Abstract
The expression of human vascular adhesion protein-1 (hVAP-1) is induced at sites of inflammation where extravasation of lymphocytes from blood to the peripheral tissue occurs. We have solved the X-ray structure of hVAP-1, a human copper amine oxidase (CAO), which is distinguished from other CAOs in being membrane-bound. The dimer structure reveals some intriguing features that may have fundamental roles in the adhesive and enzymatic functions of hVAP-1, especially regarding the role of hVAP-1 in inflammation, lymphocyte attachment, and signaling. Firstly, Leu469 at the substrate channel may play a key role in controlling the substrate entry; depending on its conformation, it either blocks or gives access to the active site. Secondly, sugar units are clearly observed at two of the six predicted N-glycosylation sites. Moreover, mutagenesis analysis showed that all of the predicted sites were glycosylated in the protein used for crystallization. Thirdly, the existence of a solvent-exposed RGD motif at the entrance to each active site in hVAP-1 suggests that it may have a functional role.
Keywords: hVAP-1, structure, amine oxidase, inflammation, glycosylation
Human vascular adhesion protein-1 (hVAP-1) was first identified in the early 1990s as an adhesion protein expressed on the surface of endothelial cells and involved in lymphocyte-endothelial cell adhesion (Salmi and Jalkanen 1992). When the cDNA for hVAP-1 was cloned in 1998 (Smith et al. 1998), it was shown to encode a member of the semicarbazide-sensitive amine oxidase/ copper-containing amine oxidase (SSAO/CAO; EC 1.4. 3.6) (for review, see Lyles 1996; Buffoni and Ignesti 2000; Klinman 2003) family of enzymes, which convert primary amines to the corresponding aldehydes with the release of hydrogen peroxide and ammonia. To date, two genes homologous to hVAP-1 have been cloned from the human genome: diamine oxidase (Chassande et al. 1994) and retina-specific CAO (Imamura et al. 1997, 1998). Two alternative splice variants of the hVAP-1 pseudogene have also been cloned (Cronin et al. 1998).
hVAP-1 is a 180-kDa dimeric membrane protein composed of a very short N-terminal cytoplasmic tail, a single membrane-spanning domain and a large extracellular part (Salmi and Jalkanen 1992; Smith et al. 1998). A soluble form of hVAP-1 has been found in man, presumably resulting from the proteolytic cleavage of membrane-bound hVAP-1 (Abella et al. 2004; Stolen et al. 2004). The expression of hVAP-1 is induced at sites of inflammation where the immune response is highly dependent on the extravasation of lymphocytes from blood to the peripheral tissue. hVAP-1 is shown to be involved in both the rolling and diapedesis steps (Salmi and Jalkanen 1992; Lalor et al. 2002; Koskinen et al. 2004). hVAP-1 is heavily glycosylated, and the interaction of hVAP-1 with an unknown counter-receptor on the lymphocyte surface is dependent on the terminal sialic acid groups of the glycans (Salmi and Jalkanen 1996). The adhesive and enzymatic functions of hVAP-1 are connected since adhesion is reduced when the enzymatic activity is inhibited (Salmi et al. 2001; Koskinen et al. 2004).
The physiological amine methylamine is a substrate of hVAP-1 in vitro (for review, see Lyles 1996; Smith et al. 1998), but little is known of the endogenous substrates in vivo. hVAP-1 is, however, able to bind the primary amino group of a lysine side chain from short peptides that fit into the active site cavity (Salmi et al. 2001; Yegutkin et al. 2004). Furthermore, the amine oxidase activity of the closely related bovine SSAO is inhibited by aminohexoses (O’Sullivan et al. 2003). Thus, a primary amino group of a lymphocyte surface protein or carbohydrate moiety might bind within the active site of hVAP-1 during the cell adhesion process (Salmi et al. 2001), leading to the formation of a transient covalent bond between the two cell types and resulting in the oxidative deamination of the bound group. In addition to the enzymatic activity of hVAP-1 and the roles of the attached carbohydrate, hVAP-1 has an RGD (arginine-glycine- aspartic acid) cell adhesion motif that is likely to be functional, since deletion of RGD diminished lymphocyte adhesion to hVAP-1 (Salmi et al. 2000).
Currently, several crystal structures have been solved for CAOs from bacteria, fungi, and plants but none from the animal kingdom: CAOs from Esherichia coli (ECAO; e.g., Protein Data Bank [PDB] code 1OAC; Parsons et al. 1995), Arthrobacter globiformis (AGAO; e.g., PDB code 1AV4; Wilce et al. 1997), Hansenula polymorpha (HPAO; e.g., PDB code 1A2V; Li et al. 1998), and Pisum sativum (PSAO; PDB code 1KSI; Kumar et al. 1996) and lysyl oxidase from Pichia pastoris (PPLO; PDB code 1N9E; Duff et al. 2003). Even though the amino acid sequence identity is only 25%–35%, all CAOs share similarities in their structures. Each is a heart-shaped dimer where the monomer is composed of three domains, named D2–D4; in ECAO there is one additional domain named D1 (Parsons et al. 1995). The two tightly bound D4 domains form the interface of the dimer and contain the active sites. The highly conserved active site in the D4 domain has several special features: (1) The unique cofactor 2,4,5- trihydroxyphenylalanine quinone (TPQ) (a post-translational modification of an intrinsic tyrosine) and a conserved catalytic aspartic acid residue are involved in the catalytic reaction; (2) a copper ion involved in TPQ biogenesis is coordinated by three conserved histidine residues; and (3) the active site is deeply buried within the protein and accessible only via a cavity formed by the D3 and D4 domains.
We have now solved the X-ray structure of the membrane bound form of hVAP-1 at 2.9 Å resolution, which is the first structure of a human CAO. hVAP-1 shares the same overall heart-shaped fold as the other CAOs but has also several unique structural features. In addition, we have confirmed the predicted glycosylation sites (Salminen et al. 1998) by mutagenesis. Our results provide new insights into the function of hVAP-1, especially regarding the role of hVAP-1 in inflammation, lymphocyte attachment, and signaling.
Results
The overall crystal structure of hVAP-1
The structure of hVAP-1, which is the first reported crystal structure of a human CAO, was refined to 2.9 Å resolution with a final Rfactor of 24.1% and Rfree of 26.6% (data collection, refinement, and validation statistics are presented in Table 1). The structure is a homodimer consisting of chains A (A55–A761) and B (B57–B761); when superimposed, they differ by a root mean square deviation (RMSD, Cα-atoms) of 0.38 Å. Each monomer contains one copper atom, two calcium atoms, and NAG units at two separate sites. The final model does not include residues A1–A54 and B1–B56 containing the few residues that extend into the intracellular space; the putative transmembrane helices; or residues A202–A204, B203, and B742–B746, where the electron density could not be interpreted.
