Abstract
Study Objectives:
(1) To describe the prevalence and prospective course of insomnia in a representative young-adult sample and (2) to describe the cross-sectional and longitudinal associations between insomnia and depression.
Design:
Longitudinal cohort study.
Setting:
Community of Zurich, Switzerland.
Participants:
Representative stratified population sample.
Interventions:
None.
Measurements and Results:
The Zurich Study prospectively assessed psychiatric, physical, and sleep symptoms in a community sample of young adults (n = 591) with 6 interviews spanning 20 years. We distinguished 4 duration-based subtypes of insomnia: 1-month insomnia associated with significant distress, 2- to 3-week insomnia, recurrent brief insomnia, and occasional brief insomnia. The annual prevalence of 1-month insomnia increased gradually over time, with a cumulative prevalence rate of 20% and a greater than 2-fold risk among women. In 40% of subjects, insomnia developed into more chronic forms over time. Insomnia either with or without comorbid depression was highly stable over time. Insomnia lasting 2 weeks or longer predicted major depressive episodes and major depressive disorder at subsequent interviews; 17% to 50% of subjects with insomnia lasting 2 weeks or longer developed a major depressive episode in a later interview. “Pure” insomnia and “pure” depression were not longitudinally related to each other, whereas insomnia comorbid with depression was longitudinally related to both.
Conclusions:
This longitudinal study confirms the persistent nature of insomnia and the increased risk of subsequent depression among individuals with insomnia. The data support a spectrum of insomnia (defined by duration and frequency) comorbid with, rather than secondary to, depression.
Citation:
Buysse DJ; Angst J; Gamma A; Ajdacic V; Eich D; Rössler W. Prevalence, Course, and Comorbidity of Insomnia and Depression in Young Adults. SLEEP 2008;31(4):473-480.
Keywords: epidemiology, insomnia, depression, prospective course
EPIDEMIOLOGIC STUDIES SHOW THAT 20% TO 35% OF THE GENERAL POPULATION REPORT INSOMNIA SYMPTOMS, AND THAT 10% TO 20% HAVE A CLINICALLY significant insomnia syndrome.1–5 Fewer data are available to address 2 other important questions regarding the epidemiology of insomnia: its natural history and its relationship with psychiatric disorders. Several studies have indicated that insomnia is often a chronic condition,6–8 but many of these studies have used retrospective designs or prospective designs with limited duration or limited number of follow-up evaluations. Results of other studies9,10 have suggested that, at least for older adults, incidence and remission rates for narrowly defined insomnia are about equal. Thus, uncertainty exists regarding the course and chronicity of insomnia. The 2005 National Institutes of Health State of the Science Conference Statement on the Manifestations and Management of Chronic Insomnia in Adults identified studies addressing this issue as an important direction for insomnia-related research: “Longitudinal observational studies are needed to identify factors affecting incidence of, natural history of, and remission from chronic insomnia.11”
A number of risk factors for chronic insomnia have been identified, including medical problems, female sex, and increasing age.4,5,12–16 However, depression and depressive symptoms are the largest and most consistent risk factors for insomnia.7,17 Conversely, insomnia is a frequent symptom of depression, and it is often hypothesized to be an antecedent or risk factor. To date, however, relatively few studies have examined prospective longitudinal data from representative community samples to define the relationship between insomnia and depression; these studies were recently reviewed.18
Data from the Epidemiologic Catchment Area Study showed that insomnia was associated with an increased risk of depression if it was present at 2 interviews over a 1-year follow-up interval but not if it was present only at the initial interview.3 Weissman and colleagues4 examined Epidemiologic Catchment Area data and found that insomnia not comorbid with a psychiatric disorder was associated with a significantly increased risk of developing first-episode major depression, alcohol abuse, and panic disorder within a 1-year follow-up period. Other studies have found similar relationships between insomnia and depression among older adults19 and younger adults 20 with 2- to 3-year follow-up intervals, even after controlling for prior depressive symptoms. Finally, Chang et al21 reported a prospective study of 1053 male medical students with annual follow-up questionnaires over a mean of 34 years. Insomnia and difficulty sleeping under stress predicted depression, as assessed by questionnaires and the general health questionnaire.
In contrast with these studies, but consistent with the results of Ford and Kamerow, were the earlier findings of the Zurich Study,22 comprising a 2- to 7-year follow-up of young adults. Although we found strong cross-sectional associations between insomnia and depression, we did not find that insomnia predicted future depression.
Although a considerable amount of data suggests an association between insomnia and subsequent depression, previous studies have been limited by the representativeness of their samples, the duration and frequency of follow-up, or the definition of insomnia. The current analyses from the Zurich Study include a representative population sample, a follow-up interval of 21 years, and the ability to examine different patterns of insomnia, including a conservative case definition based on Diagnostic and Statistical Manual (DSM)-IV criteria for primary insomnia. The aims of these analyses were (1) to describe the prevalence and prospective course of insomnia in a representative young adult sample and (2) to describe the cross-sectional and longitudinal associations between insomnia and depression, including the question of whether insomnia predicts depression or vice versa.
