Abstract
Purpose
Degradation of engulfed material is primarily mediated by lysosomal enzymes that function optimally within a narrow range of acidic pH values. RPE cells are responsible for daily degradation of photoreceptor outer segments and are thus particularly susceptible to perturbations in lysosomal pH. The authors hypothesized that elevated lysosomal pH levels could slow enzyme activity and encourage accumulation of partially digested material. Consequently, treatment to lower perturbed lysosomal pH levels may enhance degradative activity.
Methods
A high-throughput screening assay was developed to quantify the lysosomal pH of fresh mouse and cultured ARPE-19 cells. The effect of lysosomal pH on outer segment clearance was determined.
Results
Lysosomal pH is elevated in RPE cells from ABCA4 knockout mice and in cultured human ARPE-19 cells exposed to N-retinylidene-N-retinylethanolamine (A2E), tamoxifen, or chloroquine. The lysosomal pH of fresh RPE cells from ABCA4−/− mice and of chemically compromised RPE cells was reacidified by elevating intracellular cAMP directly. Compromised lysosomal pH was also restored by stimulating A2A adenosine or β-adrenergic receptors, consistent with Gs-protein coupling of these receptors. Restoring lysosomal pH with these treatments enhanced photoreceptor outer segment clearance, demonstrating functional relevance consistent with an enhancement of degradative enzyme activity.
Conclusions
Elevation of lysosomal pH in RPE cells interferes with the degradation of outer segments and may contribute to the pathologies associated with A2E. Pharmacologic elevation of cAMP can restore an acid pH and improve degradative function.
Phagocytic cells are required to clear engulfed material.1,2 This degradative activity is particularly important for retinal pigment epithelial (RPE) cells because their daily engulfing of shed photoreceptor outer segments makes them among the most phagocytotically active cells in the body.3 As such, this high degradative load makes them particularly susceptible to factors that decrease lysosomal enzyme activity. For example, the mutations in the enzyme lysosomal acid lipase in ceroid lipofuscinosis are associated with an accumulation of lipofuscin-like material in the RPE.4,5
Lysosomal enzymes function optimally over a narrow range of acidic pH values, and the primary degradative enzymes of the RPE reflect this tight pH dependence.6 For instance, the lysosomes of RPE cells have high levels of lysosomal acid lipase,7 and activity of acid lipase decreases by 60% when pH rises from 4.5 to 5.2.8 The breakdown of proteins in RPE lysosomes is largely mediated by cathepsin D, and activity of cathepsin D falls by 80% when the pH rises to pH 5.0.9,10 Similarly, decreases in N-acetyl-beta-glucosaminidase occur when the pH rises to 5.0.11,12 The sharp pH dependence of these enzymes predicts that conditions that alkalinize lysosomal pH will interfere with degradative activity.
The pH dependence of degradative enzymes is consistent with reports demonstrating that elevation of lysosomal pH in RPE cells alters the processing of phagocytosed outer segments. Chloroquine alkalinizes lysosomes, and rats treated with chloroquine demonstrated incomplete digestion of outer segments in the RPE.13,14 Chloroquine treatment also led to the accumulation of material on Bruch membrane.15 Tamoxifen can alkalinize lysosomes, and tamoxifen decreased activity of cathepsin D in RPE cells.16,17 The potential for N-retinylidene-N-retinylethanolamine (A2E) to alter lysosomal pH may be particularly relevant because A2E is increased in the RPE of patients with Stargardt disease and in the ABCA4−/− mouse model of the disease.18 The effect of A2E on lysosomal pH, however, is unclear.19,20
It has been predicted that the elevation of lysosomal pH would hasten the accumulation of partially digested outer segment fragments within RPE cells. We hypothesized that reacidification of lysosomes in compromised RPE cells could enhance the activity of lysosomal enzymes in situ. In the present study, we measured lysosomal pH in RPE cells from ABCA4−/− mice and in cultured RPE cells exposed to A2E, chloroquine, and tamoxifen. Compounds capable of acidifying compromised lysosomes were identified using a high-throughput screening procedure. Given that the goal in restoring pH is to improve the ability of lysosomal enzymes to degrade outer segments, we also investigated the relationship between lysosomal pH and outer segment clearance.
Methods
Cell Culture
The human ARPE-19 cell line was obtained from the American Type Culture Collection (Manassas, VA).21 Cells were grown for 1 to 2 weeks to encourage some differentiation, as previously described.22
Lysosomal pH Measurement from ARPE-19 Cells
Measurement of lysosomal pH from ARPE-19 cells was performed using a ratiometric lysosomal pH indicator dye (LysoSensor Yellow/Blue DND-160; Invitrogen, Carlsbad, CA). The dye exhibits pH-dependent excitation at 340 nm and 380 nm and permits the ratiometric assessment of pH changes in acidic organelles independent of dye concentration.23 This is an advantage in lysosomes because organelle volume fluctuates with the pH.24,25 The sensor colocalized with a red fluorescent lysosomal stain (LysoTracker Red DND-99; Invitrogen) in small vesicles (Fig. 1A), with a distribution consistent with lysosomal origin of the signal. Images in Figure 1A were obtained using a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Thornwood, NY) and analyzed with Zeiss software (LSM Image Browser).
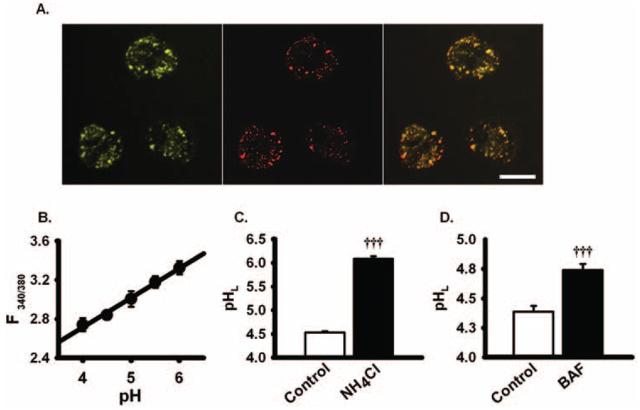
Figure 1.
Validation of dye assay. (A) RPE cells were simultaneously labeled with the lysosomal pH indicator dye (left) and the red fluorescent lysosomal stain (middle). Considerable colocalization of two dyes was found (right), indicating a predominantly lysosomal source of the pH dye signal. Scale bar, 20 μm. The lysosomal pH indicator dye was detected at 351/385 nm (excitation/emission), while the red fluorescent lysosomal stain was detected at 543/560 nm. (B) Typical calibration of lysosomal pH from ratios in the presence of ionophores monensin and nigericin. F340/380 = ratio of fluorescence excited at 340 nm/380 nm. The calibration curve was well fit with a linear regression (y = y0 + ax). In this case, y0 = 0.30, a = 1.50, and r2 = 0.99. Calibration was performed separately on each plate. (C) The assay was verified using standard alkalinizing compounds, with lysosomal pH (pHL) elevated by 10 mM NH4Cl (n = 20). (D) Bafilomycin A (BAF, 200 nM, n = 20) also elevated lysosomal pH level. †††P < 0.001 vs. control, one-way ANOVA with Tukey post hoc test. Here and throughout, bars on data plots = mean + SEM; n = number of independent wells.
