Abstract
Background:
The incidence of apnea in neonates depends on a number of factors, including sleep state and thermoregulation.
Objective:
To assess the role of thermal drive (body heat loss [BHL]) in the mechanisms underlying short episodes of central apnea during active and quiet sleep in neonates.
Material and Method:
Twenty-two neonates (postconceptional age: 36.3 ± 0.9 weeks) were exposed at thermoneutral (incubator temperature: 32.5°C), warm (34.2°C), and cool (30.4°C) conditions during 3 consecutive morning naps. Oxygen consumption (V·O2), skin and rectal temperatures, and central apnea were scored during active sleep and quiet sleep. The thermal drive was expressed as BHL calculated using indirect partitional calorimetry.
Results:
As expected, apnea occurred more frequently in active sleep than in quiet sleep (P < 0.001). The frequency of apnea in active sleep was higher in the warm condition (P < 0.05). In contrast, apnea episodes were less frequent (P < 0.05) and shorter (P < 0.05) for cool exposure, during which V·O2 and rectal temperature increased. The frequency (P < 0.001, r2 = 0.31), mean (P < 0.05, r2=0.06), and maximum (P < 0.001, r2 = 0.19) durations of apnea were correlated with the BHL: the greater the BHL (body cooling), the less frequent and the shorter the apnea episodes. In contrast, no relationship between apnea and mean skin or rectal temperature was observed.
Conclusion:
Apneic events were more closely related to BHL than to body temperatures. In cool exposure, the decreases in the duration and frequency of apneic episodes suggest that these events depend on the metabolic drive (which is proportional to energy expenditure).
Citation:
Tourneux P; Cardot V; Museux N; Chardon K; Léké A; Telliez F; Libert JP; Bach V. Influence of thermal drive on central sleep apnea in the preterm neonate. SLEEP 2008;31(4):549-556.
Keywords: apnea, neonate, temperature, sleep, metabolism
IN NEONATES, THE INCIDENCE OF APNEA DEPENDS ON A VARIETY OF FACTORS, SUCH AS BIRTH WEIGHT,1 SEX,2 GESTATIONAL AGE,3 AND POSTNATAL AGE.3,4 Central apnea is generally reported as occurring more frequently during active sleep than during quiet sleep.2,3,5–7 Many authors have pointed out that apnea incidence is also closely related to ambient temperature in both full-term8–10 and preterm neonates9,11,12 and that the rate of apneic events is increased by warm exposure (i.e., thermal drive). Although preterm neonates are more often exposed to cool stress than to warm stress, little is known about the influence of cool exposure on the incidence of apnea in the different sleep states. Bader et al11 reported a lower rate of central apnea during transient decreases in incubator temperature from warm (29°C) to thermoneutral conditions (24°C) over 30 minutes, although this was only seen during quiet sleep for preterm infants and during active sleep for term infants.
The mechanism linking thermal stress and apnea is unknown and thus warrants further investigation. On the basis of the above-cited studies, it can be supposed that suprapontine influences modify respiratory control, which must be considered as a multiple-interaction system. Abnormal functional interaction among the respiratory system, thermoregulatory system, and sleep processes may alter compensatory responses to autonomic cardiovascular or respiratory challenge and increase the likelihood of life-threatening events later in life.13
The effect of thermal stress is usually assessed by monitoring the body's internal temperature (generally esophageal or rectal temperatures, which supposedly represent the core temperature) and/or mean skin temperature.14 However, the central controller of the thermoregulatory system receives thermal inputs from thermosensitive structures distributed throughout the body. The regulated variable therefore results from a weighted sum of different body temperatures.15 Hence, to fully understand the thermal influence on apnea incidence in cool environments, it is essential to quantify the magnitude of body cooling that is proportional to the radiant, convective, conductive, and evaporative heat losses (i.e., body heat loss) on the other. Any failure to maintain thermal balance stimulates the body's thermal control mechanisms and thus triggers regulatory adjustments. This approach may help clarify a hypothesis raised by Perlstein et al,9 whereby apnea is not specifically induced by changes in air temperature but, rather, through processes controlling the overall body heat loss (BHL). Hence, in the present study, the role of thermal drive in the mechanisms underlying the genesis of central apnea in the sleeping neonate was assessed by taking into account BHL during mild warm and cool thermal exposures.
