Abstract
Study Objectives:
The upper airway compensatory response to subatmospheric pressure loading declines with age. The epidemiology of obstructive sleep apnea suggests that sex hormones play a role in modulating upper airway function. Sex hormones increase gradually during puberty, from minimally detectable to adult levels. We hypothesized that the upper airway response to subatmospheric pressure loading decreased with increasing pubertal Tanner stage in males but remained stable during puberty in females.
Design:
Upper airway dynamic function during sleep was measured over the course of puberty.
Participants:
Normal subjects of Tanner stages 1 to 5.
Measurements:
During sleep, maximal inspiratory airflow was measured while varying the level of nasal pressure. The slope of the upstream pressure-flow relationship (SPF) was measured.
Results:
The SPF correlated with age and Tanner stage. However, the relationship with Tanner stage became nonsignificant when the correlation due to the mutual association with age was removed. Females had a lower SPF than males.
Conclusions:
In both sexes, the upper airway compensatory response to subatmospheric pressure loading decreased with age rather than degree of pubertal development. Thus, changes in sex hormones are unlikely to be a primary modulator of upper airway function during the transition from childhood to adulthood. Although further studies of upper airway structural changes during puberty are needed, we speculate that the changes in upper airway function with age are due to the depressant effect of age on ventilatory drive, leading to a decrease in upper airway neuromotor tone.
Citation:
Bandla P; Huang J; Karamessinis L; Kelly A; Pepe M; Samuel J; Brooks L; Mason TA; Gallagher PR; Marcus CL. Puberty and Upper Airway Dynamics During Sleep. SLEEP 2008;31(4):534-541.
Keywords: Pcrit, obstructive sleep apnea, children
NORMAL CHILDREN HAVE FEWER OBSTRUCTIVE APNEAS DURING SLEEP THEN DO NORMAL ADULTS.1,2 CONSISTENT WITH THIS CLINICAL FINDING, WE HAVE previously shown that normal children are able to maintain near-constant inspiratory airflow despite the application of increasing subatmospheric nasal pressure loads during sleep, i.e., the pediatric upper airway appears to dynamically regulate airflow.3–5 This compensatory response to upper airway subatmospheric pressure loading declines with age.4,5 However, the upper airway response to subatmospheric pressure loading has not been studied in detail during puberty and adolescence, the transitional period from childhood to adulthood.
In children, most community-based studies have shown that the prevalence of obstructive sleep apnea syndrome (OSAS) is similar amongst boys and girls,6–9 although this is somewhat controversial, as 1 study showed a higher prevalence of obstructive apneas in infant boys than girls,10 and some studies have shown more snoring in boys than in girls.11,12 In adults, the prevalence of OSAS in men is about 3 times that of premenopausal women.13 The prevalence of OSAS then increases in women after menopause.14 This epidemiology suggests that sex hormones play a critical role in upper airway function, with male sex hormones associated with increased upper airway collapsibility and female sex hormones having a protective effect. During puberty, sex hormone levels progress from minimally detectable to adult levels, making puberty the ideal natural physiologic model with which to determine the role of sex hormones on upper airway function during sleep. We therefore studied upper airway dynamic function during sleep in males and females at different stages of puberty. Our initial hypothesis was that the upper airway compensatory response to a subatmospheric pressure load declines with increasing pubertal Tanner stage in males but remains stable during puberty in females.