For measurement of lysosomal pH, cells were loaded with the lysosomal pH indicator dye for 5 minutes at room temperature (RT; 21°C-25°C), followed by 15 minutes for internalization. Quantitative comparison was performed on parallel cells in a 96-well plate, and the fluorescence was measured with a 96-well plate reader at RT (Fluroskan; Thermo Fisher Scientific, Inc., Waltham, MA). Light emitted >520 nm in response to excitation at 340 nm and 380 nm was measured for 20 msec every 30 seconds. The ratio of light excited at 340/380 nm was converted to pH by calibration with KCl buffered to pH 4.0 to 6.0 in the presence of 10 μM H+/Na+ ionophore monensin and 20 μM H+/K+ ionophore nigericin dissolved in 20 mM 2-(N-morpholino)ethane sulfonic acid (MES), 110 mM KCl, and 20 mM NaCl and adjusted to pH 4.0 to 6.0 with HCl/NaOH. The relationship was linear over the range examined (Fig. 1B). Calibration indicated a baseline pH of 4.4 to 4.5, supporting both the lysosomal localization of the lysosomal pH indicator dye and the calibration process.6 Lysosomal pH values were elevated by NH4Cl (Fig. 1C) and by the vH+ATPase inhibitor bafilomycin-A (Fig. 1D), validating the assay. None of these compounds, or any others used later in this study, altered the signal at 340 or 380 nm in the absence of dye. Drugs tested for their ability to reverse the effects of tamoxifen or chloroquine were added to cells 5 minutes before the addition of the alkalinizing agents, and the pH was measured 20 minutes later.
Outer Segment Degradation Assay
The isolation of bovine photoreceptor outer segments was based on published protocols.26-28 Briefly, bovine retinas were homogenized in 20% sucrose with 130 mM NaCl, 20 mM Tris-HCl, 10 mM glucose, 5 mM taurine, and 2 mM MgCl2 (pH 7.2). The homogenate was placed in ultracentrifuge tubes with 20%, 27%, 33%, 41%, 50%, and 60% sucrose and was centrifuged for 70 minutes at 28,000 rpm on a SW28 rotor (4°C). The supernatant was filtered, diluted in 0.02 M Tris-HCl buffer (pH 7.2), and centrifuged at 13,000g for 10 minutes (4°C). The pellet was resuspended in 10 mM PBS, 0.1 mM NaCl, and 2.5% sucrose.
A new approach to quantifying the degradation of photoreceptor outer segments within lysosomes was developed. Outer segments have been traditionally labeled with FITC, but because this dye has a pKa of 6.8, a diminished signal in acidic organelles confuses outer segment degradation with localization and makes the fluorescent readout ambiguous.29 Outer segments were instead labeled with the pH-insensitive dye calcein. Calcein is unaffected by pH or calcium levels and has been used to determine the integrity of cellular compartments. Outer segments were loaded with 5 μM calcein-acetoxymethyl (AM) in PBS for 10 minutes and spun twice at 14,000 rpm. The AM form of the dye is nonfluorescent until cleaved by intracellular esterases, greatly attenuating the background signal. The outer segments began accumulating fluorescence immediately, with the attainment of bright, stable levels after 20 minutes, similar to the loading timeframe found in trabecular meshwork cells.30 It was not possible to determine whether staining was present within the photoreceptor disc lumen using light microscopy, although the fluorescence signal was evenly distributed and calcein does enter cellular organelles, where it can become trapped by resident esterases.31
Outer segments labeled with calcein were added to ARPE-19 cells in 96-well plates. After 2 hours, cells were washed vigorously and incubated with growth medium for another 2 hours. Baseline fluorescence levels excited at 485 nm were initially read at this point to minimize the effect of drugs on binding or internalization. Cells were then incubated with alkalinizing drugs and putative treatments or control solution for 20 hours, after which the cells were washed twice, and the final fluorescence reading was taken. This time course was based on kinetic analysis showing most outer segment degradation is complete within 18 hours.32 The “%ΔPOS” was defined as the percentage change in fluorescence from photoreceptor outer segments between final and initial reading. Changes in signal were quantified by subtracting background, calculating the proportional drop in fluorescence between the start and end of drug addition, and normalizing to controls with control ratios subtracted.
The lysosomal breakdown of outer segments involves the enzymatic degradation of the outer segment membranes, and we assume that calcein is released once the membrane is ruptured. If calcein is retained within the disc lumen, this release will require cleavage of the disc membrane as well. Trials have shown that approximately three quarters of the decline in cellular fluorescence over the 20 hours is accounted for by extracellular fluorescence, presumably reflecting pinocytosis of calcein released from the ruptured outer segment compartments. Washing of the cells before measurement was thus critical. The remaining loss may reflect degradation, quenching, or both of calcein. Some residual fluorescence in lysosome-like organelles was evident after the 24 hours, but intensity was considerably reduced. All comparisons of outer segment processing were run in parallel.
To localize outer segments (see Figure 7A), cells were cultured on glass chamber slides and costained with 100 nM LysoTracker Red after 4 hours' exposure to calcein-labeled outer segments to confirm localization. The red fluorescent lysosomal stain was imaged at 540/>590 nm (excitation /emission), whereas labeled outer segments were imaged at 480/>535 nm on a Nikon microscope (Eclipse 600; Nikon USA, Melville, NY) and imaged with a 3-CCD digital camera (Toshiba America, Irvine, CA). Images were manipulated with image processing software (Image Pro Plus; Media Cybernetics Inc., Silver Spring, MD).
Measurements of Lysosomal pH from Murine RPE Cells
ABCA4−/− mice were a kind gift from Gabriel Travis of the Jules Stein Eye Institute at the University of California at Los Angeles. Control C57BL/6 mice were obtained from Harlan (Indianapolis, IN). All mice were treated in accordance with University of Pennsylvania IACUC and the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Mice were reared at 5 to 15 lux and were killed with a CO2 overdose. Mouse eyes were isolated based on published protocols with modifications.33 Briefly, intact eyes were washed twice with PBS and incubated in 2% dispase (wt/vol) and 0.4 mg/mL collagenase intravenously for 45 minutes at 37°C with 5% CO2. Eyes were rinsed twice in growth medium (GM) that contained DMEM with 1× MEM + nonessential amino acids, 3 mM l-glutamine, 100 μg/mL streptomycin, and 2.5 mg/mL amphotericin B (Fungizone) or 50 μg/mL gentamicin (or both), plus 10% fetal bovine serum (all Invitrogen). Eyes were incubated in GM for another 20 minutes in 5% CO2 at 37°C, after which the anterior segments and retinas were removed and sheets of RPE cells were separated from the choroid with fine forceps. These sheets were transferred to a 15-mL centrifuge tube and triturated to single cells with GM. Cells from two to eight eyes were pooled and loaded with 5 μM lysosomal pH indicator dye for 5 minutes at RT. Excess dye was washed off, and cells were distributed into wells of 384-well plates (UV Star; Greiner Bio-One, Monroe, NC) and measured as described. These plates made a significant difference in our ability to detect a signal over background. Murine RPE cells loaded with the lysosomal pH indicator dye fluoresced brightly (Fig. 2A). The autofluorescence emitted from ABCA4−/− cells was slightly more intense than that from wild-type cells, consistent with elevated levels of A2E in knockout animals. However, the fluorescence signal emanating from the dye was 100-fold greater than the difference in background autofluorescence, ensuring the independence of the pH measurements. A small portion of the cell suspension was incubated in pH buffers for simultaneous calibration as described, though noncalibrated data, expressed in terms of the 340/380-nm signal, were detected in some cases. Drugs were usually added to the bath, and measurements were taken 20 minutes later. Comparisons between wild-type and ABCA4−/−, between ABCA4−/− of different ages, and between treated and untreated mice were performed on measurements made simultaneously from different wells on the same plate to control for variation. Images for Figure 2A were obtained on a Nikon microscope (Eclipse 600) with dye (LysoSensor Yellow/Blue; Invitrogen) detected at 360/515 nm and processed as described.