Table 1.
Data collection and structure determination statistics for human VAP-1
| 1PU4 | 1US1 | |
| Data collection statisticsa | ||
| Space group | P6522 | P6522 |
| Unit cell dimensions (Å,°) | a = b = 225.9, c = 218.7 α = β = 90, γ = 120 |
a = b = 226.1, c = 223.0 α = β = 90, γ = 120 |
| No. of crystals used | 3 | 1 |
| Resolution range (Å) | 20–3.2 | 20–2.9 |
| Unique reflections | 52,367 (4588) | 69,536 (6743) |
| Wavelength used (Å) | 0.81 | 0.95 |
| Matthews coefficient (Å°/Da) | 4.5 | 4.6 |
| Solvent content (%) | 72 | 73 |
| Unit cell volume (Å°) | 9,665,176 | 9,872,805 |
| Molecules per asymmetric unit | 2 | 2 |
| Completeness (%) | 95.9 (97.2) | 93.3 (95.3) |
| Redundancy | 14.1 (13.5) | 10.4 (10.4) |
| Rmerge (%) | 19.6 (46.4) | 12.1 (47.4) |
| Average I/σ | 13.2 (6.0) | 19.0 (6.4) |
| Wilson B-factor | 55.9 | 53.2 |
| Refinement statistics | ||
| Resolution range (Å) | 20–3.2 | 20–2.9 |
| Rcryst (%) | 21.7 | 24.1 |
| Rfree (%) | 25.4 | 26.6 |
| Average B-factor for all atoms (Å2) | 29.4 | 42.3 |
| Model statistics | ||
| Amino acids in modelb | 1403 | 1403 |
| Amino acids in most favoured regionsc | 987 (84.0%) | 983 (83.7%) |
| Amino acids in additional allowed regionsc | 182 (15.5%) | 185 (15.7%) |
| Amino acids in generously allowed regionsc | 0 (0%) | 1 (0.1%) |
| Amino acids in disallowed regionsc | 6 (0.5%) | 6 (0.5%) |
a Values in parentheses are for the highest resolution shell (1US1, 3.3–3.2 Å ; 1PU4, 3.0–2.9 Å). Values for 1PU4 as described previously (Nymalm et al. 2003).
b The residues A1–A54, A202–A204, A762–A763, B1–B56, B203, B742–B746, and B762–B763 were not included in the 1PU4 and 1US1 structures.
c Numbers correspond to nonglycine and nonproline residues in Ramachandran plot.
The overall structure of hVAP-1 is the heart-shaped CAO fold (Fig. 1 ▶) that consists of domains D2 (residues 55–169), D3 (residues 170–300), and D4 (residues 301– 761). The domain structure of hVAP-1 resembles the other known CAO structures, with the exception of the ECAO structure that contains the additional N-terminal D1 domain. Based on the structure-based sequence alignment (Fig. 2 ▶; Table 2), PPLO is currently the closest structural neighbor of hVAP-1 with 175 matched identical residues (24% sequence identity). The overall architecture and topology of domains D2–D4 of hVAP-1 and PPLO are strikingly similar.
Figure 1.
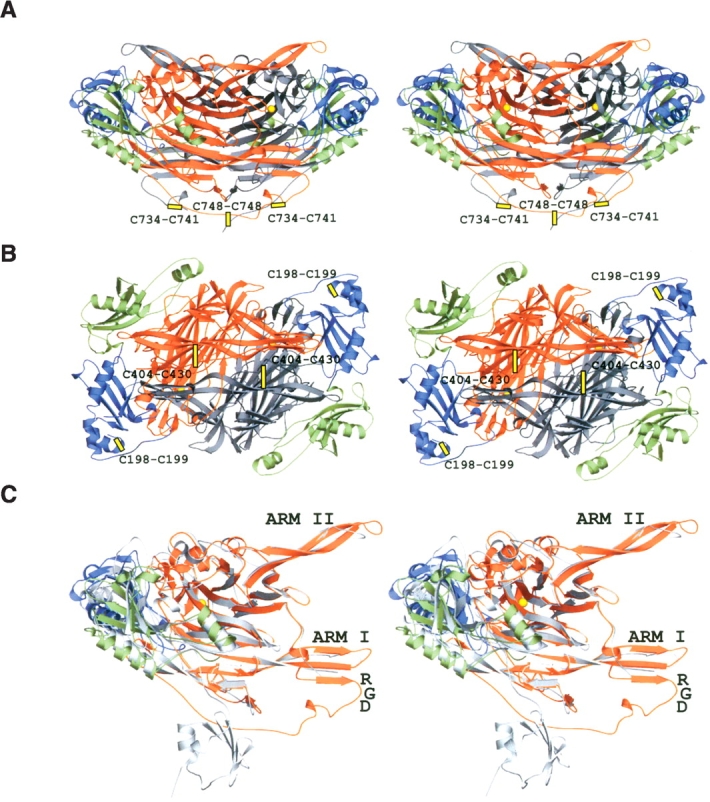
Stereo views of hVAP-1. (A) A ribbon diagram of the dimer is shown. The twofold axis of molecular symmetry lies vertically in the plane of the paper. (B) The view along the twofold axis is rotated −90° around the X-axis compared with the view in A. (C) A monomer of hVAP superimposed with ECAO (PDB code 1OAC) structure is shown in the same view as in A. The β-hairpin arms I and II, the RGD motif, and the disulfide bridges are indicated. The ribbons for hVAP-1 were drawn with domain-specific coloring: D2, green; D3, blue; D4 of subunit A, orange; and D4 of subunit B, gray. The ECAO ribbon is in gray (C). The copper atom of the active site is shown as a yellow sphere.
Figure 2.

Structural alignment of hVAP-1 with the representatives of known CAO structures. hVAP (PDB code 1US1), PPLO (PDB code 1N9E), ECAO (PDB code 1OAC), and AGAO (PDB code 1AVL) have the TPQ residue in the inactive “on-copper” conformation; TPQ in PSAO (PDB code 1KSI) and HPAO (PDB code 1A2V) is in the active “off-copper” conformation. Residues of CAOs with Cα atoms closer than 2 Å from the corresponding Cα atoms of hVAP are indicated in blue; conserved residues, in orange. Elements of secondary structure are indicated in gray (α-helices) or green (β-sheets). Alignment statistics are summarized in Table 2.
