Abstract
The pseudouridine synthase TruB is responsible for the universally conserved post-transcriptional modification of residue 55 of elongator tRNAs. In addition to the active site, the “thumb,” a peripheral domain unique to the TruB family of enzymes, makes extensive interactions with the substrate. To coordinate RNA binding and release with catalysis, the thumb may be able to sense progress of the reaction in the active site. To establish whether there is a structural correlate of communication between the active site and the RNA-sequestering thumb, we have solved the structure of a catalytically inactive point mutant of TruB in complex with a substrate RNA, and compared it to the previously determined structure of an active TruB bound to a reaction product. Superposition of the two structures shows that they are extremely similar, except in the active site and, intriguingly, in the relative position of the thumb. Because the two structures were solved using isomorphous crystals, and because the thumb is very well ordered in both structures, the displacement of the thumb we observe likely reflects preferential propagation of active site perturbations to this RNA-binding domain. One of the interactions between the active site and the thumb involves an active site residue whose hydrogen-bonding status changes during the reaction. This may allow the peripheral RNA-binding domain to monitor progress of the pseudouridylation reaction.
Keywords: RNA-modification, interdomain coupling, product release
Pseudouridine (Ψ), the C5 glycoside isomer of uridine, is the most abundant post-transcriptional modification of cellular RNAs. Sequence analyses of pseudouridine synthases showed that these enzymes belong to at least five families that share no detectable sequence similarity (Gustafsson et al. 1996; Koonin 1996; Kaya and Ofengand 2003). Nonetheless, structure determinations have demonstrated that Ψ synthases of all five families share conserved catalytic domain and active site architectures (Foster et al. 2000; Hoang and Ferré-D’Amaré 2001, 2004; Sivaraman et al. 2002, 2004; Del Campo et al. 2004; Ericsson et al. 2004; Kaya et al. 2004), and that different enzymes have divergent peripheral domains that, presumably, confer on each protein its particular substrate specificity.
TruB is the Ψ synthase responsible for the universally conserved Ψ55 in the TΨC loop of elongator tRNAs (Nurse et al. 1995). Structure determination of Escherichia coli TruB bound to a substrate RNA (Hoang and Ferré-D’Amaré 2001) demonstrated that in addition to the active site, this enzyme employs two peripheral domains to bind its substrate. These are a C-terminal PUA domain (Aravind and Koonin 1999; Ferré-D’Amaré 2003) that contacts the acceptor stem and a “thumb” that clasps the TΨC loop. Subsequent structure determinations of free TruB showed that in the absence of substrate, the thumb is either disordered (Pan et al. 2003; Chaudhuri et al. 2004) or adopts an open conformation (Phannachet and Huang 2004).
Remodeling of the structure of proteins upon binding their cognate RNAs is well documented (Williamson 2000). However, because TruB is an enzyme, its active site–distal RNA-binding moieties should be able to sense progress of the reaction so as to coordinate RNA release with completion of the isomerization reaction. To determine whether small active site perturbations are transmitted to the peripheral RNA-binding moieties of TruB, we have solved the structure of a catalytically inactive mutant of TruB bound to RNA. We find that the mutation of an active site aspartate to asparagine causes a loss of detectable enzyme activity. Comparison of the mutant complex to the wild-type protein in complex with a reaction product shows that the enzymes adopt virtually identical conformations, with the exception of the thumb that clasps the TΨC loop. This structural comparison suggests that the RNA-binding thumb senses progress of the reaction by monitoring both RNA and active site amino acid side-chain conformations.
Results
For our previous structure determination of E. coli TruB bound to RNA, we employed a chemically modified RNA and an N-terminally truncated protein. Previous work had shown that Ψ synthases are strongly inhibited by substrate RNAs in which 5-fluorouridine (f5U) (Fig. 1A ▶) replaces the uridine at the site of modification (Samuelsson 1991). Analysis of the interaction of Ψ synthase TruA with such a modified RNA led to the conclusion that that enzyme makes a covalent suicide complex with the pyrimidine ring of [f5U] in tRNA (Gu et al. 1999). However, structure determination of TruB complexed to a [f5U]RNA (Hoang and Ferré-D’Amaré 2001) revealed not a covalent complex, but TruB bound to the product of the isomerization of f5U (Fig. 1B ▶). Detailed biochemical examination of this discrepancy has shown that, whereas some Ψ synthases indeed form suicide complexes with f5U containing RNAs, others, like E. coli TruB, isomerize it efficiently, and are not inhibited by f5U (Phannachet and Huang 2004; Spedaliere and Mueller 2004).
