Abstract
The concentration of detergent in membrane protein preparations can have a critical role on protein stability, function, and the potential for crystallization. Unfortunately, dialysis or protein concentration can lead to an unknown amount of detergent in the final membrane protein preparations. Here we present a method for the determination of detergent concentration based on refractive index of the detergent solution. This method was applied to quantitate the amount of detergent remaining in solution after concentration in various concentrators. We found that the ability of the tested detergents to pass through the molecular weight cutoff membrane correlates well with detergent micelle size. Therefore, the micelle size can be used as a rough guide to estimate the retention of a given detergent in various molecular weight cutoff concentrators. The refractive index method is exceptionally informative when coupled with size exclusion chromatography and light scattering, and can be used to determine the oligomeric state of the membrane protein, the size of a protein-associated micelle, as well as the amount and size of the unbound detergent micelle.
Keywords: refractive index, detergent concentration, dn/dc, micelle size
It is estimated from the human genome that 30%–45% of human proteins are membrane-embedded or membrane- associated (Wallin and von Heijne 1998; Krogh et al. 2001) and that integral membrane proteins comprise more than 60%–70% of human drug targets (Ma and Zemmel 2002). Purification of membrane proteins requires their extraction from the plasma membrane and solubilization into detergent solution. These detergents interact with proteins to form protein–detergent complexes, thus preventing protein aggregation by coating the membrane-associated surface. In general, membrane proteins remain in the presence of detergents during subsequent purification steps, biochemical characterization, and crystallization. The concentration of detergent in membrane protein preparations can have a critical effect on protein stability (Fisher et al. 1999, 2003), function, and the potential for crystallization (Wiener 2004). Unfortunately, biochemical techniques such as dialysis or protein concentration can lead to a decrease or an increase in detergent concentration, ultimately resulting in an unknown detergent concentration in the final step of membrane protein preparations.
Several methods are available to determine the concentration of detergent in protein–detergent mixtures. These include colorimetric assays of glycosidic and bile salt-based detergents (Urbani and Warne 2005), thin layer chromatography (Eriks et al. 2003), and infrared spectroscopy (daCosta and Baenziger 2003). Here we present an additional method for the determination of detergent concentration in membrane protein preparations based on measuring the refractive index of the detergent solution. Unlike other methods, refractive index quantification is not limited to sugar-containing detergents (Urbani and Warne 2005) and does not require high concentrations of detergents (daCosta and Baenziger 2003; Eriks et al. 2003). Refractive index has been used for determination of concentrations of many compounds including glycerol, ethanol, acids, and salts (Lide 1994). Refractive index is also extensively used in the determination of guanidinium and urea concentrations used for protein denaturations. In this report, we show that refractive index can be used to characterize detergent and protein–detergent mixtures, and we apply this method to accurately quantitate the amount of detergent remaining in solution after concentration in commercially available concentrators. We also show that the refractive index method is both exceptionally informative and accurate when coupled with size exclusion chromatography and light scattering. This allows for separation of unbound detergent from a protein–detergent complex and determination of the concentration and the micelle size of the free detergent in solution.
Results and Discussion
Detergent standard curves and dn/dc values
We used refractive index measurements to directly quantitate the refractive index of several widely used detergents at various concentrations. In contrast to other methods, refractive index measurements allow the determination of standard curves for any detergent, independent of its chemical formula. Comparison of the unknown detergent sample with its standard curve yields the detergent concentration. Standard curves for polyoxyethylene(8)dodecyl ether (C12E9), n-dodecyl-β-D-maltopyranoside (DDM), n-tetradecylphosphocholine (FOS14), and lauryldimethylamine-N-oxide (LDAO) detergents are shown in Figure 1A ▶. The change of refractive index with detergent concentration appears to be linear in the concentration range used in this study. Detergent concentrations as low as 0.0075% were accurately measured for FOS14 detergent, indicating that this method is very sensitive and can be applied to detergents with low critical micelle concentration (CMC). In this work, we focused on low-to-medium CMC detergents, because high CMC detergents (such as octyl glucoside) can be generally dialyzed. Therefore, unlike low CMC detergents, the known concentration of highCMC detergent can be obtained by dialysis. It should be possible, however, to measure high CMC detergent concentrations with this method using lower-sensitivity detectors (such as the P2 cell from Wyatt Technology).
