Abstract
It has been claimed that β2-microglobulin (β2-m) interacts with type I and type II collagen, and this property has been linked to the tissue specificity of the β2-m amyloid deposits that target the osteo-articular system. The binding parameters of the interaction between collagen and β2-m were determined by band shift electrophoresis and surface plasma resonance by using bovine collagen of type I and type II and various isoforms of β2-m. Wild-type β2-m binds collagen type I with a Kd of 4.1 × 10−4 M and type II with 2.3 × 10−3 M. By the BIAcore system we monitored the binding properties of the conformers of the slow phase of folding of β2-m. The folding intermediates during the slow phase of folding do not display any significant difference with respect to the binding properties of the fully folded molecule. The affinity of β2-m truncated at the third N-terminal residue does not differ from that reported for the wild-type protein. Increased affinity for collagen type I is found in the case of N-terminal truncated species lacking of six residues. The Kd of this species is 3.4 × 10 −5 M at pH 7.4 and its affinity increases to 4.9 × 10−6 M at pH 6.4. Fluctuations of the affinity caused by β2-m truncation and pH change can cause modifications of protein concentration in the solvent that surrounds the collagen, and could contribute to generate locally a critical protein concentration able to prime the protein aggregation.
Keywords: β2-microglobulin, collagen, protein–protein interaction, amyloid
Amyloidoses are diseases caused by the aggregation of particular proteins and polypeptides that precipitate as bundles of fibrils in the extracellular spaces of selected tissues (Merlini and Bellotti 2003). The mechanism of transformation of soluble proteins, with highly heterogeneous structure, into the amyloid fibrils in which the β-fold is indefinitely repeated is largely unknown. However, even more shrouded in mystery is the molecular basis tissue of specificity of amyloid deposits. As a matter of fact, beyond a certain degree of individual variability it is well established that some amyloidogenic proteins have very pronounced tissue specificity as observed with some transthyretin variants for peripheral nerves or heart (Saraiva 1995), some apolipoprotein A-I mutants for the heart (Obici et al. 1999, 2004), and β2-microglobulin (β2-m) for the osteoarticular system (Sprague and Moe 1996). Dialysis-related amyloidosis (DRA) is the prototype of specific tissue targeting of amyloid deposition. In fact, in this case the earliest nuclei of amyloid material are generated along the tendons and in the periarticular cartilage (Jadoul et al. 1997). In a pionieristic work, Homma (1989) proposed a possible molecular explanation for the tissue-selective specificity of β2-m fibrillar deposition through the demonstration that the protein binds triple helix collagen of type I and type II in a dose-dependent manner. These results were confirmed by Moe and Chen (2001), but so far the parameters that regulate association and dissociation of β2-m and collagen have not yet been reported. On the other hand, it has been shown, by our group and others, that in the amyloid deposits of β2-m some truncated species are present, in addition to the wild-type protein. In particular, we always detect a N-terminal truncated species lacking six residues (ΔN6β2-m) that exhibits the capability of generating fibrils at a pH compatible with the biologic environment (Bellotti et al. 1998). Therefore, in this study we have accomplished the characterization of the properties of collagen/β2-m interaction by using recombinant isoforms of β2-m with particular regard to the N-terminal truncated variant identified in natural fibrils, βN6β2-m, and the intermediate naturally occurring in the folding process of the protein.
Results
Binding of β2-microglobulin to triple helix collagen
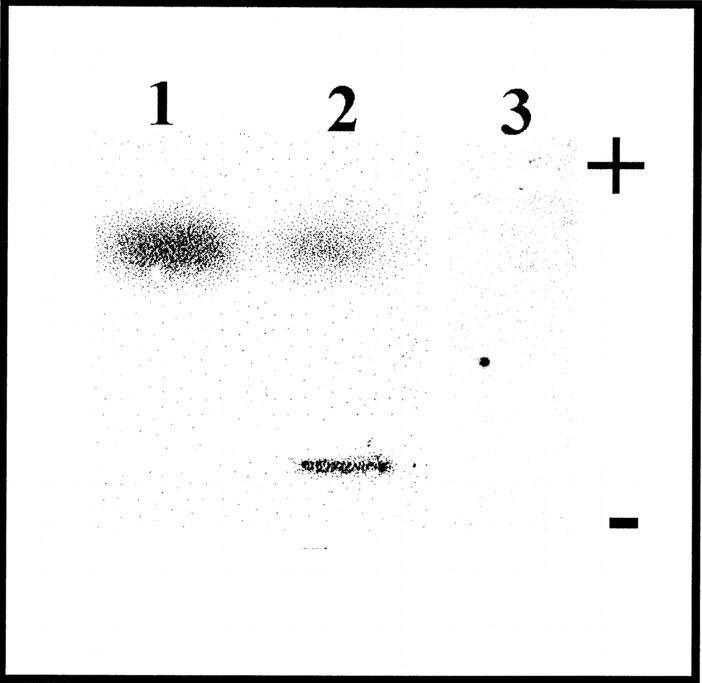
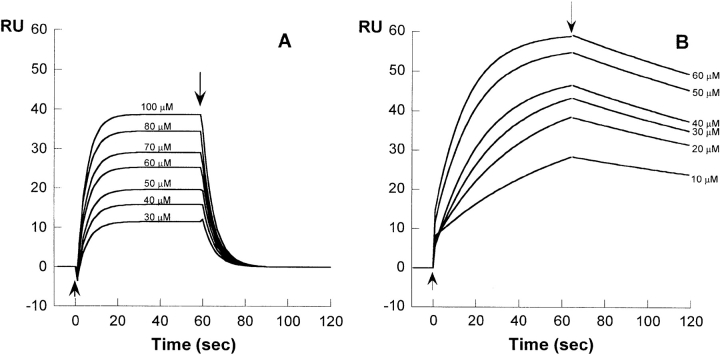
