Abstract
It has been recognized that nasal airway resistance (NAR) is elevated in patients with OSA. However, little is known regarding the influence of nasal resistance on mandibular advancement splint (MAS) treatment outcome in OSA patient. We hypothesized that nasal resistance differs between MAS responders and nonresponders and therefore may influence treatment outcome. Thirty-eight patients with known OSA underwent polysomnography while wearing a custom-made MAS. Treatment outcome was defined as follows: Responders (R) ≥50% reduction in AHI, and Nonresponders (NR) as <50% reduction in AHI. NAR was measured using posterior rhinomanometry in both sitting and supine positions, with and without MAS. The mean AHI in 26 responders was significantly reduced from 29.0 ± 2.9/h to 6.7 ± 1.2/h; P < 0.01). In 12 nonresponders there was no significant change in AHI (23.9 ± 3.0/h vs 22.0 ± 4.3/h; P=ns). Baseline NAR was significantly lower in responders in the sitting position compared to nonresponders (6.5 ± 0.5 vs 9.4 ± 1.0cm H2O; P < 0.01). There was no significant change in NAR (from baseline) with MAS in either response group while in the sitting position, but in the supine position NAR increased significantly with MAS in the nonresponder group (11.8 ± 1.5 vs 13.8 ± 1.6 cm H2O/L/s; P < 0.01). Logistic regression analysis revealed that NAR and BMI were the most important predictive factors for MAS treatment outcome. These data suggest that higher levels of NAR may negatively impact on treatment outcome with MAS.
Citation:
Zeng B; Ng AT; Qian J; Petocz P; Darendeliler MA; Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. SLEEP 2008;31(4):543-547.
Keywords: Obstructive sleep apnea, nasal airway resistance, mandibular advancement splint
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY RECURRENT NARROWING AND OCCLUSION OF THE UPPER AIRWAY DURING SLEEP, RESULTING IN sleep fragmentation and intermittent hypoxemia.1 In the context of the current epidemic of obesity, it has been estimated that approximately 17% of adults have mild or worse OSA, and 5.7% have moderate or worse OSA.2 It is associated with increased morbidity and mortality from cardiovascular complications.3 The underlying pathophysiology of OSA is complex and not fully understood. However, it is generally accepted that anatomic changes of the upper airway and functional abnormalities of upper airway dilating muscles may play important roles.1
The air flow passing through the nose and nasopharynx is limited by its shape and diameter.4,5 In OSA patients, the narrowed and collapsible upper airway facilitates a high resistance in the upstream segment of upper airway. Many studies have shown a positive association between nasal obstruction and OSA.6–9 Zwillich and associates found that artificial nasal obstruction induced by a balloon cannula was associated with a significant increase in the number of episodes of apnea and arousals in normal men.6 Other studies have also found an increase in the number of sleep related respiratory events during a night of nasal obstruction.7,8 Surgical correction of nasal obstruction has been used in the treatment OSA, however the response to such treatment is often limited and unpredictable.10–12
The growing literature regarding the benefits of oral appliances in the treatment of OSA has spawned a growing enthusiasm for their use in clinical practice.13,14 Recently updated practice parameters from the American Academy of Sleep Medicine recommend their use in the treatment of mild to moderate OSA.15 A number of predictors of treatment response have been reported, including age, obesity, gender, supine dependent OSA, baseline apnea-hypopnea index, flow-volume curve abnormalities, and a range of craniofacial characteristics such as longer maxilla, shorter soft palate, decreased distance between hyoid and mandibular plane.16–19 Since high nasal resistance is known to induce or exacerbate OSA, it is plausible that high nasal resistance could negatively affect oral appliance treatment outcome. Marklund et al noted that subjective complaints of nasal obstruction were associated with reduced efficacy of MAS amongst female patients.20 To date, no studies have evaluated this possibility by objective measurement of nasal resistance. Hence the primary aim of this study was to compare nasal resistance in MAS responders and nonresponders. Moreover, imaging studies suggest that mandibular advancement is associated with an increase in velopharyngeal caliber.21 Our own MRI work suggests that this airway change is different between responders and nonresponders to MAS treatment.22 Studies have reported that tongue protrusion in OSA patients23 and mandibular advancement in nonapneic subjects24 are associated with a reduction in nasal resistance, consistent with a decrease in retropalatal resistance. We questioned whether this change in nasal resistance could assist in the prediction of treatment outcome. Hence, a secondary aim was to assess whether the effect of mandibular advancement on nasal resistance could differentiate treatment response.