Figure 1.
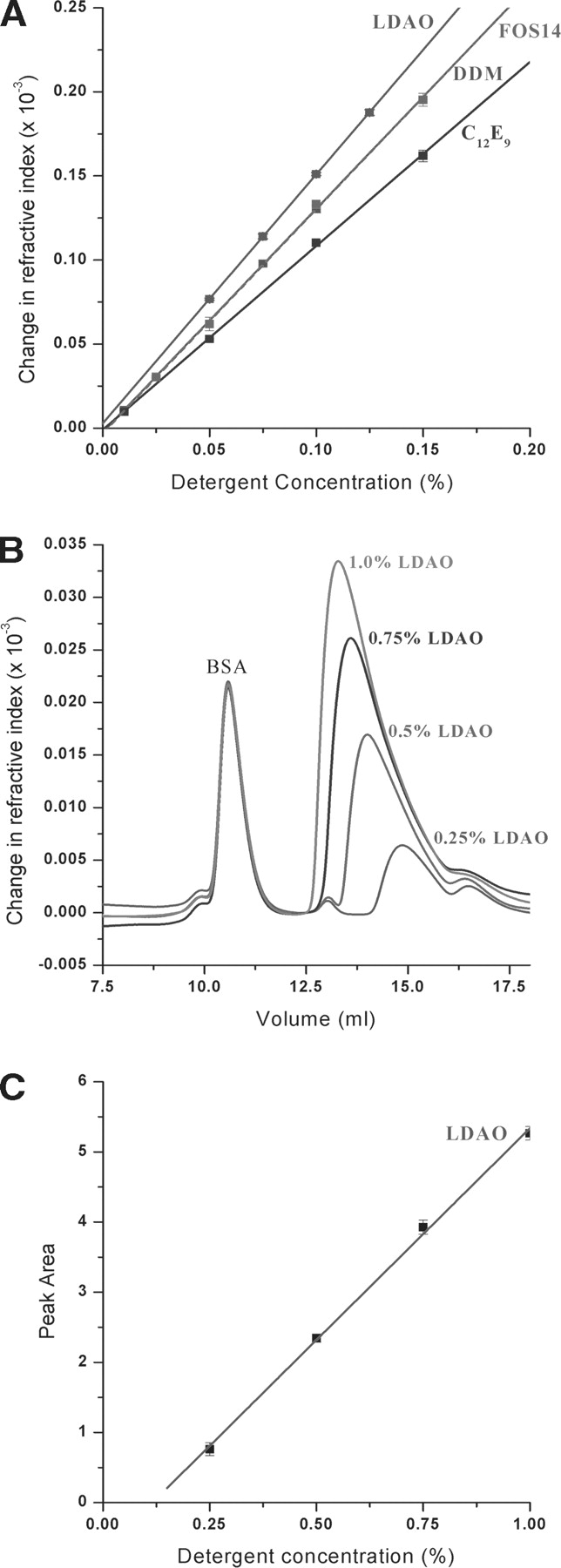
(A) Standard curves with error bars for commonly used detergents C12E9, DDM, FOS14, and LDAO. For some data points error bars are not visible, because error bars are smaller than the plotted data points. (B) Size exclusion chromatography coupled with refractive index determination. BSA was separated from excess detergent on a Shodex-804 size exclusion column, allowing for quantification of unbound detergent. Four separate chromatographs with increasing concentrations of LDAO are shown. (C) Standard curve for LDAO constructed by integrating the LDAO peak area shown in B.
Additional information provided by the slope of the standard curve of a particular detergent is the dn/dc value (change of refractive index as a function of concentration). The dn/dc value is needed in multi-angle laser light scattering (MALLS) experiments (Wyatt 1993) to determine the molecular weight of the scattering material. (For reviews of MALLS, see Wyatt 1993; Wen et al. 1996; Knobloch and Shaklee 1997; Folta-Stogniew and Williams 1999.) MALLS can be used to measure the molecular weight and oligomeric state of membrane proteins (Hayashi et al. 1989; Wen et al. 1996; Yernool et al. 2003), as well as to obtain the size of the protein-associated detergent micelle. However, to accurately analyze these measurements, it is necessary to obtain the detergent dn/dc value in addition to the known protein dn/dc value. Since the dn/dc values for most detergents are unknown, they must be determined experimentally (Fig. 1A ▶; Table 1). Once the detergent dn/dc value is obtained, it is possible to determine the relative molecular weight ratio of protein to detergent, and thus determine the size of the detergent micelle associated with the membrane protein.