METHODS
Sample
The Zurich Study (A Prospective Epidemiological Study of Depressive, Neurotic, and Psychosomatic Syndromes) was originally based on a sample of 4547 subjects (2201 men, 2346 women) representative of the canton of Zurich in Switzerland in 1978 (population 1.1 million). A 2-stage sampling procedure for epidemiologic studies, described by Dunn et al,23 was used.
In the first stage in 1978, male participants were 19 years of age (representing the age at mandatory conscription) and the women were 20 years of age (representing the age at electoral registration). With permission from the Swiss government and independent of military procedures, we were able to randomly investigate half of the male conscripts, in groups of approximately 10 persons, with questionnaires on social data and health problems. All subjects received the Symptom Checklist 90-R (SCL-90-R),24 a comprehensive self-report questionnaire of 90 questions, which has been validated against the General Health Questionnaire25 and covers a broad range of psychiatric symptoms. The refusal rate was 0.3%. Women were identified through the complete electoral register; half were randomly selected to receive mailed questionnaires, and 75% of these responded.26 A lower education level was overrepresented among nonresponding women; to correct for this bias, the female interview sample was matched by education level to the male sample.
In the second stage, a stratified sampling procedure was chosen to enrich the sample with cases at risk for the development of psychiatric syndromes, consistent with the primary aims of the larger study. Thus, 591 subjects (292 men, 299 women) were selected for interview, with two-thirds consisting of high scorers (defined by the 85th percentile or more of the SCL-90 Global Severity Index), and one-third being a random sample of those with lower scores (below the 85th percentile). Stratified sampling was used to fulfill the original aims of the Zurich Study, rather than the aims of the current paper. The stratified sample represents a weighted sample of 2600 persons of the same age from the general population. Methodologic details of the Zurich study were published in 1984.26
Six interviews were conducted, in the years 1979, 1981, 1986, 1988, 1993, and 1999. In 1980, a questionnaire identical to the screening questionnaire was mailed to participants. Across 20 years of follow-up, 62.1% (n = 367) of the original sample continued to participate in the study. Specifically 47% participated in all 6 interviews (n = 278), 63% (n = 372) in at least 5 interviews, 74% (n = 435) in at least 4 interviews, 82% (n = 486) in at least 3 interviews, and 91.4% (n = 540) in at least 2 interviews. Individuals who did not participate in the 1999 interview did not differ significantly from those who took part in the 1999 interview in terms of risk group and most demographic characteristics. Very high and very low scorers on the SCL-90-R tended to drop out at slightly higher rates, which may result in underestimation of true prevalence rates.27
Interview
The Structured Psychopathological Interview and Rating of Social Consequences of Psychic Disturbances for Epidemiology (SPIKE) was administered26 at each interview. The semistructured SPIKE interview lasts 2 to 3 hours and is conducted by trained professional interviewers. The interview starts with questions about the participant's current social situation, followed by a question regarding whether the subject had been suffering from and/or treated for any physical or psychological problems during the past 12 months. The interview includes 29 sections corresponding to major organ systems, functions, and disorders: stomach, intestines, respiration, heart, circulation, back, headache, allergies, pain, sleep, appetite, menstruation, sexuality, panic, anxiety, phobias, hypochondriasis, depression, hypomania, obsessive-compulsive syndromes, posttraumatic stress, suicidality, and substance use disorders (tobacco, alcohol, sedatives, illicit drugs). Each section starts with stem questions about the core symptoms, followed by questions on symptoms, duration, frequency and recency of episodes, days of symptom presence over the year, distress, consequences for work and social activities, and help-seeking behavior. Symptoms and treatment between interview years were also assessed retrospectively. The symptom chapter of the interview is followed by the assessment of quality of life, coping, and life events.
Validity and reliability testing have been performed with particular reference to depression and anxiety. The interviewers included clinical psychologists and psychiatrists with specific interview training. The interrater reliability of the SPIKE showed κs of 0.89 and 0.91 for the symptoms of depression (including insomnia) and anxiety and of 0.90 for the corresponding syndromal diagnoses.28 The validity of the SPIKE has been assessed by comparing physician ratings and medical records with the SPIKE among 140 patients drawn from psychiatric clinics or social-psychiatric services in the canton of Zurich29–31 and from a local hospital.32 The SPIKE rating of the diagnostic level of depression was found to have high sensitivity and modest specificity (0.95 and 0.59, respectively, for major depression and 0.83 and 0.63, respectively, for minor depression). Likewise, the SPIKE had good sensitivity for detecting subthreshold depression (κ 0.90). No corresponding data for insomnia exist. Further methodologic details, including studies on validity, have been previously published.33 Sleep behavior and insomnia were assessed at all 6 interviews as described in an earlier paper.34 Data on sleep behavior included time of going to bed and rising, sleep latency, quality of sleep, and symptoms of insomnia. The stem question for insomnia was “Have you experienced disruptions to your sleep pattern during the last 12 months, e.g., inability to fall asleep, waking up in the night, or waking up too early in the morning?” Insomnia-related distress and impairment in different social roles (work, leisure activities, relationships with partners, and other social roles) were assessed using 0-to-100 visual analog scales. Insomnia was considered to be treated if a doctor had been consulted for insomnia. Given our aim of describing the course of insomnia over time, we evaluated a spectrum of insomnia subtypes based on duration and frequency of episodes over a 12-month period. We started with a clinically relevant case definition of insomnia that captures the major features of the DSM-IV definition of primary insomnia. DSM-IV operationalizes insomnia disorder using 2 major criteria: (A) sleep difficulties for at least 1 month and (B) the insomnia or daytime sequelae cause clinically significant distress or impairment in different social roles. Criteria C through E for DSM-IV primary insomnia are exclusionary criteria relating to conditions judged to cause the insomnia. Because we specifically sought to examine the relationship between insomnia and other disorders, we did not apply Criteria C through E to our case definition. Therefore, we use the term “1-month insomnia” rather than primary insomnia, acknowledging that participants may have had comorbid conditions. Consistent with DSM-IV, no specific criterion was used regarding frequency of insomnia in terms of nights per week or per month. Significant distress was defined by a criterion score of 30 or higher on the 100-mm visual analog scale.