Central apneic events were monitored in a group of 22 near-term neonates. Indeed, there are few published studies on these infants, who are generally considered to be physiologically similar to term infants, even though their central nervous system and breathing control are less mature than those of full-term infants. Moreover, prematurity increases the risk of sudden infant death syndrome. Despite the need to study the basic physiologic processes underlying short apneic events, most studies have focused on pathologic apneic events (usually considered as those lasting more than 20 seconds).
MATERIALS AND METHODS
Subjects
Twenty-two preterm neonates (8 males and 14 females, mean ± SD gestational age: 30.6 ± 2.0 weeks, birth weight: 1.39 ± 0.37 kg) were investigated at 36.2 ± 0.9 weeks of postconceptional age, when the mean body mass was 2.20 ± 0.19 kg. The neonates were healthy and were free from infections and metabolic, neurologic, or cardiorespiratory problems. According to the questionnaires filled out by the mother and examination of their medical files, none of the neonates or their mothers received alcohol, caffeine, or any treatment that could have modified respiratory and thermal parameters.
The protocol was approved by the Picardy Regional Ethics Committee, and written informed consent was obtained from the parents.
Methods
Recordings were performed in the Neonatology Unit at Amiens University Medical Center. The nursery room temperature was monitored and held constant at 22°C to 24°C (relative air humidity: 30%–40%, air velocity ≤ 0.04 m·sec−1, natural convection). The supine neonates (wearing a diaper) lay on a mattress made out of plastic foam in a closed, convectively heated incubator (Médipréma® ISIS, Chambray-les-Tours, France).
Incubator and body temperatures
The incubator temperature (Tinc, °C) was measured with a thermocouple placed 10 cm above the center of the mattress (accuracy ± 0.10°C; model K; Bioblock, Illkirch, France). The mean temperature of the mattress (Tm, °C) surface was calculated using 5 thermistors located in the middle and the corners of the upper surface of the mattress (accuracy ± 0.10°C; YSI 402; Bioblock). The infants' rectal temperature (Tre, °C) was monitored with a thermistor probe (accuracy ± 0.10°C; YSI 402; Bioblock) inserted 2 cm beyond the anal sphincter; this core temperature is currently considered to be the major stimulus of the thermoregulatory system. The mean skin temperature (Tsk, °C) was averaged from abdominal and cheek skin temperatures, measured with thermocouples (accuracy ± 0.10°C, model K; Bioblock). Each probe was covered by an aluminum foil patch to reduce the effect of heat radiation from the incubator walls. Body and air temperatures were continuously sampled at 10-second intervals in the morning between 2 meals.
Sleep
Sleep states (active sleep, quiet sleep, and intermediate sleep) were scored according to Curzi-Dascalova and Mirmiran16 in 30-second periods, on the basis of electroencephalograms and eye movements (monitored by a piezoelectric quartz accelerometer attached to an eyelid). Limb movements (recorded using accelerometers attached to the wrist and ankle) and electrocardiogram using standard derivations and 3 patch electrodes (1 on each side of the thorax and 1 on the left flank) were also recorded. All recordings were continuously monitored on a polysomnograph (Alice 4, Respironics®, Nantes, France). The breathing rate was assessed by transthoracic impedance using the electrocardiogram patch electrodes, thus avoiding the need for an additional sensor.12,17 Oxyhemoglobin saturation was recorded from the transcutaneous pulsed oxygen saturation (SpO2), which was measured throughout the experiment using a pulse oximeter with a neonatal sensor (Oximax MAX-N, Tyco Healthcare Group LP, Nellcor Puritan Bennett Division, CA) placed on the right hand. Oxygen consumption (V·O2, mL·min−1·kg−1) was measured (using a mask gently placed on the neonate's face) in the absence of body movements for more than 30 seconds and at least twice during episodes of active sleep and quiet sleep. The O2 concentration was continuously measured during the test using a mass spectrometer (MGA-1100, GE Marquette Medical Systems, Milwaukee, WI) calibrated with standard gases of known concentrations at the beginning of each experiment. V·O2 was converted into standard temperature and pressure, dry units.