Table 1.
Detergent data
| Detergent | dn/dc value | Literature micelle size (Da) | Determined micelle size (Da) |
| C12E9 | 0.109 | n/a | 83,000 ± 1600 |
| DDM | 0.133 | 70,000a | 72,000 ± 1400 |
| FOS-14 | 0.133 | n/a | 47,000 ± 1200 |
| LDAO | 0.148 | 17,000b | 21,500 ± 700 |
Size exclusion chromatography results
Determination of detergent concentration based on refractive index can also be accomplished in protein– detergent mixtures, although this requires that the free detergent is separated from the protein–detergent complex by size exclusion chromatography. Separation of detergent micelle from a protein–detergent complex might not be always possible if they are eluting closely together. However, since many membrane proteins exist as oligomers and the protein molecular weight is increased by associated detergent, detergent micelles often elute later than equivalent molecular weight proteins (data not shown). These properties together with the wide variety of available size exclusion columns make it possible to separate many membrane proteins from detergent micelles.
In our experiments, bovine serum albumin (BSA) was mixed with a known amount of detergent and injected onto a Shodex-804 size exclusion column, which was equilibrated in buffer containing LDAO detergent slightly above itsCMC. The protein was separated from unbound detergent, and the refractive index peak of the detergent was integrated. Figure 1B ▶ shows the size exclusion chromatograms at four different detergent concentrations. Integration of these peaks yielded the detergent standard curve shown in Figure 1C ▶. The extrapolation to zero peak area does not go through the origin most likely, because the column had to be equilibrated at a detergent concentration above the CMC in order for the detergent micelles not to fall apart after injection. Separation of the membrane protein synaptobrevin from unbound detergent micelles was also accomplished in several detergents (data not shown). The ability to measure the amount of uncomplexed detergent can be used to modify the purification protocol, thus obtaining preparations with higher functionality, reproducibility, membrane protein stability, and increasing the chance of membrane protein crystallization.
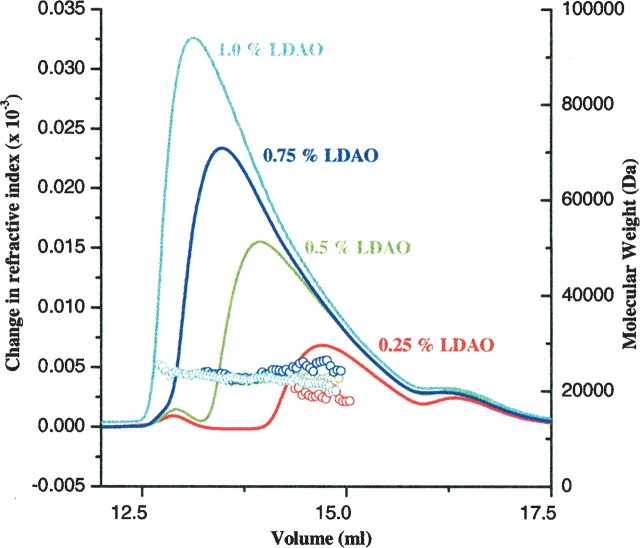
In addition to the quantification of uncomplexed detergent in protein–detergent mixtures, the molecular weight of the detergent micelle can be measured from the same experiment if the refractive index measurement is combined with light scattering (Wyatt Technology). Since C12E9 and FOS14 detergent micelle sizes are not available, we used light scattering to determine their molecular weights. Using this method and the dn/dc values obtained from the detergent standard curves (described above), we measured the molecular weights of micelles of C12E9, DDM, FOS14, and LDAO (Table 1). The micelle size reported in literature for DDM (70 kDa) (VanAken et al. 1986) and LDAO (17 kDa) (Herrmann 1962) correlate well with our determination (DDM 74 ± 1.4 kDa; LDAO 21.5 ± 0.7 kDa). The micelle sizes of C12E9 and FOS14 detergents have not been measured previously (Anatrace, pers. comm.), and are 83 ± 1.6 kDa (C12E9), and 47 ± 1.2 kDa (FOS14) in buffer containing 25 mM Tris pH 8.0 and 100 mM NaCl (Table 1). The size of some detergent micelles might be dependent on concentration and temperature (Corti et al. 1984). In our experiments, LDAO micelle size is monodisperse in molecular weight across the elution peak, and is not significantly influenced by increase in detergent concentration in the tested range (0.25%–1.0%) (Fig. 2 ▶). The MALLS-determined molecular weights were 20.2 ± 0.8 kDa, 21.8 ± 0.4 kDa, 21.7 ± 1.0 kDa, and 22.1 ± 0.9 kDa for 0.25%, 0.5%, 0.75%, and 1.0% LDAO, respectively. Although the molecular weight of the LDAO micelles does not change with concentration, the retention time is concentration-dependent. We speculate that this could be due to the interaction of detergent micelles with Shodex-804 resin. When small amounts of detergent are passed over the column, the micelles interact with the silica resin and elute at later times. As the detergent concentration increases, the interactions of micelles with column resin are shielded by other micelles, and therefore detergent micelles pass through the column more quickly and elute at earlier times (Fig. 2 ▶).
