Abstract
The human adenosine A2A receptor (A2AR) belongs to one of the largest family of membrane proteins, the G-protein coupled receptors (GPCRs), characterized by seven transmembrane (TM) helices. Little is known about the determinants of their structures, folding, assembly, activation mechanisms, and oligomeric states. Previous studies in our group showed that peptides corresponding to all seven TM domains form stable helical structures in detergent micelles and lipid vesicles. However, the peptides behave differently; TM5 is the only peptide to have a ratio [θ]222/[θ]208 obtained by circular dichroism (CD) spectroscopy>1. This finding suggested to us that TM5 might self-associate. In the present study, we investigate the unique properties of the TM5 domain. We performed detailed analyses of TM5 peptide behavior in membrane-mimetic environments using CD spectroscopy, fluorescence spectroscopy and Förster resonance energy transfer, and gel electrophoresis. We find that TM5 peptide has the ability to self-associate to form oligomeric structures in various hydrophobic milieus and that these oligomers are highly resistant to temperature and chemical denaturation. We also find that mutation of the full-length A2AR at position M193, which is located in the fifth TM domain, noticeably alters A2AR monomer: dimer ratio as observed on SDS-PAGE. Our results suggest that parallel association of TM5 dimers may play a role in the known adenosine A2A receptor dimerization. This study represents the first evidence of an individual GPCR transmembrane domain self-association.
Keywords: membrane proteins, GPCR dimerization, helix association, transmembrane peptide, FRET
The human adenosine A2A receptor (A2AR) is a member of the G-protein coupled receptor (GPCR) super family. GPCRs are integral membrane proteins characterized by seven transmembrane (TM)helices thatmediate a plethora of cellular signals across the plasma membrane via coupling to G-proteins. They modulate many physiological processes and are linked to numerous human diseases (Shichida and Imai 1998; Gether 2000; Gurrath 2001), and consequently, are the targets of an increasingly large number of drugs (Gurrath 2001). Until recently, GPCRs were believed to work as monomeric entities, activating G proteins in a 1:1 stoichiometric ratio. However, this classical model of coupling may be oversimplified, since a large body of evidence has shown that many GPCRs exist as homodimers, heterodimers, or even as higher order oligomers (Jones et al. 1998; Jordan and Devi 1999; Bai 2004; Fotiadis et al. 2004). Recent studies have also demonstrated that oligomerization has important effects on GPCRs’ functions, including ligand binding, receptor activation, desensitization, and trafficking, as well as receptor signaling (Rios et al. 2001; George et al. 2002; Breitwieser 2004).
For other receptors, such as tyrosine-kinase and steroid- hormone receptors, constitutive and ligand-induced oligomerization have long been known and documented (Schlessinger 2000), but the mechanisms of intermolecular interaction remain unclear. However, recent work has indicated that the TM helices may be the contact interfaces for receptor–receptor interaction. In some cases, glycine residues appear to be important for TM helix oligomerization. Initially, Lemmon et al. (1994) identified a seven-residue motif (LIxxGVxxGVxxT) responsible for the specific homodimerization of the TM helices of a bitopic membrane protein; glycophorin A (GpA). The combined results of site-directed mutagenesis (Lemmon et al. 1992), and solution NMR of the same TM helix dimer confirmed the location of the GxxxG motif at the dimer interface and revealed that it is stabilized by formation of favorable van der Waals surface hydrophobic interactions (MacKenzie et al. 1997).
The effect of the GxxxG motif on oligomerization has mainly been identified and characterized in other bitopic helical membrane proteins and in some multispan integral membrane proteins as well (Senes et al. 2004). GxxxG-like motifs exist in GPCR TM domains; however, their direct roles in oligomerization remain unclear. It has been determined that a GxxxG motif present in TM1 of the yeast α-factor receptor (Ste2p) is essential for oligomerization (Overton et al. 2003). However, these motifs in TM2 and TM6 of α1b-adrenergic receptor (Stanasila et al. 2003), and TM6 of β2-adrenergic receptor (Salahpour et al. 2004), do not seem to be involved in homodimerization. Also, TM6 from the Ste2p receptor, which does not contain the GxxxG motif, has been proposed to be involved in receptor oligomerization because it was found to self-aggregate and to interact with other domains using SDS-PAGE analysis (Xie et al. 2000).
In addition, it was recently demonstrated that the GxxxG motif is neither necessary nor sufficient for GpA dimerization (Doura and Fleming 2004). It has been suggested that while the presence of a (small)xxx(small) motif is a useful clue, it does not prove the existence of an interaction; the significance of GxxxG for mediating interactions must be tested in each specific case (Schneider and Engelman 2004).
Furthermore, other motifs have been shown to affect oligomerization of membrane proteins. Gurezka and coworkers (Gurezka et al. 1999) have identified a heptad motif of leucine residues, which appears to mediate homo-oligomerization of a set of transmembrane proteins. SxxSSxxT and SxxxSSxxT motifs (Dawson et al. 2002), and “polar clamp” and “serine zipper” motifs (Adamian and Liang 2002) have also been identified. Given the divergence in the different families of GPCRs, it is possible that different mechanisms of oligomerization exist. Indeed, it is not known whether all GPCRs have similar structures (Palczewski et al. 2000; Karnik et al. 2003; Rashid et al. 2004). Differences in helix orientation, helix–helix interactions, and topology may exist. Taken together, these observations indicate that multiple interfaces may contribute to receptor dimerization or that dimerization interfaces could be receptor-specific. Therefore, it is of great interest to understand the determinants for GPCR oligomerization.
