Abstract
The formation of polypeptide aggregates represents a nucleated polymerization reaction in which an initial nucleation event (lag phase) is followed by the extension of newly formed nuclei into larger aggregates, including fibrils (growth phase). The efficiencies of these reactions relate to the lag time (lag phase) and to the rate of aggregation (growth phase), which can be determined from experimental aggregation curves. Here we present a mutagenic analysis in which we replace valine 18 of the Alzheimer’s Aβ (1–40) peptide with 17 different amino acids and determine its effect on the lag time, and therefore, on the propensity of nucleation. Comparison with various physico-chemical properties shows that nucleation is affected in a predictable manner depending on the β-sheet propensity and hydrophobicity of residue 18. In addition, we observe a direct proportionality between the lag time and the rate of aggregation. These data imply that the two reactions, nucleation and polymerization, are governed by very similar physicochemical principles and that they involve the formation of the same types of noncovalent interactions.
Keywords: amyloid, conformational disease, kinetics, protein folding, prion
The formation of a nucleus is a critical step in the structural conversion of initially disordered polypeptide chains into amyloid fibrils or aggregates (Harper and Lansbury 1997). The natural slowness by which many disease-related polypeptide sequences nucleate along with the possibility to eliminate in vitro the need of self-nucleation by addition of preformed seeds or fibril fragments support proposals that a nucleated polymerization mechanism underlies the transmissibility phenomena in the conformational disorders that involve prions or amyloid-enhancing factor (Prusiner 1998; Lundmark et al. 2002). The aggregation curves arising from such nucleated polymerization reactions consist typically of two phases, namely, an initial lag phase that relates to the nucleation event and a subsequent growth phase of rapid aggregation, representing the polymerization reaction (Harper and Lansbury 1997).
The effect of amino acid mutation on the polymerization reaction, that is, the addition of further polypeptide chains onto the nucleus, is well understood. It can be expressed in terms of changes in the rate of aggregation k, which is obtained by fitting a single exponential to the growth phase of the aggregation curve. k was shown to depend on several intrinsic or environmental parameters, such as polypeptide concentration, mutation, protein stability, pH, and temperature (Chiti et al. 2000, 2003; DuBay et al. 2004; Tartaglia et al. 2004). The effect of mutation on the nucleation event, in contrast, is less well understood. However, there is evidence that genetic mutations can trigger amyloidosis or reduce the onset of such diseases, suggesting that mutation acts, at least in part, on the nucleation event (Kelly et al. 1997; Prusiner 1998; Dobson 2001; Stix et al. 2005). Nuclei represent typically an arrangement of polypeptide chains that encompasses nascent elements of the aggregated conformation (homogeneous nucleation) (Harper and Lansbury 1997), but also heterogeneous seeding reactions are known to exist where aggregation is promoted by nonproteinaceous material, such as hydrophobic surfaces (Teflon), lipids, or sugar compounds (Sluzky et al. 1991; Cohlberg et al. 2002; Hayashi et al. 2004). The efficiency of nucleation is estimated here fromthe length of the lag phase, the lag time tl. Using a total of 18 variants of oxidized Alzheimer’s Aβ(1–40) peptide and conditions under which the wildtype peptide aggregates with a well-resolved lag phase, we have been able to discern experimentally, and to rationalize, the effect of the different side chains on nucleation and polymerization.
Results
We use only the oxidized form of Aβ(1–40) peptide in which Met35 is present as sulfoxide. This form is much more redox-stable than the reduced peptide, thus minimizing the potential interference of redox reactions with our measurements (Watson et al. 1998). Within this peptide, valine 18 is replaced with all other standard amino acids, except those containing sulfur. Nuclear magnetic resonance analysis of Aβ(1–40) fibrils suggests that residue 18 is located within a β-strand and that it is not obviously involved in any specific intramolecular sidechain interactions, such as salt bridges, hydrogen bonds, or strand–strand interactions (Petkova et al. 2002). Nevertheless, it may well contribute to the intermolecular interactions between juxtaposed peptide units, because proline-scanning mutagenesis has revealed that replacement of valine 18 with proline is unfavorable to the aggregation of Aβ(1–40) and Aβ(1–42) (Morimoto et al. 2004; Williams et al. 2004). The experimental conditions used here are incubation at 37°C for 4 d in 50mM sodium phosphate buffer (pH 7.4) along with a thioflavine T (ThT) based detection system of the aggregates. Under this condition, wild-type Aβ was found previously to aggregates slow enough to enable observation of mutagenic changes (Hortschansky et al. 2005).