Figure 2.
Molecular weight distribution profile of LDAO micelles. Size exclusion chromatograms of LDAO micelles are shown at four concentrations (0.25% LDAO, red; 0.5% LDAO, green; 0.75% LDAO, blue; 1.0% LDAO, cyan). The circles indicate the molecular weight of the eluting species across the elution peak as determined by MALLS combined with refractive index measurements.
Concentrator results
Concentration of membrane proteins via protein concentrators generally leads to the undesired effect of an increase in detergent concentration in the protein sample. Excess detergent in membrane protein samples can interfere with activity assays and crystallization experiments (Wiener 2004), or destabilize native protein–protein interactions (Fisher et al. 1999, 2003). Previous reports have shown that some maltosides (dodecyl-, undecyl-, and decyl-) concentrate in 30-kDa and 50-kDa cutoff concentrators, but do not concentrate in 100-kDa concentrators (Urbani and Warne 2005). Cholate was also observed not to concentrate in a 100-kDa concentrator (daCosta and Baenziger 2003). We characterized the behavior of C12E9, DDM, FOS14, and LDAO in 30-kDa and 100-kDa cutoff concentrators using our refractive index method. Detergent solutions above the CMC (1.5–2.2-fold) were concentrated 10-fold, and both the filtrate and concentrates were analyzed. The results are shown in Table 2. C12E9, DDM, and FOS14 detergents showed increases in concentration of 3.0–4.7-fold in 30-kDa cutoff concentrators. The concentration of LDAO, however, did not increase in a 30-kDa cutoff concentrator. When 100-kDa cutoff concentrators were used, none of the tested detergents showed any increase in concentration. The ability of the tested detergents to pass through the molecular weight cutoff membrane correlates well with their micelle size. The micelle size of LDAO was reported to be 17 kDa (Herrmann 1962), and LDAO passes through both the 30-kDa and 100-kDa concentrators. DDM micelles, estimated to be 70 kDa (VanAken et al. 1986) in size, only pass through 100-kDa concentrators and are significantly retained in 30-kDa cutoff concentrators. C12E9 and FOS14 detergent micelles are 82 kDa and 46 kDa, respectively, and both are retained by the 30-kDa, but not the 100-kDa concentrators. Therefore, the micelle size determined from light scattering or obtained fromthe literature can be used as a rough guide to estimate the retention of given detergent in various molecular weight cutoff concentrators. These results should prove valuable to many laboratories working with membrane proteins.
Table 2.