Table 2.
Structural alignment statistics
| hVAP, PDB code 1US1 (704 residues) | ||||
| Length of alignment (with hangs) | Pairs | Identical residues | Identity (%)(with hangs) | |
| PPLO, P. pastoris; PDB code 1N9E (720 residues) | 732 (735) | 689 | 175 | 23.9 (23.8) |
| ECAO, E. coli; PDB code 1OAC (634 residues) | 698 (738) | 600 | 144 | 20.6 (19.5) |
| PSAO, P. sativum; PDB code 1KSI (642 residues) | 721 (733) | 613 | 116 | 16.1 (15.8) |
| HPAO, H. polymorpha; PDB code 1A2V (653 residues) | 707 (734) | 623 | 131 | 18.5 (17.9) |
| AGAO. A. globiformis; PDB code 1AVL (620 residues) | 687 (728) | 596 | 127 | 18.5 (17.5) |
The A chains of each structure were used. The number of residues corresponds to the residues used in the alignment after deleting the N-terminal sequences that did not match the hVAP sequence. All the residues (A55–A201, A205–A761) of the hVAP structure were used.
Domains of hVAP-1: Unique and conserved features
The D2 domain
The “core” of the D2 domain consists of four β-strands (β1.1–β1.4) and two α-helices (α1 and α2) and is well conserved in all known CAO structures (Figs. 1 ▶, 2 ▶). In hVAP-1 there are two additional short β-strands, β1.N and β1.C, anti-parallel to strand β1.1 and to strand β1.3, respectively. The β1.N strand is unique to hVAP-1, but β1.C is also found in PPLO but not in the other known CAO structures. Similarly to PPLO, hVAP-1 has a short helix, α1b, located between strands β1.N and β1.1, which is the only part of D2 in contact with the active site cavity. Another unique feature of the D2 domain of hVAP-1 is the insertion of helix α3 between strands β1.4 and β1.C, which determines the shape of two cavities, A2 and B2 (see below; Fig. 3 ▶). Four residues from D2— Glu67, Pro104, Arg122, and Glu140 (hVAP-1 numbering) —are conserved throughout the known CAO family structures (Fig. 2 ▶), and with the exception of Pro104, all are involved in salt bridges that function to stabilize the fold.
Figure 3.
The cavities of hVAP-1. A dot surface of the hVAP-1 dimer is shown as a side (A,B), bottom (C), and top (D) view; domains are colored as in Figure 1 ▶. The active site cavities A1 and B1 of subunits A and B, respectively, as well as the central cavities C1–C3 and the cavities A2 and B2 of the subunits A and B, respectively, are shown as light gray surfaces. The putative N-linked glycosylation sites N1–N6 are labeled, and the carbohydrate visible in the X-ray structure are drawn as yellow spheres. The locations of the RGD cell attachment sites are indicated.
The D3 domain
Both the D3 and the D2 domain share the same α–β fold, but there are considerable differences between them as reflected in a comparison of the domains where only 77 equivalent Cα-atoms (D2 vs. D3 from chain A) superimpose with an RMSD of 4.4 Å. The presence of strand β2.5 and helices α8 and α9 (Fig. 1D ▶) further distinguishes the D3 domain of hVAP-1 and PPLO from the other CAO structures. Another unique feature of D3 in hVAP-1 is the intradomain disulfide bond between two adjacent cysteines, Cys198 and Cys199, which would function to stabilize the C-terminal end of helix α6 (Fig. 1B ▶). Despite the relatively high structural similarity of D3 among the known CAO structures, in terms of the sequence the D3 domain is the least conserved domain and not one residue is completely conserved (Fig. 2 ▶).
The D4 domain
The catalytic D4 domain has 25 β-strands and seven α-helices (Fig. 1C ▶). The numerous β-strands of D4 are assembled into two extended β-sheets. In addition, a smaller β-sheet is located in the middle of D4, and the βL-strand is located in the long loop that connects the D3 and D4 domains.
D4 is the most conserved domain in the CAOs: 27 out of the 460 residues in the hVAP-1 D4 domain are completely conserved within the CAO family (Fig. 2 ▶). These conserved residues include 17 nonglycine/proline residues, e.g., all of the residues involved in topaquinone generation and/or in the catalytic reaction: (1) TPQ471; (2) the catalytic base Asp386; (3) Tyr372 and Asn470, which are involved in the proper positioning of topaquinone; and (4) His520, His522, and His684 that coordinate the copper ion, as well as Asp529 and Asp673 of metal binding site A, and His443, Thr467, and Asn470 lining the active site cavity. The D4 domain of hVAP-1 is stabilized by an intradomain disulphide bridge formed by Cys404 and Cys430 (Fig. 1B ▶), as are all structurally characterized CAOs but ECAO. Although β-hairpin arms I and II, which extend from one D4 domain to embrace the D4 domain of the other subunit, are found in all known CAO structures (Parsons et al. 1995; Kumar et al. 1996; Wilce et al. 1997; Li et al. 1998; Duff et al. 2003), the most pronounced differences in hVAP-1 are found in arm I (Fig. 1C ▶).
In hVAP-1, the β4.2- and β4.3-strands of hairpin arm I form a three-stranded β-sheet with the extra β4.4 β-strand (residues 723–726) (Fig. 1C ▶). PPLO was recently reported to contain a novel β-hairpin arm III (Duff et al. 2003), consisting of the above-mentioned β4.4 βstrand and an additional β-strand. In hVAP-1, this β-hairpin arm is not present. Instead, the β4.4 β-strand is followed by a loop and a short helix α17 (Fig. 1 ▶). Located at the end of the β4.4 β-strand is the RGD cell adhesion motif (residues 726–728) (Salminen et al. 1998), which is a unique feature of the hVAP-1 structure. The C-terminal part of the hVAP-1 dimer is also stabilized by three disulphide bridges in a novel way; in each subunit Cys734 and Cys741 form an intrasubunit bond, whereas Cys748s form an inter-subunit disulfide bond (Fig. 1A ▶).
hVAP-1 cavities
A large share of the total volume of hVAP-1 is occupied by cavities (the seven largest ones are shown in Fig. 3 ▶). These include the central cavities C1–C3 at the dimerization interface between subunits A and B, and the active site cavities A1 and B1 respectively present in subunit A and subunit B. Two additional cavities, A2 in subunit A and B2 in subunit B, are located between the D2 and the D4 domains in each monomer. Generally, the cavities of hVAP-1 have unique shapes, and only a few residues lining the cavities are structurally conserved among the CAO structures.