Figure 2.
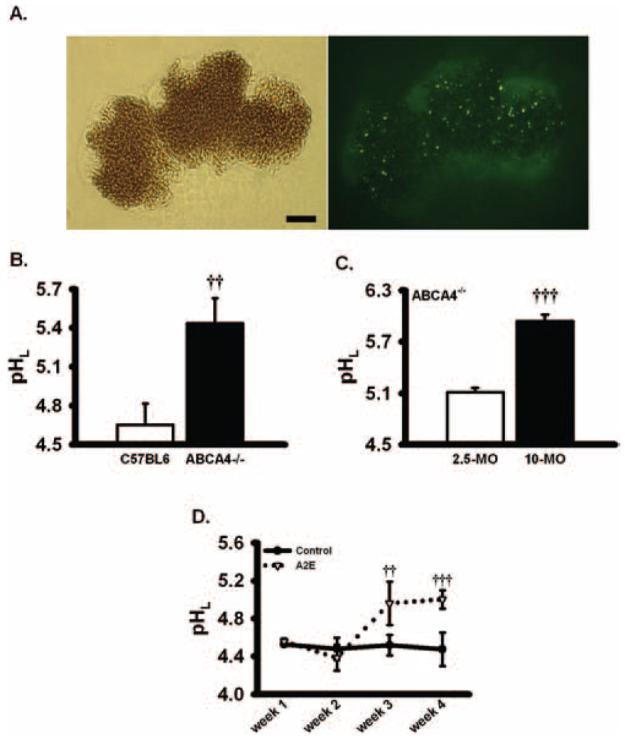
Elevated lysosomal pH in RPE cells from ABCA4−/− mice and in cultured cells exposed to A2E for extended durations. (A) The lysosomal pH indicator dye was detected in freshly isolated C57BL6 mice RPE cells. Left: bright-field image. Right: fluorescence image of the same field, excited at 360 nm (emission, 510 nm). Note that despite the pigment, the signal from the lysosomal pH indicator dye could be detected in sufficient quantity to visualize individual lysosomes. Bar, 10 μm. (B) Lysosomal pH was significantly elevated in fresh RPE cells from ABCA4−/− mice (pH, 5.5 ± 0.2; age, 213 ± 25 days) compared with RPE cells from age-matched control C57BL6 mice (pH, 4.7 ± 0.2; age 208 ± 28 days; n = 7; experiments using pooled RPE cells from a total of 28 knockout and 24 wild-type mice). Qualitatively similar increases in fluorescence ratio were found in seven additional mice of each type. (C) Lysosomal pH was higher in RPE cells from 10-month-old ABCA4−/− mice than from 2.5-month-old ABCA4−/− mice (n = 4). In two other experiments, similar increases were found in the lysosomal pH of cells from the older ABCA4−/− mouse. (D) A2E elevated pHL of ARPE-19 cells, though the treatment at this relatively low dosage took 3 weeks to have an effect (14 nM A2E given twice/wk; n = 6). Similar results were found in two additional trials. ††P < 0.01, †††P < 0.001 vs. control, one-way ANOVA with Tukey post hoc test.
RT-PCR
Although the pharmacologic identification of the A2A adenosine receptor was suggestive, this receptor has not been identified on a molecular level in human RPE cells. Receptor identity was thus confirmed in ARPE-19 and fresh human RPE cells using RT-PCR. RNA was extracted from ARPE-19 cells and fresh human RPE cells using reagent (Trizol; Invitrogen). RNA was treated with RQ1 RNase-free DNase I (Promega, Madison, WI). Reverse transcription was performed from 1 μg total RNA using first-strand DNA synthesis (SuperScript First-Strand Synthesis; Invitrogen). Primers were obtained from a previously published paper,34 as follows: sense, 5′-CAAGAAGGGCTTGGGTTCTG-3′; antisense, 5′-TGACAGCAGGAGAGGACTGC-3′; 431-base pair product. PCR was performed with 2 μL first-strand DNA synthesis (SuperScript First-Strand Synthesis; Invitrogen) product, 2 mM MgCl2, and 0.4 nM each primer with the 0.5-μL recombinant DNA polymerase (AmpliTaq Gold Polymerase; Applied Biosystems, Foster City, CA) at 95°C for 15 minutes, followed by 35 cycles at 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 1 minute, with a final extension step at 72°C for 10 minutes. First-strand DNA synthesis (SuperScript First-Strand Synthesis; Invitrogen) was omitted from the negative control. The band was visualized on a 2% agarose gel, photographed, and purified with an extraction kit (QIAEX II Gel Extraction; Qiagen, Valencia, CA). The DNA was sequenced at the University of Pennsylvania Sequencing Facility and identified with the BLAST nucleotide database (http://www.ncbi.nlm.nih.gov). Identical processing was used on RNA extracted from fresh human RPE cells. Eyes were obtained anonymously, and the research was conducted in accordance with the tenets of the Declaration of Helsinki.
Materials
Myristoylated PKI (14–22) amide was obtained from Tocris Bioscience (Ellisville, MI). A2E was a kind gift from John Dowling and Tom Jordan of Neuron Systems Inc.; samples were stored in aliquots at −80°C and were used promptly. A2E was conjugated with LDL by mixing A2E with LDL (Invitrogen) at a ratio of 14 nmol:10 mg and was incubated at 37°C for 2 hours in the dark based on published protocols,19 although we found that the inclusion of LDL did not alter the pH. Attempts to hasten the change using higher concentrations of A2E were not successful because exposure was toxic, consistent with the loss of lysosomal membrane integrity found with elevated levels.35,36 All other reagents were obtained from Sigma Chemical (St. Louis, MO) unless otherwise indicated.
Statistical Analysis
Data are reported as mean ± SEM. Statistical analysis used a one-way ANOVA with Tukey post hoc test on differences for intragroup comparisons. Results with P < 0.05 were considered significant.