MATERIALS AND METHODS
Subjects
Patients were recruited from a multidisciplinary sleep disorders clinic in a university teaching hospital. Inclusion criteria were the presence of ≥ 2 symptoms of OSA, and evidence of OSA on polysomnography. Patients were excluded if there was periodontal disease, insufficient teeth, or an exaggerated gag reflex. The study was approved by the institutional ethics committee, and written informed consent was obtained from all patients.
Oral Appliance
A custom-made 2-piece oral appliance was used (SomnoMed MAS, SomnoMed Ltd, Australia), the design features and effectiveness of which have been previously published.25–27 The appliance was initially fabricated at approximately 60% of the patient's maximal advancement, and titration screws were subsequently used to provide additional advancement. Acclimatization occurred over a period of approximately 6 weeks, during which the appliance was incrementally advanced until the maximum comfortable limit of mandibular advancement was reached.
Nocturnal Polysomnography
Standard nocturnal polysomnography was performed to determine treatment outcome and was scored according to standard criteria and blinded to the patients' treatment status, as previously described.25–27 In brief, apnea was defined as cessation of airflow for ≥ 10 sec. Hypopnea was defined as a reduction in amplitude of airflow, measured as pressure change at the nares, or thoracoabdominal wall movement of greater than 50% of the baseline measurement > 10 sec with an accompanying oxygen desaturation ≥ 3% and/or associated with an arousal.
Treatment Outcome
We defined “Responders” as patients who had a ≥ 50% reduction in AHI and “Nonresponders” as patients with a < 50% reduction in AHI. Reflecting differences in the clinical definition of treatment success, we used additional definitions for comparative purposes, as follows: ≥ 50% reduction in AHI and residual AHI ≤ 5/hr (Complete Responders); and ≥ 50% reduction in AHI and residual AHI > 5/hr (Partial Responders).
Nasal Airway Resistance (NAR)
NAR was measured by posterior rhinomanometry in a standardized fashion28 in the afternoon prior to the standard nocturnal polysomnography. This method involves measurement of posterior oral pressure via a tube placed in the back of the mouth while airflow is measured for both nasal cavities simultaneously.28 Since an aim of our study was to evaluate the effect of mandibular advancement on retropalatal resistance as a means of predicting treatment outcome, the posterior rhinomanometry technique was specifically chosen over other nasal resistance techniques because it combines transnasal and transpalatal resistances. Specifically, nasal airflow was determined via a sealed nose mask connected to a pneumotachograph. A catheter (4 mm internal diameter polyethylene tubing) was used to determine mask pressure via a sampling site in the nose mask. Mouth pressure was determined by using a similar catheter (with six 14-gauge holes at the distal end) placed as far posteriorly in the mouth as was comfortable. The pressure transducer was calibrated with a water manometer. The transducer was demonstrated to be linear to ± 20 cm H2O. Patients were coached so that reproducible pressure-flow curves were obtained. Simultaneous pressure and flow signals were recorded in real time and monitored on an X-Y plot on a breath-by-breath basis using an established in-house acquisition program, and subsequently analyzed to derive resistance measurements (at a flow of 0.5 L/s) based on the Rohrer equation: ΔP =K1 × V + K2 × V2.29 NAR was measured in both sitting and supine positions, with and without MAS in situ. For each condition, NAR measurement consisted of ≥ 5 consecutive breaths. NAR was calculated as the mean of the 3 most consistent measurements. Values of nasal resistance were expressed as cm H2O/L/s.
Statistical Analysis
Analyses were carried out using a statistical package (SPSS version 14; SPSS Inc, Chicago IL). All descriptive statistics are presented as mean ± SEM. Differences between two groups were tested by independent samples Student's t tests. One-way analysis of variance (ANOVA) was used to compare groups. Receiver operating characteristic (ROC) curves were constructed to derive cut-off predictive values of NAR. The Chi-squared test was used to compare the distribution of predictive values of NAR between the response groups. A P value < 0.05 was considered significant. Multivariable logistic regression analysis was performed, with MAS treatment outcome as the dependent variable and independent variables including sitting NAR, supine NAR, body mass index (BMI), neck circumference, age, gender, baseline AHI, and amount of mandibular advancement induced by the oral appliance. A stepwise forward selection procedure was also performed to examine the effects of different variables and identify the important explanatory variables.
RESULTS
Table 1 shows the clinical characteristics of the 38 eligible patients recruited into the study (29 men, 9 women), at baseline and after MAS treatment, as well as the results of the baseline NAR parameters. MAS treatment was well tolerated by all patients with only mild and transient side effects, including temporomandibular joint pain (10%), salivation (50%), teeth tenderness (40%), and gum irritation (8%). The mean (± SEM) mandibular advancement with MAS was 6.5 ± 0.4 mm (range 2.5–12 mm), representing a mean 70% of maximal mandibular protrusion.