The interaction between β2-m and collagen was initially studied by using band-shift electrophoresis in native state agarose gel electrophoresis. Under native conditions triple helix collagen displays a very low mobility, whereas β2-m quickly migrates toward the anode. The formation of a complex β2-m/collagen is confirmed by the positive immunostaining of the collagen/β2-m mixture reported in lane 2, where a band of β2-m coprecipitates with collagen at the site of protein deposition (Fig. 1 ▶). However, these results can be considered only qualitative because collagen and collagen β2-m complexes are not easily transferred to the immobilon sheets, and this prevents a precise quantification of the bound protein. Therefore, the determination of binding parameters was carried out by surface plasmon resonance (SPR) by using the equipment supplied by BIAcore. When exposed to the functionalized surface of the sensor chip, β2-m was found to bind, in a concentration-dependent manner, to immobilized collagen. Figure 2 ▶ reports the sensorgrams obtained with full-length wild-type protein and with the variant devoid of six residues at the N terminus, i.e., ΔN6β2-m after collagen type I being immobilized on the sensor chip. The association rate and the dissociation rate of bound β2-m were rapid, and the related curves were well fitted using a 1:1 Langmuir model.
Figure 1.
Electrophoretic mobility of β2-m (1), β2-m/collagen type I (2), and collagen type I (3) analyzed at a concentration of 5 μg/mL and 500 μg/mL for β2-m and collagen, respectively. Afterward, agarose gel electrophoresis proteins were blotted and immunodetected by using an antihuman β2-m polyclonal antibody.
Figure 2.
Overlay of sensorgrams measuring the interaction of β2-m (A) and ΔN6 β2-m (B) at different concentrations with the collagen type I immobilized on a sensor chip CM5 and recorded at pH 6.4 and 25°C. Each sensorgram has been evaluated using the BIA evaluation 3.0 software provided with the system. The arrows show the start and the end of injection, respectively.
Association and dissociation kinetic rate costants (kon and koff) and the equilibrium dissociation constant Kd (koff/kon) were calculated by nonlinear fitting using the BIAevaluation 3.0 software (Karlsson and Fält 1997).
Table 1 summarizes the results obtained with different isoforms of β2-m including point mutants, truncated variants, and the wild-type folding intermediate (I2) we previously identified (Chiti et al. 2001a). No significant differences were detected between wild-type protein and the Arg3Ala or His31Tyr, two species whose tertiary structure is very similar to the wild-type β2-m (Corazza et al. 2004; Rosano et al. 2004). The rate of association to collagen of the full-length protein (kon = 6.3 × 102 M−1 sec−1) is slightly slower than that of ΔN6β2-m (kon = 1.4 × 103 M−1 sec−1). The koff values, however, are significantly different for the two species (koff = 2.6 × 10−1 sec−1 for wild-type and 4.7 × 10−2 sec−1 for ΔN6β2-m). The koff of ΔN6β2-m is sharply influenced by a shift of pH from 7.4 to 6.4. In fact, its value changes from 4.7 × 10−2 sec−1 to 5.0 × 10−3 sec−1, and consequently, the equilibrium Kd calculated for = ΔN6β2-m is 3.4 × 10−5 M at pH 7.4 and 4.9 × 10−6 M at pH 6.4. On the contrary, the pH shift has no effect on the strength of interaction of wild-type β2-m.
Table 1.
Kinetics and thermodynamics of β2-m isoform interaction with collagen type I determined by surface plasmon resonance measurements
| Ligand | pH | kon (M−1 sec−1) | koff (sec−1) | Kd (M) |
| β2-m | 7.4 | 6.3 × 102 | 2.6 × 10−1 | 4.1 × 10−4 |
| β2-m | 6.4 | 4.1 × 102 | 9.0 × 10−2 | 2.2 × 10−4 |
| β2-m I2,T= 30″ | 7.4 | 3.4 × 10 | 2.6 × 10−1 | 7.6 × 10−3 |
| β2-m I2,T= 600″ | 7.4 | 2.8 × 10 | 2.3 × 10−1 | 4.3 × 10−3 |
| β2-m I2,T= 1200″ | 7.4 | 6.6 × 102 | 2.4 × 10−1 | 4.4 × 10−4 |
| R3Aβ2m | 7.4 | 2.1 × 102 | 1.4 × 10−1 | 6.7 × 10−4 |
| H31Yβ2m | 7.4 | 3.0 × 102 | 1.4 × 10−1 | 6.8 × 10−4 |
| ΔN6β2m | 7.4 | 1.4 × 103 | 4.7 × 10−2 | 3.4 × 10−5 |
| ΔN6β2m | 6.4 | 1.0 × 103 | 5.0 × 10−3 | 4.9 × 10−6 |
Using a similar experimental procedure we have measured a dose-dependent binding of wild-type β2-m to type II collagen even if the equilibrium Kd calculated at pH 7.4 (2.3 × 10−3 M) is lower than that determined for type I collagen according to previous data (Moe and Chen 2001).