Figure 1.
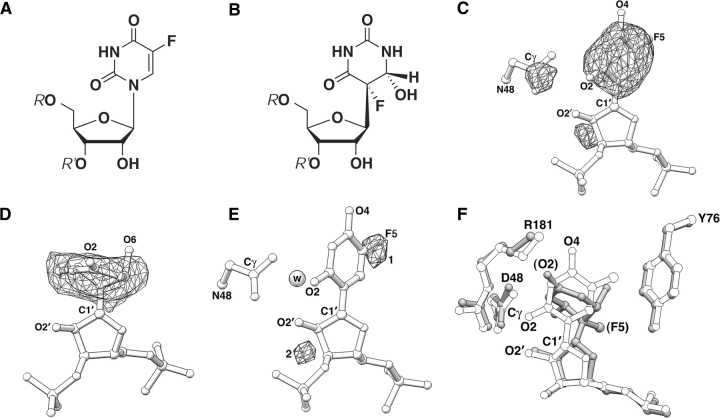
Structure of the catalytically inactive tTruB(D48N)–RNA complex. (A) Chemical structure of 5-fluorouridine (f5U). (B) Chemical structure of the enzymatic reaction product, 5,6-dihydro-6-hydroxy-5-fluoro-pseudouridine. Formally, this results from reattachment of the base to the sugar through C5, and addition of water to the C5–C6 double bond (Spedaliere et al. 2004). (C) Omit σA |Fo|−|Fc| electron density in the active site of the tTruB(D48N)–RNA complex (mesh) calculated with phases from a model missing the nucleobase of RNA residue 55 as well as the Cγ, Oδ, and Nδ atoms of residue 48. The map was contoured at 3.5 standard deviations above mean peak height (SD). The nucleobase electron density approximates an oblate ellipsoid whose long axis is parallel to the glycosidic bond, demonstrating that the base remains planar. (D) Omit σA |Fo|−|Fc| electron density around the isomerized RNA residue 55 of the wild-type tTruB complex (PDB code 1K8W). The map was calculated at 2.8 Å resolution, and contoured at 3.5 SD (cf. planar nucleobase in C). (E) Residual |Fo|−|Fc| electron density map (3.5 SD) calculated with a model from which the fluorine atom of residue 55 (in the anti conformation; torsion angle=150°) has been omitted. Once the fluorine atom is added to density feature 1, there are no residual electron density features in the active site except for the noise peak (labeled 2) next to the ribose. (F) Superposition of the two structures. The side chains and nucleotide 55 of the wild-type complex (gray) are labeled in parentheses.
In order to visualize crystallographically a precursor complex of TruB, we mutated its absolutely conserved active site aspartate (D48) to asparagine. Further, we cocrystallized the mutant protein with a [f5U]RNA, rather than unmodified RNA, for two reasons. First, the previously solved structure of the isomerization product of f5U (Fig. 1B ▶) is nonplanar. Therefore, the experimental electron density would show clear evidence of isomerization if the mutant (D48N) enzyme retained any activity. In contrast, the electron density maps for uridine and pseudouridine (both of which are planar) would be indistinguishable. Second, the presence of fluorine at position 5 of f5U would also allow us to establish unambiguously the orientation of the nucleobase. Fluorine has essentially the same X-ray scattering power as carbon, but its atomic radius is considerably smaller (1.18 Å vs. 1.54 Å) (Boyd 1977). Thus, f5U is more isosteric to uridine (hydrogen at position 5, with an atomic radius of 1.24 Å) than, for instance, thymidine (with a methyl group at position 5).