Results
Increased Lysosomal pH in ABCA4−/− Mice and with A2E
Because the RPE cells of ABCA4−/− mice accumulated A2E at rates higher than those of wild type,18 the lysosomal pH in these cells was determined. The pH of lysosomes from RPE cells obtained from ABCA4−/− mice was significantly higher than that of wild-type animals (Fig. 2B). Multiple measurements from numerous mice with a mean age of 7 months demonstrated pH levels of 4.7 in wild-type mice and 5.5 in ABCA4−/− mice. This difference lies in the most sensitive portion of the pH-dependent activity range for lysosomal enzymes and is predicted to reduce the activity of cathepsin D by 70%.10 A significant increase in pH was found in RPE cells from ABCA4−/− mice as young as 4 months of age (the earliest age compared directly with wild types). The elevation in lysosomal pH was age dependent, with lysosomes of cells obtained from older animals more alkaline (Fig. 2C). This is consistent with the age-dependent accumulation of A2E in the knockout mice.18
Although A2E has been thought to alter lysosomal pH for some time,35,37 its contribution to lysosomal alkalinization has been unclear; lysosomal pH was elevated by A2E delivered for 4 weeks,19 though a recent report did not observe this increase when A2E was given for shorter periods.20 To resolve this question with our quantitative system, ARPE-19 cells were exposed to A2E for various intervals. Lysosomal pH was not affected in cells fed 100 to 800 nM A2E for 24 or 48 hours. In contrast, chronic exposure to moderate levels of A2E (14 nM) significantly alkalinized the lysosomes, with pH levels increasing from 4.5 to 5.0. The alkalinization was first apparent after 3 weeks of treatment (Fig. 2D). This slow time course is consistent with both the qualitative increase in pH described in cultured RPE cells fed A2E for 4 weeks19 and the absence of an effect after acute exposure to A2E.20
Model for Rapid Elevation of Lysosomal pH
The alkalinized lysosomes in RPE cells from ABCA4−/− mice and in ARPE-19 cells exposed to A2E implied that restoration of lysosomal pH might be beneficial. However, rapid screening for compounds able to restore an acidic pH required a fast and reproducible method for elevating lysosomal pH above baseline, and neither fresh murine RPE cells nor A2E added to cultured cells for 3 weeks qualified as high-throughput. Because tamoxifen can elevate lysosomal pH and was previously found to be particularly effective at interfering with lysosomal enzyme activity in RPE cells,16,38 its effect was characterized. Tamoxifen led to a rapid alkalinization; lysosomal pH reached maximal levels within 10 to 12 minutes and remained elevated for the entire 30 minutes of observation (Fig. 3A). The response was dose dependent, with an EC50 of 22 μM (Fig. 3B). Because tamoxifen exhibits estrogenic and antiestrogenic activity,39 the effect of 17β-estradiol was examined. The alkalinization produced by tamoxifen was neither mimicked nor blocked by 17β-estradiol (Fig. 3C), consistent with the receptor-independent effect on lysosomal pH observed previously.16 Chloroquine also elevated lysosomal pH (Fig. 3D).
Figure 3.
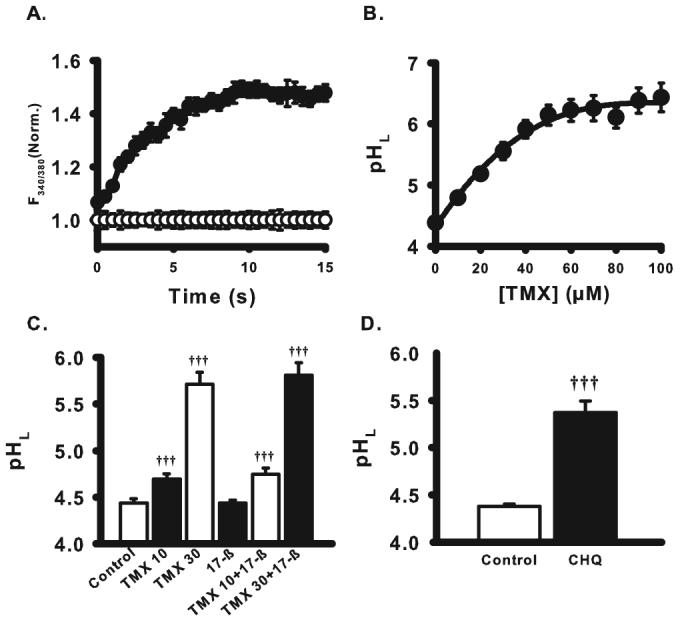
Effects of tamoxifen on lysosomal pH of ARPE-19 cells. (A) Time course of tamoxifen effect on the lysosomal pH. Tamoxifen (30 μM) elevated lysosomal pH rapidly, with levels reaching a plateau within 15 minutes (at which time point all subsequent measurements were made). Values are expressed as fluorescence ratios normalized to mean control levels at the corresponding time point. (B) Concentration dependence of tamoxifen on lysosomal pH of RPE cells. Tamoxifen (TMX; 10–100 μM) was applied to the cultured ARPE-19 cells for 15 minutes. Tamoxifen showed a concentration-dependent effect to increase the lysosomal pH (pHL), with EC50 = 22 μM. Symbols represent mean ± SEM fit with a single exponential curve (all n = 30, all different from 0 μM, P < 0.001). (C) Comparison of effects of tamoxifen and 17β-estradiol (17-β) on lysosomal pH of ARPE-19 cells. 17β-estradiol (100 nM), an estrogen receptor agonist, was applied to the cells in the absence or presence of 10 μM or 30 μM tamoxifen. The response to tamoxifen was neither mimicked nor inhibited by 17β-estradiol (n = 6), suggesting the effect of tamoxifen did not involve estrogen receptors. Here and elsewhere, numbers adjacent to drugs are concentrations in μM. (D) Lysosomal pH was also elevated by 20 μM chloroquine (CHQ, n = 20). †††P < 0.001 vs. control, one-way ANOVA with Tukey post hoc test.
Pharmacologic Treatment to Lower Lysosomal pH
Initial attempts to identify compounds that could reacidify compromised lysosomes focused on the effects of catecholamines. Substantial acidification was induced by epinephrine, norepinephrine, and the β-adrenergic receptor agonist isoproterenol, whereas the α1 receptor agonist phenylephrine had no effect (Fig. 4A). Timolol inhibited the acidifying effects of norepinephrine, though it did not increase pH in cells treated with tamoxifen alone. Norepinephrine also acidified lysosomes in ARPE-19 cells treated with chloroquine, demonstrating its acidifying effect was not specific to tamoxifen-treated cells (Fig. 4B). Together, these results implied that stimulation of the β-adrenergic receptor lowered lysosomal pH in RPE cells perturbed under two different conditions.
Figure 4.
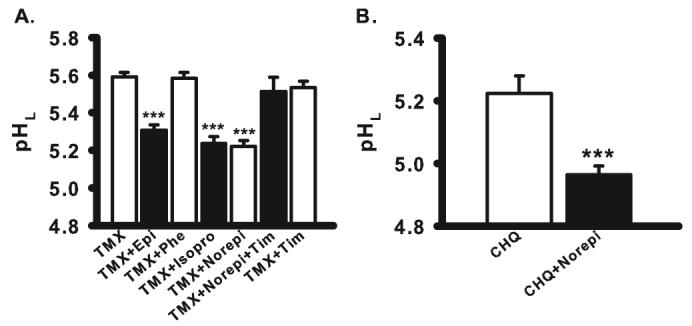
Stimulation of β-adrenoceptors lowers lysosomal pH. (A) Adrenoceptor agonists lowered pHL in tamoxifen (TMX)-treated ARPE-19 cells. Adrenoceptor agonists epinephrine (Epi, 10 μM), isoproterenol (Isopro, 10 μM), and norepinephrine (Norepi, 10 μM) helped restore pHL raised by 30 μM tamoxifen. Phenylephrine (Phe, 10 μM) did not change lysosomal pH. Acidification by norepinephrine was blocked by β-adrenoceptor inhibitor timolol (Tim, 10 μM), although timolol alone did not change pHL (n = 8–71). (B) Norepinephrine also lowered pHL in cells exposed to 20 μM chloroquine (CHQ) (n = 5–10). ***P < 0.001 vs. TMX or CHQ alone, depending on the drug used for the test; one-way ANOVA with Tukey post hoc test.