Active site cavity
In CAOs, residues from the least conserved D3 domain as well as variable residues from the most conserved D4 domain protrude into the active site cavity, altering its shape and potential for molecular interactions, thus serving to define the substrate specificity for each CAO family member. The active site cavities, A1 and B1, of hVAP-1 (Fig. 4 ▶), with approximate dimensions of 20 Å ×10 Å ×15 Å, provide access to the deeply buried active sites of subunits A and of B, respectively. Residues from the D3 domain (Phe173, Tyr176, Leu177, Asp180, Thr210, Met211, and Phe227) together with residues from the long β-hairpin arm I of the other subunit (His444, Asp446, Leu447, Tyr448, and His450) form the wall of the cavity on one side. Asp446 seems to have an important role in stabilizing the loop conformation of the β-hairpin arm I via an intrasubunit hydrogen bond to His444 and an intersubunit hydrogen bond to the main-chain nitrogen atom of Ser761. The loop conformation is further stabilized by an intrasubunit hydrogen bond between the main-chain oxygen atom of His450 and the main-chain nitrogen atom of Gly727, which is part of the putative RGDcell attachment site at themouth of the active site cavity (Fig. 4 ▶). The opposite cavity wall has contributions from the D2 domain (Ala87) and the D4 domain (Thr395, Thr396, Pro397, Phe415, Leu416, Leu417, Glu418, Ser419, Ala421, Pro422, Lys423, Thr424, Ile425, and Arg426). In addition, the main-chain oxygen atoms of residues from the C-terminal (Gly758, Gly759, Phe760, and Ser761) point toward the cavity and contribute to the shape of this wall. The bottom of the cavity is formed by the D4 domain (Thr212, Lys393, Tyr394, Thr396, Pro397, Phe398, Phe415, Leu417, Leu425, Thr467, Leu468, and Leu469). In general, the active site cavity of hVAP-1 is rich in the aromatic residues tyrosine and phenylalanine as well as hydrophobic side chains. The charged side chains of Asp180, Glu418, Lys393, and Lys423 point toward the cavity together with the polar side chains of Thr212, Thr396, Thr467, Ser419, and Ser761.
Figure 4.
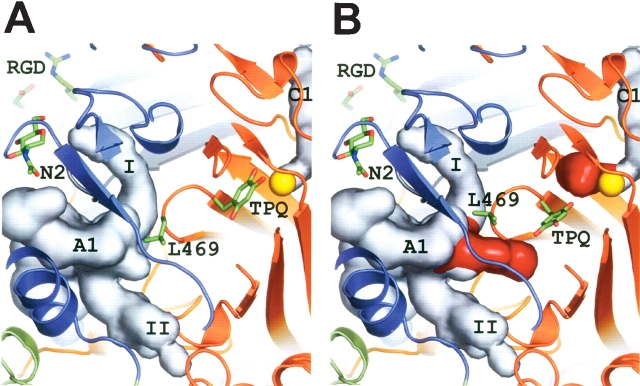
The active site architecture of hVAP-1. (A) Active site in the inactive state (crystal structure) where TPQ is in the “on-copper” conformation and where L469 serves as a “guardian” blocking entry to the substrate channel. (B) Active site in the activated state (modeled) where TPQ is in the “off-copper” conformation and where the conformation of L469 has been adjusted to allow free access to the substrate channel. The side-chains of TPQ, L469, and the RGD motif, as well as the carbohydrate at site N2 are shown as sticks. The active site cavity A1 and the central cavity C1 are seen as light gray surfaces. The red surface color indicates additional space within cavities A1 and C1 in the modeled active state structure. The copper atom of the active site is shown as a yellow sphere, and the domain colors are as in Figure 1 ▶.
Two long channels, I and II (shown for monomer A in Fig. 4 ▶), protrude from the bottom of the active site cavity A1 in monomer A, but channel II is not present in monomer B. These branching channels give an irregular overall shape for the active site cavities. Channel I is lined by residues from D2 (Phe97), D3 (Arg169, Pro170, Val171, Leu172, and Arg216), and D4 (Phe344, Gly388, and Phe389). The D2 and D4 domains form the bottom of this channel (residues Arg168 of D2, and Val647, Phe648, Gln650, and Asn651 of D4). Channel II, which in monomer A is located below the long β-hairpin arm I protruding from monomer B, is formed by D3 domain (Asn232, Ile233, Ser234, Ala236, Gly237, Phe238, and Phe239) and the β-hairpin of D4 domain (His443, Ser445, Tyr448, Ser449, and Tyr451). Neither of these channels leads to the active site.
In the X-ray structure of hVAP-1, the active site is not accessible to ligands since the hydrophobic side-chain of Leu469 blocks the entrance to the active site (Fig. 4 ▶). Therefore, we constructed a structural model of hVAP-1 where another rotamer conformation was selected for the side chain of Leu469 in order to open up the substrate channel leading to the active site. Since the cofactor TPQ471 in the X-ray structure of hVAP-1 is in the “on-copper” conformation, in which it is in direct contact with the copper ion (for review, see Dawkes and Phillips 2001), we modeled TPQ into a catalytically active “off-copper” conformation with theO2 hydrogen positioned near the catalytic base Asp386, as is observed for iminoquinone in the ECAO structure (PDB code 1D6Z; Wilmot et al. 1999). These alterations resulted in the formation of a narrow and almost circular channel with dimensions of 4.5 Å ×4.5 Å, giving ligands access to the active site (Fig. 4 ▶).
Other cavities
Cavities A2 and B2 are located between the D2 and D4 domains in monomers A and B, respectively. Each cavity is rimmed partly by the α3-helix, which is unique to the D2 domains of hVAP-1. In addition, there are three major cavities (C1–C3) located at the dimerization interface of hVAP-1 (Fig. 3 ▶), which are likely to be connected to each other. The central cavities of hVAP-1, and especially the C1 cavity, have their own characteristic shapes compared with the corresponding cavities present in the other known CAO structures. In both the on-copper and the modeled off-copper TPQ471 conformation, the C1 cavity is branched into two channels, one of which leads to TPQ471 in subunit A and the other one to TPQ471 in subunit B. The C1 cavity is solvent-exposed on the top of the heart-shaped hVAP-1 fold (Fig. 3 ▶) and could provide access to and from the active site for small molecules such as hydrogen peroxide and molecular oxygen, as has previously been suggested for HPAO (Lee et al. 2002). The C2 and C3 cavities also link the interior of hVAP-1 to the solvent. The channel protruding into the active site of subunit A is in close proximity to cavity C2, while the channel reaching to the active site of subunit B is close to cavity C3.