In addition to 1-month insomnia, we examined 3 other subtypes of insomnia based on symptoms, duration and frequency and not requiring distress or impairment. These mutually exclusive subtypes are (1) 2- to 3-week insomnia occurring at least once over the past 12 months; (2) recurrent brief insomnia (RBI), i.e., insomnia of less than 2 weeks' duration recurring at least monthly over the past 12 months; and (3) occasional brief insomnia (OBI), i.e., insomnia of less than 2 weeks' duration occurring less than monthly.34 These insomnia subtypes were derived empirically from an examination of common patterns of insomnia symptoms obtained during the SPIKE interviews. The symptom profiles of these insomnia subtypes have been described and validated in previous studies from this sample.22,34 For example, the insomnia subtypes differ not only in terms of symptom frequency and pattern, but also in terms of age of onset, subjective sleep quality, psychological symptom severity on the SCL-90, rates of positive family history, and associations with depression, anxiety, and psychosomatic symptoms. The duration-based insomnia subtypes did not differ in terms of symptoms types (i.e., sleep onset, sleep maintenance insomnia), sex ratios, or level of subjective impairment. Subtypes based on frequency and duration of symptoms may be particularly relevant to this type of longitudinal, natural-history study, where 1 of the goals is to characterize changes in pattern over time. However, data regarding these subtypes should be interpreted with caution, given that formal interrater reliability studies have not been conducted.
Depression was also defined along a spectrum of severity including the following subtypes: (1) DSM-III-R major depressive episodes (MDE), (2) minor mood disorders (DSM-III-R dysthymia, minor depression [3–-4 of the 9 symptoms of DSM-III R major depression lasting at least 2 weeks] and recurrent brief depression [DSM-IV appendix]), and (3) depressive symptoms not qualifying for a diagnosis.
Statistics
Kruskal Wallis tests and χ2 tests were used for comparisons of 2 or more groups. Prevalence rates were estimated for the general population by taking into account sampling weights and stratification into high and low scorers on the SCL-90R (as described above). Using these weighting procedures, we can report 1-year prevalence rates for the actual sample and for the entire Zurich population of the same age from which the sample was drawn. Longitudinal analyses of the stability of insomnia and the relationship between insomnia and depression across 5 interviews used generalized estimating equations,35,36 which simultaneously take into account the data from all interviews, and log-linear models,37 carried out using SAS PROC CATMOD.
In the log-linear models, we assessed the comorbidity and predictive power of insomnia and depression by examining odds ratios (OR) between and within insomnia and depression, and between interviews. To increase statistical power and to have sufficient sample sizes for the response categories in the log-linear analysis, we combined all subtypes of insomnia (insomnia symptoms, OBI, RBI, 2- to 3-week insomnia, 1-month insomnia), and all forms of depression (depressive symptoms and all more severe manifestations as described above). Based on these definitions, 3 groups were submitted to log-linear analysis: “pure” insomnia (without concurrent depressive symptoms), insomnia with comorbid depression, and “pure” depression (without concurrent insomnia symptoms). A similar set of analyses was used in a previously published paper that examined longitudinal trajectories of depression and anxiety in the Zurich study.38 Thus, in the earlier paper, as in the current analyses, ORs derived from log-linear models were used to evaluate the stability over time within a category and the switching over time from 1 category to another. Analyses were conducted in Stata 8.2 (Stata Corp., College Station, TX) and SAS 8.2 (SAS Institute, Cary, NC).
RESULTS
Prevalence Rates and Longitudinal Course of Insomnia
Table 1 shows cumulative and age-specific frequencies and prevalence rates for the various subtypes of insomnia in the study sample and in the entire population. The number of participants at each interview is also indicated. One-year prevalence rates for 1-month insomnia increased steadily from 2.3% at age 20 to 13.9% at age 35, then dropped to 5.5% at age 41, resulting in a cumulative prevalence rate across the 6 interviews of 20% (women 27%, men 12%). The brief forms of insomnia tended to decrease until age 35 and then increased at age 41, but there was considerable variability across interviews.
Table 1.