Table 1.
Clinical Characteristics of Patients and Baseline NAR According to Treatment Outcome
| Variables | Responders | Non-Responders |
|---|---|---|
| Patients | 26 (68%) | 12 (32%) |
| Sex (male/female) | 21/5 | 8/4 |
| Age (yr) | 50.9 ± 2.2 | 55.0 ± 2.1 |
| BMI (kg/m2) | 28.7 ± 0.8 | 34.3 ± 1.1* |
| Neck Circumference (cm) | 39.9 ± 0.6 | 41.8 ± 1.1 |
| AHI baseline (h-1) | 29.0 ± 2.9 | 23.9 ± 3.0 |
| AHI with MAS (h-1) | 6.7 ± 1.2 | 22.0 ± 4.3* |
| Sitting NAR (cm H2O/L/s) | 6.5 ± 0.5 | 9.4 ± 1.0* |
| Supine NAR (cm H2O/L/s) | 10.7 ± 1.2 | 11.8 ± 1.5 |
| Amount of mandibular advancement (mm) | 6.4 ± 0.4 | 7.1 ± 0.7 |
Results are presented as mean ± SEM; * P < 0.01.
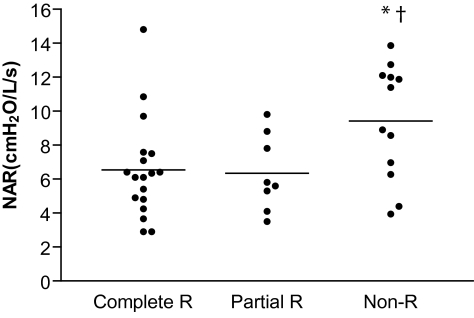
The majority of patients had an elevated NAR, with 78% of responders and 100% of nonresponders having NAR values exceeding 3.5 cm H2O/L/s, which is considered to be the upper limit of the normal range.4,5 There were significant differences in baseline NAR between MAS responders and nonresponders in the sitting position, with a lower NAR in MAS responders (6.5 ± 0.5 vs 9.4 ± 1.0 cm H2O; P < 0.01). However, there was no significant difference in baseline NAR between response groups when measured in the supine position (Table 1). Using more rigorous definitions of treatment outcome revealed a consistently lower NAR in both complete and partial responders, compared to nonresponders (Figure 1).
Figure 1.
Comparison of NAR (sitting) According to Treatment Outcome Category. Complete Responders (Complete R): >50% reduction in AHI and residual AHI <5/hr; Partial Responders (Partial R): >50% reduction and residual AHI >5/hr; and Nonresponders (Non-R): <50% reduction in AHI. Results are presented as mean ± SEM; * P < 0.01 versus Complete R; † P < 0.01 versus Partial R.
There was no significant change in NAR (from baseline) with MAS in either response group while in the sitting position. However, in the supine position NAR increased significantly with MAS in the nonresponder group (11.8 ± 1.5 vs 13.8 ± 1.6; P < 0.01), but not the responder group (Table 2).
Table 2.
Effect of Mandibular Advancement on Nasal Airway Resistance (NAR)
| NAR (cmH2O/L/s) |
||
|---|---|---|
| Baseline | With MAS | |
| Sitting position | ||
| Responders | 6.5 ± 0.5 | 6.4 ± 0.6 |
| Nonresponders | 9.4 ± 1.0 | 8.9 ± 0.9 |
| Supine position | ||
| Responders | 10.4 ± 1.2 | 11.0 ± 1.2 |
| Nonresponders | 11.8 ± 1.5 | 13.8 ± 1.6* |
Results are presented as mean ± SEM; * P < 0.01.
A NAR cut-off value of ≤ 6.4 cm H2O/L/sec was derived using ROC curves. The sensitivity and specificity of this cut-off value were 65% and 75%, respectively. Comparison of the distribution of predictive values of NAR between the response groups was significant (χ2 = 5.34, P < 0.05).
Multivariable logistic regression revealed that NAR (sitting) and BMI were significant independent predictors of MAS treatment outcome, but neck circumference, age, gender, baseline AHI, and amount of mandibular advancement were not (Table 3).
Table 3.
