Abstract
Liver X receptor (LXR) and peroxisome proliferator-activated receptor (PPAR) are two members of nuclear receptors involved in the nutrient metabolisms of dietary fatty acid and cholesterol. They are found to be of cross-talk function in that LXR regulates fatty acid synthesis and PPAR controls fatty acid degradation. LXRs (LXRα and LXRβ) function by forming obligate heterodimers with the retinoid X receptor (RXR), and subsequently binding to specific DNA response elements within the regulatory regions of their target genes. In this work, the kinetic features concerning LXR/RXR and LXR/PPAR interactions have been fully investigated using surface plasmon resonance (SPR) technology. It is found that LXRs could bind to all the three PPAR subtypes, PPARα, PPARγ and PPARδ with different binding affinities, and such receptor/receptor interactions could be regulated by ligand binding. Moreover, molecular dynamics (MD) simulations were performed on six typical complex models. The results revealed that ligands may increase the interaction energies between the receptor interfaces of the simulated receptor/receptor complexes. The MD results are in agreement with the SPR data. Further analyses on the MD results indicated that the ligand binding might increase the hydrogen bonds between the interfaces of the receptor/receptor complex.
Keywords: liver X receptor (LXR), peroxisome proliferator-activated receptor (PPAR), retinoid X receptor (RXR), surface plasmon resonance (SPR), kinetic analysis, molecular dynamics (MD) simulation
LXRs (LXRα and LXRβ) are nuclear receptors that regulate the metabolisms of several important lipids, including cholesterol and bile acids (Song et al. 1994; Willy et al. 1995). These receptors function by forming obligate heterodimers with the retinoid X receptor (RXR) and binding specific DNA sequences (response elements) within the promoters of the genes they regulate (Willy and Mangelsdorf 1997). It is already known that LXRs regulate the nutrient metabolism pathways through their interactions with specific, naturally occurring oxysterols, including 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol and 24(S),25-epoxycholesterol (Lehmann et al. 1996; Peet et al. 1998). LXRα is expressed most highly in the liver and to a lesser extent in the kidney, small intestine, spleen, and adrenal gland (Willy et al. 1995). In contrast to the restricted expression pattern of LXRα, LXRβ is ubiquitously expressed (Kainu et al. 1996). PPAR belongs to another sort of nuclear receptor family, which plays an important role in the regulation of the storage and catabolism of dietary fats (Grossman and Lessem 1997; Rutolo et al. 1998; Willson et al. 2000). PPAR contains three subtypes: PPARα, PPARδ(also termed as PPARβ), and PPARγ. PPARα is a member of the steroid/thyroid hormone receptor and regulates the expression of a number of genes critical for lipid and lipoprotein metabolism. PPARγ is a ligand-dependent transcription factor influencing the adipocyte differentiation and glucose homeostasis, while PPARδ may work as a widespread regulator in fat burning and probably be used as a potential target in the treatment of obesity and related disorders (Uppenberg et al. 1998; Willson et al. 2000; Wang et al. 2003).
The involvements of PPARs in multiple and diverse cellular functions suggest these receptors may be integrated with other cellular signaling pathways apart from the well-characterized RXR (Retinoid X receptor) pathway (Miyata et al. 1996). RXRαand its cognate ligand 9cRA appear to have a critical role in hormonal signaling. RXRα can heterodimerize with many nuclear receptors including LXR and PPAR in facilitating the binding and transactivation of receptors with appropriate DNA response elements (Willson et al. 2000). Some reports have indicated that PPAR does not interact with the thyroid hormone receptor (TR), but with LXR (Juge-Aubry et al. 1995), even though the reason of such a binding selectivity is still unknown. In fact, much attention has recently been paid to PPAR/LXR interaction (Mangelsdorf et al. 1995; Ide et al. 2003; Yoshikawa et al. 2003). By gel shift and in vitro protein/protein binding assays, it has been discovered that the interactions between LXRs and PPARα are involved in fatty acid degradation, which is a reverse of the fatty acid synthesis function of PPARα Mangelsdorf et al. 1995).
A nuclear receptor protein contains a central DNA binding domain (DBD) and a C-terminal ligand binding domain (LBD) (Zhou et al. 1998). In general, the LBD is about 200–300 amino acids in length and not conserved as well. There are at least four functions encoded in the LBD, viz. dimerization, ligand binding, binding to coactivators or co-repressors, and transactivation (Bourguet et al. 1995; Renaud et al. 1995). The LBD has thus been the focus of intense study for the determination of ligand specificity (Wurtz et al. 1996). Among the 12 helices in the LBD of PPARs, the carboxyl helices are required for heterodimerization with RXR and the N-terminal helices are involved in forming ligand binding pockets (Juge-Aubry et al. 1995). Recently, by use of varied biophysical techniques such as fluorescent and circular dichroism spectral technology, the ligand binding features for some receptors have been fully characterized (Palmer and Wolf 1998; Margeat et al. 2003; Yu et al. 2004).
Since 1991, surface plasmon resonance (SPR) technology-based Biacore has been widely used to monitor the thermodynamics and kinetics for a wide variety of macro-molecular interactions, including protein/protein, protein/DNA, and ligand/protein (DNA) interactions (Karlsson et al. 2000). SPR technology has been also successfully employed to study the interactions between ligands and nuclear receptors and the effects of ligand-binding on receptor dimerization (Cheskis et al. 1995; Myszka 1997; Robinson et al. 2000; Yu et al. 2004).
In the following, we report the in vitro study of the kinetic binding characterizations of LXR–LBDs/RXRα–LBD and LXR–LBDs/PPAR–LBDs using SPR technology-based Biacore 3000. The experimental results are quite consistent with the molecular dynamics (MD) simulations on these protein/protein interactions, which are also reported here. The results demonstrate that the protein/protein interactions exist between LXRs and PPARs, and these interactions could be regulated by the bindings of the corresponding ligands.
Results
SPR assay
To obtain the kinetic and thermodynamic features for the receptor/receptor interactions, the association (ka) and dissociation (kd) rate constants and the dissociation equilibrium constants (KDs) for the bindings of LXR–LBDs to RXRα– LBD and PPAR–LBDs were determined by using the Biacore 3000 biosensor (Biacore AB). The binding responses in resonance units (RUs) were continuously recorded and presented graphically as a function of time. The heterogeneous ligand-parallel reaction (HLPR) fit model (Cheskis and Freedman 1996) was used to calculate the ka, kd, and KD parameters. In this fit model, one analyte interacts with two independent binding sites, i.e., A + B1 + B2 = AB1 + AB2.