We are studying the folding and assembly of the human A2A receptor (A2AR), as a representative example of human GPCRs. The adenosine family of receptors belongs to the class A of GPCRs (rhodopsin-like) and four members have been identified: A1, A2A, A2B, and A3. They are linked to cardioprotective and hypertensive effects during periods of stress such as hypoxia and ischemia. A2AR activates adenylate cyclase through coupling to the GS proteins, which trigger a cascade of events including vasodilation. Adenosine receptors are important targets in the search for the molecular origins of cardiovascular disease, and numerous biomedical, clinical, and drug discovery efforts are aimed at these receptors. It is now widely accepted that A2AR form homodimers (Canals et al. 2004), as well as heterodimers with the dopamine D2 receptor (Franco et al. 2000).
In a recent study, we showed that peptides corresponding to the seven TM domains of A2AR display significant variability in their helical propensity and tendency to insert into or associate with micelles and vesicles (Lazarova et al. 2004). These initial studies suggested that all of the peptides except TM5 are monomeric: For every peptide except TM5, the ratio [θ]222/[θ]208 obtained by circular dichroism (CD) spectroscopy is <1, which is indicative of an α-helical, monomeric peptide (Lau et al. 1984; Zhou et al. 1992; Melton et al. 1995). In contrast, in membrane-mimetic environments, the CD spectra of TM5 peptide show a [θ]222/[θ]208 ratio >1; this ratio is often associated with coiled coils or other assemblies of helical peptides (Zhou et al. 1992; Dutta et al. 2001). This finding suggested to us that TM5 might self-associate in hydrophobic environments.
Here, we investigate in further detail the unique properties of the TM5 domain of the human adenosine A2A receptor. We used CD and fluorescence spectroscopy, Förster resonance energy transfer (FRET), and gel electrophoresis to study TM5 peptide behavior in membrane-mimetic environments. We also performed mutagenesis of the full-length A2A receptor and analyzed the formation of A2A dimers by SDS-PAGE and Western blot. We find that TM5 peptide forms oligomeric structures in both SDS micelles and DMPC vesicles, and thatmutation at position M193 in the fifth TM helix of the full-length receptor disrupts A2AR dimer. These findings strongly suggest that TM5 is involved in the dimerization of the adenosine A2A receptor.
Results
Secondary structure determination by CD spectroscopy
Far-UV CD spectroscopy was used to characterize the secondary structure of TM5 peptide in hydrophobic environments. Figure 1A ▶ shows representative CD spectra of TM5 peptide in SDS micelles and in DMPC vesicles. In each membrane-mimetic environment, TM5 peptide adopts an α-helical structure with characteristic double minima at 222 nm and 208 nm and a positive maximum at 192 nm. Interestingly, unlike the other A2AR TM peptides previously studied (Lazarova et al. 2004), TM5 peptide displays a ratio of [θ]222/[θ]208>1. This ratio is characteristic of oligomeric peptides. The CD spectrum of TM5 is also concentration dependent (Fig. 1B ▶); helicity increases as the peptide concentration increases. Furthermore, upon dilution, the [θ]222/[θ]208 ratio becomes<1 between 5 and 10 μM. These data suggested to us that TM5 peptide forms oligomeric structures in hydrophobic environments.
Figure 1.
CD spectra of TM5 peptide in SDS micelles and DMPC vesicles. (A) TM5 (20 μM) in SDS micelles (open circles), and in DMPC vesicles (filled squares). (B) CD spectra of TM5W in DMPC vesicles at increasing concentration: 5 μM (filled circles), 10 μM (open triangles), 15 μM (filled squares), 20 μM (open circles), and 25 μM (crosses).
We constructed two variants of the TM5 peptide, to be used in fluorescence and FRET experiments: In TM5W,Y at position 9 in the peptide is replaced with W; in the TM5Pyr peptide, pyrene is coupled to the third lysine in the sequence (Table 1). Incorporation of TM5W and TM5Pyr in DMPC vesicles and SDS micelles (data not shown) result in the formation of helical structures very similar to that obtained for TM5. These data strongly indicate that the modifications made to TM5 do not alter its secondary and higher order structural features. Using intrinsic fluorescence, acrylamide quenching and polarized FTIR spectroscopy we previously demonstrated that the TM5 peptides incorporate in SDS micelles and DMPC vesicles (Lazarova et al. 2004).
Table 1.
Sequences of the peptides corresponding to the TM5 domain of adenosine A2A receptor and its variants
| Amino acid sequencesa | Name |
| KKKMNYMVYFNFFACVLVPLLLMLGVYLRKKK | TM5 |
| KKKMNYMVWFNFFACVLVPLLLMLGVYLRKKK | TM5W |
| KKK(Pyrene)MNYMVYFNFFACVLVPLLLMLGVYLRKKK | TM5Pyr |
a The 25 residues predicted to be part of the A2AR TM5 domain (http://www.gpcr.org/7tm/) are indicated in bold. The Lys added at the N and C termini are indicated in italics. The nonnative W is underlined.
Analysis of the oligomeric states of TM5 peptide by PAGE
To determine whether TM5 peptides form oligomeric structures, we first performed gel electrophoresis experiments. The results for different peptide concentrations (5–40 μM) are presented in Figure 2A ▶. At each TM5 concentration, a band with an apparent MW ~5000 Da corresponding to the monomeric form of TM5 is evident. In addition, a band at an apparent molecular weight corresponding to twice that of the monomer (~10 kDa) can be seen at increasing peptide concentrations, strongly suggesting the existence of a dimeric species of the TM5 peptide. As observed with CD spectroscopy, the oligomeric form appears in a concentration-dependent manner, and begins to appear between 5 and 10 μM. Dimerization or higher order oligomerization is not observed with the other TM peptides from A2AR (Fig. 2B ▶).
Figure 2.