Multivariable Logistic Regression Showing the Influence of Baseline Nasal Resistance (Sitting) and Body Mass Index on Treatment Outcome
| Variables | Beta | SE | Wald X2 | P-value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Nasal resistance (sitting) | −0.54 | 0.20 | 7.08 | 0.008 | 0.58 | 0.39–0.87 |
| BMI | −0.43 | 0.15 | 8.36 | 0.004 | 0.65 | 0.49–0.87 |
| Constant | 18.46 | 5.85 | 9.94 | 0.002 |
DISCUSSION
Although oral appliances are now considered an acceptable treatment option for mild to moderate OSA, there remains uncertainty about how to reliably predict treatment outcome.13,14 We examined the influence of nasal resistance on oral appliance treatment outcome, and found that high nasal resistance may have a negative impact on treatment response. This, when combined with other reported predictors, could assist clinicians in assessing the likely response of patients to MAS treatment.
The key finding of this study is the observed significant difference in baseline NAR between MAS responders and nonresponders. These data suggest that OSA patients with lower NAR have a greater likelihood of responding to MAS treatment. This is entirely consistent with the literature on nasal resistance in OSA. Many studies have shown that nasal obstruction can induce or increase apnea frequency in OSA patients.6–9 When nasal resistance is high, a greater pressure drop is required to achieve the same flow, and hence collapse of the pharynx is facilitated and this could mitigate the effect of mandibular advancement. Moreover, high nasal resistance is associated with mouth breathing, which could potentially limit the beneficial effect of mandibular advancement. However, the therapeutic implications of this have not been realized, since many studies show limited and unpredictable efficacy of nasal surgery in the management of OSA.10–12 Whether reducing nasal resistance is a worthwhile strategy to improve the outcome of oral appliance treatment is not resolved by our study, but merits investigation. Furthermore, the potential of alternative methods of assessing nasal anatomy and function, such as acoustic rhinometry, requires exploration.
We evaluated the effect of MAS on nasal resistance, in the hope that this may serve as a discriminator of treatment outcome. We found a difference only in the supine position, whereby MAS nonresponders had higher NAR (supine) while wearing MAS. The pathophysiological significance of this response, noted only in the supine position, is uncertain. Previous studies have reported a decrease in nasal resistance in response to mandibular advancement and tongue protrusion, which is thought to reflect a reduction in transpalatal resistance.23,24 We speculate that our observed increase in resistance is due to an increase in transpalatal resistance amongst nonresponders, and is consistent with our preliminary MRI work showing a reduction in supine velopharyngeal caliber with mandibular advancement in nonresponders.22 However, there was significant interindividual variability and hence the sensitivity and specificity of this observation are inadequate for use in the clinical setting.
Logistic regression was used to examine predictors of MAS treatment outcome in this patient group. This revealed that of the variables included in the model, only nasal resistance (sitting) and BMI were significant predictors of MAS treatment success. BMI has previously been reported to be a clinical predictor of MAS treatment outcome.18 Our study, suggests that clinical evaluation of nasal airflow is also important in formulating a clinical prediction of treatment outcome. Notably, the amount of mandibular advancement was not found to be a significant predictor.
This study has a number of limitations. Our chosen definition of MAS treatment outcome can be criticized for being too liberal, but this was intended to reflect definitions used in clinical practice. The use of more rigorous definitions of complete and partial response did not change the fundamental findings of our study. Nasal resistance measurements show high inter- and intra-individual variability, and not all patients are able to produce valid pressure-flow curves with the active posterior rhinomanometry technique. This may be more difficult in the supine position, and may provide an explanation for our failure to find a significant difference in nasal resistance between the responder groups in the supine position. Whilst our choice of the posterior rhinomanometry technique was justified on the basis of our specific aims, the clinical application of this technique may be limited. Despite our finding of a significant difference in sitting NAR between responders and nonresponders, there was overlap in NAR values between patient groups, precluding the identification of a reliable cut-off value for use in the clinical setting. Furthermore, our study was not conducted as a pretreatment prediction of oral appliance outcome, and therefore it is important to undertake a prospective study in order to assess the clinical utility of our findings.
In conclusion, our study suggests that higher levels of NAR may negatively affect treatment outcome with MAS. This information may be useful to clinicians when assessing the likely outcome of treatment with an oral appliance. Further work is required to study the relationship between nasal function and treatment outcome, and particularly whether methods to lower nasal resistance can improve the outcome of oral appliance treatment.
ACKNOWLEDGMENTS
The authors are grateful to Drs Belinda Liu and Helen Gotsopoulos for the dental management of patients.