Using this setup, we observe aggregate formation in all samples and Aβ(1–40) variants studied here, except those where valine 18 is replaced with proline (Table 1). Consistent with this, when these aggregates are stained with Congo red and examined in a polarizing microscope with crossed polarizers, all but the Val18Pro variant show Congo red green birefringent properties (Table 1) and therefore characteristics that serve as a gold standard to detect amyloid during pathological or clinical examination (Westermark et al. 1999). Moreover, centrifugation shows the formation of insoluble material (data not presented). In contrast to this, there is only little or no evidence from electron microscopy that substantial amounts of highly ordered fibrils might be present after 4 d, i.e, within the time frame monitored by ThT-fluorescence. This shows that our kinetic data relate to polypeptide aggregation and amyloid formation to the extent that they involve the formation of aggregated or amyloid-like β-sheets, although further and more specific contacts need to be established before species of more linear ultrastructure can appear, such as mature amyloid fibrils.
Table 1.
Structural properties of the aggregates after 4 d of incubation
| Variant | ThT | CRGB | EM |
| Wild type | + | + | — |
| V18A | + | + | — |
| V18D | + | + | — |
| V18E | + | + | — |
| V18F | + | + | — |
| V18G | + | + | — |
| V18H | + | + | — |
| V18I | + | + | — |
| V18K | + | + | (+) |
| V18L | + | + | (+) |
| V18N | + | + | — |
| V18P | — | — | — |
| V18Q | + | + | — |
| V18R | + | + | — |
| V18S | + | + | — |
| V18T | + | + | — |
| V18W | + | + | — |
| V18Y | + | + | — |
ThT, increase of the ThT-fluorescence; CRGB, green birefringence after Congo red staining; EM, presence of fibrils by electron microscopy; +, property observed; —, property not observed; (+), presence of very small quantities of fibrils. Otherwise all samples are morphologically indistinguishable by EM.
Each Aβ(1–40) variant is represented in our analysis by a total of 10 samples for which the aggregation kinetics were recorded and the values k and tl were extracted. Individual aggregation traces can deviate substantially even when examining apparently identical samples as shown by representative examples of the raw data in Figure 1 ▶. This heterogeneity usually exceeds the uncertainty of the measurement and reflects, therefore, true differences between these samples. We believe that this variability indicates the stochastic nature of the underlying nucleation event, which is consistent with the observation of a similar heterogeneity in the aggregation curves of the reduced wild-type Aβ(1–40) (Hortschansky et al. 2005). Whereas most variants aggregate with a well-resolved lag phase, no lag or growth phase can be discerned in case of the Val18Pro mutant (Fig. 1C ▶). These data are in agreement with previous observations that replacement of Val18 with Pro disfavors the aggregation of Aβ(1–40) and Aβ(1–42) (Morimoto et al. 2004; Williams et al. 2004), and Val18Pro is the only variant studied here for which we could not determine any k and tl value. All other variants and samples aggregate with a clearly discernible growth phase, resulting in a data set containing the tl and k values from 17 variants. Out of these, only Val18Tyr was found to be associated with a growth phase that starts within the dead time of the experiment (7 min). Therefore, we have approximated, for further analysis, the tl value of Val18Tyr with 0 h, which represents only a small inaccuracy for the comparison with mutants that vary by more than 20 h (Fig. 2A, B ▶). Interestingly, from the data shown in Figure 2C, D ▶, it is evident that the heterogeneity of the individual k (or tl) measurements is correlated with the magnitude of the average values kav (or tl av); namely, mutants with a large average value tlav are also these that are associated with a pronounced heterogeneity in tl. Similar with this, mutants with a large average value kav possess very heterogeneous k values. In addition, when we compare k and tl, we find that a large heterogeneity in k is usually opposed by only a small heterogeneity in tl and vice versa. Mutation affects, therefore, the average values kav and tlav along with the heterogeneity between the individual samples.
Figure 1.