Determination of the oligomeric states of the peptides corresponding to the TM domains of A2AR by PAGE. (A) TM5 peptide was loaded on an 10% Bis-Tris gel at various concentrations as indicated at the top of the gel. (B) Peptides corresponding to other TM domains of A2A receptor were loaded at a concentration of 20 μM and run on an 18% Tricine-Glycine gel using PFO as running buffer. Position of receptor bands are denoted by arrows and molecular weight markers are indicated (in kDa) at the left of each panel.
Fluorescence and Förster resonance energy transfer (FRET)
To study TM5 peptide self-association in more detail, we performed fluorescence experiments, using the properties of two fluorescent groups: the tryptophan at position 9 on the TM5W peptide, and the pyrene attached to the third N-terminal lysine on the TM5Pyr peptide. Pyrene molecules form excited-state dimers (excimers) when two pyrene rings reside within 10 A ¢ª (Sahoo et al. 2002) of each other. This excimer state has a unique fluorescence peak at ~470 nm. It also has been shown that van der Walls contact distance (2.4–4.0 A ¢ª ) is required between pyrenes for excimer formation (Chen and Katz 2002). The fluorescence emission spectrum of Trp overlaps very well with the excitation spectrum of pyrene, leading to an excellent energy transfer between these two groups. The Förster critical distance for energy transfer between Trp and pyrene is 28 A¢ª (Lakowicz 1999), enabling us to monitor helix–helix association.
We first used TM5Pyr by itself to follow the appearance of pyrene excimers. When we titrated TM5Pyr in DMPC vesicles, a clear increase of excimer fluorescence emission at 470 nm was observed (Fig. 3 ▶), indicating that pyrene molecules come in very close proximity to each other, suggesting formation of TM5 homodimers.
Figure 3.
Excited-state dimer formation followed by fluorescence. Titration of TM5Pyr in DMPC vesicles upon excitation at 345 nm: 2.56 μM (dashed line), 8.45 μM (solid line), and 10.2 μM (dotted line). For ease of comparison, intensities of the spectra are normalized to the 380 nm peak of the pyrene monomer.
In the FRET experiments we monitored both Trp and excimer fluorescence emissions upon mixing of TM5W and TM5Pyr in DMPC vesicles. When exciting Trp at 295 nm, we observe an almost complete quenching of the Trp emission when TM5Pyr is added (Fig. 4A ▶), indicating a productive energy transfer between Trp and pyrene. On the other hand, when exciting pyrene at 345 nm, a significant decrease of excimer emission is observed (Fig. 4B ▶). These results suggest that TM5W competes with the homodimer formed by TM5Pyr, disrupting the excimer formation. Similar results were obtained in SDS and C12E8 micelles (data not shown). As described in Materials and Methods, it was necessary to premix TM5W and TM5Pyr because when preformed TM5W or TM5Pyr homooligomers were mixed, the apparent rate of heterooligomer formation (as measured by the decrease in intensity of the excimer peak, or the quenching of the Trp fluorescence) was extremely slow.
Figure 4.
TM5 peptide self-association monitored by FRET. (A) TM5W tryptophan fluorescence emission spectrum (310–500 nm) upon excitation at 295 nm in the presence or the absence of TM5Pyr: 10 μM TM5W (solid line) and 20 μM (TM5W+TM5Pyr) (dashed line). (B) TM5Pyr excimer fluorescence emission spectrum (360–550 nm) upon excitation at 345 nm in the presence or the absence of TM5W: 10 μM TM5Pyr (solid line) and 20 μM (10 μM TM5W+10 μM TM5Pyr) (dashed line). For ease of comparison, intensities of the spectra are normalized to the 380 nm peak of the pyrene monomer. Total absorbance in all cases was <0.1 at the excitation wavelength to avoid inner filter effects.
Altogether, the fluorescence data clearly show that TM5 peptides can interact and come in very close contact, confirming the idea that TM5 peptides self-associate. Moreover, the concentration range where we observe oligomerization by excimer formation and FRET appears to be the same as in the CD and PAGE experiments.
Stability of TM5 oligomeric forms
After demonstrating the existence of TM5 oligomers, we sought to assess the stability of these species to temperature and chemical denaturation using CD spectroscopy and SDS-PAGE. We first studied the thermal stability of TM5 inserted in DMPC vesicles by collecting CD spectra at temperatures up to 95°C. Figure 5A ▶ shows the relatively high thermal stability of TM5 oligomeric structures. Only at a temperature of 95°C does the [θ]222/[θ]208 ratio invert, suggesting that the oligomer is dissociating, leading to monomers that have [θ]222/[θ]208 <1. It is also important to note that this denaturation is reversible; when cooling down to 25°C, the CD spectrum exhibits a [θ]222/[θ]208 ratio >1 again.
Figure 5.
Stability of TM5 peptide to temperature and urea denaturation assessed by CD spectroscopy. (A) CD spectra of TM5 peptide in DMPC vesicles at various temperatures: 25°C (filled diamonds), 40°C (open circles), 95°C (filled circles), and 25°C return (crosses). (B) CD spectra of TM5 (20 μM) mixed in DMPC vesicles in 3 M urea (filled diamonds), in 5 M urea (open circles), and in 5 M urea+5 min at 95°C (filled circles).
We then determined the denaturing effect of urea using both SDS-PAGE and CD spectroscopy. Figure 5B ▶ shows that urea alone has very little effect on TM5 oligomeric state and structure, even at high concentrations. Indeed, oligomers are still clearly visible on SDS-PAGE even at 5 M urea (not shown). Figure 5B ▶ underlines the fact that TM5 structures retain a high helical content and a [θ]222/[θ]208 ratio >1 at high urea concentration. Only the combination of 5 M urea and 95°C is able to disrupt the oligomers as judged by CD (Fig. 5B ▶) and SDS-PAGE (not shown). Note that for the CD spectra in urea, data could only be collected down to 212 nm because the strong absorbance of urea increases noise and dynode voltage, leading to a loss of resolution. Taken together, these data demonstrate the strong resistance of TM5 oligomeric species to the denaturing effects of both temperature and urea.