Experimental Design
The measurements were performed during 3 successive morning naps (during the period from 09:00 to 12 noon). On the first morning (baseline condition: N), Tinc was set to thermoneutrality using a skin-temperature, servo-controlled system designed in our laboratory.18 This interactive device ensures the maintenance of a thermal condition that takes account of each neonate's particular thermoregulatory requirements. Hence, the thermal environment fulfilled the criteria for thermoneutrality given by Sauer et al.19
For the second (mild warm condition) and third sessions (mild cool condition), Tinc was set to TN + 2°C and TN − 2°C, respectively. This experimental sequence was chosen in order to rule out a possible effect of thermal adaptation to cool exposure, since the latter can modify thermoregulatory responses and sleep structure.20,21 Such a modification has never been reported after warm exposure, which is usually considered to be less disruptive than cool exposure.22,23 Only mild thermal influences (similar to those encountered during standard nursing procedures) were considered, in order to avoid over-complex responses.
Data Analysis
Apneic Events
Only central apnea was considered, since its incidence is closely related to the interaction (between breathing control and thermal drive) that we sought to assess. Apnea was recorded using transthoracic impedance and defined as a respiratory pause lasting for longer than 3 seconds, as recommended by Gould et al24; the latter authors emphasized that specific effects of sleep state could be observed for apneic events as short as 2 to 5 seconds. Moreover, this duration facilitates comparisons with data from previous studies using the same criteria.8,11,25 We also measured apnea occurring during periodic breathing, since this respiratory pattern is also influenced by thermal drive.25
Apneic episodes were described in terms of their frequency (events·h−1), since the relative durations of active sleep and quiet sleep are known to be influenced by the thermal condition.18,21,26 The mean duration (in seconds), 90th percentile duration (in seconds), and maximal duration (in seconds) of the events were also taken into account. Results obtained with the 90th percentile of apnea duration were consistent with those using the maximal duration and, thus, are not presented here.
Body Heat Loss
Neonates lose heat to the environment via several channels.14 The various heat exchanges (expressed in W) are defined by the following equations (partitional heat loss):
Conductive heat (K) is exchanged between the skin (Tsk, °C) and the mattress (Tm, °C) surfaces:
K = hk (Tm − Tsk) Ak AD
hk = the conductive heat transfer coefficient (= 2 W·m−2·°
C−1),27
AK = the percentage of body skin surface area through which heat is flowing (= 0.104),27
AD = Boyd's body surface area28, m2;
Convective heat is exchanged through air moving over the mucous membrane of the respiratory tract (Cresp) or over the skin surface (C):
Cresp = V·E Cp (Te − Ti) ρ
V·E = the volumetric respiratory flow (m3·h−1),
Cp = the heat capacity of the air (1.044 kJ·kg−1·°C−1),
Te − Ti = the temperature difference between expired and inspired air (°C),
The value of Te is nearly equal to the body's internal temperature,
ρ = volumetric mass of air (kg.m−3);
C = hc (Ta − Tsk) Fcl Ac AD
hc = the convective heat transfer coefficient (W·m−2·°C−1) = 2.38 (Tsk − Ta)0.25,29 as recommended for natural convection29,30,
Ac = the percentage of body surface area available for convective heat exchange (0.804).31
The value was determined by taking into account the surface area contacting the mattress (0.104) and the surface area of the upper part of the trunk covered by the diaper (0.092),31
Fcl = the heat reduction factor due to clothing, which can be calculated as follows32:
Fcl = [(hc + hr) Icl + 1/fcl]−1
hr = the radiative heat transfer coefficient = 4.7 W·m−2·°C−1,29,33
Icl = the effective clothing thermal insulation of the diaper, which ranged from 0.021 to 0.064°C·m−2·W−1 according to the actual air temperature measured in each experimental condition.31
The clothing surface area factor fcl is 1 + 1.97 Icl;
Radiant heat exchange (R) is calculated using the Stephan-Boltzmann law:
R = hr Ar [(Tr + 273)4 − (Tsk + 273)4] Fcl AD
Ar = the effective radiating area (0.77), determined for a relaxed neonate34 less the percentage of the surface area in contact with the mattress (0.104) and the upper part of the trunk surface area covered by the diaper (0.092), Ar = 0.574,
Tr = the mean radiant temperature of the environment (°C)29;
Evaporative heat losses occur through the respiratory system (Eresp) and through the skin by transepidermal water diffusion (E). The evaporative heat loss due to breathing is related to the volume of expired air (V·E, m3·h−1) and the difference in water content between expired (Me) and inspired (Mi) air (kg·m−3):
Eresp = V·E δ (Me − Mi)
δ = the latent heat of vaporization of water (2.406 kJ·g−1);
E = he ω Ac (PsH20 − PaH20) Fp,cl AD
he = the evaporative heat transfer coefficient (W·m−2·mb−1) = 1.67 hc,
ω = the skin wetness (dimensionless) = 0.06,35 since sweating activity is absent or very limited in these preterm neonates,
PsH20 − PaH20 = the water vapor partial pressure difference (mb) between the skin (PsH20) and air (PaH20),
Fp,cl = the reduction factor for water vapor calculated from the clothing insulation32:
Fp,cl = [1 + 2.22 hc (Icl − (1−1/fcl)/(hc + hr)]−1
By convention, heat loss from the body to the environment is negative. Hence, the BHL (in kJ·h−1·kg−1, 1 W = 3.608 kJ·h−1) to the environment is − (K + Cresp + C + R + Eresp + E).
The body is a system within which there is inevitably a minimal level of metabolic heat production (M); this balances the heat loss, so that the body heat storage is 0 (i.e., thermoneutral conditions). The BHL can be affected by an increase in metabolic heat production over the minimal rate (due to increased brain activity), such as that encountered in active sleep. The increment (ΔM) limits body cooling and must therefore be added to BHL in order to enable comparisons with quiet sleep.
Statistical Analyses
Effects of thermal conditions and sleep states on temperatures, heart rate, sleep state durations, and oxygen consumption were tested using an analysis of variance (Statview® 5.0). When F values were significant (P < 0.05), a Fisher posthoc protected least significant difference test was performed. Data are provided as mean ± SD. Simple linear regressions were computed to correlate the thermal parameters (independent variables) on one hand and apnea frequency, mean duration, and maximal duration (dependent variables) on the other.
RESULTS
The incubator temperature was 32.40°C ± 0.83°C at thermoneutrality, 33.91°C ± 0.74°C for the warm exposure, and 30.31°C ± 0.69°C for the cool exposure. The relative air humidity values were 36.64% ± 8.98%, 34.18% ± 6.93%, and 35.86% ± 8.64% for thermoneutrality, warm and cool thermal conditions, respectively, whereas the corresponding mean radiant temperatures were 25.67°C ± 1.13°C, 27.34°C ± 0.82°C, and 22.70°C ± 1.11°C. The temperature of the mattress surface was 28.12°C ± 1.01°C (thermoneutral), 29.74°C ± 0.73°C (warm), and 25.43°C ± 0.89°C (cool).
Total sleep time during the nap (106 ± 31 min, Table 1) did not depend on the thermal condition (F2,42 = 1.17, P = 0.32). However, active sleep increased at the expense of quiet sleep (+7.3% and −5.4% of total sleep time, respectively) during cool exposure, when compared with thermoneutrality (interaction thermal condition × sleep state: F2,84 = 2.94, P = 0.025).