Glycosylation sites
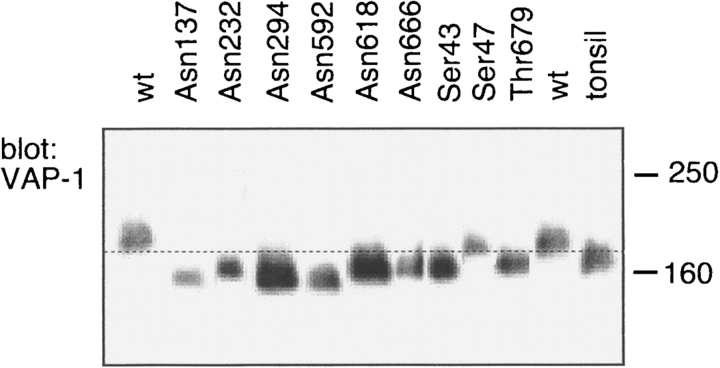
Four O-glycosylation sites on hVAP-1—Ser43, Ser47, Thr679, and Thr212—were predicted by using NetO-Glyc 2.0 (Hansen et al. 1995, 1998). Based on the mutagenesis studies Ser43 and Thr679 appear to be glycosylated in Chinese hamster ovary (CHO) cells, whereas Ser47 does not (Fig. 5 ▶). Thr212 has not yet been mutated, since the mutagenesis studies were based on earlier predictions (Salminen et al. 1998). However, in the crystal structure of hVAP-1, there is weak electron density near Thr212 in monomer B, suggesting that O-glycosylation at Thr212 has taken place.
Figure 5.
hVAP-1 contains multiple N- and O-linked oligosaccharides. Lysates from CHO cells transfected with plasmids encoding wild-type hVAP-1 (wt) or hVAP-1 molecules, in which the indicated amino acid residues were mutated into alanine residues, were separated on SDS-PAGE and subjected to immunoblotting with anti-hVAP-1 antibody. Tonsil lysate was used as the source of endogenous hVAP-1. Molecular weight standards (in kilodaltons) are indicated on the right.
hVAP-1 has six potential N-glycosylation sites: Asn137 (N1) in domain D2; Asn232 and Asn294 (N2, N3) at the edge of the active site cavity in domain D3; and Asn592, Asn618, and Asn666 (N4–N6) on top of the heart-shaped fold in domain D4 (Fig. 3 ▶). Each of these asparagine residues has been mutated individually to alanine and expressed in CHO cells. The increased mobility of all hVAP-1 mutants on SDS-PAGE (Fig. 5 ▶) indicates that carbohydrate is attached to each of the putative N-glycosylation sites in the recombinant protein. Moreover, in our X-ray structure of hVAP-1, electron density is observed around Asn137 (N1) and Asn232 (N2) in both monomers; N-acetylglucosamine (NAG) sugar units can easily be fit into the electron density at these sites, two units at N1 and one at N2. There is also weak but detectable electron density around some of the other potential N-glycosylation sites of hVAP-1, e.g., Asn592 (N4) in monomer B, indicating the likely attachment of carbohydrate at these other sites as well.
Discussion
We report the first X-ray structure of hVAP-1, a human CAO, which is distinguished from other CAOs in being membrane-bound. The structure reveals intriguing features that may have fundamental roles in the regulation and activity of the adhesive functions of hVAP-1, especially regarding the role of hVAP-1 in inflammation, lymphocyte attachment, and signaling.
Cavities provide routes into and out of the active site
Active site cavity
The architecture of the active site cavity of hVAP-1 resembles the wide active site funnel of the PPLO structure more than the narrow substrate channel of ECAO, PSAO, AGAO, and HPAO. Channels I and II are large enough to accommodate an amino acid side chain and thus might interact with peptide or protein ligands. Indeed, both hVAP-1 and PPLO are capable of using lysyl peptides as ligands (Kuchar and Dooley 2001; Salmi et al. 2001), which supports the idea that the natural substrate(s) of hVAP-1 might include larger molecules with free amino groups, such as proteins or modified carbohydrates attached to cell-surface proteins. Furthermore, in vitro experiments suggest that hVAP-1 arrests lymphocyte rolling as a consequence of binding the primary amino group prior to the oxidative deamination of the group (Salmi et al. 2001).
Substrate channel architecture—Leu469 may function to guard entry to the active site
In the X-ray structure of hVAP-1, the active site is not accessible to ligands since the hydrophobic side-chain of Leu469 blocks the entrance (Fig. 4 ▶), which suggests that in hVAP-1 this residue might function as a “guardian” regulating the access of substrate to the active site. Leu469 is one of the two variable residues (Xxx2) in the highly conserved Thr-Xxx1-Xxx2-Asn-Tyr(TPQ)- Asp/Glu sequence motif for the CAO active site (Parsons et al. 1995). Residue Xxx2 may have an important role in defining the preferred substrate since its conservation among the CAO family members varies with the substrate specificity of these enzymes. In the mammalian diamine oxidases, Xxx2 is tyrosine; in the retinaspecific amine oxidases, the corresponding residue is glycine, whereas in bovine serum amine oxidase and mouse VAP-1, which are monoamine oxidases, Xxx2 is leucine as in hVAP-1 (Salminen et al. 1998). In ECAO, HPAO, AGAO, and PPLO, Xxx2 is glycine, and in PSAO, it is alanine. In the modeled active conformation, the ligands could access the active site, which indicates an important functional role for Leu469 as the guardian of the substrate-entry channel. Therefore it would be interesting to characterize the role of Leu469 in detail.
Other cavities
The C1 cavity extends from the top of the heart-shaped fold to both active sites (Figs. 3 ▶, 4 ▶); thus a synchronized control of the turnover of reaction intermediates would be possible. The biological function of the A2 and B2 cavities is not clear, but as they are located close to the active sites, they might have a role in the efficient turnover of amine oxidation products since only minor conformational changes would be required to access the active site cavities.