Effect of reducing agent
To test whether the cysteine residue present in TM5 peptide sequence is involved in a disulfide bond, we assessed the effect of Tris(2-carboxyethyl)phosphine (TCEP), a strong reducing agent. Even with a large excess of TCEP (1 mM) the CD spectra of TM5 inserted in DMPC vesicles stayed unchanged (data not shown), indicating that no disulfide bonds are involved in the formation of TM5 oligomers. As described in Materials and Methods, the experiments were performed to ensure that the disulfide bond did not form during peptide handling. The reversibility of the [θ]222/[θ]208 ratio upon dilution (Fig. 1B ▶) and with temperature (Fig. 5A ▶) also supports the idea that a disulfide bond is not involved.
Mutagenesis of the full-length A2AR
A computational model for TM5 dimer based on a method for modeling the structures of simple TM helix homooligomers (Kim et al. 2003) indicates that N181, C185, P189, and M193 might be part of the contact interface between two associated TM5 peptides (S. Kim and J.U. Bowie, pers. comm.). To test whether these residues are involved in the dimerization of A2AR, we performed mutagenesis in the full-length A2A receptor. The M193A mutant notably and consistently disrupts the monomer: dimer receptor ratio as observed by SDS-PAGE after Western blot analysis (Fig. 6 ▶). Indeed, the relative amount of receptor dimer is reduced by at least twofold for the M193A mutant. This result indicates that M193 is involved in the dimerization of A2AR.
Figure 6.

Immunobloting of wild-type and M193A mutant A2AR expressed in HEK293E cells. Lysed cells expressing either wild-type A2AR (WT) or M193A mutant were immunoblotted following SDS-PAGE using the appropriate anti-A2AR antibody. The blot reveals immunoreactive bands corresponding to the expected monomeric form (~40 kDa) as well as a higher molecular mass species (~80 kDa). Position of receptor bands are denoted by arrows and molecular weight markers are as shown.
Discussion
The aim of this study was to investigate the unique folding and assembly properties of the peptide corresponding to the fifth transmembrane domain of the human adenosine A2A receptor. To achieve this goal, we designed and characterized peptides corresponding to the “wild-type” TM5 sequence and two variants containing fluorescence probes (a tryptophan or a pyrene). As previously described (Lazarova et al. 2004), we flanked each TM domain with several Lys residues on both termini to increase their solubility. Thus, all TM5 variants share the same chain length of 32 amino acid residues, with 25 residues representing the putative TM domain.
CD spectra of TM5 in SDS micelles and DMPC vesicles first suggested to us that TM5 may self-associate. TM5 spectra display a concentration dependent ratio of [θ]222/[θ]208>1. This ratio is one characteristic of oligomerization, and is unique among the peptides corresponding to the seven TM domains of A2AR (Lazarova et al. 2004). Since a [θ]222/[θ]208>1 is, on its own, not definitive evidence for oligomerization, further characterization was performed.
Unlike the other TM peptides, which appear monomeric on PAGE, when the TM5 peptide is subjected to gel electrophoresis at increasing concentrations, it exhibits a band with an apparent molecular weight twice that of the monomeric TM5 peptide. This finding strongly suggests the formation of TM5 dimers. Moreover, the concentrations where this higher molecular weight band appears seem to correlate well with the appearance of a ratio of [θ]222/[θ]208>1 observed in CD data; in both cases, self-association seems to occur between 5 and 10 μM. The fact that the other six TM peptides from A2AR are all monomeric on PAGE, and do not show any concentration dependence in their CD spectra, indicate that the TM5 interactions are specific. It is worth noting that the other TM peptides appear monomeric even when run on native PAGE using PFO (rather than SDS); PFO has been shown to protect interactions within protein oligomers (Ramjeesingh et al. 1999). This finding further emphasizes that the TM5 interactions are unique to this transmembrane domain peptide.
Pyrene excimers were formed in DMPC vesicles upon increase of TM5 pyrene-labeled peptide concentration, clearly indicating that TM5 peptides come into close proximity to each other since the critical distance of formation for these excimers is<10 A ¢ª . This finding is strong evidence that TM5 peptides self-associate in a parallel orientation, as would be expected for A2AR dimerization in vivo. Considering the characteristics of a typical α-helix, the transmembrane region of TM5 peptide should be around 37.5 A ¢ª in length; if two TM5 peptides were associating in an anti-parallel orientation, the pyrene molecules would be much more than 10 A ¢ª apart and no excimer formation would be possible. However, at this time we have not ruled out the possibility that a mixture of parallel and anti-parallel dimers exists, and that the observed signal is the result of an average of signals arising from parallel and anti-parallel dimers.
When TM5W and TM5Pyr are mixed together in DMPC vesicles, energy transfer occurs between Trp and pyrene. We observed a dramatic quenching of the TM5W Trp emission when TM5Pyr is added. Similarly, a significant decrease of excimer emission is observed, indicating that TM5W competes with the homodimers formed by TM5Pyr, disrupting the excimer formation. The fluorescence data clearly indicate that TM5 peptides can come into very close contact and form oligomers.
These oligomeric species are quite stable, and resist the denaturing effects of temperature and urea. Reducing agents such as TCEP have no apparent effect on TM5 oligomeric state, indicating that disulfide bonds are not involved in the formation of TM5 oligomers.