Table 1.
Sleep State Expressed as a Percentage of Total Sleep Time in the Different Thermal Conditions
| Thermoneutrality | Warm exposure | Cool exposure | |
|---|---|---|---|
| Total sleep time, min | 112 ± 20 | 107 ± 39 | 98 ± 32 |
| Sleep state | |||
| Active | 65.4 ± 8.5 | 66.9 ± 7.1 | 72.7 ± 12.2a,b |
| Quiet | 25.3 ± 6.8 | 23.5 ± 7.5 | 19.9 ± 10.0c |
| Intermediate | 9.3 ± 4.9 | 9.6 ± 5.6 | 7.4 ± 5.7 |
Thermal condition × sleep state interaction: F2,84 = 2.94, P = 0.025.
Cool exposure vs thermoneutrality in active sleep: P = 0.013
Cool vs warm exposure in active sleep: P = 0.049
Cool exposure vs thermoneutrality in quiet sleep: P = 0.032
Heart rate was affected by the thermal load (F2,34 = 3.66, P = 0.036): in contrast with the cool exposure, the warm condition increased heart rate (thermoneutral: 146 ± 10 beats·min−1, warm: 151 ± 9 beats·min−1, cool: 146 ± 9 beats·min−1, respectively; warm versus thermoneutral or cool: P was always < 0.013).
V·O2 differed according to the thermal condition (F2,22 = 10.06, P < 0.001): it was higher in the cool condition (thermoneutral: 7.25 ± 1.45 vs cool: 9.22 ± 1.46 mL·min−1·kg−1, P = 0.003) but was similar in the warm condition and for thermoneutrality (thermoneutral vs warm: 8.21 ± 1.65 mL·min−1·kg−1, P = 0.115). As expected, V·O2 was larger in active sleep than in quiet sleep (active sleep: 9.18 ± 1.34 vs quiet sleep: 7.28 ± 1.70 mL·min−1kg−1, F1,11 = 31.84, P < 0.001), whatever the thermal condition (interaction: F2,22 = 0.23, P = 0.795).
Body Temperatures and BHL
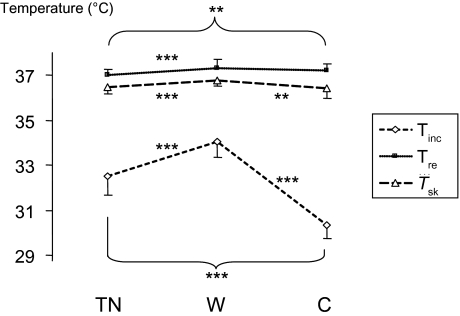
Compared with thermoneutrality, warm exposure increased the rectal (thermoneutral: 37.17°C ± 0.25°C vs warm: 37.70°C ± 0.07°C, P < 0.001) and mean skin temperatures (thermoneutral: 36.36°C ± 0.28°C vs warm 36.70°C ± 0.23°C, P < 0.001). During cool exposure, Tre increased (cool: 37.34°C ± 0.24°C, P = 0.010), whereas Tsk did not change (cool: 36.28°C ± 0.37°C, P = 0.151). The magnitudes of Tre and Tsk were lower in the cool condition than in the warm condition (P was always < 0.030) (Figure 1).
Figure 1.
Incubator (Tinc), rectal (Tre) and mean skin (Tsk) temperatures (°C) at thermoneutrality (TN) and in warm (W) and cool (C) conditions (mean values ± 1 SD). Posthoc analyses comparing warm exposure, cool exposure, and thermoneutrality are indicated: ** P < 0.01, *** P < 0.001.