LXR–LBDs/RXRα–LBD interactions
In the investigation of LXR–LBDs/RXRα–LBD interactions, two experimental strategies have been applied. During the first determination, RXRα–LBD was immobilized on the sensor chip by the standard amine coupling protocol, and LXR–LBDs (LXRα–LBD and LXRβ–LBD) were treated as analytes. A surface with approximately 800 RU of immobilized RXRα–LBD was obtained. Injections of apo-or ligand-bound LXRα (β)–LBD at concentrations from 1.28 nM to 4 μM were run over the chip surface. As two typical examples, Figure 1A ▶ shows the sensorgrams of serial injections of apo-LXRα–LBD over the immobilized RXRα–LBD surface, and Figure 1B ▶ presents the sensorgrams of serial injections of LXRα–LBD preincubated with 22RHC over the immobilized RXRα–LBD surface. The sensorgrams were then processed for computing the related kinetic and thermodynamic parameters of protein/protein interactions by the HLPR fit model, and the results are listed in Table 1.
Figure 1.
Unliganded and liganded LXRα–LBD/RXRα–LBD interactions evaluated by Biacore 3000. Sensorgrams obtained from the injections of LXRα–LBD over the immobilized RXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (A); injections of LXRα–LBD incubated with 20 μM 22RHC over the immobilized RXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (B); injections of RXRα–LBD over the immobilized LXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (C); and injections of RXRα–LBD incubated with 20 μM 9cRA over the immobilized LXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (D).
Table 1.
Association (ka) and dissociation (kd) rate constants and apparent equilibrium dissociation (KD′) constants of unliganded and liganded LXR-LBDs/RXRα-LBD interactions with RXRα-LBD immobilized (A) and LXRs-LBD immobilized (B)
| A | Immobilized receptor RXRα | ||
| Analyte | ka (M −1S−1) | kd (S−1) | KD′ (M) |
| LXRα | 57.70 ± 4.39 | 1.67 ± 0.13 × 10−5 | 2.90 ± 0.22 × 10−7 |
| LXRα + 22RHC | 1.85 ± 0.16 × 104 | 4.03 ± 0.40 × 10−5 | 2.17 ± 0.22 × 10−9 |
| LXRα + T0901317 | 1.70 ± 0.12 × 104 | 3.69 ± 0.23 × 10−7 | 2.17 ± 0.13 × 10−11 |
| LXRβ | 4.35 ± 0.24 × 103 | 4.93 ± 0.34 × 10−4 | 1.14 ± 0.08 × 10−7 |
| LXRβ + 22RHC | 2.47 ± 0.15 × 105 | 5.74 ± 0.52 × 10−4 | 2.32 ± 0.21 × 10−9 |
| LXRβ + T0901317 | 2.47 ± 0.13 × 105 | 6.95 ± 0.59 × 10−4 | 2.82 ± 0.24 × 10−9 |
| B | Immobilized receptor | |||||
| LXRα | LXRβ | |||||
| Analyte | ka (M−1S−1) | kd (S −1) | KD′ (M) | ka (M−1S−1) | kd (S −1) | KD′ (M) |
| RXRα | 63.90 ± 3.90 | 3.94 ± 0.27 × 10−5 | 6.17 ± 0.42 × 10−7 | 64.50 ± 5.71 | 4.98 ± 0.30 × 10−5 | 7.75 ± 0.47 × 10−7 |
| RXRα + 9cRA | 7.18 ± 0.51 × 103 | 3.78 ± 0.34 × 10−7 | 5.26 ± 0.47 × 10−11 | 7.25 ± 0.58 × 103 | 2.95 ± 0.24 × 10−6 | 4.07 ± 0.33 × 10−10 |
In general, the HLPR fit model may produce two sets of binding constants (Cheskis and Freedman 1996). The apo-LXRα–LBD binding to the immobilized RXRα–LBD produced association and dissociation rate constants by ka1 = 57.7 M−1sec−1 and kd1 = 1.67 × 10−5 sec−1, and ka2= 1.7 × 103 M−1sec−1 and kd2 = 1.76 × 10−3 sec−1; their corresponding equilibrium dissociation constants KD1 (kd1/ka1) and KD2 (kd2/ka2) could thus be calculated as 2.9 × 10−7 and 3.75 × 10−7 M, respectively. For the binding of 22RHC-bound LXRα–LBD to RXRα–LBD, ka1 = 2.19 × 103 M−1sec−1, kd1 = 1.43 × 10−3 sec−1, ka2 = 1.85 × 104 M−1sec−1, and kd2 = 4.03 × 10−5sec−1; the corresponding KD1 and KD2 are 6.54 × 10−7 and 2.17 × 10−9 M, respectively. Usually, the binding parameter corresponding to the smaller KD value representing the majority of the binding is considered as the apparent equilibrium dissociation constant (denoted by KD′ herein after) (Cheskis and Freedman 1996). The kinetic data and KD′ values of other apo- and ligand-bound LXR–LBDs/RXRα–LBD (immobilized) interaction are listed in Table 1A.
To determine the effect of ligand 9cRA binding to RXRα–LBD on the interactions between RXRα–LBD and LXR–LBDs, LXR–LBDs were immobilized on the sensor chips with resonance unit around 800 RU by the standard amine coupling method mentioned above, and RXRα–LBD was regarded as analyte. apo- or 9cRA-Bound RXRα–LBD was injected over the immobilized LXR–LBD surface at different concentrations from 1.28 nM to 4 μM. Figure 1C ▶ shows the sensorgrams of serial injections of RXRα–LBD to the immobilized LXRα–LBD surface, and Figure 1D ▶ presents the sensorgrams of serial injections of RXRα–LBD preincubated with 9cRA to the immobilized LXRα–LBD surface. The kinetic and thermodynamic parameters were also obtained by fitting the sensor-grams with the HLPR fit model, and the binding parameters of apo- and 9cRA-bound RXRα–LBD to the immobilized LXRβ–LBD were obtained. The determined data are listed in Table 1B.
SPR results indicate that both the LXRα–LBD and LXRβ–LDB have high binding affinities with RXR–LBD by KD′ values around 0.1 μM (Table 1). Ligands of both LXR and RXR are able to increase the binding affinities of LXR–LBDs to RXRα–LDB by two to four orders of magnitude. However, it is noticed that different ligands exert different promotion activity against one given protein/protein interaction. The binding of T0901317 increases the LXRα–LBD/RXRα–LBD interaction by four orders of magnitude, while the binding of 22RHC only enhances this interaction by two orders of magnitude (Table 1A). Both T0901317 and 22RHC promote the LXRβ–LDB/RXRα–LBD interaction by two orders of magnitude (Table 1A). Moreover, the kinetic data indicate that ligands of either LXR or RXR largely increase the association rate constant (ka) and decrease the dissociation rate constant (kd) of the LXR–LBDs/RXRα–LBD binding processes, thereby increasing the binding affinities between the receptors (Table 1).
