Abstract
Prion diseases appear to be caused by the aggregation of the cellular prion protein (PrPC) into an infectious form denoted PrPSc. The in vitro aggregation of the prion protein has been extensively investigated, yet many of these studies utilize truncated polypeptides. Because the C-terminal portion of PrPSc is protease-resistant and retains infectivity, it is assumed that studies on this fragment are most relevant. The full-length protein can be distinguished from the truncated protein because it contains a largely structured, α-helical, C-terminal region in addition to an N terminus that is unstructured in the absence of metal ion binding. Herein, the in vitro aggregation of a truncated portion of the prion protein (PrP 90–231) and a full-length version (PrP 23–231) were compared. In each case, concentration-dependent aggregation was analyzed to discern whether it proceeds by a nucleation-dependent pathway. Both protein constructs appear to aggregate via a nucleated polymerization with a small nucleus size, yet the later steps differ. The full-length protein forms larger aggregates than the truncated protein, indicating that the N terminus may mediate higher-order aggregation processes. In addition, the N terminus has an influence on the assembly state of PrP before aggregation begins, causing the full-length protein to adopt several oligomeric forms in a neutral pH buffer. Our results emphasize the importance of studying the full-length protein in addition to the truncated forms for in vitro aggregation studies in order to make valid hypotheses about the mechanisms of prion aggregation and the distribution of aggregates in vivo.
Keywords: prion protein, aggregation, full-length prion protein, N-terminal domain
Prion diseases are currently untreatable, fatal neurodegenerative illnesses apparently caused by misfolding and misassembly of the cellular prion protein, PrPC, into an aggregated form designated PrPSc (Prusiner 1982, 1998). In some cases, PrP aggregation in vitro and in vivo leads to the formation of amyloid fibrils, a primary feature of many neurodegenerative diseases. Human prion diseases include Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler syndrome (GSS), and fatal familial insomnia (FFI). Animals are also susceptible to prion diseases, such as bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep, and chronic wasting disease in deer and elk (Collinge 2001). Prion diseases are unique among the family of misfolding diseases because in addition to being inherited or sporadic, they can be transmitted by infection (Weissmann 1996; Prusiner 1998). Under rare circumstances, prion disease can cross species barriers, as demonstrated by the emergence of new variant CJD in humans, which is derived from infected cattle (Bruce et al. 1997).An important step in the development of inherited or infectious prion disease is the conversion of PrPC to PrPSc. The steps involved in this transition have been elusive, especially because they involve both misfolding and aggregation. Although PrPC and PrPSc have identical primary structures, the properties of each PrP isoform are strikingly different (Stahl et al. 1993). PrPC is soluble, monomeric under most conditions, primarily composed of α-helix, and readily digested by proteinase K. PrPSc is an insoluble oligomer of unknown structure, is composed mainly of β-sheet, and is resistant to proteinase K treatment (Oesch et al. 1985; Meyer et al. 1986; Caughey et al. 1991; Pan et al. 1993). Understanding the pathway involved in forming the infectious agent is crucial for developing therapies aimed at treating these diseases.
Investigations into the process of PrP aggregation in vitro reveal the complexity of the steps involved, some of which may play a role in disease pathogenesis. To date, most studies have focused on characterizing the in vitro misfolding/aggregation pathways of a truncated form of prion protein rather than the entire protein (Swietnicki et al. 1997, 2000; Zhang et al. 1997; Hornemann and Glockshuber 1998; Wildegger et al. 1999; Baskakov et al. 2001, 2002; Morillas et al. 2001; Sokolowski et al. 2003; Baskakov 2004). The mature, full-length cellular PrP begins at amino acid 23 and ends at residue 231 of the pro-sequence, following cleavage of both the N- and C-terminal signal sequences. TheC-terminal region encodes the addition of a glycosyl phosphatidylinositol (GPI) anchor to serine 231, which inserts into the cell membrane and directs the protein into the extracellular space (Stahl et al. 1987). Cellular PrP is also glycosylated at several sites, although previous in vitro studies and the experiments described below utilize the unglycosylated protein (Haraguchi et al. 1989). An unglycosylated, truncated form of the prion protein, PrP 90–231, is often used to study aggregation because it comprises the proteinase K-resistant portion of PrPSc from diseased brains, which retains the ability to propagate disease and thus is considered the “core” of the infectious agent (Prusiner 1998). The 121–231 portion of the protein is another fragment that is frequently used to study aggregation because it is the highly structured subdomain with three α-helices and two short β-strands, yet lacks the flexible N terminus. In contrast, the N-terminal region of the protein, comprising amino acids 23–124, is unstructured and flexible, although it can chelate copper and thus acquire some structure (Riek et al. 1996; Brown et al. 1997; Donne et al. 1997; Liu et al. 1999). Although some recent aggregation studies utilized the full-length prion protein, PrP 23–231 (Baskakov and Bocharova 2005; Bocharova et al. 2005), the aggregation properties of this construct have not been studied as extensively as those of the truncated forms, perhaps because the flexible portion of the protein is not considered important. However, it was previously shown that PrPs with N-terminal deletions form abnormal conformations of prion aggregates (Lawson et al. 2001, 2004). Indeed, although PrPSc composed of amino acids 90–231 is sufficient to transmit disease, evidence suggests that the N terminus may play a role in prion pathogenesis since transgenic mice lacking residues 32–106 are not susceptible to prion infection (Weissmann 1999).
Insight into the mechanism of PrP aggregation can be obtained by examining the dependence of its rate of polymerization on protein concentration. The aggregation of numerous proteins involved in neurodegenerative diseases is proposed to proceed by a nucleation-dependent mechanism, the most widely accepted model for this type of process (Goldstein and Stryer 1986; Harper and Lansbury 1997; Ferrone 1999). In this model, the formation of the highest energy species, the thermodynamic nucleus, from monomeric units is rate-limiting for the formation of larger aggregates. The rate of aggregation has a high-order dependence on the concentration of monomeric protein, which has been used to explain the late onset of sporadic Alzheimer’s and prion diseases (Jarrett and Lansbury 1993).
In this study, the concentration dependence of the aggregation of both full-length recombinant mouse PrP 23–231 and truncated murine PrP 90–231 was examined to compare the aggregation pathways and to determine whether aggregation by either sequence proceeds by a nucleation-dependent oligomerization. Partial denaturation utilizing GdnHCl at pH 4 initiates aggregation that can be followed on a laboratory timescale by several methods, including thioflavin T (TfT) fluorescence, circular dichroism (CD), turbidity, light scattering, and analytical gel filtration. We show that there are several differences in the aggregation properties of full-length and truncated PrP, most notably the size of the aggregates formed. These observations have implications for future studies of PrP aggregation in vitro, showing that it is imperative to verify and compare any results obtained with truncated prions with the full-length protein.
