet C., Lam J.S. Topological and functional characterization of WbpM, an inner membrane UDP-GlcNAc C6 dehydratase essential for lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2001;41:1295–1310. doi: 10.1046/j.1365-2958.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- 27.Thoden J.B., Frey P.A., Holden H.M. Crystal structures of the oxidized and reduced forms of UDP-galactose 4-epimerase isolated from Escherichia coli. Biochemistry. 1996;35:2557–2566. doi: 10.1021/bi952715y. [DOI] [PubMed] [Google Scholar]
- 28.Rizzi M., Tonetti M., Vigevani P., Sturla L., Bisso A., Flora A.D., Bordo D., Bolognesi M. GDP-4-keto-6-deoxy-d-mannose epimerase/reductase from Escherichia coli, a key enzyme in the biosynthesis of GDP-l-fucose, displays the structural characteristics of the RED protein homology superfamily. Structure. 1998;6:1453–1465. doi: 10.1016/s0969-2126(98)00144-0. [DOI] [PubMed] [Google Scholar]
- 29.Thoden J.B., Wohlers T.M., Fridovich-Keil J.L., Holden H.M. Crystallographic evidence for Tyr 157 functioning as the active site base in human UDP-galactose 4-epimerase. Biochemistry. 2000;39:5691–5701. doi: 10.1021/bi000215l. [DOI] [PubMed] [Google Scholar]
- 30.Pereira P.J., Macedo-Ribeiro S., Parraga A., Perez-Luque R., Cunningham O., Darcy K. Structure of human biliverdin IXβ reductase, an early fetal bilirubin IXβ producing enzyme. Nat. Struct. Biol. 2001;8:215–220. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 31.Gerratana B., Cleland W.W., Frey P.A. Mechanistic roles of Thr134, Tyr160, and Lys164 in the reaction catalyzed by dTDP-glucose 4,6-dehydratase. Biochemistry. 2001;40:9187–9195. doi: 10.1021/bi0108249. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Thoden J.B., Kim J., Berger E., Gulick A.M., Ruzicka F.J. Mechanistic roles of tyrosine 149 and serine 124 in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry. 1997;36:10675–10684. doi: 10.1021/bi970430a. [DOI] [PubMed] [Google Scholar]
- 33.Lamb H.K., Ren J., Park A., Johnson C., Leslie K., Cocklin S. Modulation of the ligand binding properties of the transcription repressor NmrA by GATA-containing DNA and site-directed mutagenesis. Protein Sci. 2004;13:3127–3138. doi: 10.1110/ps.04958904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stammers D.K., Ren J., Leslie K., Nichols C.E., Lamb H.K., Cocklin S. The structure of the negative transcriptional regulator NmrA reveals a structural superfamily which includes the short-chain dehydrogenase/reductases. EMBO J. 2001;20:6619–6626. doi: 10.1093/emboj/20.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinogradov E., Peppler M.S., Perry M.B. The structure of the nonreducing terminal groups in the O-specific polysaccharides from two strains of Bordetella bronchiseptica. Eur. J. Biochem. 2000;267:7230–7237. doi: 10.1046/j.1432-1327.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- 36.Dong C., Major L.L., Srikannathasan V., Errey J.C., Giraud M.F., Lam J.S. RmlC, a C3′ and C5′ carbohydrate epimerase, appears to operate via an intermediate with an unusual twist boat conformation. J. Mol. Biol. 2007;365:146–159. doi: 10.1016/j.jmb.2006.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki T., Igarashi Y., Saito N., Furumai T. TPU-0031-A and B, new antibiotics of the novobiocin group produced by Streptomyces sp. TP-A0556. J. Antibiot. (Tokyo) 2001;54:441–447. doi: 10.7164/antibiotics.54.441. [DOI] [PubMed] [Google Scholar]
- 38.Tello M., Jakimowicz P., Errey J.C., Freel Meyers C.L., Walsh C.T., Buttner M.J. Characterisation of Streptomyces spheroides NovW and revision of its functional assignment to a dTDP-6-deoxy-d-xylo-4-hexulose 3-epimerase. Chem. Commun. (Cambridge) 2006:1079–1081. doi: 10.1039/b515763c. [DOI] [PubMed] [Google Scholar]
- 39.Freitag A., Mendez C., Salas J.A., Kammerer B., Li S.M., Heide L. Metabolic engineering of the heterologous production of clorobiocin derivatives and elloramycin in Streptomyces coelicolor M512. Metab. Eng. 2006;8:653–661. doi: 10.1016/j.ymben.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Schweizer H.P., Hoang T.T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 41.Antoine R., Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol. Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 42.Preston A., Maxim E., Toland E., Pishko E.J., Harvill E.T., Caroff M., Maskell D.J. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 2003;48:725–736. doi: 10.1046/j.1365-2958.2003.03484.x. [DOI] [PubMed] [Google Scholar]
- 43.Parkhill J., Sebaihia M., Preston A., Murphy L.D., Thomson N., Harris D.E. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- 44.Simon R. A broad host range mobilizations system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 45.Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C.I. Positive selection procedure for entrapment of insertion sequence elements in Gram-negative bacteria. J. Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc. Natl Acad. Sci. USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinter N.J., Brewster G., Barth P.T. Two mechanisms necessary for the stable inheritance of plasmid RP4. Plasmid. 1989;22:203–214. doi: 10.1016/0147-619x(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 48.Hitchcock P.J., Brown T.M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preston A., Maskell D., Johnson A., Moxon E.R. Altered lipopolysaccharide characteristic of the I69 phenotype in Haemophilus influenzae results from mutations in a novel gene, isn. J. Bacteriol. 1996;178:396–402. doi: 10.1128/jb.178.2.396-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai C.M., Frasch C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 51.Allen A., Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 52.Otwinowski Z., Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 53.French S., Wilson K. On the treatment of negative intensity observations. Acta Crystallogr., sect. A. 1978;34:517–525. [Google Scholar]
- 54.Collaborative Computational Project Number 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 55.McCoy A.J., Grosse-Kunstleve R.W., Storoni L.C., Read R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 56.Adams P.D., Grosse-Kunstleve R.W., Hung L.W., Ioerger T.R., McCoy A.J., Moriarty N.W. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 57.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 58.Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 59.Perrakis A., Harkiolaki M., Wilson K.S., Lamzin V.S. ARP/wARP and molecular replacement. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2001;57:1445–1450. doi: 10.1107/s0907444901014007. [DOI] [PubMed] [Google Scholar]
- 60.Potterton E., Briggs P., Turkenburg M., Dodson E. A graphical user interface to the CCP4 program suite. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 61.Painter J., Merritt E.A. TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 2006;39:109–111. [Google Scholar]
- 62.Brunger A.T., Adams P.D., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 63.Laskowski R.A., MacArthur M.W., Moss D.S., Thornton J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 64.Hooft R.W., Vriend G., Sander C., Abola E.E. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- 65.Lovell S.C., Davis I.W., Arendall W.B., 3rd, de Bakker P.I., Word J.M., Prisant M.G. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 66.DeLano W.L. DeLano Scientific; Palo Alto, CA: 2002. The Pymol User's Manual. [Google Scholar]
- 67.Potterton E., McNicholas S., Krissinel E., Cowtan K., Noble M. The CCP4 molecular-graphics project. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2002;58:1955–1957. doi: 10.1107/s0907444902015391. [DOI] [PubMed] [Google Scholar]
- 68.Voss N.R., Gerstein M., Steitz T.A., Moore P.B. The geometry of the ribosomal polypeptide exit tunnel. J. Mol. Biol. 2006;360:893–906. doi: 10.1016/j.jmb.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.