This work was supported by a project grant (No. 300525) from the National Health and Medical Research Council (NH&MRC) of Australia.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Cistulli contributed to the development of the oral appliance used in this study, which is being commercialized by SomnoMed Ltd. He has consulted and been on the advisory board for SomnoMed and has a financial interest in the company. His department has is engaged in research funded by ResMed and has received use of equipment provided by ResMed and SomnoMed for other research. He is a board member of the ResMed Foundation, a non-profit, charitable organization. Dr. Ng has participated in research initiated and sponsored by Sanofi. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Cistulli PA, Sullivan CE. Sleep and Breathing. New York: Marcel Dekker Inc; 1994. Pathophysiology of sleep apnea; pp. 405–88. [Google Scholar]
- 2.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 3.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation. 2003;108:9–12. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 4.Warren DW, Hairfield WM, Seaton DL, Hinton VA. The relationship between nasal airway cross-sectional area and nasal resistance. Am J Orthod Dentofacial Orthop. 1987a;92:390–5. doi: 10.1016/0889-5406(87)90259-9. [DOI] [PubMed] [Google Scholar]
- 5.Warren DW, Hinton VA, Pillsbury HC, Hairfield WM. Effects of size of the nasal airway on nasal airflow rate. Arch Otolaryngol. 1987b;113:405–8. doi: 10.1001/archotol.1987.01860040067019. [DOI] [PubMed] [Google Scholar]
- 6.Zwillich CW, Pickett CK, Hansen F, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis. 1981;124:158–60. doi: 10.1164/arrd.1981.124.2.158. [DOI] [PubMed] [Google Scholar]
- 7.Olsen K, Kern E, Westbrook P. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg. 1981;89:804–10. doi: 10.1177/019459988108900522. [DOI] [PubMed] [Google Scholar]
- 8.Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest. 1986;90:324–9. doi: 10.1378/chest.90.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Rombaux P, Liistro G, Hamoir M, et al. Nasal obstruction and its impact on sleep-related breathing disorders. Rhinology. 2005;43:242–50. [PubMed] [Google Scholar]
- 10.Aubert-Tulkens G, Hamoir M, van den Eeckault J, Rodenstein DO. Failure of tonsil and nose surgery in adults with long standing severe sleep apnea syndrome. Arch Intern Med. 1989;149:2118–21. [PubMed] [Google Scholar]
- 11.Busaba N. The nose in snoring and obstructive sleep apnea. Curr Opin Otolaryngol Head Neck Surg. 1999;7:11–3. [Google Scholar]
- 12.Woodhead CJ, Allen MB. Nasal surgery for snoring. Clin Otolaryngol Allied Sci. 1994;19:41–4. doi: 10.1111/j.1365-2273.1994.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 14.Cistulli PA, Gotsopoulos H, Marklund M, Lowe AA. Treatment of snoring and obstructive sleep apnea with mandibular repositioning appliances. Sleep Med Rev. 2004;8:443–57. doi: 10.1016/j.smrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. An American Academy of Sleep Medicine Report. Sleep. 2006;29:240–43. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 16.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–35. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 17.Tsuiki S, Lowe AA, Almeida FR, Fleetham JA. Effects of an anteriorly titrated mandibular position on awake airway and obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 2004;125:548–55. doi: 10.1016/j.ajodo.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001;120:639–47. doi: 10.1067/mod.2001.118782. [DOI] [PubMed] [Google Scholar]
- 19.Zeng B, Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:726–30. doi: 10.1164/rccm.200608-1205OC. [DOI] [PubMed] [Google Scholar]
- 20.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CF, Love LL, Peat D, et al. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng B, Ng AT, Qian J, Darendeliler MA, Petocz P, Cistulli PA. Prediction of oral appliance treatment outcome in obstructive sleep apnea using upper airway magnetic resonance imaging [abstract] Sleep Biol Rhythms. 2006;4:48. [Google Scholar]
- 23.Coste A, Lofaso F, d'Ortho MP, et al. Protruding the tongue improves posterior rhinomanometry in obstructive sleep apnoea syndrome. Eur Respir J. 1999;14:1278–1282. doi: 10.1183/09031936.99.14612789. [DOI] [PubMed] [Google Scholar]
- 24.Okawara Y, Tsuiki S, Hiyama S, Hashimoto K, Ono T, Ohyama K. Oral appliance titration and nasal resistance in nonapneic subjects. Am J Orthod Dentofacial Orthop. 2004;126:620–2. doi: 10.1016/j.ajodo.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 26.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 27.Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1:374–80. [PubMed] [Google Scholar]
- 28.Clement PAR, Godts F. Rhinology. 2005. Consensus report on acoustic rhinometry and rhinomanometry; pp. 169–79. [PubMed] [Google Scholar]
- 29.Rohrer F. The resistance in the human airway and the influence of branching of bronchial systems on frequency of breathing at different lung volumes. Pfluegers Arch Physiol. 1915;162:255–99. [Google Scholar]