Results
Oligomeric state of PrP prior to aggregation
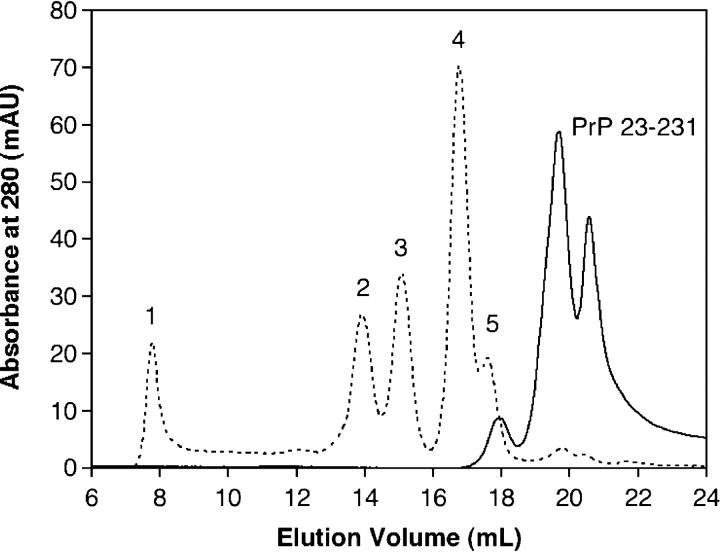
Before examining the aggregation process of the truncated (PrP 90–231) and full-length (PrP 23–231) forms of PrP, the oligomeric state of each protein was probed using analytical gel filtration in conjunction with light scattering to ensure that both constructs were initially monomeric. Neither protein showed any large aggregates that would elute in the void volume, confirming that the purification process rid the sample of aggregates (Supplemental Fig. 1 ▶). However, when a pH 7 running buffer was employed (50 mM NaPi, 150 mM NaCl), monomeric full-length PrP 23–231 eluted later than a protein of similar molecular weight and as multiple peaks spaced closely together. Figure 1 ▶ illustrates these observations with an overlay of a gel filtration chromatogram of several protein standards (dotted line) and the trace of PrP 23–231. The relative intensities of each peak and even the number of peaks (either three or four) varied for each injection. Analysis of molar mass by static light scattering did not reveal significant differences in molecular weight between the peaks; thus further examination of the characteristics of each peak was needed. To determine peak identity, they were collected, run on reverse-phase HPLC, and subjected to mass analysis. Each one had the same retention time on HPLC eluted with aqueous acetonitrile, and coinjection of all gel filtration peaks resulted in one HPLC peak. In addition, coinjection of the fractions on a mass spectrometer showed only the mass of the full-length protein. From these data, it was apparent that the protein was either forming small aggregates or adopting different conformations to elute as multiple peaks. Because a monomeric protein was desired in order to use gel filtration to monitor protein disappearance during aggregation, many different running buffers were explored to find one that supports a monomeric structure. A pH 4 buffer (20 mM NaAc, 200 mM NaCl) analogous to that previously used forNMR(Riek et al. 1996;Donne et al. 1997)was found to be the optimal buffer for analytical gel filtration analysis. To confirm that the protein was present as oligomers in the pH7 buffer, analytical ultracentrifugation (AUC) analysis was employed (Fig. 2 ▶). Sedimentation velocity studies revealed that PrP 23–231 in the phosphate buffer at pH 7 (solid line) exists mostly as a monomer, but dimer (10%) and trimer (3%) were also present. In pH 4 buffer (dotted line), only monomer is detected by sedimentation velocity (>95%), and thus the pH 4 buffer was deemed more suitable for use in the aggregation reactions discussed later, as the starting state of the protein is well defined.
Figure 1.
Gel filtration chromatogram of standards and PrP 23–231. The gel filtration traces show protein standards (dotted line) and PrP 23–231 (solid line) eluted with a mobile phase of 50 mM NaPi, 150 mM NaCl (pH 7). Numbers above each peak correspond to protein standards (1, blue dextran, 2000 kDa; 2, bovine serum albumin, 67 kDa; 3, ovalbumin, 43 kDa; 4, chymotrypsinogen, 25 kDa; 5, ribonuclease A, 13.7 kDa). The molecular weight of PrP 23–231 is 23.1 kDa.
Figure 2.
Sedimentation velocity analysis of PrP 23–231 at pH 7 (solid line) and pH 4 (dotted line). In a buffer of 50 mM NaPi and 150 mM NaCl at pH 7, sedimentation velocity experiments of full-length protein result in three peaks. Arrows above each peak correspond to monomer (1.57 Svedberg), dimer (3.79 Svedberg), and trimer (5.86 Svedberg). The trace of full-length protein in a pH 4 buffer with 50 mM NaAc and 150 mM NaCl shows one peak at 1.85 Svedberg and is composed only of monomer.
In contrast to the full-length protein, gel filtration traces of PrP 90–231 in a pH 7 buffer exhibit a single peak corresponding to that expected of a monomer (Fig. 3A ▶). Sedimentation velocity studies corroborate assignment of the truncated protein asmonomeric atpH7 and 4 (Supplemental Fig. 2 ▶). The truncated form of the protein, which has a smaller mass by about 7 kDa, has an earlier retention time in the pH 4 running buffer relative to PrP 23–231 (Fig. 3B ▶). This behavior suggests that the unstructured N terminus, comprising 67 residues, affects the way that PrP 23–231 is retained on a gel filtration column, as well as influencing the assembly state as a function of pH. Previous experiments with recombinant full-length human PrP corroborate these findings, and aggregation was attributed to a gain of structure in the N terminus at neutral pH (Zahn 2003). These data demonstrate significant differences in the two forms of PrP. Further analysis of the aggregation (see below) also indicates that there are important differences in their aggregation pathways.
Figure 3.

Gel filtration chromatograms of full-length and truncated protein. PrP 23–231 (dotted line) and PrP 90–231 (solid line) are shown. Gel filtration traces in A were collected using a running buffer of 50 mM NaPi, 150 mM NaCl (pH 7). B displays gel filtration traces with a mobile phase of 20 mM NaAc, 200 mM NaCl (pH 4).
Aggregation assay conditions and structural changes
The concentration dependence of the aggregation of both full-length recombinant mouse PrP 23–231 and truncated PrP 90–231 was investigated to gather more information about the transition of PrP to a misassembled state and the role of the N-terminal residues in this process. It is known that the transition of PrP to β-sheet-rich oligomers can be induced by acidification in the presence of chaotropes (Swietnicki et al. 2000; Morillas et al. 2001; Baskakov et al. 2002; Sokolowski et al. 2003). Optimal aggregation conditions were therefore established by measuring the change in PrP β-sheet quaternary structure using CD spectroscopy in buffers with a pH range of 4 to 7 in the presence of GdnHCl (0.25–2 M). Buffers with a pH >5 or concentrations of GdnHCl >1 M elicited structural changes that were complete in<5 min, yet the magnitude of the signal change was small and therefore difficult to analyze. An assay buffer at pH 7 was not desirable for these studies because neutral pH led to oligomerization of PrP 23–231 as described above. The optimal pH 4 aggregation buffer within the conditions explored is 0.5 M GdnHCl, 150 mM NaCl, 50 mM NaAc, 37°C. Under these conditions, PrP aggregation was complete within 1 h at most concentrations of PrP employed. Although these reaction conditions are partially denaturing, they may approximate conditions leading to PrPSc formation. Since PrPC cycles between the cell surface and endosomes, it is exposed to acidic pH environments in which the PrPC-to-PrPSc conversion could occur, albeit on an inconvenient timescale for laboratory studies in the absence of chaotropes (Shyng et al. 1993). Indeed, the cellular location of PrPSc formation is still highly debated. The appropriateness of these aggregation conditions will become clear as more becomes known about the PrPC-to-PrPSc conversion in vivo.
As shown in Figure 4A ▶, the secondary structure of PrP 23–231 before the addition of GdnHCl is typical of an α-helical protein, with minima at 222 and 208 nm in the far-UV CD spectrum. An immediate decrease (within 20 sec) in the α-helical content of the protein was observed once aggregation was initiated (Fig. 4B ▶). After the reaction was complete (3600 sec), the spectrum had a minimum at 215 nm (Fig. 4A ▶, dotted line), corresponding to a protein composed of mainly β-sheet quaternary structure.
Figure 4.

Conformational change of PrP 23–231 monitored by CD. (A) CD spectra of 25 μM PrP before (solid line) and 1 h after (dotted line) addition of GdnHCl. The spectrum before aggregation is typical of a helical protein, with minima at 222 and 208 nm. After PrP polymerization, the spectrum is a characteristic of a β-sheet quaternary structure, with a minimum at 215 nm. (B) After addition of 0.5M GdnHCl, the kinetics of the structural changes were monitored at 222 nm. The decrease in α-helix occurs immediately upon initiation of aggregation.
Electron microscopy was used to confirm the presence of aggregates in the polymerized PrP solution (Supplemental Fig. 3 ▶). Both buffer alone and monomeric protein controls lack negatively stained protein aggregates. Images of the aggregating protein solution were taken at various times, and small negatively stained aggregates are clearly present in the samples subjected to aggregation conditions.
Aggregation of PrP monitored by Tft binding
The aggregation kinetics for six different PrP concentrations (5, 10, 17.5, 25, 37.5, and 50 μM) were monitored by binding of the dye TfT, the fluorescence of which increases upon binding to aggregates with a cross-βsheet structure (LeVine 1999). This includes both fibrils and species with a nonfibrillar morphology (Hurshman et al. 2004; Zhang et al. 2004), although the fluorescence increase is often less with nonfibrillar aggregates. In this assay, TfT is used to measure the formation of nonfibrillar aggregates. Figure 5A ▶ displays the TfT-monitored aggregation traces for PrP 23–231, while Figure 5B ▶ shows those for PrP 90–231. Each trace was truncated at the point where the aggregates become large enough to settle out of solution and the mixtures become heterogeneous. Each assay was allowed to proceed until the fluorescence was constant, and subsequently vortexed before endpoint signal determination to ensure homogeneity. Notably, no lag phase is observed on the timescale of this experiment at any concentration employed. A time-dependent increase in the TfT fluorescence occurs immediately after the addition of 0.5 M GdnHCl. The rate and endpoints observed in the assays are dependent on the concentration of PrP. Both the TfT endpoint amplitude and the initial rate of aggregation increase with increasing protein concentration. The endpoint TfT fluorescence intensities at any given concentration of PrP 90–231 were comparable to those observed for PrP 23–231 (see Table 1).