Amicon concentrator data
| Detergent | CMC × 10−3 (%) | Starting concentrationa × 10−3 (%) | Amicon 30 kDa filtrate × 10−3 (%) | Amicon 30 kDa 10X concentrate × 10−3 (%) | Amicon 30 kDa 10X concentrate | Recoveryb 30 kDa |
| C12E9 | 5.8 | 10.0 | 3.5 ± 0.5 | 47.0 ± 1.0 | 4.70-fold | 79% |
| DDM | 8.7 | 13.5 | 9.0 ± 0.0 | 41.0 ± 1.0 | 3.04-fold | 90% |
| Fos-14 | 4.6 | 10.0 | 5.0 ± 0.5 | 40.5 ± 0.5 | 4.05-fold | 86% |
| LDAO | 34.3 | 50.0 | 44.0 ± 0.0 | 47.0 ± 1.0 | 0.94-fold | 89% |
| Amicon 100 kDa filtrate × 10−3 (%) | Amicon 100 kDa 10X concentrate × 10−3 (%) | Amicon 100 kDa 10X concentrate | Recoveryb 100 kDa | |||
| C12E9 | 5.8 | 10.0 | 8.0 ± 0.0 | 9.5 ± 0.5 | 0.95-fold | 82% |
| DDM | 8.7 | 13.5 | 12.0 ± 0.0 | 13.0 ± 0.0 | 0.96-fold | 89% |
| Fos-14 | 4.6 | 10.0 | 8.5 ± 0.5 | 9.5 ± 0.5 | 0.95-fold | 94% |
| LDAO | 34.3 | 50.0 | 46.0 ± 0.0 | 48.0 ± 0.0 | 0.96-fold | 93% |
a Starting concentration was chosen to be above the critical micelle concentration.
b Percentage of detergent recovered from both the filtrate and retentate.
In the concentrators that allowed detergent micelles to pass freely, the final concentration was surprisingly somewhat lower than the starting concentration. The manufacturer of A micon filter devices reports protein recovery of around 90%. The recovery of detergents in our experiments (estimated by measuring both the filtrate and retentate detergent solution) was 79%–90% for 30-kDa, and 82%–94% for 100-kDa concentrators (Table 2). The loss of detergent is most likely due to detergent binding to the concentrator membrane. When using detergent concentrations very close to the CMC value, it might be necessary to take into account the nonspecific binding of detergents to the concentrator membrane and increase the detergent concentration accordingly.
In summary, refractive index measurements can be used in membrane protein research to determine the concentration of detergents and to estimate the amount of unbound detergent in membrane protein preparations. The high accuracy and sensitivity of the refractive index can be used in membrane protein purification to improve protein preparative protocols and should yield membrane protein preparations with improved function, stability, reproducibility, and an increased chance of crystallization. Unlike other methods, refractive index detectors are highly accurate, and can be operated “in-line” with liquid chromatography systems. When used in combination with size exclusion chromatography and light scattering, the oligomeric state of the membrane protein, the size of protein-associated micelle, and the amount and size of the unbound detergent micelle can be determined. The information provided by these methods makes them a powerful tool in membrane protein research.
Materials and methods
All detergents were obtained from Anatrace. Refractive index measurements were performed using an OPTILAB DSP instrument (Wyatt Technology) with a P10 cell. For lower or higher sensitivity, P2 or P100 cells are also available from Wyatt Technology. The standard curves for C12E9, DDM, FOS14, and LDAO were obtained by injecting 5 mL of detergent solution through the refractometer at 0.5 mL/min. Due to the sensitivity of refractive index to temperature, the cell temperature was maintained at 35°C. Two measurements were obtained for each detergent concentration. For concentrator studies, initial detergent concentrations of 0.01% C12E9, 0.0135% DDM, 0.01% FOS14, and 0.05% LDAO in water were used. The solutions were concentrated 10-fold in Amicon 30-kDa and 100-kDa concentrators, and analyzed by refractometry. All measurements were performed in duplicates.
For the determination of excess detergent in protein–detergent mixtures, bovine serum albumin (1 mg/mL) was mixed with 0.25%, 0.5%, 0.75%, and 1.0% LDAO in 25 mM Tris-HCl pH 8.0, 100 mM NaCl, and 100 μL was passed over a size exclusion column (Shodex-804, 0.5 mL/min) prior to the refractive index measurement. The Shodex- 804 column was pre-equilibrated in the same buffer containing 0.05% LDAO. The standard curve was obtained by integrating the LDAO peak area and plotting the area as a function of detergent concentration (Fig. 1C ▶). A DAWN EOS (Wyatt Technology) with a K5 flow cell, and a 690-nm wavelength laser was used in light scattering experiments. Astra software (Wyatt Technology) was used to analyze the light scattering data.
Acknowledgments
We thank Timothy D. Fenn, Marija Vrljic, and Michael R. Brzustowicz for their helpful discussions and reading of this manuscript.