LXR–LBDs/PPAR–LBDs interactions
Similar to the above assays, the effects of LXR ligand-binding to the LXR–LBDs/PPAR–LBDs interactions could be analyzed by immobilizing PPAR–LBDs (PPARα–LBD, PPARβ–LBD, and PPARδ–LBD) on the sensor chips with apo- and ligand-bound LXR–LBDs as analytes. By the standard amine coupling protocol, surfaces with ~1000 RU of immobilized PPAR–LBDs were prepared. The apo- or ligand-bound LXR–LBDs at concentrations ranging from 1.28 nM to 4 μM were run over the chip surface. As two representative examples, Figure 2A ▶ shows the sensorgrams of serial injections of apo-LXRα–LBD over the immobilized PPARα–LBD surface, and Figure 2B ▶ displays the sensorgrams of serial injections of LXRα–LBD preincubated with the LXR ligand, 22RHC, over the immobilized PPARα–LBD surface. The sensorgrams were fitted by the HLPR fit model, and the obtained kinetic and thermodynamic parameters are listed in Table 2.
Figure 2.
Interaction of apo- or ligand-bound LXRα–LBD with immobilized PPARα–LBD evaluated by Biacore 3000. Sensorgrams obtained from the injections of LXRα–LBD over the PPARα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (A); injections of LXRα–LBD incubated with 20 μM 22RHC over the PPARα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (B).
Table 2.
Association (ka) and dissociation (kd) rate and apparent equilibrium dissociation (KD′) constants of unliganded and liganded LXRs-LBD/PPARs-LBD (immobilized) interactions
| Immobilized receptor | |||||||||
| PPAR α | PPARγ | PPARδ | |||||||
| Analyte | ka (M−1S−1) | kd (S−1) | KD′ (M) | ka (M−1S−1) | kd (S−1) | KD′ (M) | ka (M−1 S−1) | kd (S−1) | KD′ (M) |
| LXRα | 6.28 ± 0.52 ×104 | 1.84 ± 0.24 × 10−3 | 2.92 ± 0.038 × 10−8 | 1.45 ± 0.14 × 104 | 2.53 ± 0.32 × 10−4 | 1.74 ± 0.22 × 10−8 | 377.00 ± 35.00 | 9.55 ± 1.29 × 10−5 | 2.41 ± 0.34 × 10−7 |
| LXRα + 22RHC | 3.56 ± 0.65 ×104 | 9.44 ± 1.24 × 10−7 | 2.65 ± 0.35 × 10−11 | 2.1 ± 0.18 × 104 | 2.93 ± 0.21 × 10−7 | 1.39 ± 0.10 × 10−11 | 2.03 ± 0.19 × 104 | 4.80 ± 0.42 × 10−4 | 2.36 ± 0.21 × 10−8 |
| LXRα + T0901317 | 3.33 ± 0.43 ×104 | 3.42 ± 0.21 × 10−4 | 1.03 ± 0.063 × 10−8 | 2.04 ± 0.25 × 104 | 6.7 ± 0.52 × 10−5 | 3.28 ± 0.21 × 10−9 | 1.72 ± 0.12 × 104 | 3.76 ± 0.29 × 10−4 | 2.18 ± 0.17 × 10−8 |
| LXRβ | 187.00 ± 27.00 | 6.09 ± 0.17 × 10−5 | 3.26 ± 0.091 × 10−7 | 66.80 ± 6.45 | 8.28 ± 0.67 × 10−5 | 1.24 ± 0.10 × 10−6 | 57.70 ± 4.35 | 1.28 ± 0.12 × 10−4 | 2.22 ± 0.21 × 10−6 |
| LXRβ + 22RHC | 67.90 ± 4.30 | 1.94 ± 0.15 × 10−6 | 2.86 ± 0.22 × 10−8 | 67.30 ± 3.25 | 2.76 ± 0.14 × 10−4 | 4.10 ± 0.21 × 10−6 | 79.60 ± 6.97 | 2.58 ± 0.27 × 10−4 | 3.25 ± 0.34 × 10−6 |
| LXRβ + T0901317 | 96.60 ± 8.70 | 5.04 ± 0.62 × 10−7 | 5.21 ± 0.64 × 10−9 | 202.00 ± 18.00 | 2.47 ± 0.23 × 10−4 | 1.22 ± 0.11 × 10−6 | 96.40 ± 5.45 | 7.09 ± 0.69 × 10−5 | 7.35 ± 0.72 × 10−7 |
SPR determinations indicate that LXR–LBDs may tightly bind to PPAR–LBDs, the KD′ values range from ~1–0.01μM. Similarly, these receptor/receptor interactions may also be regulated by the ligands: (1) The binding of the LXR natural ligand, 22RHC, promotes the LXRα–LBD/PPARα–LBD and LXRα–LBD/PPARγ–LBD interactions by three orders of magnitude and the LXRα–LBD/PPARδ–LBD interaction by one order of magnitude; (2) the binding of the synthetic ligand, T0901317, exhibits little effect on the LXRα–LBD/PPAR–LBDs interactions; (3) the binding of 22RHC to LXRβ–LBD may promote the LXRβ–LBD/PPARα–LBD interaction by one order of magnitude, but has little effect on the PPARγ–LBD/PPARδ–LBD interactions; (4) the binding of T0901317 to LXRβ–LBD increases the LXRβ–LBD/PPARδ–LBD and LXRβ–LBD/PPARα–LBD interactions by one and two orders of magnitude, respectively, while it has little effect on the LXRβ–LBD/PPARγ–LBD interaction. In addition, the large increase of the LXRα–LBD/PPARα (γ)–LBD interactions by 22RHC binding to LXRα–LBD may be mainly ascribed to the high decrease of the dissociation constants (Table 2).
To investigate the effect of the PPAR ligand binding on the PPAR–LBDs/LXR–LBDs interactions, LXRα(β)–LBD was immobilized on the sensor chip with the resonance unit ~1000 RU, and PPARα (γ/β)–LBD was treated as analytes. As typical examples, the sensorgrams of the serial injections of the apo- PPARγ–LBD over the immobilized LXRα–LBD surface are shown in Figure 3A ▶, and the sensorgrams of the serial injections of PPARγ–LBD preincubated with 15dPGJ2 over the immobilized LXRα–LBD surface are shown in Figure 3B ▶. All the sensorgrams were also fitted by the HLPR fit model as described above. The obtained kinetic and thermodynamic parameters are listed in Table 3.
Figure 3.
Interaction of apo- or ligand-bound PPARγ–LBD interaction with immobilized LXRα–LBD evaluated by Biacore 3000. Sensorgrams obtained from the injections of PPARγ–LBD over the LXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (A); injections of PPARγ–LBD incubated with 20 μM 15dPGJ2 over the LXRα–LBD surface at concentrations of 4, 0.8, 0.16, 0.032, 0.0064, and 0.00128 μM (B).
Table 3.