The TruB construct used for our previous structure determination has a 10-amino acid N-terminal truncation, preceded by a His-tag (Materials and Methods). We have characterized this truncated TruB (tTruB) construct kinetically and find that the N-terminal truncation increased kcat to 0.78±0.07 s−1 and increased Km to 2441 nM±476 nM, compared to values of 0.11 s−1 and 148 nM for full-length TruB (Spedaliere et al. 2000). The mutant protein tTruB(D48N) lacked detectable activity. Even at an elevated enzyme concentration (1 μM) and prolonged incubation (2 h), the measured tritium release did not vary significantly from that of a control incubation with no enzyme. Under these conditions, wild-type tTruB releases 7500 dpm, and the system can routinely detect tritium release as low as 25 dpm. We determined the structure of tTruB(D48N) bound to [f5U]RNA at 2.8 Å resolution (Table 1). The experimental electron density maps (Fig. 1C,D,E ▶) show no signs of isomerization, and thus corroborate the kinetic finding that the D48N point mutation renders the Ψ synthase completely inactive.
Table 1.
Crystallographic statistics
| Overall | Last shell | |
| Diffraction data | ||
| Resolution (Å) | 20.0–2.8 | 2.9–2.8 |
| Unique reflections/redundancy | 10,935/6.34 | 1082/4.71 |
| Rsym (%)a | 9.1 | 34.1 |
| 〈I〉/〈σ(I) 〉 | 23.6 | 3.6 |
| Completeness (%) | 99.9 | 99.4 |
| Refinement | ||
| No. of atoms total/water/ion | 2832/26/15 | |
| Rwork/Rfreeb | 20.2/26.4 | 34.3/43.3 |
| RMSD lengths (Å)/angles(°)c | 0.0056/1.35 | |
aRsym = ∑|I − 〈I〉|/∑I, where I is the observed intensity and 〈I 〉 is the statistically weighted absolute intensity of multiple measurements of symmetry related reflections.
bRwork = 100×∑|Fo −Fc|/∑|Fc|, where Fo and Fc are the observed and calculated structure factors, respectively. Rfree is the same as Rwork, but calculated with a random 10% of the data excluded from refinement.
cRoot-mean-square (RMSD) differences of model bond lengths and angles from ideal geometry.
Discussion
Our structural analysis demonstrates that, even in the crystallization time frame (days to weeks) the D48N mutant of TruB is completely inactive. This has allowed us to visualize crystallographically a precursor form of the TruB–RNA complex. Least-squares superposition of the precursor and product forms of the enzymes results in a root-mean-square (RMS) difference of Cα positions of 0.31 Å (for 302 atom pairs). Superposition of the RNAs results in an RMS difference of 0.21 Å for 21 phosphorus atom pairs. Thus, the mutant precursor complex and the wild-type product complex are very similar. This similarity suggests that the D48N mutation inactivates TruB by ablating a mechanistically critical carboxylate group, not by drastically altering the protein structure.
The primary structural difference between the two complexes lies at the active site. This is a result of the steric difference between the nonplanar isomerization product and the planar precursor f5U (Fig. 1F ▶). Because the nucleobase at the site of isomerization needs to detach from the sugar, rotate, and reattach to the sugar during the course of the isomerization (Hamilton et al. 2005), the two structural states we have captured using f5U may mimic active site rearrangements that take place during uridine to pseudouridine isomerization. The change of nucleobase conformation results in displacement of the attached sugar (Fig. 1F ▶). During the course of the isomerization reaction, such ribose displacements may propagate to the backbone phosphates of the RNA.
Superposition of the precursor and product structures (Fig. 2A,B ▶) shows that the displacements of active site residues are transmitted anisotropically to two peripheral segments of the enzyme: motif I (Fig. 2A ▶) and the RNA-clasping thumb (Fig. 2C ▶). Neither of these segments has unusually elevated crystallographic thermal parameters (Fig. 2D ▶), suggesting that the differences we observe in these segments between the precursor and the product structures reflect propagation of active site displacements, not crystallographic disorder. In contrast, the peripheral RNA-binding PUA domain is both displaced between the two structures, and has high thermal parameters (Fig. 2A,B,D ▶).
Figure 2.