BHL differed according to the thermal condition (F2,24 = 107.86, P < 0.001) and was greater for the cool exposure (−13.79 ± 2.07 kJ·h−1·kg−1) than at thermoneutrality or in the warm condition (thermoneutral: −11.28 ± 1.84 kJ·h−1·kg−1, warm: −10.59 ± 1.96 kJ·h−1·kg−1, always P < 0.001). Furthermore, BHL was lower in active sleep (−10.45 ± 2.87 kJ·h−1·kg−1) than in quiet sleep (−13.33 ± 1.04 kJ·h−1·kg−1, F1,12 = 20.03, P < 0.001).
Apnea
About 4000 apnea episodes were scored. The overall mean apnea frequency was 36.2 ± 32.5 events per hour and the mean event duration was 5.4 ± 1.2 seconds.
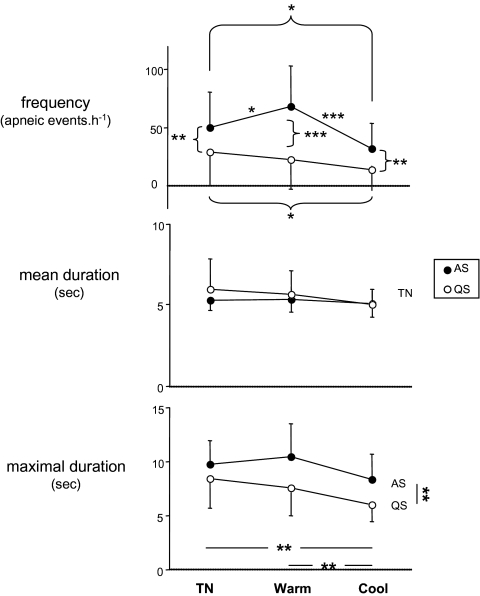
In agreement with most literature studies,2,3,5–7 the apnea frequency was higher in active sleep than in quiet sleep (47.7 ± 28.6 vs 20.3 ± 23.1 events·h−1, F1,19 = 47.78, P < 0.001, Figure 2), whatever the thermal condition (P was always < 0.024). We observed a significant interaction between thermal load and sleep state (F2,38 = 7.18, P = 0.002); the apnea frequency was lower for cool exposure than at thermoneutrality in both active sleep (thermoneutral: 45.9 ± 27.2 events·h−1 vs cool: 29.9 ± 22.2 events·h−1, P = 0.046) and quiet sleep (thermoneutral: 24.2 ± 25.7 events·h−1 vs cool: 13.2 ± 16.6 events·h−1, P = 0.042). Warm exposure increased the apnea frequency in active sleep (warm: 67.2 ± 36.4 events·h−1 vs thermoneutral: P = 0.050), whereas no difference was observed in quiet sleep (P = 0.388).
Figure 2.
The frequency (apneic events·h−1), mean and maximal duration (sec) of apnea in the 2 sleep states (AS: active sleep, QS: quiet sleep) and the 3 thermal experimental conditions (thermoneutrality [TN] and warm and cool conditions) (mean values ± SD). * P < 0.05, ** P < 0.01, *** P < 0.001
The maximal duration of apneic episodes was shortened by the cool condition (thermoneutral: 9.1 ± 2.6 sec, warm: 8.1 ± 1.9 sec, cool: 7.2 ± 1.9 sec, F2,20 = 6.27, P = 0.008; thermoneutral vs cool: P = 0.008). Active sleep was characterized by a longer maximal duration of apneic episodes (active sleep: 9.1 ± 2.2 sec vs quiet sleep: 7.2 ± 2.1 sec, F1,10 = 13.86, P = 0.004). Thermal and sleep-state effects did not reach a significant level regarding the mean apnea duration (always F2,20 < 1.03, P > 0.375).