Purinergic agents were also assessed, as increasing evidence suggests their signaling has a considerable impact on RPE physiology.40 The stable nonspecific adenosine receptor agonist 5′-(N-ethylcarboxamido)-adenosine (NECA) reduced lysosomal pH in cells treated with tamoxifen (Fig. 5A). Because NECA activates A1 and A2A adenosine receptors, more specific agents were also tested.41 The A2A receptor agonist CGS21680 acidified the lysosomes (Fig. 5B), whereas A1 adenosine receptor agonists N6-cyclopentyladenosine (CPA) and (2S)-N6-[2-endo-Norbornyl]adenosine (ENBA) were ineffective (Fig. 5C). Stimulation of the A2A adenosine receptor also lowered the lysosomal pH of RPE cells treated with chloroquine (Fig. 5D). Message for the A2A adenosine receptor was identified in ARPE-19 cells and in fresh human RPE cells (Fig. 5E). This is the first molecular identification of the A2A adenosine receptor in human RPE cells to our knowledge, and it supports the pharmacologic identification.
Figure 5.
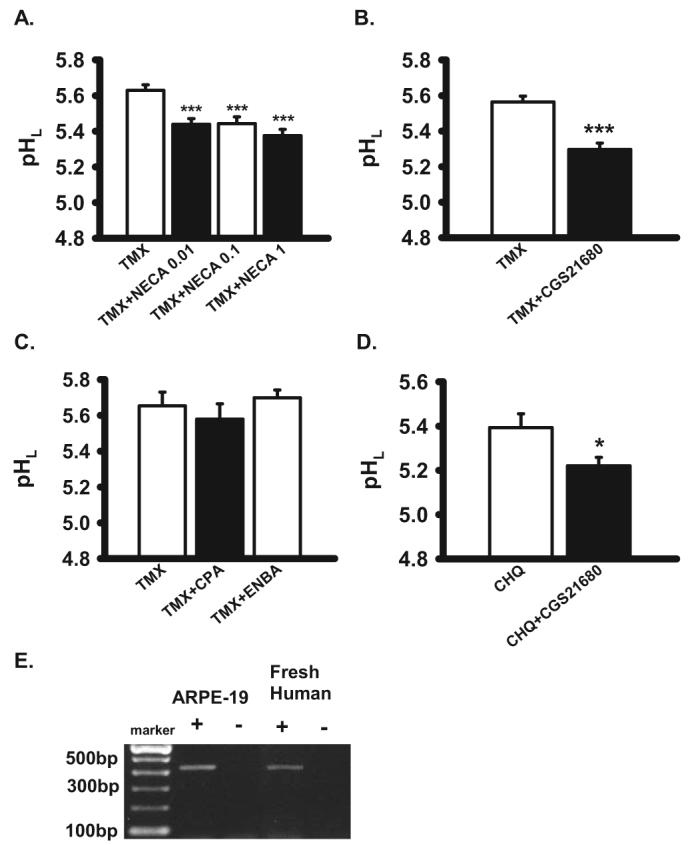
Stimulation of adenosine A2A receptors reduces pHL. (A) Non-selective adenosine receptor agonist NECA reversed lysosomal alkalinization by 30 μM tamoxifen (TMX) in ARPE-19 cells (n = 26–44). (B–D) Although A2A adenosine receptor agonist CGS21680 (100 nM) inhibited the effect of 30 μM tamoxifen (B; n = 48) and 20 μM chloroquine (D; n = 16) in ARPE-19 cells, A1 agonists CPA (100 nM) and ENBA (100 nM) had no effect (C; n = 8–28). (E) Expression of adenosine receptor subtype A2A in RPE cells. A2A receptor expression in ARPE-19 cells and postmortem human RPE cells by RT-PCR of expected 431 bp (+). No bands were seen without reverse transcriptase (−). The sequenced PCR product was verified as the A2A receptor. *P < 0.05 vs. CHQ alone, ***P < 0.001 vs. TMX alone; one-way ANOVA with Tukey post hoc test.
Given that β-adrenergic and the A2A adenosine receptors are both coupled to Gs proteins whose activation stimulates adenylate cyclase and increases intracellular cAMP, direct elevation of cAMP was examined. The cell-permeable analogues chlorophenylthio-cAMP (cpt-cAMP) and 8-bromo-cAMP lowered lysosomal pH in compromised cells (Fig. 6A). A cAMP-activating cocktail consisting of 3-isobutyl-1-methylxanthine (IBMX), forskolin, and cpt-cAMP reacidified lysosomes even more effectively. These observations indicated that cAMP was a key effector in the receptor-mediated acidification of compromised lysosomes.
Figure 6.
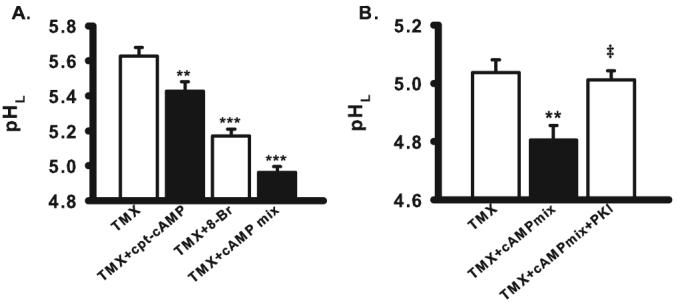
cAMP derivatives lowered pHL in tamoxifen-treated ARPE-19 cells. (A) Cell-permeant cAMP analog cpt-cAMP (500 μM), 8-Br-cAMP (8-Br, 500 μM), and a cAMP-stimulating mix of forskolin (10 μM), cpt-cAMP (500 μM), and IBMX (100 μM) acidified cells exposed to tamoxifen (30 μM, n = 16–43). (B) Myristoylated PKI (14–22) amide (100 μM), the cell-permeant inhibitor of protein kinase A, blocked the restorative effects of the cAMP cocktail on cells treated with tamoxifen (10 μM, n = 8–16), implying a role for PKA. ‡P < 0.005 vs. TMX + cAMP mix. **P < 0.01, ***P < 0.001 vs. TMX alone; one-way ANOVA with Tukey post hoc test.
The myristoylated cell-permeable version of protein kinase A (PKA) inhibitor PKI (14–22) amide was added to the cells to determine whether the effects of cAMP required the activation of PKA. Myristoylated PKI prevented the acidification mediated by the cAMP mix, implying a central role for PKA in lysosomal reacidification (Fig. 6B).
Restoration of Outer Segment Clearance
The ultimate goal of restoring lysosomal pH in RPE cells is to improve the function of the degradative enzymes. Because a central role for these enzymes is to clear engulfed outer segments, the effect of pH manipulations on outer segment clearance was explored. Outer segments were labeled with the pH-insensitive dye calcein to separate the effects of pH from degradation (see Methods). Colocalization with the red fluorescent lysosomal stain confirmed that most labeled outer segments were present in lysosomes before any pharmacologic intervention was initiated (Fig. 7A). Tamoxifen slowed the degradation of outer segments in a dose-dependent way, with the decrease proportional to its effect on lysosomal pH (Fig. 7B). Treatment with chloroquine also slowed the degradation. In contrast, pharmacologic reacidification of perturbed lysosomes increased outer segment clearance. Degradative function was improved by treatment with NECA, CGS21680, norepinephrine, and isoproterenol (Fig. 7C). These observations are consistent with the hypothesis that lysosomal enzyme activity is sensitive to lysosomal pH and that restoring acidity can improve their ability to degrade outer segments.
Figure 7.