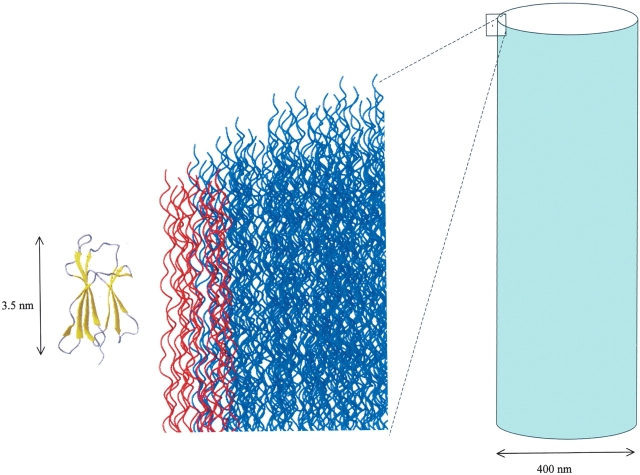
Through the BIAcore system we can mimic the dynamic condition of protein–protein interaction occurring in vivo in order to approximate the distribution of a soluble protein over an immobilized target at the putative physiologic concentration. In the case of full-length β2-m at a concentration of 20 mg/L (1.5 μM), which represents the average plasma concentration of free protein during hemodialysis, the molar ratio between collagen-bound β2-m and triple helix collagen species would be approximately 1:270. This estimate is based on the assumption that the activity of the species involved in the β2-m-collagen binding can be expressed as aqueous concentrations, disregarding any phase heterogeneity of the interaction equilibrium. In the case of ΔN6β2-m, the ratio would decrease to 1:20 at pH 7.4 and even 1:4 at pH 6.4. In vivo we expect that the collagen helix accessible for binding would be most likely restricted to the fiber surface (Fig. 3 ▶). By considering, for example, the reported diameter of a prototypic fiber, i.e., around 400 nm, and hence a circumference of 1256 nm (Nimni and Harkness 1988), we expect that the number of triple helix collagen molecules (average diameter of 1.5 nm) would be approximately 800.
Figure 3.
A collagen fiber interacting with β2-m. The actual dimensions of the fiber (blue tube) with respect to a single molecule (dot) are reported in the drawing proportions. The boxed region is magnified on the left to show the details of the collagen microfibrils (dark blue) with respect to a β2-m molecule (yellow-gray). In the figure, a single collagen microfibril, formed by assembly of five triple helix structures, is highlighted in red. The box dimensions on the right and the separation from the fiber surface of the dot inside the box have been deliberately exaggerated with respect to the magnification on the left for sake of clarity.
When circulating β2-m is at micromolar concentration we can calculate that ≅3–4 molecules of full-length β2-m could surround a mature fiber. However, in the case of ΔN6β2-m, this number could raise to ≅40 molecules at pH 7.4 and even to 200 molecules at pH 6.4.
Binding of partially folded β2-m to collagen.
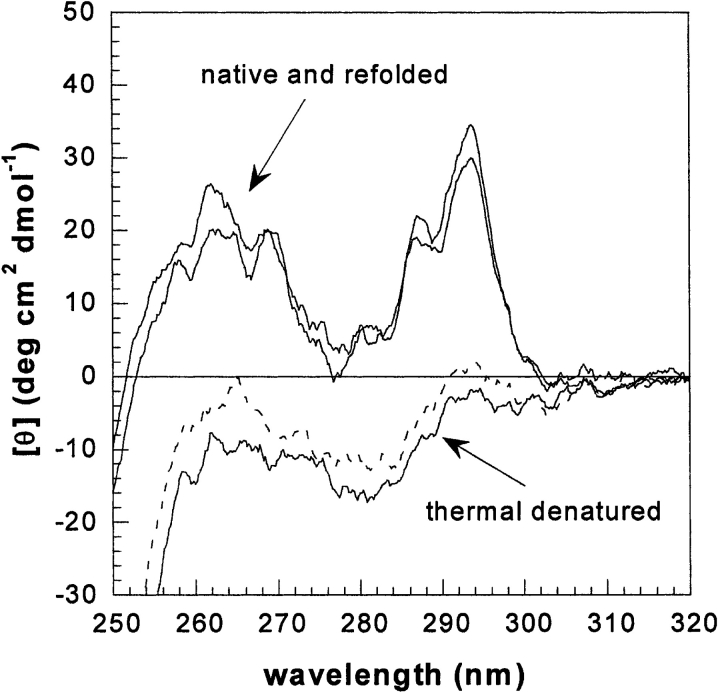
The BIAcore system allows us to perform the analysis of the binding properties in approximately 120 sec; therefore, this technique is suitable for the investigation of the binding properties of systems undergoing sufficiently slow structural conversion. Since we have previously discovered that the refolding pathway of β2-m involves a slow phase of folding (Chiti et al. 2001a,b; Corazza et al. 2004), which is fully accomplished in 20 min, under physiologic conditions, we have monitored the interaction with collagen type I of the folding intermediate previously named I2 at 30, 600, and 1200 sec from the start of the refolding reaction (Table 1). In this case, the protein had to be subjected to thermal denaturation in order to avoid technical difficulties due to buffer changes. The experimental data indicate that, throughout the slow phase of the refolding, the interaction of I2 with collagen is weaker than that displayed by the folded molecule. Only after 1200 sec the binding β2m/ collagen