Relationship Between Apneic Events and Thermal Influences
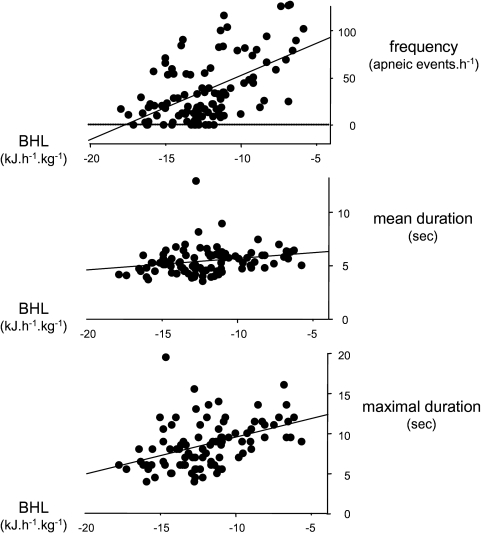
The frequency and mean and maximal duration of apneic events were not significantly related to rectal temperatures (F1,94 always < 1.83, P > 0.179) or mean skin temperatures (F1,117 always < 1.94, P > 0.167). In contrast, the frequency of apneic events was significantly related to BHL (F1,103 = 45.66, P < 0.001, r2 = 0.31, Figure 3): the greater the BHL, the less frequent the apneic events. Likewise, the mean and maximal durations of apneic events were positively correlated with BHL (mean duration: F1,91 = 5.44, P = 0.022, r2 = 0.06; maximal duration: F1,91 = 20.75, P < 0.001, r2 = 0.19). This relationship is consistent with results obtained with Tsk − Tinc, although the latter are somewhat less correlated (frequency: F1,117 = 15.84, P < 0.001, r2 = 0.12; mean duration: F1,105 = 2.77, P = 0.098, r2 = 0.03, maximal duration: F1,105 = 9.13, P = 0.003, r2 = 0.08). The correlations with BHL were consistent over the 3 thermal conditions. Moreover, these correlations were significant in both active sleep and quiet sleep (always F1,44 > 5.21, P < 0.027), enabling us to pool the data.
Figure 3.
The frequency (apneic events·h−1), mean and maximal duration (sec) of apnea as a function of body heat loss (BHL, kJ·h−1·kg−1) in the 2 sleep states (active sleep & quiet sleep) and the 3 thermal experimental conditions (thermoneutral, warm, and cool conditions)
Frequency = 112.0 + 7.68 BHL, F1,103 = 45.66, P < 0.001, r2 = 0.31
Mean duration = 6.79 + 0.13 BHL, F1,91 = 5.44, P = 0.022, r2 = 0.06
Maximal duration = 14.18 + 0.56 BHL, F1,91 = 20.76, P < 0.001, r2 = 0.19
DISCUSSION
The present study indicates that the frequency and the mean and maximum durations of apneic events were significantly related to BHL: the greater the BHL, the less frequent the apneic events and the shorter their mean and maximum durations. These correlations were consistent in both active sleep and quiet sleep and in all 3 thermal conditions.
Thermal, Metabolic, and Hypnic Parameters
Mild thermal challenges (air temperature: +1.5°C and −2.1°C around thermoneutrality) can indeed modify BHL, V·O2, sleep structure, and heart rate in near-term neonates. This observation can be explained by the fact that their thermoregulatory responses are not very effective, while their body heat exchanges with the environment are large.14 In response to a mild cool exposure, the thermal responses consist of cutaneous vasoconstriction (a Tsk decrease), as well as nonshivering thermogenesis, which occurs rapidly (inducing a Tre increase) via sympathetic activation. These responses (inducing opposite changes in internal and cutaneous temperatures and, thus, increasing the temperature gradient between core and skin) are a normal feature of thermoregulation.15 Similar findings have been reported by Silverman et al36 in extremely low-birth-weight neonates. As previously reported in the literature, cool exposure increases the relative duration of active sleep, at the expense of quiet sleep (reviewed in26). In contrast, the few studies dealing with warm challenges have demonstrated that the latter lack a strong effect on sleep structure,22 in agreement with the present results. The increases in heart rate (+6 beats·min−1) and mean skin temperature reflect the vasodilatory response to warm exposure.