Association (ka) and dissociation (kd) rate and apparent equilibrium dissociation (KD′) constants of unliganded and liganded PPARs-LBD/LXRs-LBD (immobilized) interactions
| Immobilized receptor | ||||||
| LXRα | LXRβ | |||||
| Analyte | ka (M−1S−1) | kd (S−1) | KD′ (M) | ka (M−1S−1) | kd (S−1) | KD′ (M) |
| PPARα | 69.50 ± 7.40 | 1.92 ± 0.12 × 10−5 | 2.77 ± 0.17 × 10−7 | 103.00 ± 7.30 | 1.97 ± 0.45 × 10−5 | 1.92 ± 0.44 × 10−7 |
| PPARα + WY14643 | 1.18 ± 0.27 × 104 | 1.97 ± 0.21 × 10−4 | 1.66 ± 0.18 × 10−8 | 9.88 ± 1.34 × 104 | 1.68 ± 0.23 × 10−3 | 1.70 ± 0.23 × 10−8 |
| PPARα + Bezafibrate | 37.40 ± 5.76 | 3.15 ± 0.35 × 10−6 | 8.40 ± 0.94 × 10−8 | 1.09 ± 0.97 × 105 | 2.97 ± 0.28 × 10−3 | 2.72 ± 0.26 × 10−8 |
| PPARγ | 51.10 ± 3.27 | 4.68 ± 0.47 × 10−5 | 9.17 ± 0.92 × 10−7 | 57.50 ± 5.85 | 1.40 ± 0.13 × 10−5 | 2.43 ± 0.23 × 10−7 |
| PPARγ + 15dPGJ2 | 2.15 ± 0.19 × 104 | 6.43 ± 0.78 × 10−7 | 2.99 ± 0.36 × 10−11 | 2.45 ± 1.86 × 104 | 1.18 ± 0.17 × 10−5 | 4.81 ± 0.69 × 10−10 |
| PPARγ + Troglitazone | 4.64 ± 0.38 × 103 | 1.31 ± 0.12 × 10−5 | 2.82 ± 0.26 × 10−9 | 88.10 ± 9.65 | 4.96 ± 0.45 × 10−4 | 5.62 ± 0.51 × 10−6 |
| PPARγ + GI262570 | 40.90 ± 5.89 | 2.05 ± 0.25 × 10−5 | 5.03 ± 0.61 × 10−7 | 43.40 ± 3.42 | 2.86 ± 0.35 × 10−5 | 6.58 ± 0.81 × 10−7 |
| PPARδ | 2.06 ± 0.45 × 103 | 2.98 ± 0.36 × 10−3 | 1.45 ± 0.17 × 10−6 | 1.57 ± 0.14 × 103 | 6.01 ± 0.54 × 10−4 | 3.83 ± 0.34 × 10−7 |
| PPARδ + Bezafibrate | 1.36 ± 0.085 × 103 | 1.33 ± 0.13 × 10−4 | 9.80 ± 0.96 × 10−8 | 569.00 ± 34.00 | 3.46 ± 0.31 × 10−5 | 6.10 ± 0.54 × 10−8 |
The data listed in Table 3 indicate that, compared with the interactions of apo- LXR–LBDs with the immobilized PPAR–LBDs, the binding affinities of PPAR–LBDs to the immobilized LXR–LBDs are found to decrease by about one order of magnitude. This may ascribe to the fact that the immobilization of LXR–LBDs to the sensor chip has some unfavorable effect on the formation of LXR–LBDs/PPAR–LBDs complexes (Cheskis and Freedman 1996). As indicated above, ligand regulation also exists for these receptor/receptor interactions. For example, the two PPARα ligands WY14643 and Benzafibrate promotes PPARα–LBD/LXRα (β)–LBD interactions by about one order of magnitude; the binding of 15dPGJ2 to PPARγ–LBD increases the PPARγ–LBD/LXRα–LBD and PPARγ–LBD/LXRβ–LBD interactions by four and three orders of magnitude, respectively. It is interesting that the binding of Troglitazone to PPARγ–LBD increases the PPARγ–LBD/LXRα–LBD binding affinity by two orders of magnitude but decreases the PPARγ–LBD/LXRβ–LBD interaction by about one order of magnitude. However, the binding of GI262570 to PPARγ–LBD does not affect the PPARγ–LBD/LXRα(β)–LBD interaction, while the binding of Bezafibrate to PPARδ–LBD may strengthen the PPARδ–LBD/LXRα(β)–LBD interaction by one or two orders of magnitude.
Molecular dynamics simulations for the receptor/receptor interactions
To gain insights into the detailed influence of the ligands on the receptor/receptor interactions at the molecular and atomic levels, 3-nsec molecular dynamics (MD) simulations were performed on six typical receptor/receptor binary complexes and ligand–receptor/receptor (or receptor/receptor–ligand) ternary complexes, viz. apo-RXRα–LDB/apo-LXRα–LDB, apo-RXRα–LDB/LXRα–LDB-22RHC, RXRα–LDB-9cRA/apo-LXRα–LDB, apo-PPARγ–LDB/apo-LXRα–LDB, apo-PPARγ–LDB/LXRα–LDB-22RHC, and PPARγ–LDB-15dPGJ2/apo-LXRα–LDB.
The energetic properties of the systems with respect to time give an indication of the overall stability of the MD trajectory. The profiles of total energies of the above six models versus simulation time are shown in Figure S1 in the Supplemental Material, which indicate that all the systems are stable during the simulations. The profiles of the receptor/receptor interaction energies of the complex systems versus simulation time are shown in Figure 4 ▶. For each complex, the receptor/receptor interaction energy fluctuates dramatically along the MD trajectory. Along the interaction energy profile of a complex, we can address the lowest minimum point that corresponds to the configuration with the maximum interactions. Such lowest minimum points of apo-RXRα–LDB/apo-LXRα–LDB, apo-RXRα–LDB/LXRα–LDB-22RHC, RXRα–LDB-9cRA/apo-LXRα– LDB, apo-PPARγ–LDB/apo-LXRα–LDB, apo-PPARγ–LDB/LXRα–LDB-22RHC, and PPARγ–LDB-15dPGJ2/apo-LXRα–LDB are, respectively, located at around 1080, 2710, 1970, 1380, 1110, and 740 psec in their own interaction energy profiles (Fig. 4 ▶). The interaction energies corresponding to the lowest minimum points are listed in Table 4, which indicate that these ligands (22RHC, 9cRA, and 15dPGJ2) may increase the receptor/receptor interaction strengths. This is in good agreement with the SPR determinations (Tables 2, 3). To address the reason of the ligand binding-caused increase of the receptor/receptor interactions, we monitored the hydrogen bond number and hydrophobic interaction pairs on the snapshots corresponding to the lowest interaction energies using the Ligplot program (Wallace et al. 1995). The results are listed in Table 4 and in Figure S2 in the Supplemental Material, which suggest that the ligand binding only slightly affects the hydrophobic interaction, but dramatically enhances the hydrogen-bond interaction.