Figure 5.
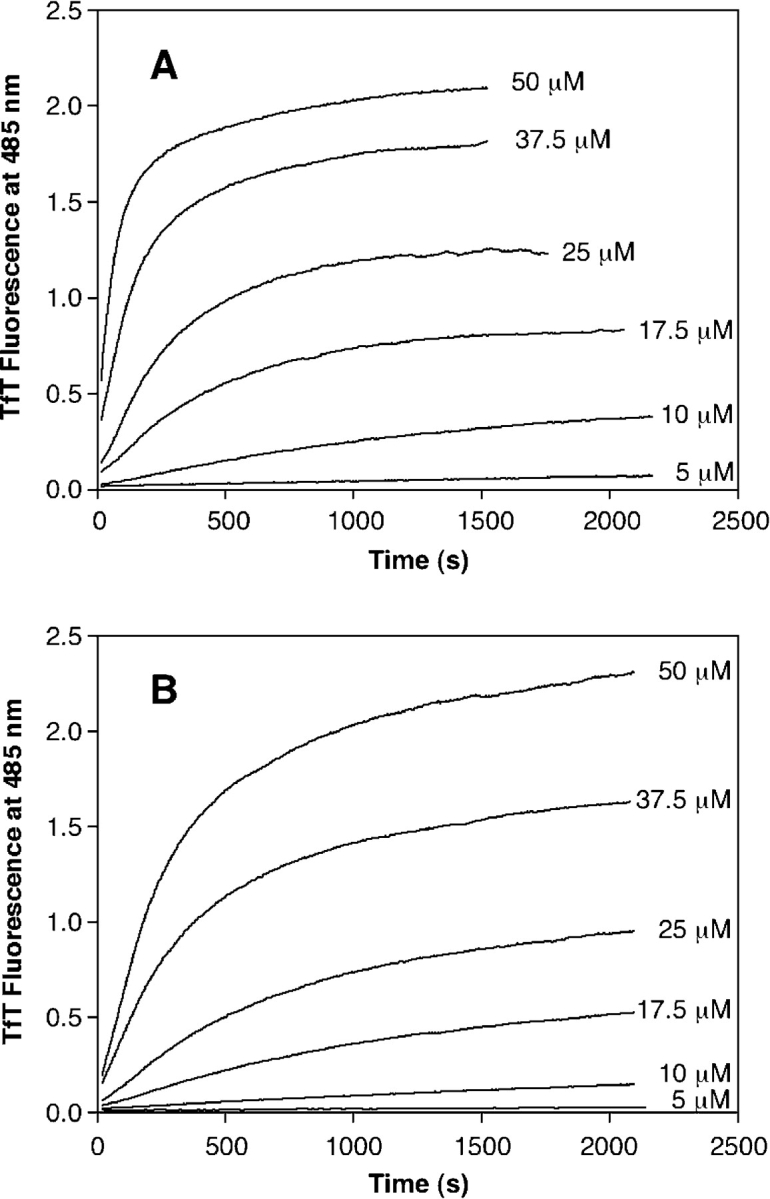
Concentration-dependent PrP aggregation monitored by TfT fluorescence. The aggregation of full-length PrP 23–231 (A) and truncated PrP 90–231 (B) was followed by TfT binding, measured by fluorescence at 485 nm (excitation at 440). GdnHCl was added to different concentrations of PrP, and the increase in signal was followed continuously. Concentrations of protein in micromolars are shown to the right of the trace.
Table 1.
Comparison of TfT and turbidity endpoints
| TfT fluorescence endpoint | Turbidity endpoint | |||
| Conc. (μM) | PrP 23–231 | PrP 90–231 | PrP 23–231 | PrP 90–231 |
| 5 | 0.21 | 0.17 | ||
| 10 | 0.45 | 0.40 | 0.102 | |
| 17.5 | 0.88 | 0.80 | 0.95 | 0.052 |
| 25 | 1.20 | 1.16 | 2.80 | 0.123 |
| 37.5 | 1.81 | 1.78 | 4.80 | 0.334 |
| 50 | 2.12 | 2.06 | 0.574 | |
Shown are aggregation endpoints measured by TfT binding and turbidity for both full-length PrP 23–231 and truncated PrP 90–231. Each aggregation reaction was allowed to proceed until completion before endpoint amplitude measurement.
To ensure that the presence of TfT in the aggregation reactions does not influence polymerization, controls where TfT was added immediately before the fluorescence was measured were conducted using a separate sample for each time point. The increase in TfT fluorescence in these controls closely resembles the continuous assays (Supplemental Fig. 4 ▶), indicating that the presence of TfT throughout the reaction neither accelerates nor slows the aggregation.
Aggregation of PrP followed by gel filtration
To further monitor aggregation of PrP, an analytical gel filtration time course was recorded to follow the disappearance of monomeric protein throughout the aggregation process. A pH 4 elution buffer was employed (20 mM NaAc, 200 mM NaCl) because a neutral pH buffer led to monomer oligomerization as previously described. An aliquot of the reaction solution was filtered through a 0.22-μm filter and then injected onto a high-resolution column at various times during the aggregation reactions, and the resulting peak areas were calculated. Figure 6 ▶ shows sample time courses for 25 μM PrP 23–231 (Fig. 6A ▶) and 50 μM PrP 90–231 (Fig. 6B ▶). Although each protein exhibits a decrease in monomer peak area over time, there are differences in the chromatograms. No soluble aggregate peaks appear in any of the PrP 23–231 assays, and thus only the monomer peak is displayed in Figure 6A ▶. Detection of the eluting peaks by light scattering was also employed to verify the absence of small, soluble oligomers. This technique is more sensitive to oligomers than turbidity, but no aggregates were detected, confirming that they are unable to enter the column. Adsorption of β-sheetrich PrP oligomers to gel filtration columns has been shown to occur with mobile phases lacking denaturants (Baskakov et al. 2004). Although the elution buffer used in this study does not contain denaturants, such adsorption of PrP 23–231 oligomers seems unlikely because no elution peaks are observed after rigorous post-analysis cleaning of the column with 0.5 M NaOH. These results show that the aggregates formed by Prp 23–231 are too large to pass through a 0.22-μm filter, even at the shortest time examined (t=40 sec).
Figure 6.
Gel filtration time courses of PrP monomer disappearance. At various times, aliquots of an aggregating solution of PrP were injected onto an analytical gel filtration column. A displays a monomer disappearance time course of 25 μM PrP 23–231. The peak at 19.4 mL corresponds to monomeric PrP, and decreases over time as the arrow indicates. Samples were injected at 40, 120, 210, 300, 600, 1800, and 3600 sec into the aggregation reaction. The inset shows the same data, plotted as peak area vs. time. B represents the analogous experiment for 50 μM PrP 90–231. The first peak, eluting between 8 and 12 mL, corresponds to a soluble aggregate, which increases over time as the arrow illustrates. The second peak corresponding to monomer, with a retention volume of 18.4 mL, decreases over time as indicated by the arrow. The traces correspond to 40, 150, 225, 300, 600, 1200, and 1800 sec of aggregation time. Shown in the inset are the data plotted as peak area vs. time.
The time course for PrP 90–231, shown in Figure 6B ▶, shows that soluble aggregate peaks are visible in the chromatogram after about 300 sec of aggregation. All concentrations >17.5 μM display these aggregate peaks. The soluble aggregate peak elutes over several minutes, indicating a distribution of oligomers. At lower concentrations this is not observed, likely because the amount of each oligomer is small, and the signal is below the detection limit. These PrP 90–231 aggregates are small enough to pass through the 0.22-μm filter and enter the column, in contrast to those from PrP 23–231.