While the ratio of [θ]222/[θ]208 alone is not definitive evidence of oligomeric structures (Holtzer and Hotlzer 1995), in our case it correlates very well with the other lines of evidence presented here. Thus, we believe that for TM5, this ratio is a useful marker.
Taken together, these many lines of evidence indicate that TM5 peptides self-associate to form oligomeric species in membrane-mimetic environments. They also show that strong interactions are involved. Although it is possible that oligomers larger than dimers may be formed, SDS-PAGE results and fluorescence data, especially the disruption of TM5Pyr excimer by TM5W, strongly support the idea of dimer formation. Furthermore, preliminary sedimentation equilibrium analytical ultracentrifugation experiments and data analysis are also consistent with dimer formation (data not shown). We conclude that TM5 peptides self-associate to form homodimers.
In addition, mutation of Met 193 to Alanine clearly alters the apparent monomer:dimer receptor ratio, indicating that M193 is involved in A2AR dimerization, as suggested by the computational model. We believe that TM5 is part of the contact interface between two adenosine A2A receptors.
Based on CD, PAGE, and fluorescence data, the KD for the TM5 dimerization appears to lie in the low micromolar range. Currently, it is difficult to determine whether affinity in this range would be sufficient to account for the dimerization of A2AR. The local effective concentration of the receptor in cell membranes is not known; we are also not aware of any reliable estimate for the receptor dimerization constant in vivo. In addition, TM5 helix contacts may not be the only interactions implicated in GPCR oligomerization; other structures such as loops may also be involved (White et al. 1998; Fotiadis et al. 2004; Giguère et al. 2004). In any case, we believe that additional studies of the interactions of TM5 peptides, and in the context of the full-length receptor, will continue to be useful in probing these important molecular events (Marti 1998; Yeagle et al. 2001).
The resistance of TM5 oligomers to temperature and urea denaturation also raises the possibility that TM5 is involved in A2AR aggregation when it is overexpressed. The well-known tendency of GPCRs to aggregate in inclusion bodies when they are expressed in such systems as Escherichia coli or yeast underlines the importance of understanding these interchain interactions. TM5 might be the interface in question in the case of A2AR. Studying the residues involved in TM5 self-association may provide better insights in how to control A2AR aggregation. Indeed, variants of TM5 peptide that would reduce oligomerization and/or aggregation may help solve the technical problems associated with the purification and solubilization of this receptor, leading to easier structural studies.
Finally, it is interesting to interpret our findings in the context of transmembrane dimerization motifs. Indeed, none of the known motifs for TM helix dimerization (GxxxG, AxxxA, SxxSSxxT, polar clamp, serine zipper, leucine zipper) appears in TM5. However, statistical analysis of amino acid patterns in TM helices using TMSTAT (Senes et al. 2004) reveals that the PM4 pair (PxxxM) is the most overrepresented doublet pattern from any combination of PxxxX doublet pattern. This analysis is consistent with our findings, and suggests that the PxxxM pattern may play a role in the dimerization of A2AR.
This study is the first reported evidence of an individual GPCR transmembrane domain self-association, and lays the groundwork for more detailed analysis of A2AR dimerization. Measurement of thermodynamic parameters for the TM5 peptide helix–helix interactions, and the identification of other potential residues involved in the association, are the subjects of ongoing experiments. The possible roles of TM5 in the dimerization of A2AR in vivo, and as a motif for aggregation, are also the focus of our current investigations.
Materials and methods
Peptide design and synthesis
Table 1 shows the amino acid sequence of the peptides used in this study. The peptides were designed to correspond to the fifth TM domain (TM5) of the human adenosine A2A receptor as described previously (Lazarova et al. 2004). Lysine residues were added at the N and C termini of the peptides (Lazarova et al. 2004). Two variants were constructed to enable us to monitor oligomerization using fluorescence spectroscopy. We substituted Y for W in TM5 (TM5W), and we labeled TM5 with a pyrene-butyric acid derivative on the third N-terminal Lysine (TM5Pyr). These changes did not alter the secondary structure of the peptides; peptides with and without these modifications displayed equivalent CD spectra (not shown). Peptides were synthesized by and purchased from SynPep. All peptides were purified to>95% purity as judged by HPLC. The identity of the purified peptides was confirmed by mass spectrometry. Peptides were stored at −20°C as solid powders.
Peptide concentration
Peptide concentrations were determined using two different approaches: by amino acid analysis (performed at Purdue University Core Facility PSAL), or by measuring UV absorbance of the peptides in 6 M Guanidine-HCl at 280 nm, using appropriate extinction coefficients for the aromatic Tryptophan and Tyrosine residues (Brandts and Kaplan 1973). Prior to measurements, peptide stock solutions (2mg/mL) were prepared in acetonitrile/water (1:1).
Preparations of vesicles and micelles
SDS micelles and DMPC vesicles were both prepared in 10 mM Tris, pH 7 as previously described (Lazarova et al. 2004). The lipid films were prepared by dissolving about 10 mg of lipid in chloroform/methanol (2:1), then drying under a stream of N2. Large unilamellar vesicles were obtained after hydrating in 10 mM Tris, pH7 and extruding through two-stacked polycarbonate membranes (with a pore size of 100 nm) using a Mini Extruder (Lipofast, Avestin). The concentration of SDS was always well above the critical micelle concentration (Henry and Sykes 1994).