shows to recover the properties characteristic of the native protein. On the other hand, circular dichroism, used to monitor the refolding process of β2-m, in the same conditions of the BIAcore analysis, allows us to confirm the complete recovery of protein structure (Fig. 4 ▶). The analysis of the interaction of collagen type I and β2-m folding intermediates shows that the reduced Kd value is generated by the equivalent contribution of a reduced rate of association, but similar dissociation rate compared to the values observed for the folded species. The partially folded species that populates the slow phase of refolding of β2-m therefore displays a different behavior with respect to the truncated ΔN6 β2-m. This is consistent with the significantly different structural properties of the two species, namely a largely folded structure for I2, not far from the minimum conformational energy of the fully folded state (Corazza et al. 2004), as opposed to the extensively destructured conformation of ΔN6β2-m (Esposito et al. 2000).
Figure 4.
CD spectra in the near UV region of native β2-m, β2-m thermally denatured at 90°C, and refolded β2-m 1200 sec after the cooling of the solution at 25°C. The figure also shows (dotted line) the spectrum of the protein before the slow phase of folding.
Discussion
Amyloidosis caused by deposits of β2-m fibrils is peculiar for its selective tissue specificity. In fact, bones and ligaments are invariably the target tissues of the disease. The common molecular constituent shared by these tissues is collagen of type I and type II, and therefore a role of this fibrous protein in the localization of these amyloid deposits, was earlier hypothesized (Homma 1989).
The parameters that describe the interaction of β2-microglobulin to type I and type II collagen are consistent with a weak interaction, most likely sufficient, however, to sequester significant amounts of β2m due to the massive occurrence of type I and type II collagen in the body. The distribution of radiolabeled β2m (Vincent et al. 1992) confirms an extensive captation of the molecule by an extravascular compartments. Collagen is not the only plausible molecular target of β2-m. Glycosaminoglycans could play a similar role. The data obtained by Ohashi et al. (2002) indicate that the interaction between β2-m and heparin has a Kd of 1 × 10−5 M, and is therefore similar to that between β2-m and collagen.
The binding properties suggest that collagen of type I bind with higher affinity than collagen of type II, and confirm previous qualitative data obtained with similar collagen preparations (Moe and Chen 2001).
By considering various isoforms of β2-m we have found that the species truncated at the N-terminal ΔN6β2-m has a stronger affinity for collagen with respect to the full-length molecule. The potential role of this species in β2-m fibrillogenesis was previously proposed by our group on the basis of its presence in natural fibrils (Bellotti et al. 1998; Stoppini et al. 2000), its high propensity to make fibrils even at physiologic pH (Esposito et al. 2000), the similarity of its conformation with that of fibrillar full-length β2-m (Monti et al. 2002), and finally, for its capability of accelerating the oligomerization of full-length protein.