Representative examples of the kinetic data. The 10 kinetic traces of V18I (A), V18Q (B), and V18P (C). Note the different time scale in each panel. Samples contain 120 μM peptide, 20 μM ThT, and 10 mM sodium azide in 50 mM sodium phosphate (pH 7.4).
Figure 2.

Variability of k and tl for different residues 18. Variability of k (A) and tl (B). Gray symbols, individual k and tl values. Black symbols, tlav and kav. Relationship between the standard deviation arising from the 10 individual measurements and kav (C) and tlav (D). In Figures 2–5 ▶ ▶ ▶, values of tl are given without the dead time of experiment (7 min).
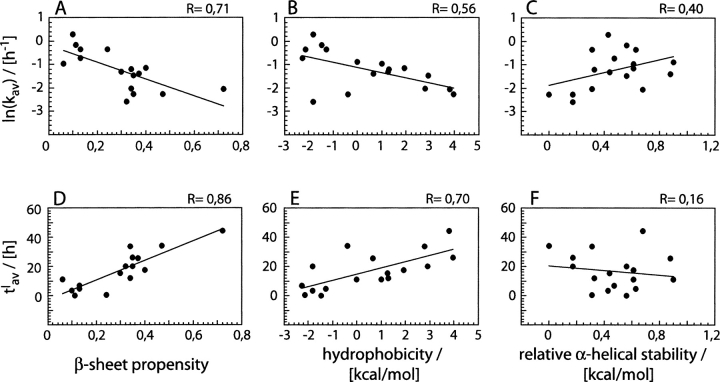
Henceforth, we have focused mainly on the average values kav and tlav and on their possible relationship with the physico-chemical properties of residue 18. This shows that the wild-type residue 18 (valine) is one of the most favorable residues to aggregation. Valine 18 possesses the fourth largest k value, indicating a very rapid growth phase, and the fourth smallest lag time, suggesting a fast nucleation event. Only Tyr, Trp, and Ile show faster polymerization or nucleation properties. Next, we analyzed the rate of aggregation in more detail. Literature evidence describes a linear dependence of the ratio ln(kMUT/kWT), in which kWT refers to the wild-type sequence and kMUT to the mutant, on factors such as hydrophobicity, charge, β-sheet or α-helical propensities (Chiti et al. 2003; Tartaglia et al. 2004). Indeed, the present data support such proposals for the β-sheet propensity and hydrophobicity (Fig. 3A, B ▶). In contrast, only a poor or no correlation can be seen when ln(kav) is compared with the van der Waals volume (Creighton 1993) or the α-helix stabilizing effect (Fig. 3C ▶). Moreover, it is difficult to differentiate between charge and hydrophobicity because the charged residues represent also the least hydrophobic residues of the plot in Figure 3B ▶. Indeed, small kav values are often associated with residues affecting the charge state of the peptide (Arg, Asp, Glu, His, Lys). Similar considerations apply for the aromaticity, which was identified also as an important factor in aggregation reactions (Porat et al. 2004; Tartaglia et al. 2004; Tracz et al. 2004).
Figure 3.
Dependence of kav and tlav on residual physico-chemical properties. (A–C) Plots of ln(kav) versus β-sheet propensity (A), hydrophobicity relative to glycine (Creighton 1993) (B), and α-helical stability relative to alanine (Fersht 1998) (C). (D–F) Plots of tlav versus β-sheet propensity (D), hydrophobicity relative to glycine (E), and α-helical stability relative to alanine (F). The β-sheet propensities are taken from Street and Mayo (1999), where Pro or Gly are not included. By definition, smaller propensity values mean that residues are more favorable for β-sheet structure. Data are fitted with the function, a+bx, where x represents β-sheet propensity, hydrophobicity, or relative α-helical stability. The corresponding correlation coefficients R are reported.
Next we examined the extent to which tlav might correlate with the various physico-chemical properties described above. Plots of tlav versus β-sheet propensity and hydrophobicity can be fit reasonably with straight lines (Fig. 3D, E ▶). In contrast, no clear dependence can be discerned in case of the α-helix stabilizing effect (Fig. 3F ▶)or for the van der Waals volume (Creighton 1993). These data show that the β-sheet propensity (Street and Mayo 1999) correlates best with ln(kav) or tlav and that both parameters respond to changes in the physicochemical properties of residue 18 in a very similar manner. This suggests that the two reactions underlying these properties, namely, the nucleation and the polymerization reaction, are mechanistically related and that they involve the formation of very similar non-covalent interactions. Strong support for this idea comes from the plot of ln(kav) versus tlav (Fig. 4 ▶), which shows a direct proportionality between these two values, that is, variants with short lag times (small value of tl av) are also these that extend their nuclei very rapidly (large value of kav).