CD measurements
Far-UV CD spectra of the peptides were recorded on an Aviv model 202 spectrometer equipped with a Peltier thermal-controlled cuvette holder. All measurements were performed at 25°C unless noted. Peptide concentrations used in CD experiments were in the range of 5–30 μM. Peptide: lipid molar ratios were 1:100 for measurements in vesicles. CD intensities are expressed in Mean Residue Molar ellipticity [θ], calculated from the equation
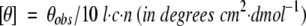 |
where θobs is the observed ellipticity in millidegrees, l is the optical path length in centimeters, c is the final molar concentration of the peptides, and n is number of amino acid residues. To minimize effects of scattering and to ensure that the CD spectra were observed from peptides in solution, several precautions were taken as previously described (Lazarova et al. 2004). The spectra were recorded using a 0.1-cm path length quartz cuvette, from 260–190 nm, at 1-nm step resolution and integration time of 3 sec. In the case of TCEP treatment, 1 mM TCEP was added to a 5 μM TM5 peptide solution, a concentration at which TM5 peptide is known to be monomeric to ensure that disulfide bonds are not formed during peptide handling. The TM5 peptide concentration is subsequently increased to 10 μM, 20 μM, and 40 μM by addition of appropriate volumes of the stock solution, in 5 μM increments.
Gel electrophoresis
Appropriate volumes of peptide stock solutions were first premixed with 10 mM HEPES buffer. The solutions were then mixed with 2 × sample buffer (100 mM Tris-Cl at pH 6.8, 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20% (v/v) glycerol). The samples were loaded on 10% Nu PAGE Bis- Tris precast polycacrilamide gels (Invitrogen). The running buffer contained 50 mM MES at pH 7.2, 50 mM Tris-HCl, 0.1% SDS, and 1 mM EDTA. Samples were run at 200 V, at room temperature. For additional experiments with the other TM peptides, using perfluorooctanoic (PFO) PAGE, the protocol described by Ramjeesingh and colleagues (Ramjeesingh et al. 1999) was followed. Peptides were first premixed with 10 mM HEPES buffer containing 0.5% PFO. The solutions were incubated at room temperature for 15 min and then mixed with 2 × sample buffer. The samples were loaded on native 18% Tris-Glycine precast gels (Invitrogen). The running buffer contained 25 mM Tris, 192 mM glycine, and 0.5% PFO; the pH was adjusted to pH 8.5 with NaOH. Samples were electrophoresed at 125 V, at 4°C. Gels were then stained and visualized using GelCode Plus Staining Reagent (Pierce).
Fluorescence measurements
Fluorescence measurements were performed on an ISS PC-1 spectrofluorimeter, operating in photon-counting mode, using 10 × 10- or 2 × 10-mm quartz cuvettes at 25°C unless specified. To minimize light scattering effects, all scans were performed with emission polarizer oriented at 0° and the excitation polarizer at 90°. If Trp emission was monitored, the samples were excited at 295 nm and the emission spectra were taken from 300 to 500 nm. In the case of pyrene emission, the excitation wavelength was 345 nm and the emission was scanned from 360 to 550 nm. Concentrations of the peptides were chosen to prevent inner filter effects.
To study the interaction between TM5W and TM5pyr, 24 mL of 1 μM of TM5W solution in water was combined with 24 mL of 1 μM of TM5pyr to give 48 mL of 1 μM (TM5+TM5pyr) solution. Aliquots of this solution were prepared, and then vacuum centrifuged overnight at 30°C. Pellets were finally resuspended in either 10 mMTris buffer or DMPC to the appropriate concentrations of (TM5W+TM5pyr) solution. This procedure was necessary because when preformed TM5W or TM5Pyr homooligomers were mixed, the apparent rate of heterooligomers formation (as measured by the decrease in intensity of the excimer peak, or the quenching of the Trp fluorescence) was extremely slow. The experiments with DMPC vesicles were performed at 35°C, which is well above the Tm of the lipid (23°C). To study the formation of pyrene excited-state dimers, TM5Pyr was titrated in the presence of DMPC vesicles, maintaining the 1:100 peptide: lipid molar ratios.
A2AR mutagenesis
Site-specific mutations were introduced in the full-length cDNA encoding for the human adenosine A2A receptor cloned into the mammalian expression vector pCEP4 (Invitrogen) using the overlap extension method (Higuchi et al. 1988). Briefly, two primary polymerase chain reactions (PCR) first produce two overlapping DNA fragments, both bearing the same mutation introduced via primer mismatched, in the region of overlap. A secondary reamplification combined the two fragments using the two flanking (outermost) primers to produce the full-length product. This product was then subcloned into pCEP4. Identity of the mutants was confirmed by DNA sequencing (University of Delaware Biotech Core Facility).
Mammalian cell culture and A2AR expression
HEK293E cell line with and without transiently transfected A2AR was grown and maintained in Dubelcco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.5 mg/mL geneticin (Invitrogen). Transient transfections were performed using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. Two days after transfections, the cells were washed twice with 5 mL of phosphate-buffered saline and collected for analysis.
Western blotting
As previously described (Berger et al. 2005), transiently transfected cells were resuspended in 2 mL of ice-cold buffer containing 5 mM Tris-HCl at pH 7.4, 2 mM EDTA, and 100 μM PMSF, and then were homogenized and lysed by sonication on ice using a Branson 450 sonicator. The samples were loaded and run on 10% NuPAGE Bis-Tris gel as described above, transferred to nitrocellulose membrane using Trans-blot transfer medium (Bio-Rad) and then blotted using 1:200 goat anti- A2AR primary and 1:2000 HRP-conjugated rabbit anti-goat antibodies (Santa Cruz Biotechnology, Inc). Visualization was achieved using SuperSignal West Pico Chemiluminiscent substrate (Pierce) and a Typhoon 8600 Variable Mode Imager (Amersham Biosciences).
Acknowledgments
This research was supported by NIH 1-P20-RR017716-01. M.F.R. was supported by the HHMI Undergraduate Research Scholars program.