Small active-site perturbations propagate preferentially to the substrate-binding thumb of TruB. (A) Worm representation of tTruB onto which the magnitude of the difference in Cα position between the superimposed wild-type and mutant RNA complexes has been mapped in shades of blue. The darkest blue (at the poorly ordered N terminus) denotes a displacement of ~1 Å. The darkest residues in the thumb have Cα displacements of ~0.7 Å. The active site loop (where the D48N mutation lies) and motif I have displacements of ~0.4 Å and ~0.5 Å, respectively. (B) View orthogonal to A. (C) Ribbon representation of the tTruB–RNA complex. The thumb is in blue, the rest of the protein in gray, the RNA in yellow, with the site of modification (nucleotide 55) in red. The active site residue R181 (A) is located at the N-terminal (upper) end of helix α5. (D) Cα representation of the tTruB–RNA complex (PDB code 1K8W), color-coded by crystallographic thermal parameter. The parameter ranges from 15 Å2 (dark blue) to 68 Å2 (red). (E) Detail of the interaction of the thumb with the active site. The thumb of the wild-type (dark blue) and mutant (light blue) complexes is shown as a ribbon. In yellow is the backbone of residues 126–128. Hydrogen bonds between backbone atoms and the active site arginine (R181), as well as the phosphate 3′ to RNA residue 55 are shown, as is a hydrogen bond between the precursor nucleobase and R181. “HFΨ” refers to the isomerization product (Fig. 1B ▶) of 5-fluorouridine. The RNA-thumb and the R181-thumb interactions highlighted are nearly identical between the E. coli (Hoang and Ferré-D’Amaré 2001) and Thermotoga maritima (Chaudhuri et al. 2004; Phannachet and Huang 2004) TruB-RNA complex structures.
Transmission of active site motions to motif I is most likely through the side chain of a conserved lysine in that motif that makes a conserved, buried hydrogen bond with the backbone carbonyl of the active site aspartate (asparagine in the D48N mutant). This lysine (K19 in TruB) is part of a sequence motif that is absolutely conserved among members of the TruB, RluA, and RsuA families of Ψ synthases (Koonin 1996). The importance of this interaction is underscored by the results of mutational analyses (Spedaliere et al. 2000). Mutations of the lysine resulted in destabilization of both TruB and RluA, and in decreases of kcat/Km by about two orders of magnitude. This suggests that the coupling between motif I and the active site seen in our structures is important, and may be maintained during catalysis.
Because the thumb domain that clasps the TΨC loop of the RNA must undergo large motions in order to allow binding and release of the substrate, we were particularly interested in seeing how it reacts to small active site perturbations that may mimic those taking place during the isomerization reaction. Superposition of the precursor and product structures shows that other than the poorly ordered N terminus and C-terminal PUA domain, the thumb is the segment of TruB that undergoes the largest displacements between our two structures (Fig. 2A,B ▶). These displacements of the thumb are distinct from the hinge-like flexing of the entire catalytic domain previously documented for Ψ synthases of the TruB (Chaudhuri et al. 2004) and TruD (Hoang and Ferré-D’Amaré2004) families. Only two direct hydrogen bonds are made between the thumb and active site atoms that move between the precursor and the product structures (Fig. 2E ▶). First, the backbone carbonyl of thumb residue 126 makes two hydrogen bonds to the guanidino group of the active site arginine. Of the three conserved active site residues of TruB (D48, Y76, and R181) the active site basic residue shows the largest Cα displacement (0.49 Å). Second, the backbone amide of thumb residue 128 makes a hydrogen bond to a nonbridging oxygen of the RNA phosphate 3′ to the site of isomerization.
Although the thumb domain protrudes considerably from the surface of TruB (Fig. 2A ▶), in our RNA-bound structures of the enzyme it is very well ordered. Except in the immediate vicinity of the mutation, the thumb-RNA interface is unchanged, and the RNA (which makes a large number of crystal contacts) is in nearly identical conformation in the two structures. Thus, it is very likely that the displacement of the thumb we observe between the precursor and product structures reflects sensing of the detailed conformation of active site residues (both RNA and protein) by the thumb. The close coupling of the thumb to the active site arginine is particularly suggestive. In the wild-type structure, this arginine makes a salt bridge with the active-site aspartate. However, this interaction would be lost when the aspartate serves as a nucleophile to make a covalent intermediate, either with the nucleobase or ribosemoiety of the substrate uridine (Hamilton et al. 2005). The loss of the salt bridge will require a structural rearrangement to accommodate the partially unsatisfied charge and hydrogen-bonding capabilities of the arginine, and the rearrangement could readily propagate to the thumb domain. The thumb-active site interactions highlighted in Figure 2E ▶ may form part of a molecular “latch” that releases the thumb once catalysis is complete. Multiple sequence alignments demonstrate that the thumb domain is highly conserved between TruB orthologs from organisms as distant as E. coli and budding yeast (Hoang and Ferré- D’Amaré 2001). Thus, the coupling of this substrate binding peripheral domain to the active site may have been conserved through several billion years of evolution.