Sites for cell adhesion
Glycosylation sites
The mutagenesis results and the X-ray structure of hVAP-1 support the prediction that all putative N-glycosylation sites are normally glycosylated in hVAP-1. Previously, the terminal sialic acid end groups of the glycans have been shown to interact with an unknown counter-receptor on the lymphocyte surface (Salmi and Jalkanen 1996), and the N-linked carbohydrates at N4– N6 have been proposed to be important for cell adhesion (Salminen et al. 1998; Maula et al. 2005). The N5 and N6 glycosylation sites are located near the entrance to the C1 cavity (Fig. 3 ▶), and thus, they might function in the regulation of the entry/exit of molecular oxygen, hydrogen peroxide, and ammonia. The N1 glycosylation site is also located on the top of hVAP-1 (Fig. 3 ▶), but the role of the attached glycans has not yet been characterized. Interestingly, the carbohydrate at sites N2 and N3 might control both enzymatic activity and cell adhesion since they are located at the active site entrance near the RGD cell adhesion motif (Figs. 3 ▶, 4 ▶). In addition, the putative O-glycan at Thr212 is located in the vicinity of Leu469 at the substrate-entry channel, and it might have an effect on ligand binding.
RGD motifs
The RGD cell adhesion motif is found in extracellular matrix proteins such as collagen and fibronectin, as well as on some picornaviruses (Pfaff 1997). The RGD motif is recognized by several members of the family of cell-surface integrins (Ruoslahti and Pierschbacher 1987), all of which function in cell–cell and cell–matrix interactions. Interestingly, hVAP-1 has an RGD motif (residues 726–728) (Fig. 2 ▶; Smith et al. 1998; Salmi et al. 2000), which is not found in the other known CAO structures.
Recognition of the RGD motif by a ligand would require the motif to adopt the correct conformation, i.e., a fingertip-like structure that protrudes into the solvent from the surface of the protein (for review, see Pfaff 1997). In hVAP-1, the RGD motif is well defined and is in an ideal conformation for interactions with a ligand, since it is located on the surface of the molecule at the tip of a loop (Fig. 1C ▶). The deletion of the RGD sequence from hVAP-1 decreases lymphocyte adhesion (Salmi et al. 2000), which is in agreement with the crystal structure, suggesting that hVAP-1 might indeed interact through its RGD sequence. Interestingly, the RGD motif of monomer A is located near the active site of monomer B, and vice versa (Figs. 1 ▶, 4 ▶). Consequently, the binding of a ligand to one of the RGD motifs could alter the structure of hVAP-1 and function to synchronize and control the CAO activity or other functions of hVAP-1. Altogether, the structural data presented in this study propose a biological function for the RGD motif of hVAP. However, further experiments are needed to clarify the role of the RGD triplet in vivo.
Materials and methods
Expression, purification, and crystallization
The structure of hVAP-1 was solved from two crystals resulting in two separate structures (PDB codes 1PU4 and 1US1). The 3.2 Å structure (1PU4) was solved first and was used to solve the 2.9 Å structure (1US1). hVAP-1 was expressed, purified, and crystallized as described previously (Nymalm et al. 2003). Briefly, the full-length protein was expressed in CHO cells and purified by using monoclonal antibodies against hVAP-1. The purified protein was confirmed to be enzymatically active by using benzylamine as the substrate (Nymalm et al. 2003). The best crystals were obtained by using hanging drops, including 2 μL of 1.0 M potassium/ sodium tartrate, 100 mM imidazole (pH 7.8), and 150–300 mM sodium chloride as the precipitant and 2 μL of hVAP-1 (1 mg/mL) in 10 mM potassium phosphate buffer (pH 7.2).
Data collection and structure determination
Data for the 1PU4 structure were collected by using beamline X11 at EMBL/DESY, Hamburg, and for the 1US1 structure by using beamline ID14.1 at ESRF. The data were processed with the program XDS (Kabsch 1993) in space group P6522 (Table 1).
The 3.2 Å 1PU4 structure was solved by using molecular replacement and the program AMORE (Navaza 1994) of the CCP4i program suite (Collaborative Computational Project Number 4 1994), which confirmed the space group with one biological unit, a dimer, per asymmetric unit. The backbone from P. sativum CAO (residues 7–634; PDB code 1KSI) was chosen for molecular replacement and used as a template for initial model building of hVAP-1. The structure was rebuilt manually by using the program O (Jones et al. 1991) and refined with REFMAC 5.1.24 (Murshudov et al. 1997) of the CCP4i suite. Side chains were added to the structure in between cycles of refinement. Coordinates for TPQ were taken from the Hetero-compound Information Centre (Kleywegt and Jones 1998). The 1US1 structure was solved by using phase information from the 1PU4 structure and refined and built as described above. The stereochemical qualities of the final hVAP-1 models were assessed with PROCHECK (Laskowski et al. 1993) and WHATIF (Vriend 1990). The summary of the structure determination statistics is presented in Table 1.
Since the 3.2 Å and 2.9 Å structures are practically identical (their Cα-atoms superimpose with an RMSD of 0.21 Å ) and because of the resolution and structure determination statistics (Table 1), the 2.9 Å structure (1US1) is reported in the text.
Mutational analysis
Site-directed mutagenesis of the full-length cDNA for hVAP-1 was used to eliminate potential N-glycosylation sites (Asn137Ala, Asn232Ala, Asn294Ala, Asn592Ala, Asn618Ala, and Asn666Ala) and O-glycosylation sites (Ser43Ala, Ser 47Ala, and Thr679Ala), using either the unique site elimination system (U.S.E. Mutagenesis Kit, Pharmacia) or by PCR (Maula et al. 2005). All hVAP-1 constructs were subcloned into a eukaryotic expression vector pcDNA3.1, and the mutations were verified by sequencing the whole insert.
The plasmids were transfected into CHO cells by using nucleofection (Nukleofector Solution T optimized for CHO cells, Amaxa). Transfected cells were plated in α-MEM medium with 10% FCS and ribonucleosides (Gibco) and cultured for 2 d. The cells were then washed and lysed in a lysis buffer (1% NP-40, 150 mM NaCl, 10 mM Tris-base, 1.5 mM MgCl2 [pH 7.0]). Lysate from human tonsil was made in the same lysis buffer, and it served as a source of natural hVAP-1. Protein concentrations in the clarified supernatants were determined by using the Bio-Rad DC Protein Assay.
Equal amounts of protein (10 μg/lane) were loaded on 5%– 12.5% SDS-PAGE gels. The gels were run for 48 h to allow maximal separation of proteins. The proteins were then transferred onto an ECL nitrocellulose membrane, and hVAP-1 was immunodetected by using a rabbit anti-hVAP-1 polyclonal antibody at 1:10,000 dilution (a prebleed serum at the same concentration was used as a negative control), peroxidase-conjugated anti-rabbit second-stage antibody, and ECL detection system.