For this study, we measured the upper airway pressure-flow relationship during sleep, using techniques similar to those previously used to evaluate the upper airway in children and adults.3–5,15–20 This approach is based on the concept that the upper airway functions as a simple collapsible tube, as predicted by the Starling resistor model.21 According to this model, under conditions of flow limitation, maximal inspiratory airflow is determined by the pressure changes upstream (nasal) to a collapsible locus of the upper airway and is independent of the downstream (hypopharyngeal) pressure generated by the diaphragm. The upper airway can be represented as a tube with a collapsible segment, the resistance of which is 0. The segments upstream and downstream from the collapsible segment have fixed diameters and resistances. Upstream (nasal) resistance (RN) can be determined by calculating the reciprocal of the slope of the pressure-flow curve. In this model of the upper airway, inspiratory pressure at the nares is atmospheric and downstream pressure is equal to hypopharyngeal/tracheal pressure. Collapse would occur when the pressure surrounding the collapsible segment of the upper airway (critical tissue pressure, Pcrit) becomes greater than the pressure within the collapsible segment of the airway. In the normal subject with low upstream resistance or subatmospheric Pcrit, who is breathing at atmospheric pressure, the downstream pressure never drops to Pcrit; thus, airflow is not limited and is largely determined by negative tracheal (inspiratory) pressure. However, if the downstream pressure falls below Pcrit, inspiratory flow (VImax) reaches a maximum (inspiratory airflow limitation) and becomes independent of downstream pressure swings. Under these circumstances, RN and Pcrit determine maximal inspiratory flow, as described by the following equation: VImax = (PN − Pcrit)/RN. The slope of the pressure-flow curve (SPF) represents the conductance of the upper airway (1/RN). Airflow will become 0 (i.e., the airway will occlude) when PN falls below Pcrit. Thus, both Pcrit and SPF can be used to characterize the flow response to changes in PN. This is analogous to using both the slope and X-intercept of the minute ventilation-Pco2 curve in order to characterize the sensitivity of the ventilatory response to hypercapnia. Previous studies in children have found a very flat SPF. This precludes the determination of Pcrit in many subjects, as airway collapse does not occur even at maximal subatmospheric PN.3–5 Therefore, pediatric studies have used the SPF as the primary outcome parameter.
METHODS
Study Group
Normal healthy subjects aged 8 to 18 years were recruited from the general community by means of advertisements. Subjects with a history of nightly snoring, adenoidectomy and/or tonsillectomy, obesity (body mass index > 95th percentile for age, race and sex22), or with medical conditions requiring daily medications were excluded. The Children's Hospital of Philadelphia Institutional Review Board for human studies approved the protocol. Informed consent was obtained from 18-year-old subjects and the parents or guardians of subjects younger than 18 and assent from those subjects younger than 18 years.
Study Design
Subjects underwent baseline polysomnography to ensure that they did not have a sleep disorder, using standard pediatric techniques,23–25 as previously described for our laboratory.15 Pubertal staging was performed by trained male and female investigators using the method of Tanner of genital development and pubic hair distribution for boys and breast development for girls.26 Tanner stage was corroborated with serum hormone levels, which were obtained first thing in the morning following the polysomnogram in order to minimize circadian variability. Ultrasensitive luteinizing hormone levels were obtained in all subjects, follicular stimulating hormone and estradiol in females, and testosterone in males. On a separate night, the subject underwent repeat monitoring with determination of upper airway pressure-flow responses.
Pressure-Flow Measurements
Pressure-flow relationships were measured during a second overnight polysomnogram, using previously published techniques.4,5,15 Routine polysomnographic measurements were obtained. In addition, the subject wore a gel continuous positive airway pressure nasal mask (Respironics, Murrysville, PA) attached to a heated pneumotachometer (Hans Rudolph, Inc., Kansas City, MO) and pressure transducer (Validyne Engineering Corp., Northridge, CA). A thermistor measured airflow at the mouth in order to assess for mouth breathing. Nasal pressure (PN) was measured at the mask, using a differential pressure transducer referenced to atmosphere. Signals were acquired on a PowerLab system (ADInstruments, Colorado Springs, CO) and simultaneously displayed on a Rembrandt polysomnography system (Embla, Denver, CO). PN was altered in either a positive or subatmospheric direction using a device provided by Respironics.4,5,15 A toggle switch allowed the patient to be switched rapidly between positive and negative nasal pressure, ranging from −25 to +30 cm H2O.
Measurements were performed preferentially during slow wave sleep, as subjects are least likely to arouse during this stage. If this was not possible due to insufficient amounts of slow wave sleep during the night, measurements were performed during stage 2 sleep. It has been shown that there is no difference in pressure-flow relationships using these techniques between stage 2 and slow wave sleep.4 Studies were initiated with the subject breathing continuous positive airway pressure of 2 cm H2O, which in all cases was sufficient to overcome inspiratory airflow limitation. Flow limitation was determined by the characteristic waveform pattern, consisting of increasing inspiratory flow followed by a midinspiratory plateau,3,5,15,27 rather than by using invasive esophageal pressure measurements in these young volunteers.28 PN was decreased in a stepwise fashion by 2-cm H2O decrements every 30 seconds until flow approached 0 or the subject aroused from sleep.