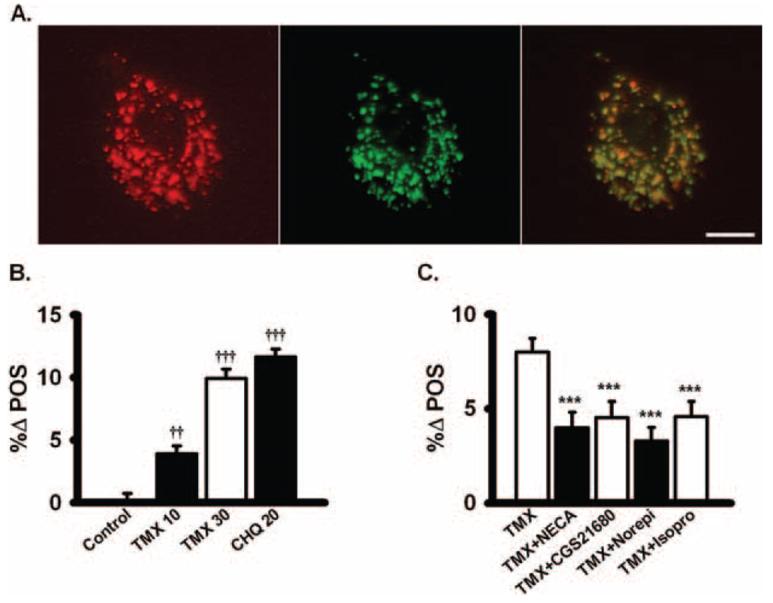
Increase in clearance of photoreceptor outer segments. (A) Colocalization of the red fluorescent lysosomal stain (left) and calcein-labeled photoreceptor outer segments (middle) in ARPE-19 cells. Overlap (right) indicates most outer segments were in lysosomes. Scare bar, 10 μm. (B) Tamoxifen (TMX) and chloroquine (CHQ) both impaired the ability of ARPE-19 cells to degrade photoreceptor outer segments. The reduction in the clearance of outer segments by tamoxifen was dose dependent and proportional to the effect of tamoxifen on pHL (n = 12). Measurements were made 20 hours after the addition of drugs. %Δ POS is the normalized percentage change in outer segment fluorescence. (C) The nonselective adenosine agonist NECA (100 nM) and the specific A2A receptor agonist CGS21680 (100 nM) both lowered the retention of outer segments that was increased by 30 μM tamoxifen, consistent with an increased enzymatic function with lowered pHL. Norepinephrine (Norepi, 10 μM) and isoproterenol (Isopro, 10 μM) also restored outer segment clearance slowed by tamoxifen (n = 20). ††P < 0.01 vs. control, †††P < 0.001 vs. control, ***P < 0.001 vs. TMX alone, one-way ANOVA with Tukey post hoc test.
Restoration of Lysosomal pH in Cells Exposed to A2E
Although the mechanisms underlying the increase in pH initiated by tamoxifen, chloroquine, and chronic exposure to A2E are likely different, it was possible that the pathways used to reacidify the lysosomes were relevant regardless of the original insult. Direct elevation of intracellular cAMP with the cAMP-activating mix reduced lysosomal pH in 6-month-old ABCA4−/− mice from 5.2 to 4.8 (Fig. 8A). The lysosomal pH of ARPE-19 cells challenged with A2E was significantly decreased by the A2A adenosine agonist CGS21680, with levels restored near those in control cells (Fig 8B). Similar acidification was found with 10 μM norepinephrine, suggesting that cAMP-mediated acidification and relevant receptors were functioning in cells exposed to A2E for extended times.
Figure 8.
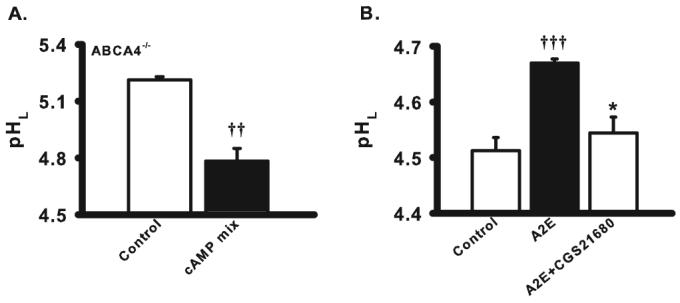
Restoration of lysosomal pH in cells exposed to A2E. (A) The lysosomal pH of RPE cells from 6-month-old ABCA4−/− mice was decreased by the cAMP-activating mix to 4.78 ± 0.07 (n = 4). (B) The A2A adenosine receptor agonist CGS21680 (100 nM) acidified the pHL in ARPE-19 cells treated with A2E (14 nM twice a week for 4 weeks; n = 4–12). The lysosomal pH in treated cells was not significantly different from that in control. ††P < 0.01 vs. control, †††P < 0.001 vs. control, *P < 0.05 vs. TMX alone, one-way ANOVA with Tukey post hoc test.
Discussion
Because pH can modulate the activity of lysosomal enzymes and because the dysregulation of lysosomal enzymes may be detrimental to the health of postmitotic cells, the observations described herein offer a reasonable approach to improving lysosomal enzyme activity compromised by challenges to pH. The development of a high-throughput screening assay to identify compounds capable of restoring lysosomal pH, combined with a protocol to assess outer segment clearance independent of pH, have enabled examination of this fundamental problem on a quantitative scale. The pharmacologic identification of intracellular cAMP as a key mediator of lysosomal acidification offers a variety of potential options to correct a compromised pH level. The extension of these approaches to RPE cells from ABCA4−/− mice demonstrates for the first time that elevated lysosomal pH is a specific defect in these animals, with the magnitude of the lysosomal alkalinization in adult mice predicted to substantially lower lysosomal enzyme activity in situ. Furthermore, the restoration of lysosomal pH in RPE cells from ABCA4−/− mice by cAMP is predicted to increase enzyme activity twofold to fourfold.10
Validity and Relevance of the Present Approach
Several considerations support the validity of these findings. First, great care was taken in developing the protocol for lysosomal pH measurements. Precise control over dye-loading parameters, coupled to the use of multiwell plates and stringent calibration techniques, led to a highly reproducible method. Second, acidification by A2A adenosine receptor agonists, β-adrenergic receptor agonists, and cAMP are all consistent with the existence of a common second-messenger system. Third, the ability of drugs to produce similar changes in fresh mouse and cultured human RPE cells suggests a general application of our findings. This also demonstrates that RPE cells from older ABCA4−/− mice are amenable to treatment. Fourth, the tight correlation among lysosomal pH, outer segment degradation rate, and predicted activity of lysosomal enzymes based on biochemical analysis indicates that the interventions identified above can have significant physiological consequences.8,10,12
Some caution is, of course, necessary in interpreting these results. Our method measured only the mean levels of lysosomal pH, and it is possible that subpopulations of lysosomes containing less digestible oxidized material could be relatively unresponsive. However, Figure 8A demonstrates that the mean lysosomal pH in the 6-month-old ABCA4−/− mice was restored to 4.8 by the cAMP-stimulating mix. This level is very close to that seen in wild-type animals, suggesting that the pH of most lysosomes had to be improved. Although the pH changes we measured in lysosomes may differ from those of the phagolysosome, the related shifts in pH and outer segment degradation imply that the response in these two organelles is at least qualitatively similar. There are many differences between fresh and cultured cells, with the absence of melanosomes in the latter particularly relevant. Still, the lysosomal acidification of older ABCA4−/− mice by cAMP implies merit in using the cultured RPE cells to rapidly identify compounds that can modulate lysosomal pH in fresh cells.