The interaction of ΔN6β2-m with collagen is significantly influenced by the pH, a decrease by a single unit, from 7.4 to 6.4, corresponding to a 10-fold affinity enhancement. The stronger affinity could be related to an improved electrostatic interaction, as ΔN6β2-m lacks an Arg in position 3 and a Lys in position 6 and its isoelectric point lowers by about 1 pH unit with respect to the parent species. However, the pH effect would contradict this simple interpretation. According to pKa predictions of Table 2 that were performed using the available structural data (Esposito et al. 2000), following a pH change from 7.4 to 6.4, ΔN6β2-m is expected to decrease its overall charge from −4.29 to −2.34 electron charge units. Within the same pH interval, the corresponding overall charge span of the full-length folded sequence goes from −2.41 to −1.41. The same calculations performed by considering only the actual amino acid sequences would give overall charge extrema of −4.16/−3.01 for ΔN6β2-m, and −2.16/−1.01 for full-length β2-m. Since the experimental pH variation is expected to affect the immobilized collagen molecule to the same extent during both SPR experiments with ΔN6β2-m and full-length protein, the affinity enhancement observed with the former cannot be attributed to a generic electrostatic effect ensuing from the decrease of its overall negative charge at lower pH because an even larger decrease takes place also for full-length β2-m that experimentally, however, does not exhibit any collagen binding affinity increase upon pH lowering. Hence, we conclude that the result obtained with ΔN6β2-m should be attributed to specific local effects, involving the meaningful titration changes occurring in the considered pH range in terms of actual loss of negative charge and/or gain of positive charge. Inspection of Table 2 shows that for several titrable groups a ΔpKa ≥ |0.4| units is obtained. Upon lowering pH from 7.4 to 6.4, however, nonnegligible effects should involve only the four His residues. With the exception of His51, at pH = 6.4, the extent of positive charge on the imidazole groups of the His residues should be larger for ΔN6β2-m than for the full-length species. However, while only a threefold increase of the positive charge amount is expected for His13 and His31, the increment becomes 55 times for His84 where, most significantly, the population ratio of the charged-to-uncharged species is 3.0 × 10−1 when pH = 6.4. This is quite different compared to the protonation state of His84 in wild-type β2-m, where the charged-to-uncharged species ratio reaches only 5.5 × 10−3 under the same conditions. Therefore, the collagen affinity increase, experimentally observed for ΔN6β2-m on pH lowering, could reflect the specific involvement of the region encompassing His84, i.e., the C-terminal end of strand F. Interestingly, the NMR data of ΔN6β2-m (Esposito et al. 2000) show that His84 resonances retain most of the chemical shift values and the local NOE pattern with Leu87, at the start of strand G, which are observed with the wild-type full-length molecule. This is consistent with a somehow conserved local secondary and tertiary structure at His84 of ΔN6β2-m, although the pKa change (Table 2) and a deviation of the Hα chemical shift (Esposito et al. 2000) suggest some accessibility increase for this residue compared to the wild-type molecule. An involvement of His31 and His13 in pH-dependent stability, such as that pointed out previously for β2-m (Verdone et al. 2002), seems unlikely with ΔN6β2-m where both residues are largely exposed to the solvent and no longer arranged in a compact local geometry with neighboring residues that can be affected by their charge (Esposito et al. 2000). Likewise, an involvement of His51 such as that recently proposed by the group of Goto (Villanueva et al. 2004) that, at higher pH, could account for a flexibility increase, and hence, a reduced overall conformational stability, in turn possibly related to binding affinity changes, would not apply to ΔN6β2-m whose conformation is anyway rather perturbed around His51 with respect to native full-length β2-m (Esposito et al. 2000).
Table 2.
Calculated pKa values of ionisable residues of wild-type β2-m and ΔN6β2-m species at 310 K
| Residue type | Residue number | Titred group | β2-m | ΔN6β2-m |
| MET | 0 | NT | 7.36 | |
| ARG | 3 | CZ | 12.79 | |
| LYS/MET | 6 | NZ/NT | 10.90 | 7.29 |
| TYR | 10 | OH | 10.17 | 9.49 |
| ARG | 12 | CZ | 13.25 | 12.21 |
| HIS | 13 | ND1 | 5.72 | 6.18 |
| GLU | 16 | CD | 3.76 | 4.00 |
| LYS | 19 | NZ | 11.46 | 10.65 |
| TYR | 26 | OH | 10.29 | 10.10 |
| HIS | 31 | ND1 | 5.41 | 5.85 |
| ASP | 34 | CG | 3.59 | 3.39 |
| GLU | 36 | CD | 3.97 | 4.17 |
| ASP | 38 | CG | 1.96 | 3.55 |
| LYS | 41 | NZ | 14.28 | 10.19 |
| GLU | 44 | CD | 3.39 | 4.08 |
| ARG | 45 | CZ | 13.46 | 12.77 |
| GLU | 47 | CD | 3.83 | 3.77 |
| LYS | 48 | NZ | 11.42 | 11.18 |
| GLU | 50 | CD | 3.92 | 3.87 |
| HIS | 51 | ND1 | 6.64 | 6.47 |
| ASP | 53 | CG | 3.41 | 3.70 |
| LYS | 58 | NZ | 10.62 | 10.74 |
| ASP | 59 | CG | 3.43 | 3.81 |
| TYR | 63 | OH | 10.62 | 9.80 |
| TYR | 66 | OH | 11.60 | 10.26 |
| TYR | 67 | OH | 10.63 | 10.36 |
| GLU | 69 | CD | 3.68 | 3.94 |
| GLU | 74 | CD | 4.03 | 3.96 |
| LYS | 75 | NZ | 10.98 | 10.87 |
| ASP | 76 | CG | 2.06 | 3.58 |
| GLU | 77 | CD | 3.23 | 3.72 |
| TYR | 78 | OH | 9.56 | 11.25 |
| ARG | 81 | CZ | 13.60 | 12.54 |
| HIS | 84 | ND1 | 4.14 | 5.87 |
| LYS | 91 | NZ | 10.50 | 10.63 |
| LYS | 94 | NZ | 11.53 | 11.21 |
| ASP | 96 | CG | 3.62 | 3.29 |
| ARG | 97 | CZ | 13.62 | 13.04 |
| ASP | 98 | CG | 4.00 | 3.86 |
| MET | 99 | CT | 3.41 | 3.86 |
UHBD calculations on β2-m were performed using the NMR solution structure ensemble of β2-m (Verdone et al. 2002) and ΔN6β2-m (Esposito et al. 2000). Values in bold indicate ΔpKa ≥ |0.4| of the variant with respect to wild-type protein. The reference side-chain pKa values in unfolded protein used for calculation are: Arg = 12.0, Lys = 10.4, His = 6.3, Asp = 4.0, Glu = 4.4, Tyr = 9.6.