Data Analysis
The average midinspiratory flow was measured from the lowest 2 consecutive breaths at each level of PN.5,15 Pressure-flow curves were constructed by plotting maximal inspiratory airflow (VImax) of flow-limited breaths against PN. PN versus V· curves were fitted by least squares linear regression. The SPF was the primary outcome parameter. The critical closing pressure (Pcrit) was defined as the X-axis intercept of the regression line (VImax = 0). Because many pediatric subjects are able to maintain airflow even at markedly subatmospheric pressures, the X-intercept cannot always be determined without extreme extrapolation.3–5,15 Therefore, as in previous studies, we arbitrarily assigned a threshold value of −25 cm H2O (the lowest PN deliverable by our equipment) to Pcrit data that were extrapolated to less than −25 cm H2O.5,15 This allowed us to apply statistical methods to the Pcrit data, although it resulted in a floor effect and, hence, an underestimation of differences between groups. For this reason, SPF rather than Pcrit was the primary outcome measure.
Statistical Analysis
Histograms and 1-sample Kolmogorov-Smirnov tests indicated that SPF and Pcrit did not have normal distributions; thus, nonparametric statistics were used. In order to delineate the effects of age, Tanner stage and height on SPF and Pcrit, 0-order Spearman correlation coefficients were calculated, followed by partial correlation coefficients. A partial correlation is the correlation that remains between 2 variables after removing the correlation that is due to their mutual association with some other variable or variables. Differences in groups with Pcrit values greater than −25 cm H2O or less than −25 cm H2O were determined using Mann-Whitney rank sum tests, as were differences in SPF, Pcrit, and height between males and females To examine possible sex and age interaction effects, SPF and Pcrit data were ranked, and separate analysis of covariance (ANCOVA) models were used on the ranked data. In these “nonparametric” ANCOVAs on ranked data, sex was included as the factor and age (measured continuously) as a covariate and the factor × covariate interaction term was included in the initial models. Differences in SPF and Pcrit among the different races, as well as differences among adolescents and other age groups from previous studies, were compared using the Kruskal-Wallis 1-way analysis of variance on ranks, and Mann-Whitney tests were used for pairwise comparisons.
RESULTS
Study Group
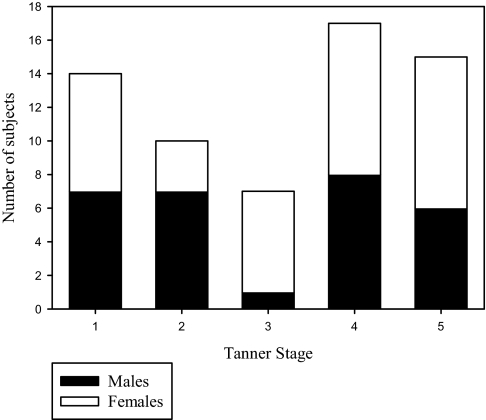
Sixty-nine subjects were recruited. Five subjects did not return for the pressure-flow response studies (2 were excluded due to an elevated number of periodic limb movements on the baseline polysomnogram, 1 was excluded due to very poor sleep efficiency at baseline, and 2 declined). A further subject was excluded because she was taking oral contraceptives. Thus, 63 subjects completed the study. Subject characteristics are shown in Table 1. The number of subjects of each sex at each Tanner stage are shown in Figure 1. Tanner staging was consistent with hormone levels in 97% of subjects (the remaining 3% were assigned using the clinical Tanner staging). There was no significant difference in age (13 ± 3 vs 13 ± 3 years, P = 0.588), Tanner stage (P = 0.405), or height (160 ± 18 vs 154 ± 11 cm, P = 0.215) between males and females.
Table 1.
Subject Demographics and Baseline Polysomnographic Characteristics
| Total, no. | 63 |
|---|---|
| Age, y, mean ± SD (range) | 13 ± 3 (8 – 18) |
| Females, no. (%) | 33 (52) |
| Race, no. (%) | |
| African American | 32 (51) |
| Caucasian | 19 (30) |
| Asian | 7 (11) |
| Hispanic | 2 (3) |
| Mixed | 3 (5) |
| BMI, Z-score | 0.6 ± 0.6 |
| AHI, no/h | 0.4 ± 0.5 |
| Sao2 nadir, % | 93 ± 2 |
| Peak end-tidal Pco2, mm Hg | 49 ± 5 |
Data are presented as mean ± SD unless otherwise indicated. BMI refers to body mass index; AHI, apnea-hypopnea index.
Figure 1.
The number of male and female subjects at each Tanner stage is shown.