Prevalence of Duration-Based Insomnia Subtypes
| 1-month insomnia | 2- to 3-week insomnia | RBI | OBI | No insomnia | |
|---|---|---|---|---|---|
| Cumulative prevalence observed in study samplea | |||||
| Total (n = 591) | 144 (24.4) | 78 (13.2) | 143 (24.2) | 94 (15.9) | 132 (22.3) |
| Men (n = 292) | 51 (17.5) | 35 (12.0) | 83 (28.4) | 48 (16.4) | 75 (25.7) |
| Women (n = 299) | 93 (31.1) | 43 (14.4) | 60 (20.1) | 46 (15.4) | 57 (19.1) |
| Cumulative weighted prevalenceb | |||||
| Total | 19.8 | 9.7 | 20.6 | 17.5 | 32.4 |
| Men | 12.0 | 7.6 | 22.6 | 19.8 | 38.0 |
| Women | 27.4 | 11.9 | 18.6 | 15.2 | 27.0 |
| Weighted prevalence by age, in yearsc | |||||
| 20 or 21 (n = 591)d | 2.3 | 7.0 | 15.8 | 15.3 | 59.5 |
| 22 or 23 (n = 456)d | 4.2 | 4.2 | 14.8 | 13.2 | 63.5 |
| 27 or 28 (n = 457)d | 5.8 | 5.8 | 6.0 | 17.3 | 65.0 |
| 29 or 30 (n = 424)d | 6.2 | 4.1 | 18.9 | 18.7 | 52.1 |
| 34 or 35 (n = 407)d | 13.9 | 2.7 | 11.4 | 7.5 | 64.4 |
| 40 or 41 (n = 367)d | 5.5 | 8.4 | 24.7 | 12.4 | 49.0 |
Cells refer to the number (%) of subjects having this as their most persistent insomnia duration subtype observed across all interviews.
Weighted population prevalence corrected for stratified sampling. Numbers in each cell represent the estimated percentage of the total population having this as the most persistent insomnia duration subtype observed across all interviews.
Weighted population prevalence corrected for stratified sampling. Numbers in each cell represent the estimated percentage of the total population having this insomnia duration subtype at a particular age.
n = Actual number of subjects included in interviews at each age.
RBI refers to recurrent brief insomnia; OBI, occasional brief insomnia.
Table 2 presents changes of 1-month insomnia from and to other insomnia-duration subtypes, as observed in previous and subsequent interviews. Categories are not mutually exclusive, as they summarize all observations across several interviews cumulatively. Among 131 cases of 1-month insomnia at their last interview, 30% had suffered from the same condition during at least 1 previous interview. Likewise, among 111 incident cases of 1-month insomnia, 35% had the same diagnosis at a later interview. Other duration-based insomnia subtypes were also common during previous and subsequent interviews. Individuals with 1-month insomnia had some type of insomnia in 99% of previous interviews and in 82% of subsequent interviews. Thus, whereas the specific subtype of insomnia was variable over time, the presence of some type of insomnia was highly persistent.
Table 2.
Previous and Subsequent Insomnia Diagnoses Among Subjects with 1-Month Insomnia
| Insomnia duration subtype | Insomnia subtype at any previous interview (n = 131)a,b | Insomnia subtype at any subsequent interview (n = 111)b,c |
|---|---|---|
| 1-month insomnia | 39 (29.8) | 39 (35.1) |
| 2- to 3-week insomnia | 33 (25.2) | 31 (27.9) |
| RBI | 68 (51.9) | 40 (36.0) |
| OBI | 54 (41.2) | 30 (27.0) |
| Any insomnia subtype | 129 (98.5) | 91 (82.0) |
| No insomnia | 1 (0.8) | 20 (18.0) |
Data are presented as number (%). RBI refers to recurrent brief insomnia; OBI, occasional brief insomnia.
Percentages do not sum to 100% because subjects could have different diagnoses at different assessment times. Each cell indicates the number (%) of subjects who had a given diagnosis in at least 1 previous (column 1) or subsequent (column 2) interview.
Based on subjects having 1-month insomnia at any time and previous interview data
Based on subjects having 1-month insomnia at any time and subsequent interview data
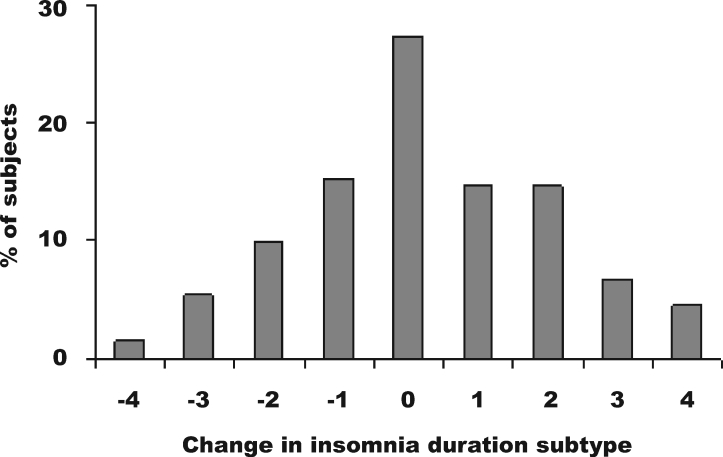
We also investigated changes of insomnia-duration subtype between the first and last completed interview for each individual subject. The first interview for all subjects was the 1 completed at age 20 or 21; the last completed interview differed for different subjects according to their patterns of participating and dropping out. The change was quantified in terms of ordinal units corresponding to insomnia duration subtypes. Subtypes in order of ascending duration were “no insomnia,” “OBI,” “RBI,” “2- to 3-week insomnia,” and “1-month insomnia.” As an example, a subject who changed from OBI at his or her first interview to RBI at his or her last interview would be assigned a change score of +1. A subject who changed from 1-month insomnia at the first interview to OBI at the last interview would be assigned a change score of −3. Figure 1 shows the histogram of these changes. Descriptively, the number of increases and decreases in duration subtype was roughly similar, but there were more increases of 2 to 4 levels in insomnia duration than decreases of 2 to 4 levels in insomnia duration. The mean change score was 0.25 (SD 1.81) indicating that, overall, more subjects experienced increasing duration of insomnia over time than decreasing duration. This is also demonstrated descriptively in the number of subjects who developed more persistent or briefer insomnia subtypes between the first and last completed interview: 188 (41%) subjects developed more persistent forms of insomnia, 149 (32%) developed briefer forms, and 127 (27%) showed no change.