Abbreviations
GPCR, G-protein coupled receptor
TM, transmembrane
A2AR, adenosine A2A receptor
SDS, sodium dodecyl sulfate
DMPC, dimyristoyl phosphatidylcholine
CD, circular dichroism
PAGE, Polyacrylamide gel electrophoresis
FRET, Förster resonance energy transfer
PFO, perfluorooctanoic acid
Tm, temperature of phase transition
TCEP, Tris(2-carboxyethyl)phosphine
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051409205.
References
- Adamian, L. and Liang, J. 2002. Interhelical hydrogen bonds and spatial motifs in membrane proteins: Polar clamps and serine zippers. Proteins 47 209–218. [DOI] [PubMed] [Google Scholar]
- Bai, M. 2004. Dimerization of G-protein-coupled receptors: Roles in signal transduction. Cell Signal. 16 175–186. [DOI] [PubMed] [Google Scholar]
- Berger, B.W., Garcia, R.Y., Lenhoff, A.M., Kaler, E.W., and Robinson, C.R. 2005. Relating surfactant properties to activity and solubilization of the human adenosine A3 receptor. Biophys. J. (in press). [DOI] [PMC free article] [PubMed]
- Brandts, J.F. and Kaplan, L.J. 1973. Derivative spectroscopy applied to tyrosyl chromophores. Studies on ribonuclease, lima bean inhibitors, insulin, and pancreatic trypsin inhibitor. Biochemistry 12 2011–2024. [DOI] [PubMed] [Google Scholar]
- Breitwieser, G.E. 2004. G protein-coupled receptor oligomerization: Implications for Gprotein activation and cell signaling. Circ. Res. 94 17–27. [DOI] [PubMed] [Google Scholar]
- Canals, M., Burgueno, J., Marcellino, D., Cabello, N., Canela, E.I., Mallol, J., Agnati, L., Ferre, S., Bouvier, M., Fuxe, K., et al. 2004. Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Neurochem. 88 726–734. [DOI] [PubMed] [Google Scholar]
- Chen, M.M.Y. and Katz, A. 2002. Steady-state fluorescence-based investigation of the interaction between protected thiols and gold nanoparticles. Langmuir 18 2413–2420. [Google Scholar]
- Dawson, J.P., Weinger, J.S., and Engelman, D.M. 2002. Motifs of serine and threonine can drive association of trans-membrane helices. J. Mol. Biol. 316 799–805. [DOI] [PubMed] [Google Scholar]
- Doura, A.K. and Fleming, K.G. 2004. Complex interactions at the helix– helix interface stabilize the glycophorin A transmembrane dimer. J. Mol. Biol. 343 1487–1497. [DOI] [PubMed] [Google Scholar]
- Dutta, K., Alexandrov, A., Huang, H., and Pascal, S.M. 2001. pH-induced folding of an apoptotic coiled coil. Protein Sci. 10 2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis, D., Liang, Y., Filipek, S., Saperstein, D.A., Engel, A., and Palczewski, K. 2004. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 564 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, R., Ferré, S., Agnati, L., Torvinene, M., Ginés, S., Hillion, J., Casadó, V., Lledó, P.-M., Zoli, M., Lluis, C., et al. 2000. Evidence for Adenosine/Dopamine receptor interactions: Indications for heterodimerization. Neuropsychopharmacology 23 S50–S59. [DOI] [PubMed] [Google Scholar]
- George, S.R., O’Dowd, B.F., and Lee, S.P. 2002. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1 808–820. [DOI] [PubMed] [Google Scholar]
- Gether, U. 2000. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21 90–113. [DOI] [PubMed] [Google Scholar]
- Giguère, V., Gallant, M.A., de Brum-Fernandes, A.J., and Parent, J.-L. 2004. Role of extracellular cysteine residues in dimerization/oligomerization of the human prostacyclin receptor. Eur. J. Pharmacol. 494 11–22. [DOI] [PubMed] [Google Scholar]
- Gurezka, R., Laage, R., Brosig, B., and Langosch, D. 1999. A heptad motif of leucine residues found in membrane proteins can drive self assembly of artificial transmembrane segments. J. Biol. Chem. 274 9265–9270. [DOI] [PubMed] [Google Scholar]
- Gurrath, M. 2001. Peptide-binding G protein-coupled receptors: New opportunities for drug design. Curr. Med. Chem. 8 1605–1648. [DOI] [PubMed] [Google Scholar]
- Henry, G.D. and Sykes, B.D. 1994. Methods to study membrane protein structure in solution. Methods Enzymol. 239 515–535. [DOI] [PubMed] [Google Scholar]
- Higuchi, R., Krummel, B., and Saiki, R.K. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 16 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer, M.E. and Holtzer, A. 1995. The use of spectral decomposition via the convex constraint algorithm in interpreting the CD-observed unfolding transitions of coiled coils. Biopolymers 36 365–379. [DOI] [PubMed] [Google Scholar]
- Jones,K.A.,Borowsky, B., Tamm, J.A., Craig, D.A., Durkin, M.M., Dai, M., Yao, W.J., Johnson, M., Gunwaldsen, C., Huang, L.Y., et al. 1998. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396 674–679. [DOI] [PubMed] [Google Scholar]
- Jordan, B.A. and Devi, L.A. 1999. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik, S.S., Gogonea, C., Patil, S., Saad, Y., and Takezako, T. 2003. Activation of G-protein-coupled receptors: A common molecular mechanism. Trends Endocrinol. Metab. 14 431–437. [DOI] [PubMed] [Google Scholar]
- Kim, S., Chamberlain, A.K., and Bowie, J.U. 2003. A simple method for modeling transmembrane helix oligomers. J. Mol. Biol. 329 831–840. [DOI] [PubMed] [Google Scholar]
- Lakowicz, J.R. 1999. Principles of fluorescence spectroscopy, 2nd ed. Kluwer Academic/Plenum Publishers, New York.