Aggregation of PrP followed by turbidity
The aggregation reactions were also assessed by turbidity increases monitored at 400 nm. It was necessary to modify the initial PrP concentrations to best utilize the turbidity assays. The lowest protein concentration used in TfTmonitored aggregation, 5 μM, shows no turbidity increases over several hours of reaction for either PrP construct, and the errors in the measurements at this concentration are high. Further analysis showed that the concentrations leading to aggregation that could be accurately followed by turbidity were different for the two PrP constructs. Although a PrP 23–231 concentration of 10 μM exhibited turbidity (Fig. 7A ▶), the same concentration of truncated PrP showed no turbidity increases. Additionally, the highest PrP 23–231 concentration used in the TfT aggregations, 50 μM, exceeded the linear range of the instrument in <2 min, precluding any kinetic analysis. In fact, saturation eventually occurred using concentrations as low as 25 μM, and the most concentrated PrP 23–231 solution that could accurately be followed by turbidity was 37.5 μM. In contrast, the lowest PrP 90–231 concentration for which aggregation could be measured by turbidity was 17.5 μM, and saturation of the instrument was not reached even with 50 μM PrP 90–231 (Fig. 7B ▶).
Figure 7.
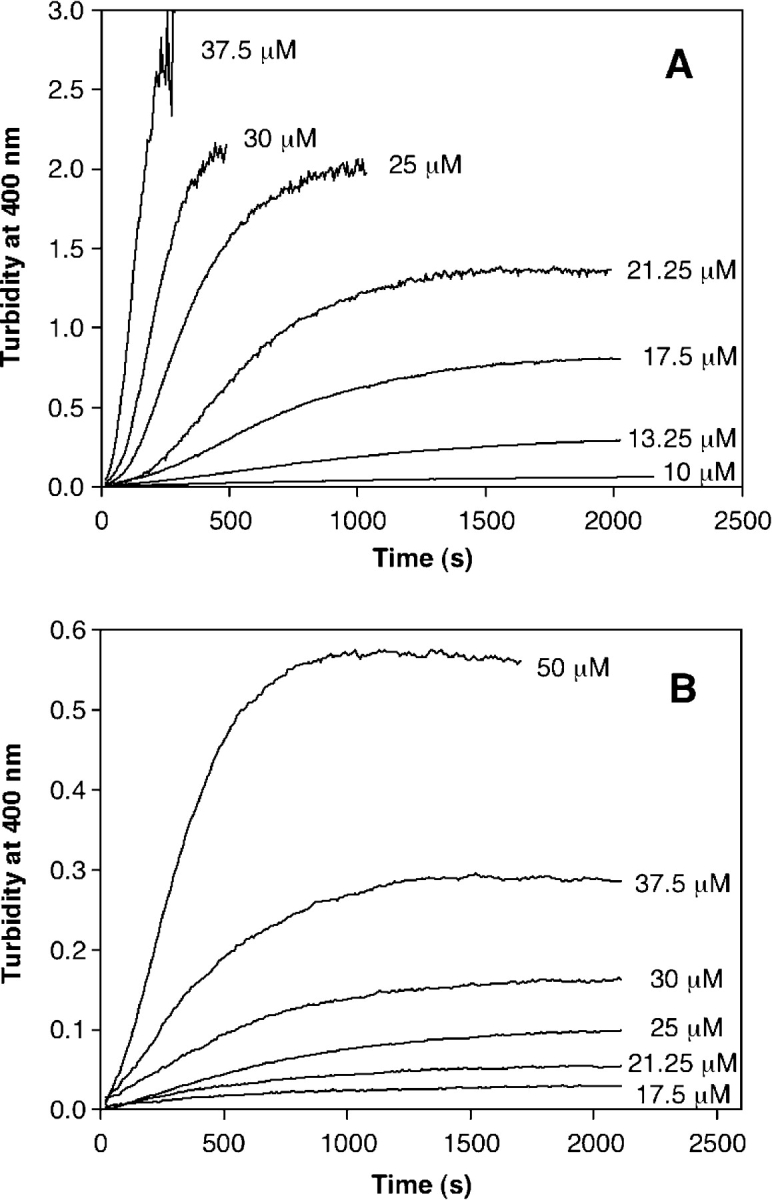
Concentration-dependent PrP aggregation followed by turbidity. The aggregation of full-length PrP 23–231 (A) and truncated PrP 90–231 (B) was followed by turbidity increases at 400 nm. GdnHCl was added to different concentrations of PrP, and the increase in signal was followed as a function of time. Concentrations of protein are shown to the right of the trace.
Clearly, the concentration differences in the turbidity measurements show that there are large disparities in the sizes of the aggregates formed by these proteins, as turbidity readily detects large but not small aggregates.
In contrast to the increase in TfT fluorescence, the turbidity traces of PrP (Fig. 7 ▶) have a sigmoidal shape. We note that this phenomenon is not due to an actual lag phase because there are immediate increases in signal, unlike a typical lag phase, which should show no aggregation initially (Harper and Lansbury 1997). Since turbidity is sensitive to both the size and concentration of aggregates, it is likely that the small amplitude observed at short time points is due to the low turbidity of small aggregates.
A comparison of the turbidity traces of truncated PrP 90–231 in Figure 7B ▶ to the aggregation of PrP 23–231 followed by turbidity in Figure 7A ▶ shows that although the shape of the curves are similar, a lower turbidity signal is obtained even at high PrP 90–231 concentration, implying that the size of the aggregates is smaller, a hypothesis that will be further explored by light-scattering analysis.
Aggregation of PrP followed by batch mode light scattering
To determine the molar masses and hydrodynamic radii of the aggregates formed over time, the aggregation of PrP was followed by light scattering. Comparison of the aggregation of each construct at 10 μMPrP shows that PrP 23– 231 forms aggregates that are about 2.5 times larger than the PrP 90–231 aggregates (Fig. 8A ▶). Additionally, the hydrodynamic radius of the PrP 23–231 endpoint aggregates is ~10 nm larger than PrP 90–231 aggregates (Fig. 8B ▶). Comparison of aggregation at 10 μM was optimal because PrP 23–231 aggregation at higher concentrations eventually saturated the detectors on the instrument, preventing accurate molar mass and radius determinations. Similar to the turbidity experiments, PrP 90–231 aggregation did not saturate the detectors at higher concentrations (up to 25 μM). These data confirm that PrP 23–231 forms larger aggregates than PrP 90–231, even at a concentration as low as 10 μM.
Figure 8.
PrP aggregation followed by batch mode light scattering. The aggregation of 10 μM PrP was measured using static and dynamic light scattering, and the molar mass (A) and hydrodynamic radius (B) were calculated. The PrP construct is labeled on each trace. The endpoint molar mass for PrP 23–231 was about 2.1 × 106 g/mol, and for PrP 90–231, the molar mass at the endpoint (measured at 18 h) was 8.8 × 105 g/mol. The hydrodynamic radius of the PrP 23–231 aggregates reached ~23.3 nm, and the radius of PrP 90–231 aggregates was ~13.6 nm.
Timescales and nucleus sizes for aggregation
Kinetic analysis of aggregating solutions is difficult because of the many reactions occurring concurrently and the distribution of products formed. Kinetic traces at different PrP concentrations utilizing turbidity, TfT fluorescence, and monomer disappearance to monitor the aggregations were analyzed using the time to reach half-maximal signal (t50), shown in Table 2. This parameter allows aggregation reactions monitored by different methods at different protein concentrations to be compared. It also can be used to characterize the mechanism by which aggregation occurs. Plots of the logarithm of t50 against the logarithm of the protein concentration should yield a straight line with a negative slope that becomes steeper as the nucleus size increases; in fact, it has been shown that the slope is equal to −n*/2, where n* is the number of monomers in the nucleus (Goldstein and Stryer 1986).
Table 2.
Timescales of aggregation
| PrP 23–231 t50 (sec) | PrP 90–231 t50 (sec) | |||||
| Conc. (μM) | TfT f1. | Gel filt. | Turbidity | TfT f1. | Gel filt. | Turbidity |
| 5 | 3551 | 3424 | 12091 | 12783 | ||
| 10 | 1003 | 962 | 1707 | 3210 | 4551 | |
| 17.5 | 402 | 351 | 715 | 1195 | 1553 | 1310 |
| 25 | 221 | 169 | 405 | 623 | 797 | 732 |
| 37.5 | 120 | 152 | 250 | 311 | 327 | 420 |
| 50 | 61 | 137 | 187 | 301 | 290 | |
PrP aggregation was monitored by three methods: TfT binding, monomer disappearance by gel filtration, and turbidity. For each method, the t50 of aggregation, or the time to reach half-maximal completion, is displayed for both full-length (PrP 23–231) and truncated (PrP 90–231) protein.