Materials and methods
Protein expression, crystallization, structure determination, and refinement
The TruB crystallization construct (tTruB), differs from full-length E. coli TruB in having an N-terminal His-tag followed by residue 10 of the full-length protein (thus omitting the N-terminal sequence MSRPRRRGR). The plasmid encoding tTruB(D48N) was prepared from that encoding tTruB using the QuikChange kit (Stratagene). Sequencing of both DNA strands confirmed that the only mutation introduced was the desired one. Proteins were expressed and purified as described (Hoang and Ferré-D’Amaré 2001). The sequence, synthesis and purification of the 22-mer [f5U]RNA have been described (Hoang and Ferré-D’Amaré 2001).
tTruB(D48N) was cocrystallized with [f5U]-RNA essentially as described previously for tTruB. Initially, crystals were obtained by seeding with fragments of tTruB–RNA cocrystals. The resulting crystals were crushed and used as seeds for six subsequent sequential rounds of seeding into tTruB(D48N)– RNA complex crystallization experiments. The sequential rounds of seeding ensured that the crystal from which X-ray data were collected was comprised entirely of the mutant protein. Diffraction data (Table 1) were measured at beamline 5.0.1 of the Advanced Light Source (ALS) (Lawrence Berkeley National Laboratory), and reduced with the HKL package (Otwinowski and Minor 1997). The tTruB(D48N) cocrystals are isomorphous with the tTruB complex crystals (space group C2, a=145.4 Å, b=40.8 Å, c=78.8 Å, β=110.6°, one protein–RNA complex per asymmetric unit). A crystallographic model consisting of the tTruB complex (Protein Data Bank [PDB] code 1K8W) from which the nucleobase of RNA residue 55 and the side chain of amino acid 48, as well as all solvent and sulfate molecules were omitted, was refined against the mutant complex structure factor amplitudes using rigid body refinement and a maximum likelihood target using CNS (Brünger et al. 1998). This produced an Rfree factor of 29% (Rwork=22%). The model was subjected to a round of torsion-angle simulated annealing to minimize bias toward the tTruB structure. After manual rebuilding, solvent and sulfate ions were added, and the model refined by conjugate-gradient energy minimization. Individual isotropic temperature factor refinement was carried out with restraints optimized to minimize the Rfree factor. Finally, RNA residue 55 and protein residue 48 were built to produce a model with an Rfree factor of 26.4% (Table 1). Throughout the refinement, an overall anisotropic temperature factor correction and a solvent mask were used. As in the case in the tTruB cocrystal structure, the sugar pucker of nucleotides 54–57, and 59–60 was restrained to the C2′-endo conformation. Ramachandran analysis (Laskowski et al. 1993) of the crystallographic model shows 89.2% of the protein residues in the most favored region, 10.4% in additional allowed region, and 0.4% (one residue) in a generously allowed region. There are no residues in disallowed regions. Atomic coordinates and structure factor amplitudes have been deposited with the PDB (code 1ZL3). Figures were prepared with GRASP (Nicholls et al. 1993) and RIBBONS (Carson 1997). Superposition of the wild-type and mutant structures was carried out with the LSQ functions of the program O (Jones et al. 1991) using all Cα pairs. Superposition of the proteins omitting the thumb and PUA domains, as well as the poorly ordered two N-terminal Cα pairs does not markedly affect the conclusions. The RMS differences for all Cα atoms of the thumb calculated with the two superposition schemes differ by only ~2.5%.
Kinetic characterization of the TruB(D48N) mutant
The assay for Ψ synthase activity measures the release of tritium from [5-3H]uridine in RNA upon its isomerization to Ψ. Complete procedures have been published elsewhere (Ramamurthy et al. 1999). The substrate for all of the experiments was the in vitro transcript of E. coli tRNAPhe containing [5-3H]uridine ([5-3H]tRNA), which was prepared as described earlier (Ramamurthy et al. 1999). A standard assay mixture contained HEPES-KOH buffer (pH 7.5) (50 mM), NH4Cl (100 mM), EDTA (0.1 mM), DTT (5 mM), Prime RNase inhibitor (30 units), and [5-3H]tRNA (0.1–3.5 μM, 1.2 μCi/nmol tRNA); reaction was initiated by addition of tTruB (20 nM). To increase sensitivity in order to determine if tTruB(D48N) had low levels of activity, the assay was run at high concentrations of [5-3H]tRNA (1 μM) and tTruB(D48N) (1 μM) with a prolonged incubation time (2 h). All assays were run at 37°C in duplicate or triplicate, and the rates of tritium release varied less than 10%, which is within experimental error. Initial velocities were fit to the Briggs-Haldane equation using Delta-Graph 4.5 (SPSS, Inc.) to determine Vmax (and hence kcat) and Km. Errors in the kinetic parameters were estimated by fitting the extreme values of initial velocity and substrate concentration to the Briggs-Haldane equation.