Figure 1.
Histogram of changes in insomnia-duration subtype between first and last completed interview. One unit corresponds to a change into the next more- or less-persistent insomnia subtype. Categories are no insomnia, occasional brief insomnia, recurrent brief insomnia, 2- to 3-week insomnia, and 1-month insomnia. See text for further details.
Stability of 1-Month Insomnia
As reported above, 35% of 111 incident cases of 1-month insomnia had the same diagnosis at a later interview. The corresponding ORs for 1-month insomnia predicting the same diagnosis at any later time ranged from 1.6 to 4.7 and were all significant (P < .05) except for the first interview (age 20 or 21, P = 0.37). When examining immediately adjacent interviews, we found that 12% to 32% of those with 1-month insomnia also had 1-month insomnia at the subsequent interview, with no obvious time trend across the course of the study. These ORs ranged from 1.8 to 5.6 but were not significant for the transitions between the first (age 20 or 21) and second (age 22 or 23) and second and third (age 27 or 28) interview. As a summary measure of the stability of 1-month insomnia, we report the OR from a longitudinal general estimating equation analysis of the association between 1-month insomnia in any 2 consecutive interviews, simultaneously incorporating data from all interviews. Stability was high, with an OR of 2.9 (95% confidence interval [CI] 1.8–4.7, P = 0.0001).
Cross-sectional and Longitudinal Associations Between Insomnia and Depression
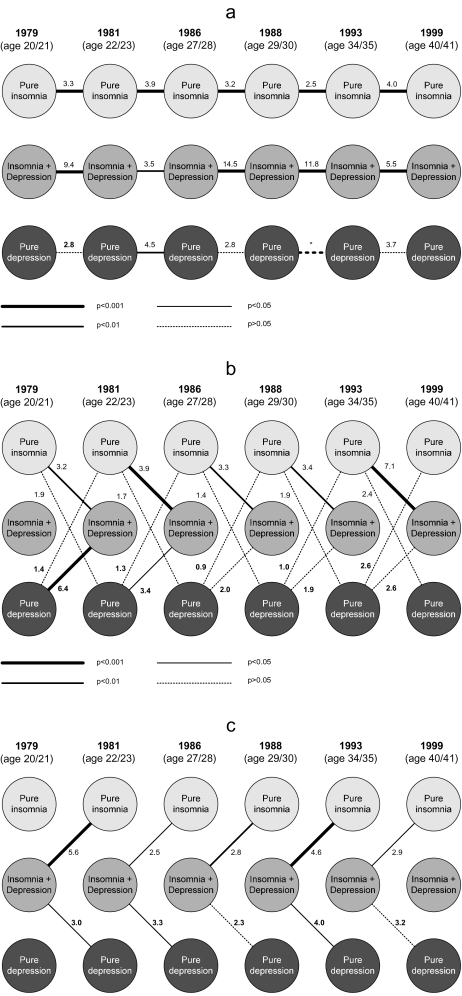
We used log-linear models39 to assess the comorbidity and predictive power of insomnia relative to depression. This approach yielded ORs between and within insomnia and depression at a given interview and between insomnia and depression across adjacent interviews (Figure 2). To increase statistical power, insomnia and depression included all subjects who manifested symptoms of any duration or severity (See Methods). Thus, this group of analyses focused on 3 groups of subjects: those having insomnia of any duration, but without concurrent depression (“pure” insomnia); those having depression of any severity but without concurrent insomnia (“pure” depression); and those having insomnia comorbid with depression. Associations within the group of pure insomnia across interview waves were strong and highly significant (ORs 2.5–4.0), indicating high stability and predictive power of this broad definition of insomnia (Figure 2a). Large ORs were also found for insomnia comorbid with depressive symptoms (ORs 3.5–14.5). However, pure depression was less consistent longitudinally: although the ORs were in the range of 2.8 to 4.5, only 1 association (between the second and third interview) was statistically significant. Associations between pure insomnia at a given interview and insomnia comorbid with depression at the next interview were strong and highly significant (Figure 2b), as were associations in the reverse direction, i.e., between insomnia comorbid with depression at a given interview and pure insomnia at the next interview (Figure 2c). All 10 of the longitudinal associations between pure insomnia and insomnia comorbid with depression were statistically significant at the P < 0.05 level. The corresponding longitudinal relationships between pure depression and insomnia comorbid with depression tended to be weaker and fewer in number; only 5 of 10 such associations were statistically significant (Figures 2b and 2c). Finally, none of the longitudinal associations between pure insomnia and pure depression, in either direction, were statistically significant (Figure 2b).