Plots of ln t50 versus ln [PrP 23–231] for both the turbidity and TfT data are shown in Figure 9 ▶. Each group of data generates a straight line, and as explained above, the slope of each line can be related to the nucleus size for the aggregation. The slope of the log– log plot of t50 values from TfT-binding aggregation time courses versus PrP 23–231 concentration is about −1.6, a weak concentration dependence that corresponds to a nucleus size of approximately three monomeric units (Fig. 9A ▶, circles). The log–log plots derived from both the turbidity and gel filtration data have slopes of about −1.5, similar to the TfT reactions (Fig. 9A ▶, squares and triangles). The data for PrP 90–231 are shown in Figure 9B ▶, and the calculated slope for the TfT reactions is about −1.8. Gel filtration data also yield a slope of about −1.8, and the slope of the log–log plot for the turbidity data is about −1.5. These data also exhibit a weak concentration dependence, indicating a small nucleus size of three or perhaps four monomeric units.
Figure 9.
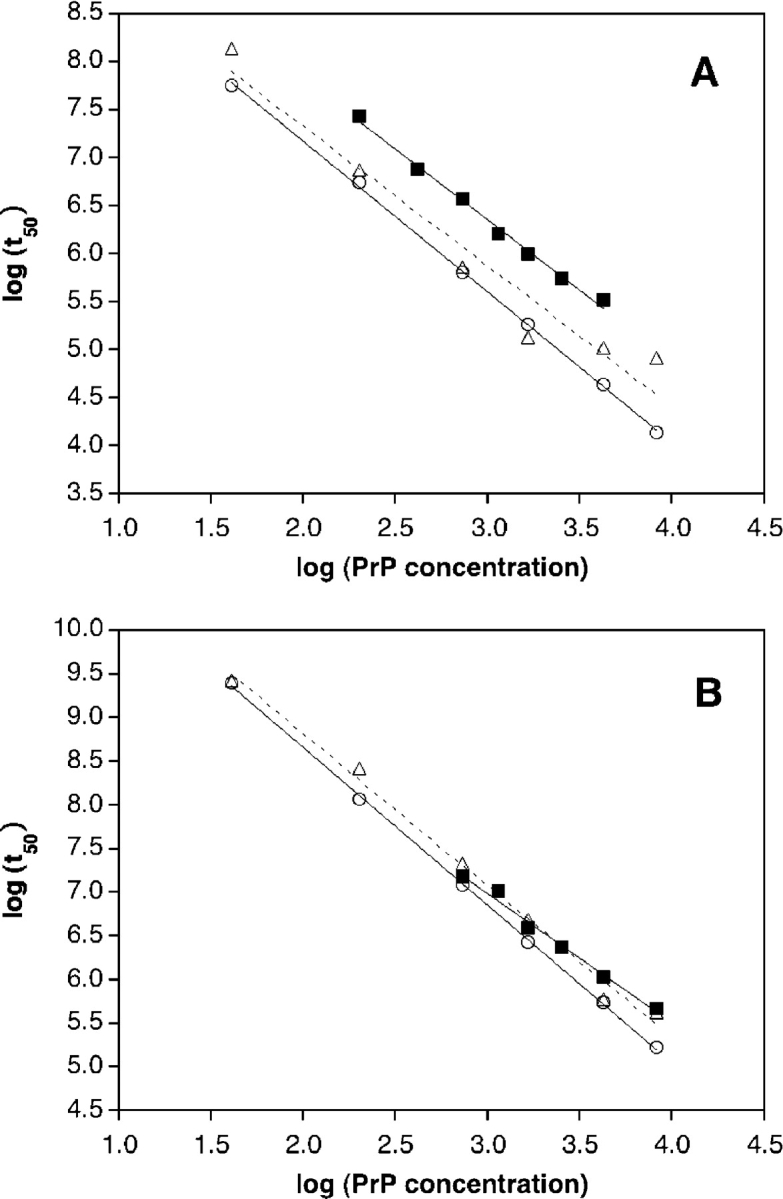
Determination of nucleus sizes. The nucleus size of aggregation can be determined from the slope of the log–log plot of t50 vs. PrP concentration. Shown in A are representative data points from a fulllength PrP 23–231 aggregation experiment, and B plots the data gathered using the PrP 90–231 construct. Data monitored by TfT binding (open circles), monomer disappearance by gel filtration (open triangles), and turbidity (filled squares) are displayed in both plots, and each set of data was fit to a linear function. The curves for TfT fluorescence and turbidity are shown as solid lines, and the curve corresponding to the gel filtration data is displayed as a dotted line. For full-length PrP 23–231, the slope of the plots are TfT fluorescence, −1.57; gel filtration, −1.46; and turbidity, −1.47. The truncated PrP 90–231 slopes are as follows: TfT fluorescence, −1.80; gel filtration, −1.75; and turbidity, −1.49.
The apparent reaction order of the aggregation of PrP 23–231 and 90–231 can be determined from the TfT fluorescence data in Figure 5 ▶ by plotting the logarithm of the initial rate at each protein concentration against the logarithm of the protein concentration and determining the slope of the line of best fit (Baskakov and Bocharova 2005). This procedure yields apparent reaction orders of 2.8 and 3.2 for PrP 23–231 and PrP 90– 231, respectively (data not shown). The concept of a reaction order for nucleated polymerization reactions is not necessarily as clear as it is for simpler reactions (for example, dimerization). Nevertheless, it is reassuring that the apparent reaction orders determined for PrP 23–231 and PrP 90–231 are consistent with the nucleus sizes determined from the ln t50 versus ln [PrP] plots.
Seeding with PrP
To assess the susceptibility of PrP 23–231 to seeding, preformed aggregates were added to reaction solutions. Since there is no observable lag phase, the t50 values were used to determine seeding efficiency. The calculated t50 values in the seeded reactions were found to be similar to those obtained without the addition of seeds. Different amounts of seeds (from 1% to 10% w/w) were added to the reactions, as well as seeds formed using different reaction times ranging from 100 to 3600 sec). None of the conditions employed for seed formation resulted in a significant change in the t50 values, suggesting an inability to seed these reactions.
Discussion
Differences in the behavior of the full-length PrP 23–231 construct and the truncated PrP 90–231 construct are immediately apparent from their oligomerization states at pH 7; PrP exists as a mixture of monomers, dimers, and trimers under these conditions, while PrP 90–231 is monomeric. This observation is consistent with the finding that the N-terminal residues in recombinant human PrP 23–230 mediate oligomerization at neutral pHs (Zahn 2003). The differences between the two constructs become even more pronounced upon examining the results described above regarding the pathways by which they aggregate.
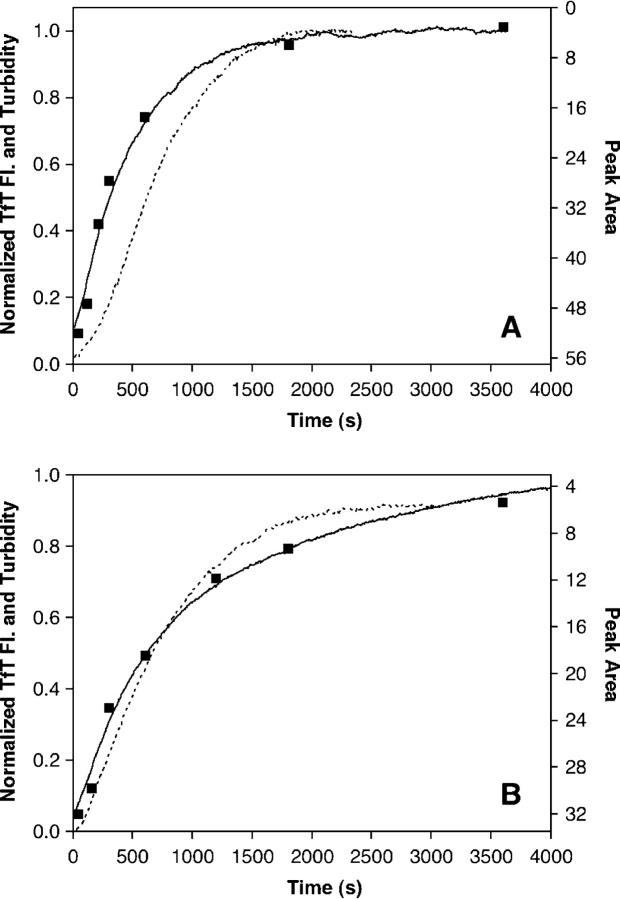
Aggregation by PrP 23–231 occurs in two overlapping stages. In the first stage, TfT-positive aggregates are produced as monomeric protein is depleted. The aggregates formed during this stage are too large to pass through a 0.22-μm filter, but the turbidity increases very little during the beginning of this stage (Fig. 10A ▶). These observations are consistent with the existence of a few large aggregates early in the first stage of the aggregation process. Based on the slope of ln t50 versus ln [PrP 23–231] plots and the apparent reaction order, the first stage of aggregation appears to occur by a nucleated polymerization in which the nucleus is a trimer. Such a small nucleus suggests that nucleation does not present a high energetic barrier to be overcome. Consistent with this assertion, seeding does not accelerate PrP 23–231 aggregation. The first stage ends when the monomer is depleted, but as it approaches completion, a second stage begins. In the second stage, existing aggregates become larger, signaled by a dramatic increase in turbidity. By the end of the second stage, the assay solutions are visibly cloudy, consistent with the formation of very large aggregates. This observation suggests that the second stage involves the direct association of aggregates to form larger aggregates, since aggregates large enough to precipitate are unlikely to form on the observed timescale by monomer addition steps alone. The t50 values for the aggregation process measured by turbidity are about three times larger than those measured by monomer depletion or TfT fluorescence (see Table 2), but they depend on protein concentration in the same way (slope of ln t50 vs. ln [PrP 23– 231]= −1.5 for turbidity, −1.6 for TfT fluorescence, and −1.5 for monomer depletion). This indicates that the nucleation step exerts some control over the kinetics of the second stage as well as the first.