The fluctuation of the binding affinity as a function of pH might have a very important effect on the concentration of ΔN6β2-m in proximity of collagen fibers. An increased binding of this species onto the collagen surface would create a local concentration gradient due to the slower release of the surface-bound protein molecules. This situation may easily approach the critical concentration for protein oligomerization in the solvent that surrounds the collagen fibers, and therefore generate the conditions for local formation of amyloid fibrils by recruiting any amyloidogenic species with proper affinity for ΔN6β2-m seeds. The whole model entails a definite fibril priming role of ΔN6β2-m, whereas full-length β2-m should contribute the bulk of fibril growth. A slight local pH lowering, which matches the physiology of articular inflammatory states, besides triggering the collagen affinity change of ΔN6β2-m, should also favor the partial unfolding of the wild-type protein for fibril recruitment, by inducing enhanced protonation of His31, and hence, a destabilizing increase of the positive charge of full-length native β2-m (Verdone et al. 2002).
Materials and methods
Extraction and fractionation of type I and II collagens
To purify type I collagen, calf skin was obtained from a slaughterhouse immediately after sacrifice. It was freed from hair, fat, and subcutaneous tissues, and dilipidized with several changes of chloroform-methanol (1:1 v/v). After vacuum drying skin was homogenized in 0.5 M acetic acid and stirred at 4°C for 24 h. The suspension was then centrifuged and the supernatant containing acid soluble collagen was discharged; the pellet was digested with 100 mg/mL pepsin in 0.5 M acetic acid at 4°C for 24 h. At the end of the digestion the suspension was centrifuged and the pellet was reextracted with pepsin in the same conditions. Supernatants from the two extractions were pooled and type I collagen was precipitated by selective salt precipitation as described previously (Miller and Rhodes 1982). Purified type I collagen was exhaustively dialyzed against 0.1 M acetic acid and lyophilized.
Calf costal cartilage was finely minced and resuspended in 4 M guanidinium chloride, 50 mM Tris-HCl (pH 7.4), and protease inhibitors for 48 h to extract proteoglycans. After extraction the insoluble material was digested with 1 mg/mL pepsin in 0.2 M NaCl and 0.5 M acetic acid for 24 h at 4°C. The digest was centrifuged, the supernatant was recovered, and the pellet was reextracted with pepsin and centrifuged. The two supernatants were pooled and type II collagen was selectively precipitated by salt precipitation as previously described (Reese and Mayne 1981). Purified type II collagen was exhaustively dialyzed against 0.1 M acetic acid and lyophilized.
The purity of both collagens was checked by SDS-PAGE and the concentration of the collagen solutions used in the binding assays were determined by the hydroxyproline assay according to Huszar et al. (1980).
Preparation of recombinant β2-m
Expression and purification of recombinant proteins was carried out as previously reported for β2m and ΔN6β2m (Esposito et al. 2000). Mutagenesis of His31 into Tyr and of Arg3 into Ala were performed by using the QuikChange site-directed mutagenesis kit, supplied by Stratagene, according to the procedure previously described (Corazza et al. 2004). The concentration of protein sample was determined spectrophotometrically at 280 nm: A1%1 cm = 16.17 β2m), 16.29 (R3Aβ2m), 17.21(H31Yβ2m), and 17.22 (ΔN6β2m).
Electrophoretic analysis of binding
Agarose gels electrophoresis was performed under native condition according to Jeppsson et al. (1979) in 75 mM sodium barbiturate (pH 8.6), 2 mM calcium lactate, and 0.1% sodium azide. The electrophoretic apparatus was equipped with a cooling bath adjusted to 10°C. Electrophoresis was performed at 25 V/cm (gel length 10.7 cm) for 60 min. A protein mixture of β2m (5 μg/mL) and collagen type I (500 μg/mL) was prepared in 100 mM sodium phosphate pH 7.4 and incubated for 1 h at room temperature. Five microliters of this mixture were used for the electrophoretic analysis. Samples of β2m and collagen type I at the same concentration used in the mixture were separately loaded as reference standards.
For Western analysis, the agarose gel was blotted to PVDF membrane, and probed with rabbit polyclonal antihuman β2m antibody (Dako).
Refolding after thermal denaturation
Circular dichroism (CD) spectra were recorded on a JASCO 710 spectropolarimeter equipped with a temperature control system using a 10-mm path length quartz cuvette over the wavelength range of 250–320 nm. The protein concentration was 600 μg mL−1 in sodium phosphate, pH 7.4 (and pH 6.4), and the CD data were expressed as mean residue ellipticity (θ). The protein was initially denatured at equilibrium after heating to 90°C. Reversibility of the thermal unfolding was determined by measuring the recovery of the CD native spectrum upon cooling protein samples to 25°C.