- Lau, S.Y., Taneja, A.K., and Hodges, R.S. 1984. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded α-helical coiled-coils. J. Biol. Chem. 259 13253–13261. [PubMed] [Google Scholar]
- Lazarova, T., Brewin, K.A., Stoeber, K., and Robinson, C.R. 2004. Characterization of peptides corresponding to the seven transmembrane domains of human adenosine A(2)a receptor. Biochemistry 43 12945–12954. [DOI] [PubMed] [Google Scholar]
- Lemmon, M.A., Flanagan, J.M., Treutlein, H.R., Zhang, J., and Engelman, D.M. 1992. Sequence specificity in the dimerization of transmembrane α-helices. Biochemistry 31 12719–12725. [DOI] [PubMed] [Google Scholar]
- Lemmon, M.A., Treutlein, H.R., Adams, P.D., Brunger, A.T., and Engelman, D.M. 1994. Adimerizationmotif for transmembrane α-helices.Nat. Struct. Biol. 1 157–163. [DOI] [PubMed] [Google Scholar]
- MacKenzie, K.R., Prestegard, J.H., and Engelman, D.M. 1997. A transmembrane helix dimer: Structure and implications. Science 276 131–133. [DOI] [PubMed] [Google Scholar]
- Marti, T. 1998. Refolding of bacteriorhodopsin from expressed polypeptide fragments. J. Biol. Chem. 273 9312–9322. [DOI] [PubMed] [Google Scholar]
- Melton, L.G., Church, F.C., and Erickson, B.W. 1995. Designed polyanionic coiled-coil proteins: Acceleration of heparin cofactor II inhibition of thrombin. Int. J. Pept. Protein Res. 45 44–52. [DOI] [PubMed] [Google Scholar]
- Overton, M.C., Chinault, S.L., and Blumer, K.J. 2003. Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J. Biol. Chem. 278 49369–49377. [DOI] [PubMed] [Google Scholar]
- Palczewski, K., Kumasaka, T., Hori, T., Behnke, C.A., Motoshima, H., Fox, B.A., Trong, I.L., Teller, D.C., Okada, T., Stenkamp, R.E., et al. 2000. Crystal strcuture of rhodopsin: A G protein-coupled receptor. Science 289 739–745. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh, M., Huan, L.J., Garami, E., and Bear, C.E. 1999. Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem. J. 342(Pt. 1): 119–123. [PMC free article] [PubMed] [Google Scholar]
- Rashid, A.J., O’Dowd, B.F., and George, S.R. 2004. Minireview: Diversity and complexity of signaling through peptidergic G protein-coupled receptors. Endocrinology 145 2645–2652. [DOI] [PubMed] [Google Scholar]
- Rios, C.D., Jordan, B.A., Gomes, I., and Devi, L.A. 2001. G protein-coupled receptor dimerization: Modulation of receptor function. Pharmacol. Ther. 92 71–87. [DOI] [PubMed] [Google Scholar]
- Sahoo, D., Weers, P.M., Ryan, R.O., and Narayanaswami, V. 2002. Lipid-triggered conformational switch of apolipophorin III helix bundle to an extended helix organization. J. Mol. Biol. 321 201–214. [DOI] [PubMed] [Google Scholar]
- Salahpour, A., Angers, S., Mercier, J.F., Lagace, M., Marullo, S., and Bouvier, M. 2004. Homodimerization of the β2-adrenergic receptor as a prerequisite for cell surface targeting. J. Biol. Chem. 279 33390– 33397. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103 211–225. [DOI] [PubMed] [Google Scholar]
- Schneider, D. and Engelman, D.M. 2004. Motifs of two small residues can assist but are not sufficient to mediate transmembrane helix interactions. J. Mol. Biol. 343 799–804. [DOI] [PubMed] [Google Scholar]
- Senes, A., Engel, D.E., and DeGrado, W.F. 2004. Folding of helical membrane proteins: The role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 14 465–479. [DOI] [PubMed] [Google Scholar]
- Shichida, Y. and Imai, H. 1998. Visual pigment: G-protein-coupled receptor for light signals. Cell. Mol. Life Sci. 54 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanasila, L., Perez, J.B., Vogel, H., and Cotecchia, S. 2003. Oligomerization of the α 1a- and α 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J. Biol. Chem. 278 40239– 40251. [DOI] [PubMed] [Google Scholar]
- White, J.H., Wise, A., Main, M.J., Green, A., Fraser, N.J., Disney, G.H., Barnes, A.A., Emson, P., Foord, S.M., and Marshall, F.H. 1998. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396 679–682. [DOI] [PubMed] [Google Scholar]
- Xie, H., Ding, F.X., Schreiber, D., Eng, G., Liu, S.F., Arshava, B., Arevalo, E., Becker, J.M., and Naider, F. 2000. Synthesis and biophysical analysis of transmembrane domains of a Saccharomyces cerevisiae G protein-coupled receptor. Biochemistry 39 15462– 15474. [DOI] [PubMed] [Google Scholar]
- Yeagle, P.L., Choi, G., and Albert, A.D. 2001. Studies on the structure of the G protein-coupled receptor rhodopsinincluding the putative G protein binding site in unactivated and activated forms. Biochemistry 40 11932–11937. [DOI] [PubMed] [Google Scholar]
- Zhou, N.E., Kay, C.M., and Hodges, R.S. 1992. Synthetic model proteins. Positional effects of interchain hydrophobic interactions on stability of two-stranded α-helical coiled-coils. J. Biol. Chem. 267 2664–2670. [PubMed] [Google Scholar]