Figure 10.
Twenty-five micromolar PrP aggregation followed by TfT binding, monomer disappearance, and turbidity. A displays plots of aggregation for PrP 23–231, and B displays aggregation data for PrP 90–231. Both turbidity and TfT-binding traces were normalized to a value of 1 and correspond to the Y-axis on the left, while the Y-axis on the right corresponds to the peak area calculated from gel filtration (not normalized). The peak area axis is inverted such that the monomer disappearance data overlays with data from the other methods used. The solid line displays aggregation monitored by TfT binding, the dotted line corresponds to turbidity, and the square dots represent the gel filtration monomer disappearance data.
Aggregation by PrP 90–231 follows a different pathway than aggregation by PrP 23–231. Monomer depletion and increases in TfT fluorescence occur more slowly (by a factor of about three) for PrP 90– 231 than for PrP 23–231 (Table 2), yet the same amounts of TfT-positive aggregates are formed by both proteins, as indicated by endpoint fluorescence (Table 1). The process detected by these techniques appears to be a nucleated polymerization with a trimeric nucleus, like that observed in the first stage of aggregation by PrP 23–231. However, with PrP 90– 231, either there is no second stage or it is very inefficient. Instead, smaller increases in turbidity are observed that occur on a similar timescale to monomer depletion or increases in TfT fluorescence (Fig. 10B ▶). The concentration dependence of the t50 values measured by turbidity is similar to those measured by monomer depletion or TfT fluorescence (see Fig. 9B ▶). The final turbidity values reached in the aggregation of PrP 90–231 are smaller than those reached in the aggregation of PrP 23–231 (see Table 1), and the solutions of PrP 90–231 aggregates never become visibly cloudy. These observations, along with molecular weight and hydrodynamic radius data obtained from light scattering (see Fig. 8 ▶), indicate that aggregates formed by PrP 90–231 are much smaller than those formed by PrP 23–231, and that direct association of aggregates does not occur or occurs very slowly. This interpretation is also consistent with the observation of soluble aggregates by gel filtration during the aggregation of PrP 90–231.
The differences in the aggregation pathways of PrP 23–231 and PrP 90–231 can plausibly be attributed to the unstructured N-terminal tail that is present in PrP 23–231 but not in PrP 90–231. For example, interactions mediated by the N terminus between PrP monomers or a PrP monomer and an aggregate could easily be responsible for the faster aggregation rate of PrP 23–231. Furthermore, it has been shown that the N terminus of PrP 23–231 is susceptible to proteolysis, which suggests that it is at or near the surface of PrP aggregates. This location would enable exposed N termini in a PrP aggregate to mediate interactions between aggregates, explaining the existence of a second phase of aggregation for PrP 23–231 but not for PrP 90–231.
It is instructive to compare the results described above with previous work focusing on the aggregation of fulllength or truncated PrP constructs. We have found that both PrP 23–231 and 90–231 form aggregates that are rich in β-sheet structure, but are not mature amyloid fibrils. This is consistent with the observation of Baskakov and coworkers (Baskakov et al. 2002) and Bocharova and coworkers (Bocharova et al. 2005) that β-sheet-rich oligomers, which they have termed β-oligomers, are formed by both truncated and full-length PrP constructs at pH values around 4.0. The oligomers observed in this study, however, aremuch larger than β-oligomers. The light-scattering data in Figure 8 ▶ show that the aggregates formed by PrP 90–231 under our conditions have an average molar mass of 8.8 × 105 g/mol at the endpoint of the aggregation reaction, consistent with an oligomer made up of ~50 subunits. The aggregates formed by PrP 23–231 are even larger (2.1 × 106 g/mol). In contrast, β-oligomers are reported to be octameric (Baskakov et al. 2002). The endpoint sizes of the aggregates formed by PrP 90–231 in this study are consistent with the endpoint sizes found by Sokolowski and coworkers (Sokolowski et al. 2003) with Syrian hamster PrP 90–232. In addition, the slopes of the plots of ln t50 versus ln [PrP 90–231] fromthe TfT, gel filtration, and turbidity data (−1.8, −1.8, and −1.5, respectively) found herein are similar to that (−2.0) determined by Sokolowski and coworkers (Sokolowski et al. 2003) from light-scattering data. This similarity suggests that the mechanism of aggregation in both cases should be similar. Sokolowski and coworkers (Sokolowski et al. 2003) have suggested that their construct of PrP aggregates via “critical oligomers,” i.e., oligomers that self-associate to form larger aggregates. These critical oligomers, like the β-oligomers described above, appear to be octameric. While we have not presented any direct evidence for the existence of critical oligomers, our data are not inconsistent with this mechanism. If the PrP constructs described herein aggregate through critical oligomers, then our results could be interpreted as follows: (1) The first stage of PrP 23–231 aggregation (and the only stage of PrP 90–231 aggregation) involves the formation of critical oligomers; (2) the formation of critical oligomers by both PrP 23–231 and 90–231 proceeds by a process that involves a particularly unstable trimer, which corresponds to a nucleus; (3) the N-terminal domain that is included in PrP 23–231 causes critical oligomers formed by this PrP construct to have much higher self-affinity, which in turn causes large aggregates to form rapidly; and (4) finally, Baskakov and coworkers (2002) have shown that β-oligomers are not on the pathway to mature amyloid fibrils. Assuming that the critical oligomers identified by Sokolowski and coworkers (2003) are β-oligomers, this suggests that the β-sheetrich aggregates observed by us in this study and by Sokolowski are not on the pathway to fibril formation. The role that fibrillar and nonfibrillar aggregates play in prion and amyloid diseases is under debate (Caughey and Lansbury 2003) and is beyond the scope of this study. It seems likely, however, that both species could be important in disease pathogenesis, perhaps through different mechanisms.
In conclusion, by whatever pathway PrP aggregates, its N-terminal domain has a clear effect on its aggregation kinetics and the properties (in particular, the size) of the aggregates formed. These effects could have physiological significance; indeed, it is known that mice expressing N-terminally truncated PrP develop disease more slowly and have lower prion titers than mice expressing full-length PrP (Flechsig et al. 2000). These results suggest that studying N-terminally truncated PrP constructs could lead to erroneous correlations between in vitro and in vivo observations if aggregation of the full-length protein is prominent in neurodegeneration. Our results emphasize the importance of studying fulllength protein constructs whenever possible.
Materials and methods
Expression and purification of PrP
The expression and purification of mouse PrP 23–231 and PrP 90–231 was carried out as previously described (Zahn et al. 1997). The protein was stored in 10 mM sodium acetate (NaAc) (pH 5.0), at 4°C.