Figure 2.
Longitudinal relationships between insomnia and depression. Cross-sectional and longitudinal relationships between “pure” insomnia (including insomnia symptoms and occasional, recurrent brief, 2- to 3-week insomnia, and 1-month insomnia) “pure” depression (including major depressive episonde, minor depression, dysthymia, and depressive symptoms) and insomnia comorbid with depression (insomnia + depression) were analyzed using log-linear models. The strength of the associations is represented by the different line styles, as indicated in the figure. Numbers represent odds ratios. *Indicates that the odds ratio between depression in 1988 and 1993 was 0 and thus not interpretable. Panel a: longitudinal associations within pure insomnia, insomnia with comorbid depression, and pure depression conditions. Panel b: Longitudinal associations between pure insomnia and the other 2 conditions, as well as longitudinal associations between pure depression and the other 2 conditions. Panel c: Longitudinal associations between insomnia comorbid with depression and the other 2 conditions.
Does Insomnia Predict Depression?
To increase the number of cases and statistical power, we combined subjects with 2- to 3-week and 1-month insomnia into a group of “2+-weeks insomnia.” Between 17% and 50% of cases with “2+-week insomnia” without concurrent MDE in a given interview developed MDE at some later interview. Conversely, 8% to 29% of incident MDE cases in any given interview were preceded by “2+ week insomnia” at the previous interview, with no apparent increasing or decreasing trend across interviews. Prediction analyses used generalized estimating equations to determine whether “2+-week insomnia” predicted MDE in the following interview or in any subsequent interview, while controlling for concurrent MDE at the time of insomnia diagnosis. “Two-plus-week insomnia” significantly predicted MDE in the immediately following interview (OR 1.9, 95% CI 1.3–2.6, P = 0.001) or in any subsequent interview (OR 1.6, 95% CI 1.1–2.1, P = 0.005). Current MDE significantly predicted “2+-week insomnia” in any subsequent interview (OR 1.5, 95% CI 1.1–2.1, P = 0.02) but not in the immediately following interview.
DISCUSSION
In this longitudinal epidemiologic study, the overall prevalence of insomnia increased slightly from ages 20 to 40. Insomnia tended to become more persistent within individuals, as indicated by changes from briefer to longer insomnia-duration subtypes. In longitudinal analyses, pure insomnia and insomnia comorbid with depression were associated with future episodes of the same diagnosis and with future episodes of the other diagnosis. Pure depression, however, was less strongly associated with future episodes of any type, including pure depression, pure insomnia, or insomnia comorbid with depression. Insomnia greater than 2 weeks in duration predicted MDE at the next and any subsequent interview. Conversely, MDE predicted subsequent insomnia in a general sense, but not specifically in the next interview. These data complement prior findings regarding the prevalence, chronicity, and functional importance of insomnia and add further evidence to its important relationship with depression.
Prevalence and Course
The 20% prevalence for all insomnia subtypes, the 5% to 10% prevalence of more narrowly defined insomnia, and the higher rate among women, as compared with men, are compatible with the results of previous epidemiologic studies conducted in Europe and the US. For instance, an interview study in 5 European countries found point prevalence rates of DSM-IV insomnia ranging from 4% to 22%,6 and questionnaire and telephone studies have reported a 1-month insomnia prevalence of 10% to 19%.1,2 The Epidemiologic Catchment Area study in the US, which used a 2-week definition of insomnia, found prevalence rates of 8.5% to 10.7% in adults aged 18 to 44 years.3,4 The wide variety of insomnia definitions used in epidemiologic studies clearly affects reported prevalence rates.5 However, more recent studies40,41 using a 1-month duration with evidence of distress or impairment yield more consistent estimates and may be more relevant to insomnia seen in clinical practice.
Some previous studies have shown that insomnia becomes more chronic with increasing age.42 We found this to be true for about 40% of our young-adult sample. Courses seen in follow-up and follow-back evaluations were characterized by multiple changes in symptom duration. Nevertheless, the presence of some type of insomnia symptom was remarkably consistent over time, again demonstrating the generally chronic, intermittent course of insomnia. This persistence also suggests that these subtypes can indeed be viewed as existing on a single insomnia continuum varying primarily in duration.