Figure 4.
Correlation between the nucleation and the polymerization propensities. The polymerization propensity is expressed here as ln(kav) and the nucleation propensity as tl av.
Discussion
In the present manuscript we have elucidated the effect of single-site mutagenesis on the nucleation and polymerization propensity of oxidized Aβ(1–40) peptide. Single-site mutagenesis avoids problems with different structural contexts and allows direct comparisons between the residues. The nonfibrillar ultrastructure, ThT fluorescence, and Congo red green birefringence of the resulting species demonstrate that these encompass aggregated β-sheets and nascent elements of amyloid structure within their core. This type of β-sheet structure has been described previously to differ generally from the one in native, globular proteins (Zandomeneghi et al. 2004). Furthermore, since these species aggregate with a well-resolvable lag phase, observation of the latter cannot be used as an indicator of the formation of highly ordered fibrils. Instead, a lag phase can occur also in the formation of nonfibrillar aggregates.
It has been well established that genetic mutation can initiate the early onset of amyloidotic diseases, predisposing an organism to spontaneous amyloid formation. Examples of such behavior are the familial cases of diseases involving the prion protein, keratoepithelin, transthyretin, and others (Kelly et al. 1997; Prusiner 1998; Dobson 2001; Stix et al. 2005). Although the biological consequences of these mutations can be very heterogeneous, possibly affecting a wide range of cellular functions, for example, proteolytic degradation, protein unfolding, subcellular transport, and interaction with lipids or other cellular factors, all of these changes result in the stabilization of a nidus that promotes aggregation and accumulation of amyloid within the tissue (Röcken and Shakespeare 2002). The present in vitro data show that the propensity of nucleation is strongly dependent on mutation and that it correlates with the physico-chemical properties of the residue subject to mutagenesis. All but four mutants aggregate more slowly than the wild-type peptide, which contains valine at position 18. The most dramatic effect is observed for Val18Pro, where mutation affects the structure of the backbone and interferes with its ability to form hydrogen bonds (Moriarty and Raleigh 1999; Morimoto et al. 2004; Williams et al. 2004). A less pronounced, but still considerable effect is observed upon insertion of a charged residue, thus leading to electrostatic repulsion. These data are further support for the concept that charges are difficult to tolerate within the core of aggregates (Fändrich and Dobson 2002). In addition, we observe a direct proportionality between tlav and the properties β-sheet propensity and hydrophobicity (Fig. 3D, E ▶), that is, residues with a high propensity to form β-sheet structure or with significantly hydrophobic properties are the fastest to aggregate under the present set of conditions. Nevertheless, hydrophobicity is not fully independent from charge and aromaticity. No significant correlation is observed for the side-chain volume or α-helical stabilizing effect. Furthermore, the aggregation kinetics is known to depend also on the structural or sequential context, patterns, or different physico-chemical conditions (DuBay et al. 2004; Fernandez-Escamilla et al. 2004). However, these parameters are not considered here, for they are kept constant in our analysis. We have also compared our data with the output from two algorithms that predict changes in ln(kMUT/kWT) arising from single- site replacements (Chiti et al. 2003) or that estimate aggregation behavior from sequential or environmental properties (TANGO) (Fernandez-Escamilla et al. 2004). Although there is a correlation of our data with the prediction of ln(kMUT/kWT), the slope of the fit deviates substantially from 1.0 (Fig. 5A ▶). The program TANGO (Fernandez-Escamilla et al. 2004) fits our data with correlation coefficients of 0.53 [ln(kav)] and 0.64 (tl av) (Fig. 5B ▶), demonstrating that both algorithms can capture trends in the aggregation propensity of different mutants. We have tried to predict changes in ln(kav) or tlav by combining several of the above properties in a linear regression analysis, but this did not result in any improvement compared with the β-sheet propensity only.
Figure 5.