Aggregation assays
Aggregation reactions were initiated by adding GdnHCl (0.5M final concentration) to a solution of PrP (final concentration between 5 and 65 μM) in 50 mM NaAc, 150 mM NaCl (pH 4). All assays were performed at 37°C and each solution was equilibrated to 37°C before mixing. Assay volumes were 200 μL unless otherwise noted. After the initial mixing, assays were left stagnant, without stirring or agitation. The fluorescence, turbidity, or CD signal was monitored as quickly as possible, which was usually 20 sec after the addition of GdnHCl. Each assay was allowed to proceed until completion (1 h for most reactions), but the endpoint of aggregation reactions containing lower concentrations of PrP was determined at 24 h. Beyond 24 h, no further aggregation took place for any concentration examined. Before determination of the final signal amplitude of each reaction, the assays were gently vortexed to ensure homogeneity. For accuracy, aggregation solutions with signal amplitude that saturated the instrument were diluted with assay buffer immediately prior to measurement of the endpoint.
CD
Aggregation assays were monitored by an AVIV 202-SF CD spectrometer (AVIV Instruments). Cells with a path length of 0.2 cm were used, and assay volumes were 600 μL. Wavelength scans both preceding and following the aggregation assays were collected every 0.5nmfrom 200 to 250 nm. To follow aggregation over time, the ellipticity was measured at 222 nm every 10 sec for 1 h.
TfT binding and fluorescence
Aggregation reactions were prepared as described above except that TfT (50 μM final concentration) was added to the protein solution prior to GdnHCl addition. The increase in TfT fluorescence was monitored on an AVIV ATF-105 fluorometer (AVIV Instruments) with excitation at 440 nm (2-nm bandwidth) and emission at 485 nm (6-nm bandwidth). Data points were collected every 5 sec for 1 h, or every 10 sec for 2 h for the lower concentrations of protein. Buffer control data without PrP was collected, and traces for every protein concentration were corrected by subtracting the control data.
Turbidity
Increases in turbidity at 400 nm were monitored on an HP 8453 UV-vis spectrophotometer (Agilent Technologies). Data were collected continuously every 6 sec for 1 h, except for the lowest PrP concentration assays, where data were monitored for up to 2 h.
Analytical gel filtration
Aliquots of aggregation assays (100 μL) at various time points were analyzed by analytical gel filtration at 25°C on a Superdex 200 10/300 GL column (10 × 300 mm, 13 μm; Amersham Biosciences) with detection at 280 nm. The pH 7 mobile phase used in the initial experiments and for the protein standards (0.2 mg/mL each) consisted of 50 mM sodium phosphate (NaPi) and 150 mM NaCl. The running buffer used for the aggregation experiments was 20 mM NaAc, 200 mM NaCl, at pH 4. PrP aggregation samples were filtered through 0.22-μm PVDF syringe filters before injection. The first time point monitored after initiation of aggregation was 40 sec.
Light scattering
Analytical gel filtration in conjunction with dynamic and static light scattering was also employed to monitor the aggregation reactions using a DAWN EOS model light-scattering photometer with Wyatt QELS (Wyatt Technology) with 18 angles of detection. Molar mass and radius determinations were performed using the ASTRA software package (Wyatt Technology). A dn/dc value of 0.185 mL/g was used for all measurements, and toluene was employed for calibration of the instrument. For flow mode experiments, the assays were completed in the same manner as those described above for gel filtration, except all running buffers contained 0.02% sodium azide to prevent bacterial growth and the column was connected in-line to the lightscattering photometer. The molar mass and hydrodynamic radius were calculated for each eluted peak.
Batch mode experiments were performed in the same manner as described above for the turbidity experiments, except all solutions were filtered with inorganic 0.02-μm filters (Whatman). The aggregation reactions were performed in scintillation vials with a reaction volume of 3.2 mL. Data were collected continuously, and molar mass and hydrodynamic radius were calculated over the entire length of the reaction with the ASTRA software package (Wyatt Technology).
AUC
Analyses of PrP 23–231 and PrP 90–231 were performed on a Beckman XL-I analytical ultracentrifuge with interference and absorbance optics. Sedimentation velocity experiments of each protein at different pH values were performed at 50,000 rpm for 4 h. Data analysis was performed by Sedfit program using a c(s) analysis. The proteins were either buffered by 50 mM NaPi, 150 mM NaCl (pH 7), or 50 mM NaAc, 150 mM NaCl (pH 4).
HPLC
Fractions of PrP 23–231 from the analytical gel filtration experiments were analyzed by HPLC. The column was a Beta- Basic-C18 reverse-phase column (3 μm, 4.6 × 30 mm; Thermo Hypersil-Keystone). The HPLC consisted of a Waters 600 pump and 486 detector measuring absorbance at 280 nm. The mobile phase was a gradient of 0%–100% B over 9 min with a flow rate of 1.25 mL/min (A: 0.05% trifluoroacetic acid in water; B: 0.05% trifluoroacetic acid in acetonitrile). Peaks collected from gel filtration were directly injected onto the HPLC column and retention times compared.
Mass spectrometry
Peaks from analytical gel filtration were injected into an Agilent MSD 1100 electrospray LC/MS, and the mass spectrum was measured.
Transmission electron microscopy
Carbon-coated copper grids (Electron Microscopy Services) were placed onto 20-μL aliquots of aggregation reactions and allowed to incubate for 2 min. Following the removal of excess liquid from the grid, the grids were washed two times with water and stained for 1 min with aqueous uranyl acetate (2% w/v). Excess stain was removed by absorption into filter paper. Controls of buffer alone (0.5 mM GdnHCl, 50 mM NaAc, 150 mM NaCl [pH 4]) and monomeric protein (10 μM PrP in 10 mM NaAc [pH 5.0]) were prepared in the same manner as above. Samples were visualized with a Phillips CM-100 transmission electron microscope with an accelerating voltage of 100 kV.
Seeding experiments
PrP aggregation assays that had progressed for various times were added to fresh aggregation reactions to assess their seeding ability. The seeded reactions were identical to the polymerization assays described above, except that a range of seed concentrations were added immediately before the addition of GdnHCl. The total concentration of PrP was identical to the unseeded assays, but a small amount of the protein in the final assay originated from the seed solution.
Acknowledgments
We thank Ted Foss for obtaining the sedimentation velocity data.We also thank Prof. Anthony Williamson for the generous gift of the protein plasmids and Dr. Laura Solforosi for initial help with protein purification. This research was supported by NIH grant AG004342, the Skaggs Institute for Chemical Biology, and the Lita Annenberg Hazen Foundation.
Abbreviations
PrP, prion protein
PrPC, cellular form of the prion protein
PrPSc, scrapie/infective form of the prion protein
PrP 23–231, full-length recombinant mouse prion protein
PrP 90–231, truncated recombinant mouse prion protein
CJD, Creutzfeldt-Jakob disease
GSS, Gerstmann-Sträussler syndrome
FFI, fatal familial insomnia
BSE, bovine spongiform encephalopathy
GPI, glycosyl phosphatidylinositol
GdnHCl, guanidine hydrochloride
TfT, thioflavin T
CD, circular dichroism
AUC, analytical ultracentrifugation
n*, nucleus size
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051434005.