Figure 4.

Profiles for the receptor/receptor interactions of the six typical complexes derived from the MD simulations. (A) Unliganded and liganded RXRα–LBD/LXRα–LBD interactions. (B) Unliganded and liganded PPARγ–LBD/LXRα–LBD interactions.
Table 4.
Interaction energies, hydrogen bond number, and hydrophobic interaction pairs for the receptor/receptor interaction derived from the molecular dynamics simulations
| Plota | Complex | Interaction energy (kJ/mol) | Hydrogen bond number | Hydrophobic interaction pairs |
| A | apo-RXRα-LBD/apo-LXRα-LBD | −1935.63 | 3 | 28 |
| B | apo-RXRα-LBD/LXRα-LBD-22RHC | −2310.15 | 10 | 29 |
| C | RXRα-LBD-9cRA/apo-LXRα-LBD | −2130.29 | 6 | 23 |
| D | apo-PPARγ-LBD/apo-LXRα-LBD | −1954.81 | 6 | 26 |
| E | apo-PPARγ-LBD/LXRα-LBD-22RHC | −2037.07 | 14 | 26 |
| F | PPARγ-LBD-15dPGJ2/apo-LXRα-LBD | −2008.41 | 11 | 22 |
a The Ligplot pictures for the interactions are shown in Figure S2 in the Supplemental Material. A–F correspond to Figures S2A–F.
Discussion
RXR is an obligate heterodimeric partner of LXRs, and it has been reported that the LXR/RXR complex is a permissive heterodimer that can be activated by the binding of RXR ligand 9cRA (Gampe et al. 2000), and the binding of the synthetic LXR ligand T0901317 and natural ligand 22RHC to LXR is able to reinforce the LXR/RXR heterodimerization at the cellular level (Bethany et al. 1999), even though no more detailed quantitative data have been obtained. Other facts regarding the activation of LXRα/RXRα interaction by LXR and RXR ligands were proved by using the GST-pulldown assay or other protein/protein interaction detecting methods (Song et al. 1995; Lehmann et al. 1996). In the present work, we have comprehensively investigated the interactions of LXRs with RXRα and the effects of the ligand binding on the receptor/receptor interactions by using the SPR technique and MD simulation. Our SPR and MD indicate that both LXR and RXR ligands can greatly regulate the LXR–LBDs/RXRα–LBD interactions. From the determined binding data, we can tentatively propose that LXR–LBD and RXRα–LBD may form a complex, and the liganded receptors may form a tighter one.
The above conclusions may also be true for the interactions between LXRs and PPARs. Since it has been known that LXRs/PPARs complexes have no specific DNA binding elements, they cannot directly modulate the transcription activity of the related genes (Miyata et al. 1996). Protein/protein interaction with ligand modulation may therefore be pivotal for their role in vivo. The SPR determination indicates that Bezafibrate can enhance both the PPARα–LBD/LXRs–LBD and PPARδ–LBD/LXRs–LBD interactions. This is in agreement with the fact that Bezafibrate is a dual agonist of PPARα and PPARδ (Willson et al. 2000). It is reported that 15dPGJ2 can strengthen the association of PPARγ with its coactivator, but Troglitazone cannot, indicating that the mode of coactivator interacting with PPARγ is ligand type-specific (Kodera et al. 2000). We found that 15dPGJ2 can enhance the PPARγ/LXRα(β) interactions; Troglitazone reinforces the PPARγ/LXRα heterodimerization, but slightly inhibits the formation of the PPARγ/LXRβ complex (Table 3). Surprisingly, GI262570, the PPARγ agonist with high binding affinity, does not affect on the PPARγ/LXRα and PPARγ/LXRβ interactions (Table 3). These data demonstrate that the ligand regulated binding mode of LXRs to PPARγ is also ligand type-specific.
In addition, the MD simulations on six typical complex models indicate that ligand binding to the receptors does not affect the hydrophobic interactions but increases the number of hydrogen bonds (Table 4; Fig. S2 [Supplemental Material]), which may be the major reason that the ligand-binding increases most of the receptor/receptor interactions in this study (Tables 2, 3).
Materials and methods
Chemicals and enzymes
The restriction and modifying enzymes were purchased from TaKaRa. The bacterial strains BL21pLysS and DH5α, as well as the vector pET15b, were from Qiagen. The chelating affinity column and lower molecular weight (LMW) marker were purchased from Amersham Pharmacia Biotech, Inc. Isopropyl β-D-thiogalactoside (IPTG) was purchased from Promega. All other chemicals were from Sigma in their analytical grade. The ligands of LXRα and LXRβ, T0901317 (N-methyl-N-[3-(2,2,2-trifluoro-1-hydroxy-1- trifluoromethylethyl ) -phenyl ]-benzenesulfonamide ) and 22RHC (22(R)-hydroxycholesterol), as well as the PPARs ligands WY14643, 15dPGJ2 (15-deoxy-Δ12,14-prostaglandin J2), Troglitazone, GI262570, and Bezafibrate were from CAYMAN Pharmaceutical Co. Ltd.
Plasmids
All the recombinant DNA manipulation methods were performed according to the standard method (Maniatis 1989). The plasmids of pCMX-hLXRα and pCMX-hLXRβ were kindly provided by Dr. Mangelsdorf (Howard Hughes Medical Institute, University of Texas Southwestern Medical Center), pAdTrack-PPARδ by Dr. Vogelstein (Howard Hughes Medical Institute, University of Texas Southwestern Medical Center), pET15b-hPPARγ–LBD (aa 204–477) by Dr. Uppenberg (Department of Structural Chemistry, Pharmacia and Upjohn, Sweden), pSG-hPPARα by Dr. Lu (Shenzhen Chipscreen Biosciences Ltd.), and pGEX-KG-RXRα by Dr. Christopher (University of California, San Diego). The cDNAs of LXRα–LBD (aa 200–447), LXRβ–LBD (aa 213–461), PPARα–LBD (aa 199–468) and PPARδ–LBD (aa 287–477) were amplified by PCR, and subcloned into vector pET15b followed by sequencing.