Comparison of the experimental data with predictions. (A) Comparison of calculated (Chiti et al. 2003) and experimental ln(kMUT/kWT) values. (B) Comparison of ln(kav) (open symbols) and tlav (filled symbols) with the difference in the TANGO score (Fernandez-Escamilla et al. 2004) between mutant and wild-type sequence. A reduction in the TANGO score means a smaller propensity to aggregate. A large TANGO score means a high propensity to aggregate. Data are fitted with the function, a+bx. In A the fit is y=−0.3+0.32x; R=0.69. In B the fits are y=−0.58+0.0024x; R=0.53 (kav); and y=4.72−0.044x; R=0.63.
The correlation coefficients of tlav versus β-sheet propensity, hydrophobicity, and TANGO score are better than the ones of ln(kav). This could indicate a higher accuracy in the measurement of tlav and would be consistent with our observations that fitting the growth part of the aggregation curve with a single exponential (the common method to define k) does not always represent a very accurate description of our experimental data. Hence, the lag time data appear to be more reliable. The fact that the lag time can be related directly to the rate of aggregation (Fig. 4 ▶) suggests that the two reactions underlying these kinetic properties, that is, the nucleation and the polymerization reaction, involve the formation of very similar noncovalent interactions. This finding is consistent, of course, with the common view that homogeneous nucleation leads to a nucleus that contains a structural scaffold in which the final aggregate structure is preformed. When further polypeptide chains are added onto this seed, they adopt and propagate the preformed structural arrangement of the nucleus. An alternative possibility to explain these observations is, however, that the Aβ variants with a high propensity to nucleate are also these that form larger quantities of nuclei. In consequence, this leads to larger average values of k. And, indeed, k-values were found to increase when seeds are added to solutions of wild-type Aβ(1–40) peptide (Hortschansky et al. 2005).
While most of the above discussion concerns the dependence of the average values kav and tl av, individual k and tl measurements were found sometimes to differ substantially from these averages. The present analysis shows that the mutants that aggregate most readily are also these that do so in the most homogeneous way, i.e., mutants with larger average values tlav deviate more substantially in tl. Henceforth, the present data support ideas that nucleation-limited reactions can be very heterogeneous because of the stochastic factor inherent in the formation of seeds with appropriate structure. Consistent with our previous proposal, it might also contribute to differences in the extent or onset of disease among patients with apparently identical genetic backgrounds (Hortschansky et al. 2005). These data provide further experimental information to understand the principles and mechanism of polypeptide aggregation as well as to support the development of methods to predict the kinetics of aggregation reactions from intrinsic or external parameters.
Materials and methods
Mutagenesis and oxidation of Aβ (1–40)
Site-directed mutagenesis of a gene coding for the human Aβ (1–40) was carried out using a QuikChange XL Site-Directed Mutagenesis Kit kit (Stratagene). Codon usage was optimized for Escherichia coli as recommended by Stratagene. The expression system was constructed and peptide was purified according to published methods (Gellermann et al. 2005). Based on a Coomassie-stained protein gel, the purity of the peptide was better than 95%. This was confirmed in representative samples by the UV-trace after reversed-phase chromatography. The identity of all peptides was verified by mass spectrometry before and after oxidation. Oxidation was performed by incubation of 5 mg/mL peptide in 3% H2O2 for 4 h at 20°C (Watson et al. 1998). The oxidized peptide was repurified with reversed-phase chromatography and lyophilized.
Aggregation assay and data analysis
All kinetic data were recorded using a BMG FLUOstar Galaxy reader (BMG) and black-wall 96-well plates (Nunc). The peptide was disaggregated by dissolving 1 mg in 2 mL of a 1:1 mixture of trifluoroacetic acid (Roth) and 1,1,1,3,3,3-hexafluoro- 2-propanol (Fluka). This solution was incubated at room temperature for 4 h, after which the solvent was dried off under a steady steam of nitrogen. The dry peptide was redissolved in 5 mL of 0.15% ammonium hydroxide, sonicated for 3 min, and lyophilized. Immediately before analysis, the peptide was dissolved in analysis buffer and filtered through a 0.22-μm acetate PLUS filter (Roth). Peptide concentrations were determined from aliquots of this solution that were diluted 1:100 and by using a Coomassie Plus kit (Pierce) calibrated with a wild-type Aβ(1–40) standard. The standard concentration was determined with UV-absorption and using the theoretical molar extinction coefficient at 280 nm (1490 M−1 cm−1) (Pace et al. 1995). The concentration of the Aβ stock solution was adjusted to 240 μM. Then 50 μL of this solution was transferred into a well of the 96-well plate containing 50 μL of a 40 μM ThT and 20 mM sodium azide solution in phosphate buffer. These procedures led to a final peptide concentration of 120 μM and an effective dead time of 7 min, measured between dissolving the peptide and acquisition of the first data point. The lag time was obtained by fitting a straight line to the initial slope of the growth phase. The time point of its intersection with the baseline was taken as the lag time. Fits of the aggregation rates with the formula y=a+b×exp(−kx) were carried out using only the growth part of the curve and assuming an exponential shape. The value of k was taken as the rate of aggregation. A more detailed description has been given in our previous manuscript (Hortschansky et al. 2005).