Pressure-Flow Relationships
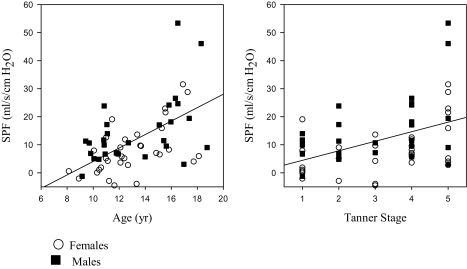
Seventy-three percent of runs were performed in slow wave sleep; the remainder were in stage 2. The mean PN at which flow limitation first occurred was 0 ± 2 cm H2O for both males and females. Results are shown in Table 2 and Figure 2.
Table 2.
Correlation Coefficients and P Values for the Pressure-Flow Relationships
| Pressure-flow relationship | Age |
Tanner stage |
||
|---|---|---|---|---|
| r | P value | r | P value | |
| SPF | 0.46 | 0.000* | 0.34 | 0.007* |
| Pcrit | 0.34 | 0.007* | 0.31 | 0.015* |
SPF refers to the slope of the pressure-flow relationship; Pcrit, critical closing pressure
P value is significant. See text for partial correlation results.
Figure 2.
The correlations between the slope of the pressure-flow responses (SPF) and age and Tanner stage are shown. See text for significance.
The SPF had a statistically significant linear relationship with both age and Tanner stage. However, the correlation between SPF and age remained significant even after removing the correlation that was due to their mutual association with Tanner stage (r = 0.35, P = 0.005), whereas the correlation between SPF and Tanner stage became nonsignificant when the correlation that was due to their mutual association with age was removed (r = −0.13, P = 0.315).
Fifty-six percent of subjects had a Pcrit less than −25 cm H2O; i.e., Pcrit was too negative to be extrapolated, indicating that the subjects were able to maintain near-constant inspiratory airflow despite increasing subatmospheric nasal pressure. When subjects were dichotomized as having either a Pcrit greater than −25 cm H2O or less than or equal to −25 cm H2O, there was a trend for subjects with a Pcrit less than −25 cm to be younger (12.6 ± 2.7 vs 13.9 ± 2.8 years), but this did not reach statistical significance (P = 0.079).
Pcrit had a statistically significant linear relationship with both age and Tanner stage (Table 2). However, the partial correlation between Pcrit and age became statistically nonsignificant (r = 0.158, P = 0.219) after removing the correlation that was due to their mutual association with Tanner stage. Likewise, the correlation between Pcrit and Tanner stage disappeared when the correlation due to their mutual association with age was removed (r = 0.024, P = 0.851). It should be noted, however, that these results were influenced by the floor effect of assigning a Pcrit of −25 cm H2O to those subjects with very negative extrapolated Pcrit values. To further assess this, analyses were repeated using an extrapolated Pcrit threshold of −100 cm H2O (realizing that this was nonphysiologic, in that it far exceeded the subatmospheric pressure deliverable by the study equipment). Only 14% of subjects (all less than 14 years of age; two-thirds at Tanner stage 1) still exceeded the cutoff Pcrit. Using this cutoff, there was a statistically significant linear relationship between Pcrit and both age and Tanner stage (r = 0.43, P < 0.0005 for age and r = 0.35, P = 0.005 for Tanner stage). However, the correlation between Pcrit and age remained even after removing the correlation that was due to their mutual association with Tanner stage (Spearman partial correlation = 0.274, P = 0.031), whereas the correlation between Pcrit and Tanner stage disappeared when the correlation that was due to their mutual association with age was removed.
Effect of Sex
When males and females were evaluated separately, the partial correlations between Tanner stage, age, and pressure-flow relationship parameters were not significant, probably because of the smaller numbers. However, females had a significantly lower SPF than males (Table 3). There were no statistically significant differences in Pcrit (using either the −25 cm H2O or the −100 cm H2O cutoffs) between the sexes. There were no significant correlations between hormone levels and pressure-flow response parameters in either males or females.
Table 3.
Pressure-Flow Relationships by Sex
| Parameter | Females | Males | P values |
|---|---|---|---|
| SPF, mL·s−1·cm H2O−1 | 5.9 (−7.2, 31.5) | 11.0 (−1.2, 64.3) | 0.005* |
| Pcrit, cm h2o | −25.0 (−25.0, −5.7) | −23.5 (−25.0, −2.8) | 0.352 |
Data are presented as median (range). SPF refers to the slope of the pressure-flow relationship; Pcrit, critical closing pressure.
P value is significant.