Protein expression and purification
LXRα(β)–LBD protein was expressed and purified according to the method in the literature (Causevic et al. 1999) with some modifications. Escherichia coli BL21pLysS cells transformed with plasmids of pET15b-LXRα(β)–LBD were grown in 100 mL of LB medium containing 100 mg/L of amphicillin at 37°C overnight, and then incubated into 1 L of LB supplemented with amphicilin. The expression of LXRα(β)–LBD was induced by the addition of 0.5 mM of isopropyl β-D-thiogalactoside (IPTG). After induction for 5 h at 25°C, the cells were harvested by centrifugation at 4000g, 4°C for 30 min. The pellet was washed, frozen, and then disrupted by sonication against Buffer A (20 mM Tris-HCl, 0.5 M NaCl, 5 mM imidazole [pH 7.5]). The lysed cells were centrifuged at 14,000g at 4°C for 30 min. The supernatant was kept and the pellet discarded. One milliliter (1 mL) of HiTrap Ni2+ chelating column was equilibrated with 10 mL of sterile deionized water, 50 mM NiSO4, and finally 10 mL of Buffer A. The supernatant was passed over the column at a flow rate of 5 mL/min, followed by washing it with 20 mL of Buffer A and 20 mL of Buffer B (60 mM imidazole in Buffer A), respectively. The protein of interest was thus eluted with 10 mL of Buffer C (20 mM Tris-HCl, 0.5 M NaCl, 0.5 M imidazole [pH 7.5]).
The expression and purification of the PPAR–LBDs (PPARα–LBD, PPARγ–LBD, and PPARγ–LBD) and RXRδ–LBD proteins were carried out using similar procedures to those of LXRα(β)–LBD proteins. The protein concentration was measured by use of the standard Bradford method.
Surface plasmon resonance (SPR) determinations
The binding affinities of LXR–LBDs with PPAR–LBDs and RXRα–LBD were evaluated by using a Biacore 3000 instrument (Biacore AB). Immobilization of the corresponding proteins to the hydrophilic carboxymethylated dextran matrix of the sensor chip CM5 (Biacore) was performed by the standard primary amine coupling reaction according to the supplied manual by Biacore Co. The proteins to be covalently bound to the matrix were diluted in 10 mM sodium acetate buffer (pH 5.0) to a final concentration of 0.30 mg/mL. Equilibration of the baseline was completed by a continuous flow of HBS running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% [v/v] surfactant P20 [pH 7.4]) through the chip for 1–2 h. Samples were automatically injected into the flow cells with concentration increased gradually. All the Biacore data were collected at 25°C with HBS as a running buffer at a constant flow of 20 μL/min. The sensorgrams were processed using automatic correction for nonspecific bulk refractive index effects.
In general, the equilibrium dissociation constant (KD) evaluating the protein/protein binding affinity could be determined by the 1:1 Langmuir binding fit model or the HLPR fitting analysis model (http://www.biacore.com). To assess the two models for analyzing the sensor data in this work, several parameters such as the standard error (SE) value, the residual plot and χ2 were analyzed by the Biacore evaluation software package. The residual plot describes the differences between the measured and calculated data. The χ2 value denotes the fitting degree between the estimative and the experimental curves. The smaller the χ2, the smaller their variation.
For both the 1:1 Langmuir binding fit model and HLPR fit model, the association rate constant (ka) and dissociation rate constant (kd) were fitted simultaneously by rate equation 1, and the equilibrium dissociation constant KD was calculated according to equation 2; the difference between these two models exists in that for HLPR fit model it has two association and two dissociation reactions as shown in equation 3
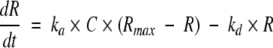 |
(1) |
where C is the concentration of the ligand, R represents the response unit (RU), for heterogeneous ligand parallel reaction fit model, R (observed) = R1 + R2.
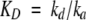 |
(2) |
 |
(3) |
The SPR data collection and process regarding the ligand binding to the receptors were also according to the same strategies as the above-mentioned. The ligands were preincubated with the proteins for 30 min before flowing over the sensor chip surfaces. When LXRα(β)–LBD protein was immobilized on the CM5 sensor chip (Biacore), the PPARα(γ/δ)–LBD protein alone or incubated with the corresponding PPAR ligand was let to flow through the chip surface. When PPARα(γ/δ)–LBD protein was immobilized on the CM5 sensor chip, the LXRα(β)–LBD protein alone or incubated with the corresponding LXR ligand was let to flow through the chip surface.
The assay for the interactions between LXRα(β)–LBD and RXRα–LBD is similar to the above procedures.
Molecular dynamics simulations
The monomer structures of PPARγ–LDB, RXRα–LDB, and LXRα–LDB were isolated from the crystal structures of PPARγ–LDB/RXRα–LDB (PDB entry 1FM6) and RXRβ–LDB/LXRα–LDB (PDB entry 1UHL) complexes. Missing residues and atoms in the crystal structures were repaired by using the Biopolymer module encoded in Sybyl 6.8 (Tripos Inc.). The models of RXRα–LDB/LXRα–LDB and PPARγ–LDB/LXRα–LDB were constructed as follows: By replacing the coordinates of RXRβ–LDB in the crystal structure of the RXRβ–LDB/LXRα–LDB complex with those of the RXRα–LDB crystal structure, we obtained a 3D model of the RXRα–LDB/LXRα–LDB complex. Similarly, substituting the coordinates of RXRα–LDB in the crystal structure of the PPARγ–LDB/RXRα–LDB complex with those of LXRα–LDB crystal structure resulted in a 3D model of PPARγ–LDB/LXRα–LDB complex. To construct the 3D models of ligand/receptor/receptor complexes, 22RHC and 15dPGJ2 were separately docked into the binding pockets of LXRα–LDB and PPARγ–LDB of the above receptor/receptor complexes using the FlexX program (Rarey et al. 1996). The X-ray crystal structure of the 9cRA/RXRα–LDB complex was directly used in constructing the 3D models of ligand/receptor/receptor complexes. Thus we obtained the 3D models of six complexes, viz. apo-RXRα–LDB/apo-LXRα–LDB, apo-RXRα–LDB/LXRα–LDB-22RHC, RXRα–LDB-9cRA/apo-LXRα–LDB, apo-PPARγ–LDB/apo-LXRα–LDB, apo-PPARγ–LDB/LXRα–LDB-22RHC, and PPARγ–LDB-15dPGJ2/apo-LXRα–LDB, for further molecular dynamics (MD) simulations.
All MD simulations were performed using the parallelized MD program GROMACS 3.1.4 (Berendsen et al. 1995; Erik et al. 2001). Before MD simulations, explicit polar and aromatic hydrogen atoms were added for all models. The GROMOS87 force field was used for the proteins (van Gunsteren and Berendsen 1987). The geometries of the ligands (22RHC, 9cRA, and 15dPGJ2) were optimized at the B3LYP/6-31G** level and then their partial atomic charges were determined using the CHelpG algorithm encoded in the Gaussian 98W package. Topology file and other force field parameters except the charges of the ligands were generated using the PRODRG program (van Aalten et al. 1996; http://davapc1.bioch.dundee.ac.uk/programs/prodrg).