Supplemental material: see www.proteinscience.org
References
- Baskakov, I.V. 2004. Autocatalytic conversion of recombinant prion proteins displays a species barrier. J. Biol. Chem. 279 7671–7677. [DOI] [PubMed] [Google Scholar]
- Baskakov, I.V. and Bocharova, O.V. 2005. In vitro conversion of mammalian prion protein into amyloid fibrils displays unusual features. Biochemistry 44 2339–2348. [DOI] [PubMed] [Google Scholar]
- Baskakov, I.V., Legname, G., Prusiner, S.B., and Cohen, F.E. 2001. Folding of prion protein to its native α-helical conformation is under kinetic control. J. Biol. Chem. 276 19687–19690. [DOI] [PubMed] [Google Scholar]
- Baskakov, I.V., Legname, G., Baldwin, M.A., Prusiner, S.B., and Cohen, F.E. 2002. Pathway complexity of prion protein assembly into amyloid. J. Biol. Chem. 277 21140–21148. [DOI] [PubMed] [Google Scholar]
- Baskakov, I.V., Legname, G., Gryczynski, Z., and Prusiner, S.B. 2004. The peculiar nature of unfolding of the human prion protein. Protein Sci. 13 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharova, O.V., Breydo, L., Parfenov, A.S., Salnikov, V.V., and Baskakov, I.V. 2005. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrPSc. J. Mol. Biol. 346 645–659. [DOI] [PubMed] [Google Scholar]
- Brown, D.R., Qin, K., Herms, J.W., Madlung, A., Manson, J., Strome, R., Fraser, P.E., Kruck, T., von Bohlen, A., Schulz-Schaeffer, W., et al. 1997. The cellular prion protein binds copper in vivo. Nature 390 684–687. [DOI] [PubMed] [Google Scholar]
- Bruce, M.E., Will, R.G., Ironside, J.W., McConnell, I., Drummond, D., Suttie, A., McCardle, L., Chree, A., Hope, J., Birkett, C., et al. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389 498–501. [DOI] [PubMed] [Google Scholar]
- Caughey, B. and Lansbury, P.T. 2003. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26 267–298. [DOI] [PubMed] [Google Scholar]
- Caughey, B.W., Dong, A., Bhat, K.S., Ernst, D., Hayes, S.F., and Caughey, W.S. 1991. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30 7672–7680. [DOI] [PubMed] [Google Scholar]
- Collinge, J. 2001. Prion diseases of humans and animals: Their causes and molecular basis. Annu. Rev. Neurosci. 24 519–550. [DOI] [PubMed] [Google Scholar]
- Donne, D.G., Viles, J.H., Groth, D., Mehlhorn, I., James, T.L., Cohen, F.E., Prusiner, S.B., Wright, P.E., and Dyson, H.J. 1997. Structure of the recombinant full-length hamster prion protein PrP(29–231): The N terminus is highly flexible. Proc. Natl. Acad. Sci. 94 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone, F. 1999. Analysis of protein aggregation kinetics. Methods Enzymol. 309 256–274. [DOI] [PubMed] [Google Scholar]
- Flechsig, E., Shmerling, D., Hegyi, I., Raeber, A.J., Fischer, M., Cozzio, A., von Mering, C., Aguzzi, A., and Weissmann, C. 2000. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron 27 399–408. [DOI] [PubMed] [Google Scholar]
- Goldstein, R.F. and Stryer, L. 1986. Cooperative polymerization reactions. Analytical approximations, numerical examples, and experimental strategy. Biophys. J. 50 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi, T., Fisher, S., Olofsson, S., Endo, T., Groth, D., Tarentino, A., Borchelt, D.R., Teplow, D., Hood, L., and Burlingame, A. 1989. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch. Biochem. Biophys. 274 1–13. [DOI] [PubMed] [Google Scholar]
- Harper, J.D. and Lansbury Jr., P.T. 1997. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66 385–407. [DOI] [PubMed] [Google Scholar]
- Hornemann, S. and Glockshuber, R. 1998. A scrapie-like unfolding intermediate of the prion protein domain PrP(121–231) induced by acidic pH. Proc. Natl. Acad. Sci. 95 6010–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurshman, A.R., White, J.T., Powers, E.T., and Kelly, J.W. 2004. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry 43 7365–7381. [DOI] [PubMed] [Google Scholar]
- Jarrett, J.T. and Lansbury Jr., P.T. 1993. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73 1055–1058. [DOI] [PubMed] [Google Scholar]
- Lawson, V.A., Priola, S.A., Wehrly, K., and Chesebro, B. 2001. N-terminal truncation of prion protein affects both formation and conformation of abnormal protease-resistant prion protein generated in vitro. J. Biol. Chem. 276 35265–35271. [DOI] [PubMed] [Google Scholar]
- Lawson, V.A., Priola, S.A., Meade-White, K., Lawson, M., and Chesebro, B. 2004. Flexible N-terminal region of prion protein influences conformation of protease-resistant prion protein isoforms associated with cross-species scrapie infection in vivo and in vitro. J. Biol. Chem. 279 13689–13695. [DOI] [PubMed] [Google Scholar]
- LeVine 3rd, H. 1999. Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 309 274–284. [DOI] [PubMed] [Google Scholar]
- Liu, H., Farr-Jones, S., Ulyanov, N.B., Llinas, M., Marqusee, S., Groth, D., Cohen, F.E., Prusiner, S.B., and James, T.L. 1999. Solution structure of Syrian hamster prion protein rPrP(90–231). Biochemistry 38 5362–5377. [DOI] [PubMed] [Google Scholar]
- Meyer, R.K., McKinley, M.P., Bowman, K.A., Braunfeld, M.B., Barry, R.A., and Prusiner, S.B. 1986. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. 83 2310–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillas, M., Vanik, D.L., and Surewicz, W.K. 2001. On the mechanism of α-helix to β-sheet transition in the recombinant prion protein. Biochemistry 40 6982–6987. [DOI] [PubMed] [Google Scholar]
- Oesch, B.,Westaway, D.,Walchli,M., McKinley,M.P., Kent, S.B., Aebersold, R., Barry, R.A., Tempst, P., Teplow, D.B., Hood, L.E., et al. 1985. A cellular gene encodes scrapie PrP 27–30 protein. Cell 40 735–746. [DOI] [PubMed] [Google Scholar]
- Pan, K.M., Baldwin, M., Nguyen, J., Gasset, M., Serban, A., Groth, D., Mehlhorn, I., Huang, Z., Fletterick, R.J., Cohen, F.E., et al. 1993. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. 90 10962–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S.B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216 136–144. [DOI] [PubMed] [Google Scholar]
- ———. 1998. Prions. Proc. Natl. Acad. Sci. 95 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek, R., Hornemann, S., Wider, G., Billeter, M., Glockshuber, R., and Wuthrich, K. 1996. NMR structure of the mouse prion protein domain PrP(121–321). Nature 382 180–182. [DOI] [PubMed] [Google Scholar]
- Shyng, S.L., Huber, M.T., and Harris, D.A. 1993. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem. 268 15922–15928. [PubMed] [Google Scholar]
- Sokolowski, F., Modler, A.J., Masuch, R., Zirwer, D., Baier, M., Lutsch, G., Moss, D.A., Gast, K., and Naumann, D. 2003. Formation of critical oligomers is a key event during conformational transition of recombinant Syrian hamster prion protein. J. Biol. Chem. 278 40481–40492. [DOI] [PubMed] [Google Scholar]
- Stahl, N., Borchelt, D.R., Hsiao, K., and Prusiner, S.B. 1987. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51 229–240. [DOI] [PubMed] [Google Scholar]
- Stahl, N., Baldwin, M.A., Teplow, D.B., Hood, L., Gibson, B.W., Burlingame, A.L., and Prusiner, S.B. 1993. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 32 1991–2002. [DOI] [PubMed] [Google Scholar]
- Swietnicki, W., Petersen, R., Gambetti, P., and Surewicz, W.K. 1997. pH-dependent stability and conformation of the recombinant human prion protein PrP(90–231). J. Biol. Chem. 272 27517–27520. [DOI] [PubMed] [Google Scholar]
- Swietnicki, W., Morillas, M., Chen, S.G., Gambetti, P., and Surewicz, W.K. 2000. Aggregation and fibrillization of the recombinant human prion protein huPrP90–231. Biochemistry 39 424–431. [DOI] [PubMed] [Google Scholar]
- Weissmann, C. 1996. The Ninth Datta Lecture. Molecular biology of transmissible spongiform encephalopathies. FEBS Lett. 389 3–11. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Molecular genetics of transmissible spongiform encephalopathies. J. Biol. Chem. 274 3–6. [DOI] [PubMed] [Google Scholar]
- Wildegger, G., Liemann, S., and Glockshuber, R. 1999. Extremely rapid folding of the C-terminal domain of the prion protein without kinetic intermediates. Nat. Struct. Biol. 6 550–553. [DOI] [PubMed] [Google Scholar]
- Zahn, R. 2003. The octapeptide repeats in mammalian prion protein constitute a pH-dependent folding and aggregation site. J. Mol. Biol. 334 477–488. [DOI] [PubMed] [Google Scholar]
- Zahn, R., von Schroetter, C., and Wuthrich, K. 1997. Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS Lett. 417 400–404. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Stockel, J., Mehlhorn, I., Groth, D., Baldwin, M.A., Prusiner, S.B., James, T.L., and Cohen, F.E. 1997. Physical studies of conformational plasticity in a recombinant prion protein. Biochemistry 36 3543–3553. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Powers, E.T., Nieva, J., Huff, M.E., Dendle, M.A., Bieschke, J., Glabe, C.G., Eschenmoser, A., Wentworth Jr., P., Lerner, R.A., et al. 2004. Metabolite-initiated protein misfolding may trigger Alzheimer’s disease. Proc. Natl. Acad. Sci. 101 4752–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]