Miscellaneous methods
Figures 1 ▶ through 4 ▶ ▶ ▶ were created with the PyMOL Molecular Graphics System (DeLano Scientific), Bodil (Lehtonen et al. 2004), and/or Corel Draw11 software. Secondary structure elements were assigned with DSSP (Kabsch and Sander 1983). A structure-based sequence alignment (Fig. 2 ▶; Table 2) was constructed by superimposing the Cα-atoms of hVAP-1, PPLO (PDB code 1N9E; Duff et al. 2003), ECAO (PDB code 1OAC; Parsons et al. 1995), PSAO (PDB code 1KSI; Kumar et al. 1996), HPAO (PDB code 1A2V; Li et al. 1998), and AGAO (PDB code 1AVL; Wilce et al. 1997) with the program VERTAA in Bodil (Lehtonen et al. 2004). Cavities were calculated with the program Surfnet (Laskowski 1995) by using 1.4 Å and 3.0 Å radii for minimum and maximum gap spheres, respectively.
Acknowledgments
The research was supported from grants from the Academy of Finland, National Technology Agency of Finland (TEKES), the National Graduate School in Informational and Structural Biology, the Foundation of Åbo Akademi, the Sigrid Juselius Foundation, Svenska Kulturfonden, Magnus Ehrnrooths Stiftelse, and the European Community—Access to Research Infrastructure Action of the Improving Human Potential Programme to the EMBL Hamburg Outstation, contract no. HPRI-CT-1999-00017. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities, and we thank the staff at beamline ID14.1 for assistance. We also thank the staff at EMBL X11 beamline at the DORIS storage ring, DESY, Hamburg. We thank Riikka Sjöroos and Sari Mäki for technical assistance and Sanna Maula and Sam Kaitaniemi for providing the mutant plasmids.
Competing interest statement
Three of the authors declare a financial interest related to this work: D.J.S. and M.P., as participants in the stock option program of Biotie Therapies Corp., and S.J., as an owner of stocks from BioTie Therapies Corp.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051438105.
References
- Abella, A., García-Vicente, S., Viguerie, N., Ros-Baró, A., Camps, M., Palacín, M., Zorzano, A., and Marti, L. 2004. Adipocytes release a soluble form of VAP-1/SSAO by a metalloprotease-dependent process and in a regulated manner. Diabetologia 47 429–438. [DOI] [PubMed] [Google Scholar]
- Buffoni, F. and Ignesti, G. 2000. The copper-containing amine oxidases: Biochemical aspects and functional role. Mol. Genet. Metab. 71 559– 564. [DOI] [PubMed] [Google Scholar]
- Chassande, O., Renard, S., Barbry, P., and Lazdunski, M. 1994. The human gene for diamine oxidase, an amiloride binding protein: Molecular cloning, sequencing, and characterization of the promoter. J. Biol. Chem. 269 14484–14489. [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4, 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50 760–763. [DOI] [PubMed] [Google Scholar]
- Cronin, C.N., Zhang, X., Thompson, D.A., and McIntire, W.S. 1998. cDNA cloning of two splice variants of a human copper-containing monoamine oxidase pseudogene containing a dimeric Alu repeat sequence. Gene 220 71–76. [DOI] [PubMed] [Google Scholar]
- Dawkes, H.C. and Phillips, S.E. 2001. Copper amine oxidase: Cunning cofactor and controversial copper. Curr. Opin. Struct. Biol. 11 666–673. [DOI] [PubMed] [Google Scholar]
- Duff, A.P., Cohen, A.E., Ellis, P.J., Kuchar, J.A., Langley, D.B., Shepard, E.M., Dooley, D.M., Freeman, H.C., and Guss, J.M. 2003. The crystal structure of Pichia pastoris lysyl oxidase. Biochemistry 42 15148– 15157. [DOI] [PubMed] [Google Scholar]
- Hansen, J.E., Lund, O., Engelbrecht, J., Bohr, H., and Nielsen, J.O. 1995. Prediction of O-glycosylation of mammalian proteins: Specificity patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Biochem. J. 308 (Pt. 3): 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J.E., Lund, O., Tolstrup, N., Gooley, A.A., Williams, K.L., and Brunak, S. 1998. NetOglyc: Prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj. J. 15 115–130. [DOI] [PubMed] [Google Scholar]
- Imamura, Y., Kubota, R., Wang, Y., Asakawa, S., Kudoh, J., Mashima, Y., Oguchi, Y., and Shimizu, N. 1997. Human retina-specific amine oxidase (RAO): cDNA cloning, tissue expression, and chromosomal mapping. Genomics 40 277–283. [DOI] [PubMed] [Google Scholar]
- Imamura, Y., Noda, S., Mashima, Y., Kudoh, J., Oguchi, Y., and Shimizu, N. 1998. Human retina-specific amine oxidase: Genomic structure of the gene (AOC2), alternatively spliced variant, and mRNA expression in retina. Genomics 51 293–298. [DOI] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.Y., Cowan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47 (Pt. 2): 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch, W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26 795–800. [Google Scholar]
- Kabsch, W. and Sander, C. 1983. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22 2577–2637. [DOI] [PubMed] [Google Scholar]
- Kleywegt, G.J. and Jones, T.A. 1998. Databases in protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 54 1119–1131. [DOI] [PubMed] [Google Scholar]
- Klinman, J.P. 2003. The multi-functional topa-quinone copper amine oxidases. Biochim. Biophys. Acta 1647 131–137. [DOI] [PubMed] [Google Scholar]
- Koskinen, K., Vainio, P.J., Smith, D.J., Pihlavisto, M., Ylä-Herttuala, S., Jalkanen, S., and Salmi, M. 2004. Granulocyte trans-migration through the endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (VAP-1). Blood 103 3388–3395. [DOI] [PubMed] [Google Scholar]
- Kuchar, J.A. and Dooley, D.M. 2001. Cloning, sequence analysis, and characterization of the “lysyl oxidase” from Pichia pastoris. J. Inorg. Biochem. 83 193–204. [DOI] [PubMed] [Google Scholar]