Longitudinal Relationships Between Insomnia and Depression
One of the strengths of this data set was the ability to use several duration subtypes of insomnia and depression to test longitudinal relationships. We found that insomnia predicted future MDE and that MDE tended to predict future insomnia. Moreover, pure insomnia and insomnia comorbid with depression were strongly associated with each other longitudinally, whereas pure depression and insomnia comorbid with depression were less consistently related to each other longitudinally. Finally, pure insomnia and pure depression were not related to each other in any longitudinal analysis. Thus, our findings provide some support for previous findings regarding the predictive relationship between insomnia and depression in young adults21,43 and suggest that insomnia comorbid with depression is an important intermediate phenotype. In aggregate, this evidence supports the view that insomnia and depression are commonly comorbid, but distinguishable, conditions. Other types of evidence also support the notion of insomnia being a common comorbidity of depression, rather than simply a symptom of depression. For instance, sleep disturbance is associated with worse clinical outcomes in depression,44 insomnia is one of the most frequent residual symptoms of depression,45 and insomnia responds to different treatments than does depression.46,47 The National Institutes of Health State of the Science Conference on the Manifestations and Management of Chronic Insomnia in Adults11 endorsed a similar concept.
Limitations
Although the representative sample and longitudinal within-subjects design of this study are strengths, the total number of identified cases was relatively small, which may limit the power of some analyses, particularly those examining insomnia-duration subtypes. We used different insomnia definitions for different analyses to ensure adequate statistical power. The small numbers, in turn, relate to the stratified sampling method, with enrichment for those at risk for psychiatric disorders. Statistical methods were used to provide prevalence estimates for the general population, but these estimates may also be affected by the relatively small sample size. The restricted age range may also limit generalizability of our findings. Although the systematic interviews provide reliable data, they are cross-sectional and may be subject to bias. In particular, reliability of the duration-based insomnia subtypes used in this study has not been formally assessed.
Prospective sleep-wake diaries may provide a more accurate estimate of subjects' actual sleep patterns and the variability in those patterns. Likewise, including a diagnostic criterion for frequency of insomnia symptoms (i.e., number of nights per week or per month) may have led to more precise case definitions. However, our criteria were consistent with those in classifications such as the International Classification of Sleep Disorders, the International Classification of Sleep Disorders-2, and the DSM-IV, which do not specify a particular frequency per week.
Diagnostic manuals and criteria for insomnia and depression changed over the 20 years of the study. For longitudinal analysis of any given syndrome, we used the most recent definitions that could be applied to all interviews. The broad consistency of our findings across various insomnia subtypes and depression subtypes suggests that specific disorder definitions were not a major limitation. Our analyses also did not take into account current medication use by subjects, which could have affected symptom reporting and the definition of symptoms. However, this concern may be mitigated by the fact that disorders were identified based on past 1-year history rather than current symptoms alone. Finally, our data relied exclusively upon self-report, which may itself be influenced by psychiatric state. Polysomnographic sleep studies may also provide additional findings relevant to longitudinal change in sleep patterns and their association with mood disorders.
Future studies of the natural history of insomnia and its relationship with depression and other psychiatric disorders should consider other methods of defining syndrome severity. For instance, it is not known whether insomnia symptoms become more severe, or if episodes become more frequent, over time. Likewise, future studies should systematically collect treatment data to determine whether successful intervention affects natural history. Finally, studies examining familial aggregation and genetic associations could be usefully combined with longitudinal symptom-based studies to more specifically define core insomnia subtypes.
ACKNOWLEDGMENTS
This work was supported by Grants 3200–050881.97/1 from the Swiss National Science Foundation, AG00972 and AG20677 from the National Institute of Aging, and MH24652 from the National Institute of Mental Health
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Buysse has consulted for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Stress Eraser, and Takeda. Dr. Angst has consulted for Lundbeck and has received honoraria from GlaxoSmithKline, Eli Lilly, AstraZeneca, Lindbeck, and Pfizer. Dr. Rössler has received honorarium from Janssen-Cilag. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31(3):333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9(1):35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19(4):245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 6.Chevalier H, Los F, Boichut D, et al. Evaluation of severe insomnia in the general population: results of a European multinational survey. J Psychopharmacol. 1999;13(4) Suppl 1:S21–4. doi: 10.1177/026988119901304S04. [DOI] [PubMed] [Google Scholar]
- 7.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998 25;158(10):1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 8.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24(4):425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 9.Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22(Suppl 2):S373–8. [PubMed] [Google Scholar]