Each complex was solvated into a rectangular periodic box filled with SPC water molecules (Berendsen et al. 1981). The minimum distance between the protein atoms and the box walls was set to be >10 Å. Counterions were added to neutralize the net charges of the systems. Then each system was subjected to an energy minimization using the steepest descent method until energy convergence to 100 kJ/(mol•nm). Afterward, each system was equilibrated for 100 psec. Finally, a 3-nsec MD simulation was performed on each system. During the MD simulations, the Lincs and Settle methods (Miyamoto and Kollman 1992; Hess et al. 1997) were applied to constrain covalent bond lengths, allowing an integration step of 2 fsec. The Particlemesh Ewald (PME) method was used to calculate the electrostatic interactions (Darden et al. 1993). The simulated systems were coupled into an external temperature bath at 300 K with a coupling constant of τT = 0.1 psec and isotropic pressure coupling with time constant of τP = 1 psec was applied to keep the pressure at 1.0 bar (Berendsen et al. 1984). The structures for analysis were saved every 500 steps (1 psec). MD simulations were run on a 128-CPU Silicon Graphics Origin3800 server. Analyses were performed using facilities within the GROMACS package and the Ligplot program (Wallace et al. 1995).
Acknowledgments
This work was supported by the State Key Program of Basic Research of China (grants 2003CB514125, 2002CB512807, 2002CB512802, and 2004CB518905), the National Natural Science Foundation of China (grants 20372069, 20472095, and 29725203), Shanghai Basic Research Project from the Shanghai Science and Technology Commission (grants 02DJ14070, 03DZ19212, and 03DZ19228), and the 863 Hi-Tech Program (grants 2002AA104270 and 2002AA233011).
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04951405.
Supplemental material: see www.proteinscience.org
References
- Berendsen, H.J., Postma, J.P., van Gunsteren, W.F., and Hermans, J. 1981. Intermolecular forces. Intermolecular forces (ed. B. Pullman), pp. 331–342. Reidel Publishing Company, Dordrecht, The Netherlands.
- Berendsen, H.J., Postma, J.P., van Gunsteren, W.F., DiNola, A., and Haak, J.R. 1984. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81 3684–3690. [Google Scholar]
- Berendsen, H.J., van der Spoel, D., and van Drunen, R. 1995. GROMACS: A message-passing parallel molecular dynamics implementation. Comp. Phys. Commun. 91 43–56. [Google Scholar]
- Bethany, A., Janowski, M.J., Grogan, S.A., Jones, G., Bruce, W., Kliewer, S.A., Corey, E.J., and Mangelsdorf, D.J. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. 96 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet, W., Ruff, M., Chambon, P., Gronemeyer, H., and Moras, D. 1995. Crystal structure of the ligand binding domain of the human nuclear receptor RXR α. Nature 375 377–382. [DOI] [PubMed] [Google Scholar]
- Causevic, M., Wolf, C.R., and Palmer, C.N. 1999. Substitution of a conserved amino acid residue alters the ligand binding properties of peroxisome proliferator activated receptors. FEBS Lett. 463 205–210. [DOI] [PubMed] [Google Scholar]
- Cheskis, B. and Freedman, L.P. 1996. Modulation of nuclear receptor interactions by ligands: Kinetic analysis using surface plasmon resonance. Biochemistry 35 3309–3318. [DOI] [PubMed] [Google Scholar]
- Cheskis, B., Lemon, B.D., Uskokovic, M., Lomedico, P.T., and Freedman, L.P. 1995. Vitamin D3-retinoid X receptor dimerization, DNA binding, and transactivation are differentially affected by analogs of 1,25-dihydroxyvitamin D3. Mol. Endocrinol. 9 1814–1824. [DOI] [PubMed] [Google Scholar]
- Darden, T., York, D., and Pedersen, L. 1993. Particle mesh Ewald—An N. log(N) method for Ewald sums in large systems. J. Chem. Phys. 98 10089–10092. [Google Scholar]
- Erik, L., Berk, H., and van der Spoel, D. 2001. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 7 306–317. [Google Scholar]
- Gampe, R.T., Montana, V.G., Lambert, M.H., Miller, A.B., Bledsoe, R.K., Milburn, M.V., Kliewer, S.A., Willson, T.M., and Xu, H.E. 2000. Asymmetry in the PPARγ/RXRα crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol. Cell 5 545–555. [DOI] [PubMed] [Google Scholar]
- Grossman, S.L. and Lessem, J. 1997. Mechanisms and clinical effects of thiazolidinediones. Expert Opin. Invest. Drugs 6 1025–1040. [DOI] [PubMed] [Google Scholar]
- Hess, B., Bekker, H., Berendsen, H.J., and Fraaije, J.G. 1997. LINCS: A linear constraint solver for molecular simulations. J. Comp. Chem. 18 1463–1472. [Google Scholar]
- Ide, T., Shimano, H., Yoshikawa, T., Yahagi, N., Amemiya-Kudo, M., Matsuzaka, T., Nakakuki, M., Yatoh, S., Iizuka, Y., Tomita, S., et al. 2003. Cross-talk between peroxisome proliferator-activated receptor (PPAR) α and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol. Endocrinol. 17 1255–1267. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry, C.E., Gorla-Bajszczak, A., Pernin, A., Lemberger, T., Wahli, W., Burger, A.G., and Merier, C.A. 1995. Peroxisome proliferator-activated receptor mediates cross-talk with thyroid hormone receptor by competition for retinoid X receptor. Possible role of a leucine zipper-like heptad repeat. J. Biol. Chem. 270 18117–18122. [DOI] [PubMed] [Google Scholar]
- Kainu, T., Kononen, J., Enmark, E., Gustafsson, J.A., and Pelto-Huikko, M. 1996. Localization and ontogeny of the orphan receptor OR-1 in the rat brain. J. Mol. Neurosci. 7 29–39. [DOI] [PubMed] [Google Scholar]
- Karlsson, R., Kullman-Magnusson, M., Hamalainen, M.D., Remaeus, A., Andersson, K., Borg, P., Gyzander, E., and Deinum, J. 2000. Biosensor analysis of drug–target interactions: Direct and competitive binding assays for investigation of interactions between thrombin and thrombin inhibitors. Anal. Biochem. 278 1–13. [DOI] [PubMed] [Google Scholar]
- Kodera, Y., Takeyama, K., Murayama, A., Suzawa, M., Masuhiro, Y., and Kato, S. 2000. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J. Biol. Chem. 275 33201–33204. [DOI] [PubMed] [Google Scholar]