Abbreviations
C12E9, polyoxyethylene(8)dodecyl ether
DDM, n-dodecyl-β-D-maltopyranoside
FOS14, n-tetradecylphosphocholine
LDAO, lauryldimethylamine-N-oxide
CMC, critical micelle concentration
dn/dc, change of refractive index as a function of concentration
MALLS, multi-angle laser light scattering
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051543805.
References
- Corti, M., Minero, C., and Degiorgio, V. 1984. Cloud point transition in nonionic micellar solutions. J. Phys. Chem. 88 309. [Google Scholar]
- daCosta, C.J. and Baenziger, J.E. 2003. A rapid method for assessing lipid:protein and detergent:protein ratios in membrane–protein crystallization. Acta Crystallogr. D Biol. Crystallogr. 59 77–83. [DOI] [PubMed] [Google Scholar]
- Eriks, L.R., Mayor, J.A., and Kaplan, R.S. 2003. A strategy for identification and quantification of detergents frequently used in the purification of membrane proteins. Anal. Biochem. 323 234–241. [DOI] [PubMed] [Google Scholar]
- Fisher, L.E., Engelman, D.M., and Sturgis, J.N. 1999. Detergents modulate dimerization, but not helicity, of the glycophorin A transmembrane domain. J. Mol. Biol. 293 639–651. [DOI] [PubMed] [Google Scholar]
- Fisher, L.E., Engelman, D.M., and Sturgis, J.N. 2003. Effect of detergents on the association of the glycophorin a transmembrane helix. Biophys. J. 85 3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta-Stogniew, E. and Williams, K.R. 1999. Implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 10 51–63. [PMC free article] [PubMed] [Google Scholar]
- Hayashi, Y., Matsui, H., and Takagi, T. 1989. Membrane protein molecular weight determined by low-angle laser light-scattering photometry coupled with high-performance gel chromatography. Methods Enzymol. 172 514–528. [DOI] [PubMed] [Google Scholar]
- Herrmann, K.W. 1962. Non-ionic-cationic micellar properties of dimethyldodecylamine oxide. J. Phys. Chem. 66 295–300. [Google Scholar]
- Knobloch, J.E. and Shaklee, P.N. 1997. Absolute molecular weight distribution of low-molecular-weight heparins by size-exclusion chromatography with multiangle laser light scattering detection. Anal. Biochem. 245 231–241. [DOI] [PubMed] [Google Scholar]
- Krogh, A., Larsson, B., von Heijne, G., and Sonnhammer, E.L. 2001. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 305 567–580. [DOI] [PubMed] [Google Scholar]
- Lide, D.R. 1994. Handbook of chemistry and physics, 74th ed. CRC Press, Boca Raton, LA.
- Ma, P. and Zemmel, R. 2002. Value of novelty? Nat. Rev. Drug Discov. 1 571–572. [DOI] [PubMed] [Google Scholar]
- Urbani, A. and Warne, T. 2005. A colorimetric determination for glycosidic and bile salt-based detergents: Applications in membrane protein research. Anal. Biochem. 336 117–124. [DOI] [PubMed] [Google Scholar]
- VanAken, T., Foxall-VanAken, S., Castleman, S., and Ferguson-Miller, S. 1986. Alkyl glycoside detergents: Synthesis and applications to the study of membrane proteins. Methods Enzymol. 125 27–35. [DOI] [PubMed] [Google Scholar]
- Wallin, E. and von Heijne, G. 1998. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 7 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J., Arakawa, T., and Philo, J.S. 1996. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal. Biochem. 240 155–166. [DOI] [PubMed] [Google Scholar]
- Wiener, M.C. 2004. A pedestrian guide to membrane protein crystallization. Methods 34 364–372. [DOI] [PubMed] [Google Scholar]
- Wyatt, P.J. 1993. Light scattering and the absolute characterization of macromolecules. Analytica Chimica Acta 272 1–40. [Google Scholar]
- Yernool, D., Boudker, O., Folta-Stogniew, E., and Gouaux, E. 2003. Trimeric subunit stoichiometry of the glutamate transporters from Bacillus caldotenax and Bacillus stearothermophilus. Biochemistry 42 12981–12988. [DOI] [PubMed] [Google Scholar]