Polarization and electron microscopy analysis
A 10-μL aliquot from a 120 μM peptide sample incubated for 4 d without ThT was dried on a glass slide. The dry material was stained with a filtered, saturated Congo red solution in 70% ethanol/water for 5 min. The Congo red solution was discarded, and the slides were washed with water and 70% ethanol. All slides were analyzed using a Leica DM/DR 450× polarizing microscope with parallel (bright field) or crossed polarizers (dark field). Electron microscopy was carried out after 4 d of incubation as described previously (Fändrich et al. 2003) and by using an EM 400 T Philips electron microscope.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG). M.F. acknowledges a BioFuture grant from the Bundesministerium für Bildung und Forschung (BMBF). The authors thank B. Beck, S. Fricke, U. Knüpfer, and R. Wendroth for technical assistance.
Conflict of interest
The authors claim a conflict of interest arising from a commercial partner that precludes free distribution of any DNA constructs described here. Basis vectors are available from commercial vendors.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051470405.
References
- Chiti, F., Taddei, N., Bucciantini, M., White, P., Ramponi, G., and Dobson, C.M. 2000. Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J. 19 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti, F., Stefani, M., Taddei, N., Ramponi, G., and Dobson, C.M. 2003. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424 805–808. [DOI] [PubMed] [Google Scholar]
- Cohlberg, J.A., Li, J., Uversky, V.N., and Fink, A.L. 2002. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro. Biochemistry 41 1502–1511. [DOI] [PubMed] [Google Scholar]
- Creighton, T. 1993. Proteins, structure and molecular properties, 2nd ed. W.H. Freeman, New York.
- Dobson, C.M. 2001. The structural basis of protein folding and its links with human disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 356 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBay, K.F., Pawar, A.P., Chiti, F., Zurdo, J., Dobson, C.M., and Vendruscolo, M. 2004. Prediction of the absolute aggregation rates of amyloidogenic polypeptide chains. J. Mol. Biol. 341 1317–1326. [DOI] [PubMed] [Google Scholar]
- Fändrich, M. and Dobson, C.M. 2002. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J. 21 5682–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fändrich, M., Forge, V., Buder, K., Kittler, M., Dobson, C.M., and Diekmann, S. 2003. Myoglobin forms amyloid fibrils by association of unfolded polypeptide segments. Proc. Natl. Acad. Sci. 100 15463–15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Escamilla, A.M., Rousseau, F., Schymkowitz, J., and Serrano, L. 2004. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22 1302–1306. [DOI] [PubMed] [Google Scholar]
- Fersht, A. 1998. Structure and mechanism in protein sciences: A guide to enzyme catalysis and protein folding. W.H. Freeman, New York.