- Lehmann, J.M., Kliewer, S.A., Moore, L.B., Smith-Oliver, T.A., Oliver, B.B., Su, J.L., Sundseth, S.S., Winegar, D.A., Blanchard, D.E., Spencer, T.A., et al. 1996. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272 3137–3140. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf, D.J., Thummel, C., Beatro, M., Herrlich, P., Schurtz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P., et al. 1995. The nuclear receptor superfamily: The second decade. Cell 83 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis, J.S. 1989. Molecular cloning: A laboratory manual, 2nd ed. Cold spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Margeat, E., Bourdoncle, A., Margueron, R., Poujol, N., Cavailles, V., and Royer, C. 2003. Ligands differentially modulate the protein interactions of the human estrogen receptors α and β. J. Mol. Biol. 326 77–92. [DOI] [PubMed] [Google Scholar]
- Miyamoto, S. and Kollman, P.A. 1992. Settle—An analytical version of the shake and rattle algorithm for rigid water models. J. Comput. Chem. 13 952–962. [Google Scholar]
- Miyata, K.S., McCaw, S.E., Patel, H.V., Rachubinski, R.A., and Capone, J.P. 1996. The orphan nuclear hormone receptor LXR α interacts with the peroxisome proliferator-activated receptor and inhibits peroxisome proliferator signaling. J. Biol. Chem. 271 9189–9192. [DOI] [PubMed] [Google Scholar]
- Myszka, D.G. 1997. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechem. 8 50–57. [DOI] [PubMed] [Google Scholar]
- Palmer, C.N. and Wolf, C.R. 1998. cis-Parinaric acid is a ligand for the human peroxisome proliferator activated receptor γ: Development of a novel spectrophotometric assay for the discovery of PPARγ ligands. FEBS Lett. 431 476–480. [DOI] [PubMed] [Google Scholar]
- Rarey, M., Kramer, B., Lengauer, T., and Klebe, G. 1996. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 261 470–489. [DOI] [PubMed] [Google Scholar]
- Peet, D.J., Janowski, B.A., and Mangelsdorf, D.J. 1998. The LXRs: A new class of oxysterol receptors. Curr. Opin. Gen. Dev. 8 571–575. [DOI] [PubMed] [Google Scholar]
- Renaud, J.P., Rochel, N., Ru, M., Vivat, V., Chambon, P., Gronemeyer, H., and Moras, D. 1995. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature 378 681–689. [DOI] [PubMed] [Google Scholar]
- Robinson, J.C., Kerjan, P., and Mirande, M. 2000. Macromolecular assemblage of aminoacyl-tRNA synthetased: Quantitative analysis of protein–protein interactions and mechanism of complex assembly. J. Mol. Biol. 304 983–994. [DOI] [PubMed] [Google Scholar]
- Rutolo, G., Ericsson, C.G., Tettamanti, C., Karpe, F., Grip, L., Svane, B., Nilsson, J., De Faire, U., and Hamsten, A. 1998. Treatment effects on serum lipoprotein lipids, apolipoproteins and lowdensity lipoprotein particle size and relationships of lipoprotein variables to rogression of coronary artery disease in the bezafibrate coronary atherosclerosis intervention trial. J. Am. Coll. Cardiol. 32 1648–1656. [DOI] [PubMed] [Google Scholar]
- Song, C., Kokontis, J.M., Hiipakka, R.A., and Liao, S. 1994. Ubiquitous receptor: A receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc. Natl. Acad. Sci. 91 10809–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C., Hiipakka, R.A., Kokontis, J.M., and Liao, S. 1995. Ubiquitous receptor: Structures, immunocytochemical localization, and modulation of gene activation by receptors for retinoic acids and thyroid hormones. Ann. N.Y. Acad. Sci. 761 38–49. [DOI] [PubMed] [Google Scholar]
- Uppenberg, J., Svensson, C., Jaki, M., Bertilsson, G., Jendeberg, L., and Berkenstam, A. 1998. Crystal structure of the ligand binding domain of the human nuclear receptor PPARγ. J. Biol. Chem. 273 31108–31112. [DOI] [PubMed] [Google Scholar]
- van Aalten, D.M., Bywater, R., Findlay, J.B., Hendlich, M., Hooft, R.W., and Vriend, G. 1996. PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Design 10 255–262. [DOI] [PubMed] [Google Scholar]
- van Gunsteren, W.F. and Berendsen, H.J.C. 1987. Gromos-87 manual. Biomos BV, Groningen, The Netherlands.
- Wallace, A.C., Laskowski, R.A., and Thornton, J.M. 1995. LIGPLOT: A program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 8 127–134. [DOI] [PubMed] [Google Scholar]
- Wang, Y.X., Lee, C.H., Tiep, S., Yu, R.T., Ham, J., Kang, H., and Evans, R.M. 2003. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113 159–170. [DOI] [PubMed] [Google Scholar]
- Willson, T.M., Brown, P.J., Sternbach, D.D., and Henke, B.R. 2000. The PPARs: From orphan receptors to drug discovery. J. Med. Chem. 43 527–550. [DOI] [PubMed] [Google Scholar]
- Willy, P.J. and Mangelsdorf, D.J. 1997. Unique requirements for retinoid dependent transcriptional activation by the orphan receptor LXR. Genes & Dev. 11 289–298. [DOI] [PubMed] [Google Scholar]
- Willy, P.J., Umesono, K., Ong, E.S., Evans, R.M., Heyman, R.A., and Mangelsdorf, D.J. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes & Dev 9 1033–1045. [DOI] [PubMed] [Google Scholar]
- Wurtz, J.M., Bourguet, W., Renaud, J.P., Vivat, V., Chambon, P., Moras, D., and Gronemeyer, H. 1996. A canonical structure for the ligand-binding domain of nuclear receptors. Nat. Struct. Biol. 3 87–94. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, T., Ide, T., Shimano, H., Yahagi, N., Amemiya-Kudo, M., Matsuzaka, T., Yatoh, S., Kitamine, T., Okazaki, H., Tamura, Y., et al. 2003. Cross-talk between peroxisome proliferator-activated receptor (PPAR)α and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. I. PPARs suppress sterol regulatory element binding protein-1c promoter through inhibition of LXR signaling. Mol. Endocrinol. 17 1240–1254. [DOI] [PubMed] [Google Scholar]
- Yu, C.Y., Chen, L.L., Luo, H.B., Chen, J., Cheng, F., Gui, C.S., Zhang, R.H., Shen, J.H., Chen, K.X., Jiang, H.L., et al. 2004. Binding analyses between human PPARγ-LBD and ligands: Surface plasmon resonance biosensor assay correlating with circular dichroic spectroscopy determination and molecular docking. Eur. J. Biochem. 271 386–397. [DOI] [PubMed] [Google Scholar]
- Zhou, G., Cummings, R., Li, Y., Mitra, S., Wilkinson, H.A., Elbrecht, A., Hermes, J.D., Schaeffer, J.M., Smith, R.G., and Moller, D.E. 1998. Nuclear receptors have distinct affinities for coactivators: Characterization by fluorescence resonance energy transfer. Mol. Endocrinol. 12 1594–1604. [DOI] [PubMed] [Google Scholar]