The ANCOVA for age × sex interaction was not statistically significant for either SPF (P = 0.520) or Pcrit (using a cutoff of −25 cm H2O) (P = 0.927). When the interaction terms were removed from these models, there was a statistically significant main effect of age on both SPF (P < 0.0005) and Pcrit (P = 0.015), and there was a significant main effect of sex on SPF (P = 0.004) but not on Pcrit (P = 0.440).
Effect of Other Factors
There were no changes in any of the above relationships when SPF was corrected for height. There was no difference in SPF or Pcrit among the different races, although it should be noted that the number of subjects of each race was low, with 81% of the subjects being either African American or Caucasian.
Comparison with Other Age Groups
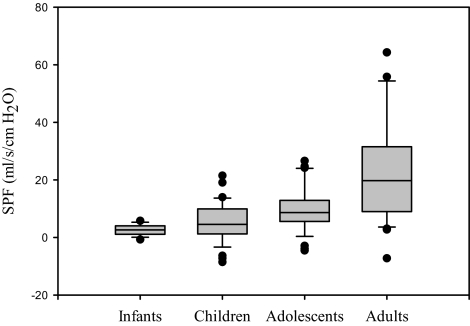
The SPF data from this study were compared with data on infants, school-aged children, and adults, acquired by the investigators in previous studies using identical methods.5 Tanner 1 subjects were grouped with the school-aged children from the previous study, Tanner 2 to 4 subjects were considered adolescents, and Tanner 5 subjects were grouped with the adults. Box plots of the data are shown in Figure 3. Kruskal-Wallis tests indicated a statistically significant difference among the age groupings (P < 0.0005). Pairwise tests were conducted with Mann-Whitney tests using the Bonferroni correction factor (0.05/6), which would require a P value of less than 0.0083. Results indicated that adults had a significantly greater SPF than any of the pediatric age groups (P < 0.0005, P < 0.0005, and P = 0.001, respectively, for infants, children, and adolescents). Adolescents had a greater SPF compared with infants (P < 0.0005), but the difference between adolescents and children did not reach statistical significance based on the Bonferroni correction factor (P = 0.009). The difference between infants and children did not reach statistical significance (P = 0.142).
Figure 3.
The slopes of the pressure-flow responses (SPF) for infants, children, adolescents, and adults are shown. Data are derived from results from the current study and a previously published study.5 The boundaries of the boxes indicate the 25th and 75th percentiles, the line within the boxes marks the medians, the whiskers indicate the 90th and 10th percentiles, and the points represent the outliers. Adults had a significantly greater SPF than any of the pediatric age groups (P < 0.0005, P < 0.0005, and P = 0.001, respectively, for infants, children, and adolescents). Adolescents had a greater SPF compared with infants (P < 0.0005) but not compared with children.
DISCUSSION
This study has shown that age, rather than Tanner stage, is the prime determinant of the upper airway response to subatmospheric pressure loads during sleep. The finding of a flatter SPF in younger children indicates that the subjects are able to maintain near constant inspiratory airflow despite increasingly subatmospheric nasal pressure, i.e., that their upper airway appeared to dynamically regulate airflow. Males in the pubertal years have less of a response to subatmospheric pressure loading than do females.
Causes of Changes in Upper Airway Function During Development
Children snore less than do adults and have fewer obstructive apneas during sleep.1 Consistent with this clinical finding, the upper airway in normal children is very resistant to collapse during sleep, compared with that of adults.4 Theoretically, puberty would be a logical time for the transition from the pediatric to the adult pattern of upper airway collapsibility. This transition could be due to a number of factors: hormone-related changes, growth-related changes, or other changes related to neural development, such as changes in the ventilatory drive.
Effects of Sex Hormones on Upper Airway Function During Sleep
Sex hormones influence the ventilatory drive, apneic threshold, upper airway structure, and upper airway collapsibility.29–35 In prepubertal children, most studies indicate that OSAS occurs equally among boys and girls.6 To the best of our knowledge, there have been no population-based studies evaluating the relationship between the prevalence of OSAS and sex in adolescents. The flatter SPF noted in adolescent girls in this study suggests that sex-related differences in upper airway function during sleep may begin during adolescence. In adults, OSAS is 3 times as common in men as in women.13 The prevalence of OSAS then increases in women after menopause.14 The administration of exogenous testosterone results in OSAS33 and increased upper airway closing pressures.34 In 1 study, childhood OSAS recurred in a small number of males during adolescence, but not in females.36 These facts all suggest that testosterone promotes upper airway collapse, whereas female sex hormones are protective. Nevertheless, attempts to treat OSAS with female sex hormones have been disappointing.37,38 One problem with previous studies is that hormone levels were manipulated by administering exogenous hormones. Sex hormones and gonadotrophins are secreted in a pulsatile fashion, and secretion is modulated by circadian and monthly cycles.39 Thus, exogenous administration is quite different from the physiologic condition and results in nonphysiologically fluctuating levels that may be subphysiologic or supraphysiologic.32 In contrast, puberty provides a natural intervention. During pubertal development, hormone levels increase from minimally detectable to adult levels.