Mechanisms of pH Change
Although this study demonstrates that lysosomal pH can be raised by a variety of toxins and restored by manipulating intracellular cAMP, the mechanisms underlying both events remain to be fully elucidated. A2E is a quaternary amine, with complex effects in RPE cells caused, at least in part, by its ability to alter lipid composition and produce a detergentlike effect on intracellular membranes.20,35,37 A2E decreases activity of the vH+ATPase on isolated lysosomes and reduces the translocation of protons into these isolated lysosomes, indicating that it may alter lipid-protein interactions in the lysosomal membrane.42 Tamoxifen is a tertiary amine; its protonation in acidic organelles neutralizes pH while trapping it within the vesicle. However, tamoxifen can also alter the lipid composition of vesicular membranes within RPE cells43 and can act within the membrane of other cells to mediate electroneutral transport of proton/chloride transport and dissipate the proton gradient.44 There may thus be more similarities in the alkalinizing actions of tamoxifen and A2E than originally thought. Regardless of these considerations, it is clear that the pH of RPE cells challenged with either A2E or tamoxifen can be restored by cAMP, implying either that both disturb the same mechanism or that the cAMP- sensitive effector can sidestep both original insults.
Increased levels of cAMP could acidify perturbed lysosomes through one or more of several routes. Proton delivery to lysosomes and late endosomes is primarily mediated by the vH+ATPase,45 creating both a proton gradient and an electrical potential across vesicular membranes.46,47 Excess positive charge can be neutralized by the influx of Cl− into the vesicle.48 In this regard, the cAMP-dependent Cl− channel CFTR contributes to lysosomal acidification and degradative activity in macrophages.49 Functional and immunochemical evidence indicates CFTR is present on RPE cells, though its contribution to intracellular organelles is not yet clear.50-52 Alternatively, cAMP could act on the proton pump. cAMP can lead to insertion of the plasma membrane variant of vH+ATPase into the plasma membrane of clear cells and an increase in proton secretion.53 Whether cAMP can regulate proton pump levels in lysosomes is not known, though increased insertion may be partially responsible for the increase in endosomal acidification it triggers in liver cells.54
Implications for RPE Health
The actions of tamoxifen, β-adrenergic receptors, and A2E on lysosomal pH raise questions about RPE health. Typical clinical doses of tamoxifen lead to a plasma concentration of 0.5 to 2 μM, but tissue concentration can be considerably higher.55 The incidence of retinopathies with moderate doses of tamoxifen treatment is low, but the rare problems that do occur at higher doses are consistent with lysosomal failure in the RPE.56,57 Extended exposure to tamoxifen can also lead to decreased levels of enzyme activity independent of pH, possible because of an altered delivery of lysosomal enzymes.17,38,58 Our results are also consistent with the small increase in risk for age-related maculopathy in patients treated long term with systemic β-blockers.59 With tamoxifen and timolol, however, the lack of extensive ocular damage in spite of widespread use implies the threat is minimal for most people using moderate doses.
The effects of A2E on lysosomal pH may have more direct implications for ocular health. Evidence has been put forth that A2E accumulates in the RPE of patients with Stargardt's disease.18,35 The disorder is associated with elevated levels of lipofuscin in RPE cells, consistent with the reduced degradation of phagocytosed outer segments and the impaired activity of lysosomal enzymes. Although A2E can reduce the activity of lipases and proteases within RPE cells,19,60 A2E does not have a direct effect on enzyme activity.20,61 This suggests A2E may have indirect effects on enzyme activity, and the sharp pH dependence of RPE lysosomal enzymes makes pH a prime candidate. The effects of A2E in vivo are predicted to be complex; chemical variants and oxidation products of A2E also lead to cell disease, whereas exposure to light produces additional toxic effects.62,63 However, changes in lysosomal pH alone can mimic pathology in the diseased eye, with chloroquine treatment leading to the accumulation of material on Bruch membrane.15 Combined with the alkalinizing effects of chronic A2E exposure, this suggests that lowering lysosomal pH may be beneficial when pathologic levels of undigested material accumulate in the RPE and Bruch membrane.
Acknowledgments
The authors thank John Dowling and Tom Jordan of Neuron Systems Inc. for the A2E, Gabriel Travis for the ABCA4−/− mice, and HuiLing Hu for assistance with dissections.
Supported by National Institutes of Health Grants EY013434 (CHM), EY015537 (CHM), and EY017045 (AML), Vision Research Core Grant EY001583 (CHM, AML), Research to Prevent Blindness (AML), the Paul and Evanina Bell Mackall Foundation Trust (AML), and Fight for Sight (JL, AML).
Footnotes
Presented in part at the annual meetings of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2006 and May 2007.
Disclosure: J. Liu, None; W. Lu, None; D. Reigada, None; J. Nguyen, None; A.M. Laties, (P); C.H. Mitchell, (P)
References
- 1.Massey AC, Zhang C, Cuervo AM. Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol. 2006;73:205–235. doi: 10.1016/S0070-2153(05)73007-6. [DOI] [PubMed] [Google Scholar]
- 2.Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol. 2000;35:119–131. doi: 10.1016/s0531-5565(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 3.LaVail MM. Rod outer segment disc shedding in relation to cyclic lighting. Exp Eye Res. 1976;23:277–280. doi: 10.1016/0014-4835(76)90209-8. [DOI] [PubMed] [Google Scholar]
- 4.Elner VM. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans Am Ophthalmol Soc. 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- 5.Siakotos AN, Armstrong D, Koppang N, Connole E. Studies on the retina and the pigment epithelium in hereditary canine ceroid lipofuscinosis, II: the subcellular distribution of lysosomal hydro-lases and other enzymes. Invest Ophthalmol Vis Sci. 1978;17:618–633. [PubMed] [Google Scholar]
- 6.Geisow MJ, Evans WH. pH in the endosome: measurements during pinocytosis and receptor-mediated endocytosis. Exp Cell Res. 1984;150:36–46. doi: 10.1016/0014-4827(84)90699-2. [DOI] [PubMed] [Google Scholar]
- 7.Hayasaka S, Hara S, Mizuno K. Partial purification and properties of acid lipase in the bovine retinal pigment epithelium. Exp Eye Res. 1977;25:317–324. doi: 10.1016/0014-4835(77)90099-9. [DOI] [PubMed] [Google Scholar]
- 8.Ameis D, Merkel M, Eckerskorn C, Greten H. Purification, characterization and molecular cloning of human hepatic lysosomal acid lipase. Eur J Biochem. 1994;219:905–914. doi: 10.1111/j.1432-1033.1994.tb18572.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayasaka S, Hara S, Mizuno K. Degradation of rod outer segment proteins by cathepsin D. J Biochem. 1975;78:1365–1367. doi: 10.1093/oxfordjournals.jbchem.a131034. [DOI] [PubMed] [Google Scholar]
- 10.Barrett A. In: Proteinases in Mammalian Cells and Tissues. Dingle JT, editor. Elsevier/North-Hollard Biomedical Press; New York: 1977. [Google Scholar]
- 11.Essner E, Gorrin GM, Griewski RA. Localization of lysosomal enzymes in retinal pigment epithelium of rats with inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 1978;17:278–288. [PubMed] [Google Scholar]
- 12.Lentrichia BB, Bruner WE, Kean EL. Glycosidases of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:884–895. [PubMed] [Google Scholar]
- 13.Krogstad DJ, Schlesinger PH. The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH. Am J Trop Med Hyg. 1987;36:213–220. doi: 10.4269/ajtmh.1987.36.213. [DOI] [PubMed] [Google Scholar]