BIAcoreX analysis
All experiments were performed at 25°C on a BIAcoreX instrument (Pharmacia Biosensor AB). Purified types I–II collagens were covalently immobilized on the dextran matrix sensorchip surface (CM5 chip) by using a protein solution (30 μg/mL in sodium acetate buffer at pH 4.0) in a 1:1 dilution with N-hydroxy-succinimide and N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide hydrochloride. The excess active groups on the dextran matrix were blocked with ethanolamine (1 M) (Johnsson et al. 1991). The immobilized level of the two types of collagen were 4000–6000 RU (1000 RU = 1 ng/mm2). The running buffer used was 10 mM HEPES (pH 7.4 or pH 6.4), containing 150 mM NaCl, 5 mM EDTA, and 0.05% Tween 20.
β2 isoforms (β2 wild type, ΔN6β2m, R3Aβ2m, and H31Yβ2 m) were injected over the surface at the flow rate of 5 μL/min at different concentrations from 10 to 100 μM in the corresponding running buffer. The surface was regenerated with a pulse of 25 mM NaOH if necessary.
This technique was also used to monitor the interaction between collagen type I and β2-m conformers that populate the slow phase of folding. For this specific purpose different concentrations of wild-type isoform were denatured, through a 2-h incubation at 90°C, then allowed to refold upon cooling at room temperature. Each sample was injected after 30, 600, and 1200 sec form the beginning of the refolding process.
Sensorgrams from three sets of data for each of the protein concentrations were collected and averaged for each β2m isoform. Association and dissociation kinetic rate costants (kon and koff) and the equilibrium dissociation costant Kd were calculated by nonlinear fitting using the BIAevaluation 3.0 software (Karlsson and Fält 1997).
pKa calculations
Calculation of pKa values for ΔN6β2-m and β2-m were performed imposing a temperature of 310 K as previously described (Corazza et al. 2004), using the methodology and the routines developed by Antosiewicz and coworkers (Antosiewicz et al. 1994). The structures employed for the calculations were the result of NMR-restrained torsion angle dynamics modeling performed by means of DYANA software (Güntert et al. 1997) on either ΔN6β2-m (Esposito et al. 2000) and β2-m (Verdone et al. 2002).
Acknowledgments
We thank Prof. Fabrizio Chiti and Dr. Antonella Forlino for illuminating discussion on this matter. This study was funded by MIUR through FIRB (RBNE01S29H_002) and COFIN (2002058218_003, 2003051399_004); Ministero della Salute grant project no. 020ALZ00/01.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.041194005.
This paper is dedicated to the memory of our unforgettable mentor Prof. Giuseppina Ferri.
References
- Antosiewicz, J., McCammon, J.A., and Gilson, M.K. 1994. Prediction of pH-dependent properties of proteins. J. Mol. Biol. 238 415–436. [DOI] [PubMed] [Google Scholar]
- Bellotti, V., Stoppini, M., Mangione, P., Sunde, M., Robinson, C., Asti, L., Brancaccio, D., and Ferri, G. 1998. β2-microglobulin can be refolded into a native state from ex vivo amyloid fibrils. Eur. J. Biochem. 258 61–67. [DOI] [PubMed] [Google Scholar]
- Chiti, F., Mangione, P., Andreola, A., Giorgetti, S., Stefani, M., Dobson, C.M., Bellotti, V., and Taddei, N. 2001a. Detection of two partially structured species in the folding process of the amyloidogenic protein beta; 2-microglobulin. J. Mol. Biol. 307 379–391. [DOI] [PubMed] [Google Scholar]
- Chiti, F., De Lorenzi, E., Grossi, S., Mangione, P., Giorgetti, S., Caccialanza, G., Dobson, C.M., Merlini, G., Ramponi, G., and Bellotti, V. 2001b. A partially structured species of beta;2-microglobulin is significantly populated under physiological conditions and involved in fibrillogenesis. J. Biol. Chem. 276 46714–46721. [DOI] [PubMed] [Google Scholar]
- Corazza, A., Pettirossi, F., Viglino, P., Verdone, G., Garcia, J., Dumy, P., Giorgetti, S., Mangione, P., Raimondi, S., Stoppini, M., et al. 2004. Properties of some variants of human beta;2-microglobulin and amyloidogenesis. J. Biol. Chem. 279 9176–9189. [DOI] [PubMed] [Google Scholar]
- Esposito, G., Michelutti, R., Verdone, G., Viglino, P., Hernandez, H., Robinson, C.V., Amoresano, A., Dal Piaz, F., Monti, M., Pucci, P., et al. 2000. Removal of the N-terminal hexapeptide from human beta;2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci. 9 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntert, P., Mumenthaler, C., and Wüthrich, K. 1997. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273 283–298. [DOI] [PubMed] [Google Scholar]
- Homma, N. 1989. Collagen-binding affinity of B2-microglobulin, a preprotein of hemodialysis-associated amyloidosis. Nephron 53 37–40. [DOI] [PubMed] [Google Scholar]