- Kumar, V., Dooley, D.M., Freeman, H.C., Guss, J.M., Harvey, I., McGuirl, M.A., Wilce, M.C., and Zubak, V.M. 1996. Crystal structure of a eukaryotic (pea seedling) copper-containing amine oxidase at 2.2 Å resolution. Structure 4 943–955. [DOI] [PubMed] [Google Scholar]
- Lalor, P.F., Edwards, S., McNab, G., Salmi, M., Jalkanen, S., and Adams, D.H. 2002. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J. Immunol. 169 983–992. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A. 1995. SURFNET: A program for visualizing molecular surfaces, cavities, and intermolecular interactions. J. Mol. Graph. 13 323–330. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., Macarthur, M.W., Moss, D.S., and Thornton, J.M. 1993. Procheck: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26 283–291. [Google Scholar]
- Lee, M., Willingham, K., Langley, D., Maher, M.J., Cohen, A.E., Ellis, P.J., Kuchar, J.A., Dooley, D.M., Freeman, H.C., and Guss, J.M. 2002. Crystallization of Pichia pastoris lysyl oxidase. Acta Crystallogr. D Biol. Crystallogr. 58 2177–2179. [DOI] [PubMed] [Google Scholar]
- Lehtonen, J., Still, D.-J., Rantanen, V.-V., Ekholm, J., Björklund, D., Iftikhar, Z., Huhtala, M., Repo, S., Jussila, A., Jaakkola, J., et al. 2004. BODIL: A molecular modeling environment for structure- function analysis and drug design. J. Comput. Aided Mol. Des. 18 401–419. [DOI] [PubMed] [Google Scholar]
- Li, R., Klinman, J.P., and Mathews, F.S. 1998. Copper amine oxidase from Hansenula polymorpha: The crystal structure determined at 2.4 Å resolution reveals the active conformation. Structure 6 293–307. [DOI] [PubMed] [Google Scholar]
- Lyles, G.A. 1996. Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: Biochemical, pharmacological and toxicological aspects. Int. J. Biochem. Cell. Biol. 28 259–274. [DOI] [PubMed] [Google Scholar]
- Maula, S.-M., Salminen, T.A., Kaitaniemi, S., Nymalm, Y., Smith D.J., and Jalkanen, S. 2005. Carbohydrates located on the top of th “cap” contribute to the adhesive and enzymatic functions of vascular adhesion protein-1 (VAP-1). Eur. J. Immunol. (in press). [DOI] [PubMed]
- Murshudov, G.N., Vagin, A.A., and Dodson, E.J. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53 240–255. [DOI] [PubMed] [Google Scholar]
- Nymalm, Y., Kidron, H., Söderholm, A., Viitanen, L., Kaukonen, K., Pihlavisto, M., Smith, D., Veromaa, T., Airenne, T.T., Johnson, M.S., et al. 2003. Crystallization and preliminary X-ray analysis of the human vascular adhesion protein-1. Acta Crystallogr. D Biol. Crystallogr. 59 1288–1290. [DOI] [PubMed] [Google Scholar]
- O’Sullivan, J., O’Sullivan, M., Tipton, K.F., Unzeta, M., Del Mar Hernandez, M., and Davey, G.P. 2003. The inhibition of semicarbazide-sensitive amine oxidase by aminohexoses. Biochim. Biophys. Acta 1647 367–371. [DOI] [PubMed] [Google Scholar]
- Parsons, M.R., Convery, M.A., Wilmot, C.M., Yadav, K.D., Blakeley, V., Corner, A.S., Phillips, S.E., McPherson, M.J., and Knowles, P.F. 1995. Crystal structure of a quinoenzyme: Copper amine oxidase of Escherichia coli at 2 Å resolution. Structure 3 1171–1184. [DOI] [PubMed] [Google Scholar]
- Pfaff, M. 1997. Recognition sites of RGD-dependent integrins. In Integrin– ligand interaction (eds. J.A. Eble and K. Kuhn), pp. 101–121. R.G. Landes Co., Austin, TX.
- Ruoslahti, E. and Pierschbacher, M.D. 1987. New perspectives in cell adhesion: RGD and integrins. Science 238 491–497. [DOI] [PubMed] [Google Scholar]
- Salmi, M. and Jalkanen, S. 1992. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 257 1407– 1409. [DOI] [PubMed] [Google Scholar]
- ———. 1996. Human vascular adhesion protein 1 (VAP-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J. Exp. Med. 183 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi, M., Tohka, S., and Jalkanen, S. 2000. Human vascular adhesion protein-1 (VAP-1) plays a critical role in lymphocyte-endothelial cell adhesion cascade under shear. Circ. Res. 86 1245–1251. [DOI] [PubMed] [Google Scholar]
- Salmi, M., Yegutkin, G.G., Lehvonen, R., Koskinen, K., Salminen, T., and Jalkanen, S. 2001. A cell surface amine oxidase directly controls lymphocyte migration. Immunity 14 265–276. [DOI] [PubMed] [Google Scholar]
- Salminen, T.A., Smith, D.J., Jalkanen, S., and Johnson, M.S. 1998. Structural model of the catalytic domain of an enzyme with cell adhesion activity: Human vascular adhesion protein-1 (HVAP-1) D4 domain is an amine oxidase. Protein Eng. 11 1195–1204. [DOI] [PubMed] [Google Scholar]
- Smith, D.J., Salmi, M., Bono, P., Hellman, J., Leu, T., and Jalkanen, S. 1998. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J. Exp. Med. 188 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolen, C.M., Yegutkin, G.G., Kurkijärvi, R., Bono, P., Alitalo, K., and Jalkanen, S. 2004. Origins of serum semicarbazide-sensitive amine oxidase. Circ. Res. 95 50–57. [DOI] [PubMed] [Google Scholar]
- Vriend, G. 1990. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 8 52–56, 29. [DOI] [PubMed] [Google Scholar]
- Wilce, M.C., Dooley, D.M., Freeman, H.C., Guss, J.M., Matsunami, H., McIntire, W.S., Ruggiero, C.E., Tanizawa, K., and Yamaguchi, H. 1997. Crystal structures of the copper-containing amine oxidase from Arthrobacter globiformis in the holo and apo forms: Implications for the biogenesis of topaquinone. Biochemistry 36 16116– 16133. [DOI] [PubMed] [Google Scholar]
- Wilmot, C.M., Hajdu, J., McPherson, M.J., Knowles, P.F., and Phillips, S.E. 1999. Visualization of dioxygen bound to copper during enzyme catalysis. Science 286 1724–1728. [DOI] [PubMed] [Google Scholar]
- Yegutkin, G.G., Salminen, T., Koskinen, K., Kurtis, C., McPherson, M.J., Jalkanen, S., and Salmi, M. 2004. A peptide inhibitor of vascular adhesion protein-1 (VAP-1) blocks leukocyte-endothelium interactions under shear stress. Eur. J. Immunol. 34 2276–2285. [DOI] [PubMed] [Google Scholar]