- 10.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–72. [PubMed] [Google Scholar]
- 11.National Institutes of Health. Bethesda, MD: 2005. Jun, NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. [PubMed] [Google Scholar]
- 12.Ohayon MM. Epidemiological study on insomnia in a general population. Sleep. 1996;19(3):S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 13.Gleason PP, Schulz R, Smith NL, et al. Correlates and prevalence of benzodiazepine use in community-dwelling elderly. J Gen Intern Med. 1998;13(4):243–50. doi: 10.1046/j.1525-1497.1998.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–7. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- 15.Morrison DN, McGee R, Stanton WR. Sleep problems in adolescence. J Am Acad Child Adolesc Psychiatry. 1992;31(1):94–9. doi: 10.1097/00004583-199201000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Olson LG. A community survey of insomnia in Newcastle. Aust N Z J Public Health. 1996;20(6):655–7. doi: 10.1111/j.1467-842x.1996.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry. 1998;39(4):185–97. doi: 10.1016/s0010-440x(98)90059-1. [DOI] [PubMed] [Google Scholar]
- 18.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 19.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43(376):445–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Public Health. 1997;87(10):1649–53. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146(2):105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 22.Vollrath M, Wicki W, Angst J. The Zurich study. VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239(2):113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 23.Dunn G, Pickles A, Tansella M, Vazquez-Barquero JL. Two-phase epidemiological surveys in psychiatric research. Br J Psychiatry. 1999;174:95–100. doi: 10.1192/bjp.174.2.95. [DOI] [PubMed] [Google Scholar]
- 24.Derogatis LR. Baltimore: Johns Hopkins; 1977. SCL-90: Administration, Scoring and Procedure Manual. [Google Scholar]
- 25.Goldberg DP. London: Oxford University Press; 1972. The Detection of Psychiatric Illness by Questionnaire. [Google Scholar]
- 26.Angst J, Dobler-Mikola A, Binder J. The Zurich Study: A prospective epidemiological study of depressive, neurotic and psychosomatic syndromes I. Problem, methodology. Eur Arch Psychiatry Clin Neurosci. 1984;234:13–20. doi: 10.1007/BF00432878. [DOI] [PubMed] [Google Scholar]
- 27.Eich D, Ajdacic-Gross V, Condrau M, et al. The Zurich Study: participation patterns and Symptom Checklist 90-R scores in six interviews, 1979–99. Acta Psychiatr Scand Suppl. 2003;(418):11–4. doi: 10.1034/j.1600-0447.108.s418.3.x. [DOI] [PubMed] [Google Scholar]
- 28.Hochstrasser B, Angst J. The Zurich Study: XXII. Epidemiology of gastrointestinal complaints and comorbidity with anxiety and depression. Eur Arch Psychiatry Clin Neurosci. 1996;246(5):261–72. doi: 10.1007/BF02190278. [DOI] [PubMed] [Google Scholar]
- 29.Meier R. Klinik Zurich: University of Zurich; 1985. Katamnese von 40 jugendlichen patienten nach einem suizidversuch bei verschiedenen behandlungsmoglichkeiten im Anschluss an eine somatische. [Google Scholar]
- 30.Busslinger M. Zurich: University of Zurich; 1984. Validierung des psychiatrisch-epidemiologischen Fragebogens SPIKE. [Google Scholar]
- 31.Illes P. Zurich: University of Zurich; 1981. Validierung des fragebogens ≪SPIKE≫ an diagnosen der krankengeschichten des sozialpsychiatrischen dienstes oerlikon (Klinik Hard) [Google Scholar]
- 32.Pfortmuller J. Zurich: University of Zurich; 1983. Beitrag zur validierung des depressionsratings im fragebogen SPIKE IV an psychiatrisch hospitalisierten patienten. [Google Scholar]
- 33.Angst J, Gamma A, Neuenschwander M, et al. Prevalence of mental disorders in the Zurich Cohort Study: a twenty year prospective study. Epidemiol Psichiatr Soc. 2005;14(2):68–76. doi: 10.1017/s1121189x00006278. [DOI] [PubMed] [Google Scholar]
- 34.Angst J, Vollrath M, Koch R, Dobler-Mikola A. The Zurich Study: VII. Insomnia: symptoms, classification and prevalence. Eur Arch Psychiatry Clin Neurosci. 1989;238:285–93. doi: 10.1007/BF00449810. [DOI] [PubMed] [Google Scholar]
- 35.Hardin JW, Hilbe JM. Boca Raton: Chapman & Hall / CRC; 003. Generalized Estimating Equations. [Google Scholar]
- 36.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 37.Kumar A, Shipley JE, Eiser AS, et al. Clinical correlates of sleep onset REM periods in depression. Biol Psychiatry. 1987;22:1473–7. doi: 10.1016/0006-3223(87)90107-7. [DOI] [PubMed] [Google Scholar]
- 38.Merikangas KR, Zhang H, Avenevoli S, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: The Zurich Cohort Study. Arch Gen Psychiatry. 2003;60(10):993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 39.Fienberg SE. Cambridge, MA: MIT Press; 1980. The analysis of cross-classified categorical data. [Google Scholar]
- 40.Ohayon MM, Caulet M, Guilleminault C. How a general population perceives its sleep and how this relates to the complaint of insomnia. Sleep. 1997;20(9):715–23. doi: 10.1093/sleep/20.9.715. [DOI] [PubMed] [Google Scholar]
- 41.Ohayon MM, Caulet M, Philip P, Guilleminault C. How sleep and mental disorders are related to complaints of daytime sleepiness. Arch Intern Med. 1997;157(22):2645–52. [PubMed] [Google Scholar]
- 42.Dodge R, Cline MG, Quan SF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med. 1995;155:1797–800. [PubMed] [Google Scholar]
- 43.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 44.Buysse DJ, Tu XM, Cherry CR, et al. Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry. 1999;45:205–13. doi: 10.1016/s0006-3223(98)00198-x. [DOI] [PubMed] [Google Scholar]
- 45.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221–5. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 46.Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60(10):668–76. doi: 10.4088/jcp.v60n1005. [DOI] [PubMed] [Google Scholar]
- 47.Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15(2):232–40. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]