- Huszar, G., Maiocco, J., and Naftolin, F. 1980. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal. Biochem. 105 424–429. [DOI] [PubMed] [Google Scholar]
- Karlsson, R. and Falt, A. 1997. Experimental design for kinetic analysis of protein–protein interactions with surface plasmon resonance biosensors. J. Immunol. Methods 200 121–133. [DOI] [PubMed] [Google Scholar]
- Jadoul, M., Garbar, C., Noel, H., Sennesael, J., Vanholder, R., Bernaert, P., Rorive, G., Hanique, G., and van Ypersele de Strihou, C. 1997. Histological prevalence of beta; 2-microglobulin amyloidosis in hemodialysis: A prospective post-mortem study. Kidney Int. 51 1928–1932. [DOI] [PubMed] [Google Scholar]
- Jeppsson, J.O., Laurell, C.B., and Franzen, B. 1979. Agarose gel electrophoresis. Clin. Chem. 25 629–638. [PubMed] [Google Scholar]
- Johnsson, B., Lofas, S., and Lindquist, G. 1991. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 198 268–277. [DOI] [PubMed] [Google Scholar]
- Merlini, G. and Bellotti, V. 2003. Molecular mechanism of amyloidosis. N. Eng. J. Med. 349 583–596. [DOI] [PubMed] [Google Scholar]
- Miller, E.D. and Rhodes, R.K. 1982. Preparation and characterization of the different types of collagen. Methods Enzymol. 8233–64. [DOI] [PubMed] [Google Scholar]
- Moe, S.M. and Chen, N.X. 2001. The role of the synovium and cartilage in the pathogenesis of beta; (2)-microglobulin amyloidosis. Semin. Dial. 14 127–130. [DOI] [PubMed] [Google Scholar]
- Monti, M., Principe, S., Giorgetti, S., Mangione, P., Merlini, G., Clark, A., Bellotti, V., Amoresano, A., and Pucci, P. 2002. Topological investigation of amyloid fibrils obtained from beta;2-microglobulin. Protein Sci. 11 2362–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimni, M.E. and Harkness, R.D. 1988. Molecular structure and function of collagen. In Collagen (ed. M.E. Nimni), Vol. I Biochemistry, pp. 3–77. CRC Press Inc., Boca Raton, FL.
- Obici, L., Bellotti, V., Mangione, P., Stoppini, M., Arbustini, E., Verga, L., Zorzoli, I., Anesi, E., Zanotti, G., Campana, C., et al. 1999. The new apolipoprotein A-I variant leu(174)→Ser causes hereditary cardiac amyloidosis, and the amyloid fibrils are constituted by the 93-residue N-terminal polypeptide. Am. J. Pathol. 155 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici, L., Palladini, G., Giorgetti, S., Bellotti, V., Gregorini, G., Arbustini, E., Verga, L., Marciano, S., Donadei, S., Perfetti, V., et al. 2004. Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated Italian families. Gastroenterology 126 1416–1422. [DOI] [PubMed] [Google Scholar]
- Ohashi, K., Kisilevsky, R., and Yanagishita, M. 2002. Affinity binding of glycosaminoglycans with beta; (2)-microglobulin. Nephron 90 158–168. [DOI] [PubMed] [Google Scholar]
- Reese, C.A. and Mayne, R. 1981. Minor collagens of chicken hyaline cartilage. Biochemistry 20 5443–5448. [DOI] [PubMed] [Google Scholar]
- Rosano, C., Zuccotti, S., Mangione, P., Giorgetti, S., Bellotti, V., Pettirossi, F., Corazza, A., Viglino, P., Esposito, G., and Bolognesi, M. 2004. beta;2-micro-globulin H31Y variant 3D structure highlights the protein natural propensity towards intermolecular aggregation. J. Mol. Biol. 335 1051–1064. [DOI] [PubMed] [Google Scholar]
- Saraiva, M.J. 1995. Transthyretin mutations in health and disease. Hum. Mutat. 5 191–196. [DOI] [PubMed] [Google Scholar]
- Sprague, S. and Moe, S. 1996. Clinical manifestations and pathogenesis of dialysis-related amyloidosis. Semin. Dial. 9 360–369. [Google Scholar]
- Stoppini, M., Arcidiaco, P., Mangione, P., Giorgetti, S., Brancaccio, D., and Bellotti, V. 2000. Detection of fragments of β2-microglobulin in amyloid fibrils. Kidney Int. 57 349–350. [DOI] [PubMed] [Google Scholar]
- Verdone, G., Corazza, A., Viglino, P., Pettirossi, F., Giorgetti, S., Mangione, P., Andreola, A., Stoppini, M., Bellotti, V., and Esposito, G. 2002. The solution structure of human β2-microglobulin reveals the prodromes of its amyloid transition. Protein Sci. 11 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva, J., Hoshino, M., Katou, H., Kardos, J., Hasegawa, K., Naiki, H., and Goto, Y. 2004. Increase in the conformational flexibility of beta;2-microglobulin upon copper binding: A possible role for copper in dialysis related amyloidosis. Protein Sci. 13 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, C., Chanard, J., Caudwell, V., Lavaud, S., Wong, T., and Revillard, J.P. 1992. Kinetics of 125I-β 2-microglobulin turnover in dialyzed patients. Kidney Int. 421434–1443. [DOI] [PubMed] [Google Scholar]