Acknowledgments
We thank the staff of ALS beamline 5.0.1 and D. Goetz for help with diffraction data collection, and A. Roll-Mecak for discussions. This work was funded by the NIH (GM63576 to A.R.F. and GM59636 to E.G.M.) and the Rita Allen Foundation (A.R.F.) Access to ALS beamline 5.0.1 was made possible by general funds from the Fred Hutchinson Cancer Research Center. A.R.F. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051493605.
References
- Aravind, L. and Koonin, E.V. 1999. Novel predicted RNA-binding domains associated with the translation machinery. J. Mol. Evol. 48 291–302. [DOI] [PubMed] [Google Scholar]
- Boyd, R.J. 1977. The relative size of atoms. J. Phys. B At. Mol. Phys. 10 2283–2291. [Google Scholar]
- Brünger, A.T., Adams, P.D., Clore, G.M., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N.S., Read, R.J., et al. 1998. Crystallography and NMR system: A new software system for macromolecular structure determination. Acta Crystallogr. D 54 905–921. [DOI] [PubMed] [Google Scholar]
- Carson, M. 1997. Ribbons. Methods Enzymol. 277 493–505. [PubMed] [Google Scholar]
- Chaudhuri, B.N., Chan, S., Perry, L.J., and Yeates, T.O. 2004. Crystal structure of the apo forms of Ψ55 tRNA pseudouridine synthase from Mycobacterium tuberculosis: A hinge at the base of the catalytic cleft. J. Biol. Chem. 279 24585–24591. [DOI] [PubMed] [Google Scholar]
- Del Campo, M., Ofengand, J., and Malhotra, A. 2004. Crystal structure of the catalytic domain of RluD, the only rRNA pseudouridine synthase required for normal growth of Escherichia coli. RNA 10 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson, U.B., Nordlund, P., and Hallberg, B.M. 2004. X-ray structure of tRNA pseudouridine synthase TruD reveals an inserted domain with a novel fold. FEBS Lett. 565 59–64. [DOI] [PubMed] [Google Scholar]
- Ferré-D’Amaré, A.R. 2003. RNA-modifying enzymes. Curr. Opin. Struct. Biol. 13 49–55. [DOI] [PubMed] [Google Scholar]
- Foster, P.G., Huang, L., Santi, D., and Stroud, R.M. 2000. The structural basis for tRNA recognition and pseudouridine formation by pseudouridine synthase I. Nat. Struct. Biol. 7 23–27. [DOI] [PubMed] [Google Scholar]
- Gu, X., Yu,M., Ivanetich, K.M., and Santi, D.V. 1998. Molecular recognition of tRNA by tRNA pseudouridine 55 synthase. Biochemistry 37 339–343. [DOI] [PubMed] [Google Scholar]
- Gu, X., Liu, Y., and Santi, D.V. 1999. The mechanism of pseudouridine synthase I as deduced from its interaction with 5-fluorouracil-tRNA. Proc. Natl. Acad. Sci. 96 14270–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, C., Reid, R., Greene, P.J., and Santi, D.V. 1996. Identification of new RNA modifying enzymes by iterative genome search using known modifying enzymes as probes. Nucleic Acids Res. 24 3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, C.S., Spedaliere, C.J., Ginter, J.M., Johnston, M.V., and Mueller, E.G. 2005. The roles of the essential Asp-48 and highly conserved His-43 elucidated by the pH dependence of the pseudouridine synthase TruB. Arch. Biochem. Biophys. 433 322–334. [DOI] [PubMed] [Google Scholar]
- Hoang, C. and Ferré-D’Amaré, A.R. 2001. Cocrystal structure of a tRNA Ψ55 pseudouridine synthase: Nucleotide flipping by an RNA-modifying enzyme. Cell 107 929–939. [DOI] [PubMed] [Google Scholar]
- ———. 2004. Crystal structure of the highly divergent pseudouridine synthase TruD reveals a circular permutation of a conserved fold. RNA 10 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.Y., Cowan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47 110–119. [DOI] [PubMed] [Google Scholar]