The results of the current study indicate that direct effects of sex hormones, such as their effect on ventilatory drive or on the nasal mucosa,40 are unlikely to be a primary cause of increased upper airway collapsibility. However, this study does not rule out the possibility that the effect of sex hormones on structural factors over many years may affect upper airway collapsibility. In addition to changes in size, the upper airway shape and composition change during puberty in males.41 For example, 1 study demonstrated that pubertal and postpubertal males have larger tongues than females, although the study was not controlled for overall body size.42
Effects of growth on upper airway function during sleep
It is possible that the changes in upper airway function demonstrated in this study were due to structural changes in the upper airway, such as changes in airway size, composition, or stiffness. The upper airway widens with growth,43 and a wider airway results in less collapse and less obstructive apnea.44 Thus, the changes in upper airway caliber with growth would be predicted to result in increased flow in response to subatmospheric pressure, rather than the decreased flow demonstrated in this study. Another possibility is that upper airway dynamics during sleep are related to tracheal length. Tracheal length is related to height,43 and a longer tracheal length results in a more collapsible upper airway.45 We consider this to be an unlikely explanation for our study results. Although upper airway length increases progressively from ages 1 to 11 years,46 with a greater tracheal growth velocity during early childhood than during puberty,47 upper airway collapsibility is the same in infants as in school-aged children.15 In addition, although females in the current study had a greater SPF than males, there was no significant difference in height between the sexes. Data are lacking in regard to changes in upper airway composition or stiffness during the adolescent years.
It is also possible that the changes in the SPF were simply a reflection of height-related changes in pulmonary function, as height is a predictor of lung volume. This is unlikely because the relationship between SPF and age remained significant when SPF was corrected for height. In addition, previous data have shown that infants and school-aged children have a similar SPF, despite a large difference in height.5
Effects of Changes in Ventilatory Control on Upper Airway Function During Sleep
The drive to the upper airway muscles is affected by the overall central nervous system ventilatory drive.48–50 Previously, it has been shown that the occlusion pressure in 100 milliseconds (P0.1) during sleep correlates with the SPF, indicating that ventilatory drive affects upper airway collapsibility.4 Children are known to have a higher ventilatory drive than adults51–53; the ventilatory drive decreases during adolescence to adult levels.53 Previous studies have shown that school-aged children have active upper airway reflexes in response to subatmospheric pressure and that these reflexes help maintain airway patency during sleep.5 In contrast, upper airway reflexes during sleep are blunted in adults.5 Thus, we speculate that 1 cause for the decrease in the upper airway compensatory responses to subatmospheric pressure loading with aging may be the depressant effect of age on the ventilatory drive, leading to a decrease in upper airway neuromotor tone during sleep.
Effect of Sex on Upper Airway Function During Sleep
In this study, females had a flatter SPF than males. Females do have some subtle differences in ventilatory control compared with males (such as a lower co2 threshold),31,54 which may account for this difference. Previous studies of upper airway collapsibility during sleep in adults have probably included too few females to allow for comparison between the sexes, although 1 study noted increased collapsibility in adult men during wakefulness.55
In this study, we used pubertal development (i.e., Tanner stage) as a marker of exposure to physiologic amounts of sex hormones to assess the effects of sex hormones on upper airway collapsibility. The serum sex hormone levels were obtained only to corroborate Tanner stage. Because sex hormone levels fluctuate with both a circadian (males and females) and monthly (females) rhythm, a single level is not indicative of baseline status. Furthermore, there is a wide range of normal values. Thus, it was expected that the sex hormone levels would not correlate with the pressure-flow response parameters.