- 14.Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004;28:277–284. doi: 10.1076/ceyr.28.4.277.27835. [DOI] [PubMed] [Google Scholar]
- 15.Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt KU, Schraermeyer U. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Res. 2006;38:83–88. doi: 10.1159/000090268. [DOI] [PubMed] [Google Scholar]
- 16.Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toimela T, Salminen L, Tahti H. Effects of tamoxifen, toremifene and chloroquine on the lysosomal enzymes in cultured retinal pigment epithelial cells. Pharmacol Toxicol. 1998;83:246–251. doi: 10.1111/j.1600-0773.1998.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 18.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holz FG, Schutt F, Kopitz J, et al. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 20.Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci USA. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn KC, Marmorstein AD, Bonilha VL, et al. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci. 1998;39:2744–2749. [PubMed] [Google Scholar]
- 22.Reigada D, Lu W, Mitchell C. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol. 2006;575:707–720. doi: 10.1113/jphysiol.2006.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurwitz SJ, Terashima M, Mizunuma N, Slapak CA. Vesicular anthracycline accumulation in doxorubicin-selected U-937 cells: participation of lysosomes. Blood. 1997;89:3745–3754. [PubMed] [Google Scholar]
- 24.Pothos EN, Mosharov E, Liu KP, et al. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J Physiol. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Wang T, Zhao Z, Weinman SA. The ClC-3 chloride channel promotes acidification of lysosomes in CHO-K1 and Huh-7 cells. Am J Physiol Cell Physiol. 2002;282:C1483–C1491. doi: 10.1152/ajpcell.00504.2001. [DOI] [PubMed] [Google Scholar]
- 26.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall MO, Abrams T. Kinetic studies of rod outer segment binding and ingestion by cultured rat RPE cells. Exp Eye Res. 1987;45:907–922. doi: 10.1016/s0014-4835(87)80105-7. [DOI] [PubMed] [Google Scholar]
- 28.Hall MO, Abrams TA, Mittag TW. The phagocytosis of rod outer segments is inhibited by drugs linked to cyclic adenosine mono-phosphate production. Invest Ophthalmol Vis Sci. 1993;34:2392–2401. [PubMed] [Google Scholar]
- 29.Geisow MJ. Fluorescein conjugates as indicators of subcellular pH: a critical evaluation. Exp Cell Res. 1984;150:29–35. doi: 10.1016/0014-4827(84)90698-0. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell CH, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Civan MM. Human trabecular meshwork cell volume regulation. Am J Physiol Cell Physiol. 2002;283:C315–C326. doi: 10.1152/ajpcell.00544.2001. [DOI] [PubMed] [Google Scholar]
- 31.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy CJ, Rakoczy PE, Robertson TA, Papadimitriou JM, Constable IJ. Kinetic studies on phagocytosis and lysosomal digestion of rod outer segments by human retinal pigment epithelial cells in vitro. Exp Cell Res. 1994;210:209–214. doi: 10.1006/excr.1994.1031. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs D, Williams DS. Isolation and culture of primary mouse retinal pigmented epithelial cells. Adv Exp Med Biol. 2003;533:347–352. doi: 10.1007/978-1-4615-0067-4_44. [DOI] [PubMed] [Google Scholar]
- 34.Schlotzer-Schrehardt U, Zenkel M, Decking U, et al. Selective upregulation of the A3 adenosine receptor in eyes with pseudo-exfoliation syndrome and glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2023–2034. doi: 10.1167/iovs.04-0915. [DOI] [PubMed] [Google Scholar]
- 35.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 37.Eldred GE. Lipofuscin fluorophore inhibits lysosomal protein degradation and may cause early stages of macular degeneration. Gerontology. 1995;2:15–28. doi: 10.1159/000213722. [DOI] [PubMed] [Google Scholar]
- 38.Toimela T, Tahti H, Salminen L. Retinal pigment epithelium cell culture as a model for evaluation of the toxicity of tamoxifen and chloroquine. Ophthalmic Res. 1995;1:150–153. doi: 10.1159/000267861. [DOI] [PubMed] [Google Scholar]
- 39.MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- 40.Mitchell CH, Reigada D. Purinergic signalling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signalling. doi: 10.1007/s11302-007-9054-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredholm BB, Abbracchio MP, Burnstock G, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 42.Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- 43.Engelke M, Tykhonova S, Zorn-Kruppa M, Diehl H. Tamoxifen induces changes in the lipid composition of the retinal pigment epithelium cell line D407. Pharmacol Toxicol. 2002;91:13–21. doi: 10.1034/j.1600-0773.2002.910103.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- 45.Sun-Wada GH, Wada Y, Futai M. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct. 2003;28:455–463. doi: 10.1247/csf.28.455. [DOI] [PubMed] [Google Scholar]
- 46.Schneider DL. ATP-dependent acidification of intact and disrupted lysosomes: evidence for an ATP-driven proton pump. J Biol Chem. 1981;256:3858–3864. [PubMed] [Google Scholar]
- 47.Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 48.Faundez V, Hartzell HC. Intracellular chloride channels: determinants of function in the endosomal pathway. Science Stke. 2004;233:18. doi: 10.1126/stke.2332004re8. [DOI] [PubMed] [Google Scholar]
- 49.Di A, Brown ME, Deriy LV, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 50.Reigada D, Mitchell CH. Release of ATP from RPE cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol. 2005;288:C132–C140. doi: 10.1152/ajpcell.00201.2004. [DOI] [PubMed] [Google Scholar]
- 51.Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol. 2003;106:43–50. doi: 10.1023/a:1022514031645. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Marmorstein AD, Peachey NS. Functional abnormalities in the retinal pigment epithelium of CFTR mutant mice. Exp Eye Res. 2006;83:424–428. doi: 10.1016/j.exer.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastor-Soler N, Beaulieu V, Litvin TN, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Dyke RW. Cholera and pertussis toxins increase acidification of endocytic vesicles without altering ion conductances. Am J Physiol Cell Physiol. 1997;272:C1123–C1133. doi: 10.1152/ajpcell.1997.272.4.C1123. [DOI] [PubMed] [Google Scholar]
- 55.Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51:4837–4844. [PubMed] [Google Scholar]
- 56.Lazzaroni F, Scorolli L, Pizzoleo CF, et al. Tamoxifen retinopathy: does it really exist? Graefes Arch Clin Exp Ophthalmol. 1998;236:669–673. doi: 10.1007/s004170050139. [DOI] [PubMed] [Google Scholar]
- 57.Noureddin BN, Seoud M, Bashshur Z, Salem Z, Shamseddin A, Khalil A. Ocular toxicity in low-dose tamoxifen: a prospective study. Eye. 1999;13:729–733. doi: 10.1038/eye.1999.217. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Noriega A, Grubb JH, Talkad V, Sly WS. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980;85:839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Leeuwen R, Tomany SC, Wang JJ, et al. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111:1169–1175. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 60.Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Nat Acad Sci USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bermann M, Schutt F, Holz FG, Kopitz J. Does A2E, a retinoid component of lipofuscin and inhibitor of lysosomal degradative functions, directly affect the activity of lysosomal hydrolases? Exp Eye Res. 2001;72:191–195. doi: 10.1006/exer.2000.0949. [DOI] [PubMed] [Google Scholar]
- 62.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 63.Shamsi FA, Boulton M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest Ophthalmol Vis Sci. 2001;42:3041–3046. [PubMed] [Google Scholar]