- Kaya, Y. and Ofengand, J. 2003. A novel unanticipated type of pseudouridine synthase with homologs in bacteria archaea and eukarya. RNA 9 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, Y., Del Campo, M., Ofengand, J., and Malhotra, A. 2004. Crystal structure of TruD, a novel pseudouridine synthase with a new protein fold. J. Biol. Chem. 279 18107–18110. [DOI] [PubMed] [Google Scholar]
- Koonin, E.V. 1996. Pseudouridine synthases: Four families of enzymes containing a putative uridine-binding motif also conserved in dUTP-ases and dCTP deaminases. Nucleic Acids Res. 24 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski, R.J., Macarthur, M.W., Moss, D.S., and Thornton, J.M. 1993. PROCHECK: A program to check stereochemical quality of protein structures. J. Appl. Crystallogr. 26 283–290. [Google Scholar]
- Nicholls, A., Bharadwaj, R., and Honig, B. 1993. GRASP-graphical representation and analysis of surface properties. Biophys. J. 64 A116– A125. [Google Scholar]
- Nurse, K., Wrzesinski, J., Bakin, A., Lane, B.G., and Ofengand, J. 1995. Purification, cloning, and properties of the tRNA Ψ55 synthase from Escherichia coli. RNA 1 102–112. [PMC free article] [PubMed] [Google Scholar]
- Otwinowski, Z. and Minor, W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276 307–326. [DOI] [PubMed] [Google Scholar]
- Pan, H., Agarwalla, S., Moustakas, D.T., Finer-Moore, J., and Stroud, R.M. 2003. Structure of tRNA pseudouridine synthase TruB and its RNA complex: RNA recognition through a combination of rigid docking and induced fit. Proc. Natl. Acad. Sci. 100 12648–12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phannachet, K. and Huang, R.H. 2004. Conformational change of pseudouridine 55 synthase upon its association with RNA substrate. Nucleic Acids Res. 32 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy, V., Swann, S.L., Spedaliere, C.J., and Mueller, E.G. 1999. Role of cysteine residues in pseudouridine synthases of different families. Biochemistry 38 13106–13111. [DOI] [PubMed] [Google Scholar]
- Samuelsson, T. 1991. Interactions of transfer RNA pseudouridine synthases with RNAs substituted with fluorouracil. Nucleic Acids Res. 19 6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman, J., Sauve, V., Larocque, R., Stura, E.A., Schrag, J.D., Cygler, M., and Matte, A. 2002. Structure of the 16S rRNA pseudouridine synthase RsuA bound to uracil and UMP. Nat. Struct. Biol. 9 353–358. [DOI] [PubMed] [Google Scholar]
- Sivaraman, J., Iannuzzi, P., Cygler, M., and Matte, A. 2004. Crystal structure of the RluD pseudouridine synthase catalytic module, an enzyme that modifies 23S rRNA and is essential for normal cell growth of Escherichia coli. J. Mol. Biol. 335 87–101. [DOI] [PubMed] [Google Scholar]
- Spedaliere, C.J. and Mueller, E.G. 2004. Not all pseudouridine synthases are potently inhibited by RNA containing 5-fluorouridine. RNA 10 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedaliere, C.J., Hamilton, C.S., and Mueller, E.G. 2000. Functional importance of motif I of pseudouridine synthases: Mutagenesis of aligned lysine and proline residues. Biochemistry 39 9459–9465. [DOI] [PubMed] [Google Scholar]
- Spedaliere, C.J., Ginter, J.M., Johnston, M.V., and Mueller, E.G. 2004. The pseudouridine synthases: Revisiting a mechanism that seemed settled. J. Am. Chem. Soc. 126 12758–12759. [DOI] [PubMed] [Google Scholar]
- Williamson, J.R. 2000. Induced fit in RNA-protein recognition. Nat. Struct. Biol. 7 834–837. [DOI] [PubMed] [Google Scholar]