Study Limitations
A limitation of this study was the cross-sectional rather than longitudinal design, and future studies evaluating a large sample of children longitudinally throughout puberty are desirable. Another limitation is the fact that studies in females were not performed at a set point of the menstrual cycle. Menstruation typically does not begin until Tanner stage 3, and, once menstrual cycles begin, they are often very irregular in pubertal girls. Thus, it was not practical to schedule subjects at a particular phase of the menstrual cycle.
Summary
In summary, this study has shown that upper airway compensatory responses to subatmospheric pressure loading during sleep increase in a continuous fashion from the prepubertal to adult years, irrespective of hormonal status. We speculate that this is due to age-related changes in the ventilatory drive, although studies evaluating changes in upper airway structure during adolescence are needed.
ACKNOWLEDGMENTS
The authors thank all of the children and their families for their enthusiastic participation in this study. We thank the sleep technologists who assisted with this study for their dedication and professionalism; the lab clinical supervisor, Joanne Elliott R.Psg.T., for making this study possible; and Mary Anne Cornaglia for assistance with manuscript preparation.
Support: Dr. Marcus was supported by grants MO1-RR-000240, U54 RR023567 and RO1 HL58585. Respironics, Inc., provided modified CPAP equipment to provide subatmospheric pressure, as well as unrestricted research support that paid for a research technician.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Brooks has received research support from Cephalon. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146(5 Pt 1):1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 2.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
- 3.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77(2):918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Fernandes do Prado LB, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 7.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 8.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance and behaviour in 4-5 year olds. Arch Dis Child. 1993;68:360–6. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castronovo V, Zucconi M, Nosetti L, et al. Prevalence of habitual snoring and sleep-disordered breathing in preschool-aged children in an Italian community. J Pediatr. 2003;142(4):377–82. doi: 10.1067/mpd.2003.118. [DOI] [PubMed] [Google Scholar]
- 10.Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23(4):487–92. [PubMed] [Google Scholar]
- 11.Liu X, Ma Y, Wang Y, et al. Brief report: An epidemiologic survey of the prevalence of sleep disorders among children 2 to 12 years old in Beijing, China. Pediatrics. 2005;115(1) Suppl:266–8. doi: 10.1542/peds.2004-0815I. [DOI] [PubMed] [Google Scholar]
- 12.Chng SY, Goh DY, Wang XS, Tan TN, Ong NB. Snoring and atopic disease: a strong association. Pediatr Pulmonol. 2004;38(3):210–6. doi: 10.1002/ppul.20075. [DOI] [PubMed] [Google Scholar]
- 13.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 14.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57(1):99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 16.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173(8):902–09. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudewyns A, Punjabi N, Van de Heyning PH, et al. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–41. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 18.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64(2):535–42. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 20.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91(5):2248–54. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 21.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64(2):789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr. 1998;132(2):211–22. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual of standardized terminology: Techniques and scoring systems for sleep stages of human subjects. Bethesda: NINDB Neurological Information network; 1968. [Google Scholar]
- 25.EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 26.Tanner JM. Growth at adolescence. 2nd ed. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 27.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 28.Chervin RD, Ruzicka DL, Wiebelhaus JL, et al. Tolerance of esophageal pressure monitoring during polysomnography in children. Sleep. 2003;26(8):1022–6. doi: 10.1093/sleep/26.8.1022. [DOI] [PubMed] [Google Scholar]
- 29.Dutton K, Blanksby BA, Morton AR. co2 sensitivity changes during the menstrual cycle. J Appl Physiol. 1989;67(2):517–22. doi: 10.1152/jappl.1989.67.2.517. [DOI] [PubMed] [Google Scholar]