- Gellermann, G.P., Appel, T.R., Tannert, A., Radestock, A., Hortschansky, P., Leisner, C., Lütkepohl, T., Shtrasburg, S., Röcken, C., Pras, M., et al. 2005. Raft lipids as novel and common components of human, extracellular amyloid fibrils. Proc. Natl. Acad. Sci. 102 6297–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.D. and Lansbury Jr., P.T. 1997. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66 385–407. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., Kimura, N., Yamaguchi, H., Hasegawa, K., Yokoseki, T., Shibata, M., Yamamoto, N., Michikawa, M., Yoshikawa, Y., Terao, K., et al. 2004. A seed for Alzheimer amyloid in the brain. J. Neurosci. 24 4894–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortschansky, P., Schroeckh, V., Christopeit, T., Zandomeneghi, G., and Fändrich, M. 2005. The aggregation kinetics of Alzheimer’s β-amyloid peptide is controlled by stochastic nucleation. Protein Sci. 14 1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, J.W., Colon, W., Lai, Z., Lashuel, H.A., McCulloch, J., McCutchen, S.L., Miroy, G.J., and Peterson, S.A. 1997. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv. Protein Chem. 50 161–181. [DOI] [PubMed] [Google Scholar]
- Lundmark, K., Westermark, G.T., Nystrom, S., Murphy, C.L., Solomon, A., and Westermark, P. 2002. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc. Natl. Acad. Sci. 99 6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty, D.F. and Raleigh, D.P. 1999. Effects of sequential proline substitutions on amyloid formation by human amylin20–29. Biochemistry 38 1811–1818. [DOI] [PubMed] [Google Scholar]
- Morimoto, A., Irie, K., Murakami, K., Masuda, Y., Ohigashi, H., Nagao, M., Fukuda, H., Shimizu, T., and Shirasawa, T. 2004. Analysis of the secondary structure of β-amyloid (Aβ42) fibrils by systematic proline replacement. J. Biol. Chem. 279 52781–52788. [DOI] [PubMed] [Google Scholar]
- Pace, C.N., Vajdos, F., Fee, L., Grimsley, G., and Gray, T. 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4 2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova, A.T., Ishii, Y., Balbach, J.J., Antzutkin, O.N., Leapman, R.D., Delaglio, F., and Tycko, R. 2002. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. 99 16742–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat, Y., Mazor, Y., Efrat, S., and Gazit, E. 2004. Inhibition of islet amyloid polypeptide fibril formation: A potential role for heteroaromatic interactions. Biochemistry 43 14454–14462. [DOI] [PubMed] [Google Scholar]
- Prusiner, S.B. 1998. Prions. Proc. Natl. Acad. Sci. 95 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röcken, C. and Shakespeare, A. 2002. Pathology, diagnosis and pathogenesis of AA amyloidosis. Virchows Arch. 440 111–122. [DOI] [PubMed] [Google Scholar]
- Sluzky, V., Tamada, J.A., Klibanov, A.M., and Langer, R. 1991. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. 88 9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stix, B., Leber, M., Bingemer, P., Gross, C., Rueschoff, J., Fandrich, M., Schorderet, D.F., Vorwerk, C.K., Zacharias, M., Roessner, A., et al. 2005. Hereditary lattice corneal dystrophy is associated with corneal amyloid deposits enclosing C-terminal fragments of kerato-epithelin. Invest. Ophthalmol. 46 1133–1139. [DOI] [PubMed] [Google Scholar]
- Street, A.G. and Mayo, S.L. 1999. Intrinsic β-sheet propensities result from van der Waals interactions between side chains and the local backbone. Proc. Natl. Acad. Sci. 96 9074–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, G.G., Cavalli, A., Pellarin, R., and Caflisch, A. 2004. The role of aromaticity, exposed surface, and dipole moment in determining protein aggregation rates. Protein Sci. 13 1939–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracz, S.M., Abedini, A., Driscoll, M., and Raleigh, D.P. 2004. Role of aromatic interactions in amyloid formation by peptides derived from human amylin. Biochemistry 43 15901–15908. [DOI] [PubMed] [Google Scholar]
- Watson, A.A., Fairlie, D.P., and Craik, D.J. 1998. Solution structure of methionine-oxidized amyloid β-peptide (1–40). Does oxidation affect conformational switching? Biochemistry 37 12700–12706. [DOI] [PubMed] [Google Scholar]
- Westermark, G.T., Johnson, K.H., and Westermark, P. 1999. Staining methods for identification of amyloid in tissue. Methods Enzymol. 309 3–25. [DOI] [PubMed] [Google Scholar]
- Williams, A.D., Portelius, E., Kheterpal, I., Guo, J.T., Cook, K.D., Xu, Y., and Wetzel, R. 2004. Mapping Aβ amyloid fibril secondary structure using scanning proline mutagenesis. J. Mol. Biol. 335 833–842. [DOI] [PubMed] [Google Scholar]
- Zandomeneghi, G., Krebs, M.R., McCammon, M.G., and Fändrich, M. 2004. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 13 3314–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]