- 30.Zwillich CW, Natalino MR, Sutton FD, Weil JV. Effects of progesterone on chemosensitivity in normal men. J Lab Clin Med. 1978;92(2):262–9. [PubMed] [Google Scholar]
- 31.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol. 1983;54(4):874–9. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- 32.White DP, Schneider BK, Santen RJ, et al. Influence of testosterone on ventilation and chemosensitivity in male subjects. J Appl Physiol. 1985;59(5):1452–7. doi: 10.1152/jappl.1985.59.5.1452. [DOI] [PubMed] [Google Scholar]
- 33.Schneider BK, Pickett CK, Zwillich CW, et al. Influence of testosterone on breathing during sleep. J Appl Physiol. 1986;61(2):618–23. doi: 10.1152/jappl.1986.61.2.618. [DOI] [PubMed] [Google Scholar]
- 34.Cistulli PA, Grunstein RR, Sullivan CE. Effect of testosterone administration on upper airway collapsibility during sleep. Am J Respir Crit Care Med. 1994;149:530–2. doi: 10.1164/ajrccm.149.2.8306057. [DOI] [PubMed] [Google Scholar]
- 35.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol. 2003;94(1):101–7. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]
- 36.Guilleminault C, Partinen M, Praud JP, Quera-Salva MA, Powell N, Riley R. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr. 1989;114(6):997–9. doi: 10.1016/s0022-3476(89)80447-0. [DOI] [PubMed] [Google Scholar]
- 37.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orr WC, Imes NK, Martin RJ. Progesterone therapy in obese patients with sleep apnea. Arch Intern Med. 1979;139:109–11. [PubMed] [Google Scholar]
- 39.Grumbach MM, Styne DM. Puberty. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia: W.B. Saunders Co; 1998. pp. 1509–1626. [Google Scholar]
- 40.Haeggstrom A, Ostberg B, Stjerna P, Graf P, Hallen H. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL J Otorhinolaryngol Relat Spec. 2000;62(1):39–42. doi: 10.1159/000027713. [DOI] [PubMed] [Google Scholar]
- 41.Kahane JC. Growth of the human prepubertal and pubertal larynx. J Speech Hearing Res. 1982;25:446–55. doi: 10.1044/jshr.2503.446. [DOI] [PubMed] [Google Scholar]
- 42.Kerr WJS, Kelly J, Geddes DAM. The areas of various surfaces in the human mouth from nine years to adulthood. J Dent Res. 1991;70(12):1528–30. doi: 10.1177/00220345910700121001. [DOI] [PubMed] [Google Scholar]
- 43.Griscom NT, Wohl ME. Dimensions of the growing trachea related to body height. Length, anteroposterior and transverse diameters, cross-sectional area, and volume in subjects younger than 20 years of age. Am Rev Respir Dis. 1985;131(6):840–4. doi: 10.1164/arrd.1985.131.6.840. [DOI] [PubMed] [Google Scholar]
- 44.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166(10):1388–95. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 46.Arens R, McDonough JM, Corbin AM, et al. Linear dimensions of the upper airway structure during development: assessment by magnetic resonance imaging. Am J Respir Crit Care Med. 2002;165(1):117–22. doi: 10.1164/ajrccm.165.1.2107140. [DOI] [PubMed] [Google Scholar]
- 47.Wailoo MP, Emery JL. Normal growth and development of the trachea. Thorax. 1982;37(8):584–7. doi: 10.1136/thx.37.8.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiner D, Mitra J, Salamone J, Cherniack NS. Effects of chemical stimuli on nerves supplying upper airway muscles. J Appl Physiol. 1982;52:530–6. doi: 10.1152/jappl.1982.52.3.530. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz AR, Thut DC, Brower RG, et al. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of co2. J Appl Physiol. 1993;74(4):1597–605. doi: 10.1152/jappl.1993.74.4.1597. [DOI] [PubMed] [Google Scholar]
- 50.Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med. 2000;162(2 Pt 1):559–65. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- 51.Springer C, Wasserman K. Evidence that maturation of the peripheral chemoreceptors is not complete in childhood. Resp Physiol. 1988;74:55–64. doi: 10.1016/0034-5687(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 52.Gozal D, Arens R, Omlin KJ, Marcus CL, Keens TG. Maturational differences in step vs. ramp hypoxic and hypercapnic ventilatory responses. J Appl Physiol. 1994;76(5):1968–75. doi: 10.1152/jappl.1994.76.5.1968. [DOI] [PubMed] [Google Scholar]
- 53.Marcus CL, Glomb WB, Basinski DJ, Davidson SL, Keens TG. Developmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthood. J Appl Physiol. 1994;76(1):314–20. doi: 10.1152/jappl.1994.76.1.314. [DOI] [PubMed] [Google Scholar]
- 54.Patrick JM, Howard A. The influence of age, sex, body size and lung size on the control and pattern of breathing during co2 inhalation in Caucasians. Respir Physiol. 1972;16:337–50. doi: 10.1016/0034-5687(72)90063-1. [DOI] [PubMed] [Google Scholar]
- 55.Brooks LJ, Strohl KP. Size and mechanical properties of the pharynx in healthy men and women. Am Rev Respir Dis. 1992;146(6):1394–7. doi: 10.1164/ajrccm/146.6.1394. [DOI] [PubMed] [Google Scholar]