Our results show that BHL is a more sensitive indicator of thermal stress than is rectal or mean skin temperature, since the typical increase in V·O2 occurring on cool exposure is observed when BHL is greater, despite a higher rectal temperature and a nonsignificant change in mean skin temperature. This is easily explained by the fact that the internal temperature is not the same throughout body, and, therefore, determination of the loss of body heat is a more accurate estimation of thermal drive than are body temperatures themselves. This finding agrees with those based on pooled37,38 or individual data39 and explains the fact that, in most studies performed on neonates, the expected negative correlation with V·O2 is obtained with air temperature rather than body temperatures. Thus, the body temperatures should be viewed as the output of the thermoregulatory system instead of its input—the latter being more related to BHL.
Apneic Events and BHL
Of the 4000 recorded breathing pauses, only 75 lasted longer than 10 seconds and would be considered as clinically relevant (probably because the neonates were near term). The overall apnea frequency (36.2 ± 32.5 events·h−1) is consistent with previous reports on preterm neonates.3,40
The apnea frequency varies in both cool and warm conditions—evidencing an interaction between thermoregulatory and respiratory processes. As shown in Figure 2, the apnea frequency increased during warm exposure in active sleep only (in contrast with cool exposure, which decreased the frequency of apneic events in both active sleep and quiet sleep), evidencing a functional interaction between respiratory control and the sleep process. Disparities between active sleep and quiet sleep have already been reported: Franco et al8 found that the apnea frequency significantly increased with a warmer ambient temperature during active sleep in older neonates (between 37 to 41 weeks of gestation, median age: 11.5 weeks). Likewise, the mean duration of apnea was decreased by cool exposure in active sleep only.22 In contrast, Bader et al11 reported that the rate of apneic events in active sleep increased with warmer ambient air temperature in full-term neonates but not in preterm neonates.
In warm exposure, an increased frequency of apneic events has been extensively reported.8,9,11,41 With the exception of the work by Bader et al11, most of these studies did not look for (or did not demonstrate) significant warm thermal effects on apnea duration, as confirmed by our results when comparing thermoneutrality and warm exposure. The mechanisms of the impact of warm stimuli on apneic episodes have not been elucidated. Several hypothesis have be put forward: changes in autonomic control8,42 or bulbopontine mechanisms,43 hypocapnia due to thermally induced hyperventilation,44,45 and the impact of more effective inhibitory inputs on breathing patterns.46,47
BHL is the sum of conductive, convective, radiant, and evaporative heat losses. It is balanced by the metabolic heat production so that heat storage is nil. During active sleep, metabolic heat production (M) is equal to metabolic heat production during quiet sleep plus ΔM, extra heat production due to increased brain activity during this sleep stage. In the cool condition, metabolic heat production is increased through nonshivering thermogenesis and increased body activity. However, ΔM is assumed to be constant. Adding ΔM to BHL in active sleep does not bias the results. Indeed, the significant correlation between apneic event parameters and BHL (see below) was still significant when active sleep and quiet sleep were analyzed separately (always, F1,44 > 5.21, P < 0.027).
Interestingly, our present results indicate that apneic event rates or durations decrease when neonates are exposed to a cool thermal challenge (i.e., low BHL negative values).
In our study, the relationship between apneic parameters and the thermal drive is not related to body temperatures but, rather, to the BHL (for each thermal condition as well as in each sleep state), confirming the hypothesis of Perlstein et al.9 Although the regression coefficients for the different relationships were significant, they only explain between 6% and 31% of the observed variance. This emphasizes the physiologic variability commonly found in the growing organism, due to differences in the ontogenesis adjustments resulting from neuronal and functional maturation. Prenatal and postnatal influences may also increase interindividual variability. Moreover, the absence of a warm thermal effect on apnea events during quiet sleep may also explain the low r2 values observed. Lastly, partitional calorimetry does not quantify the strength of the dependence of body heat exchanges on convective (hc) and radiative (hr) heat transfer coefficients, which are currently assumed to be constant—despite the fact that their values depend on body shape and the curvature of the body segments, which differ from one neonate to another. Moreover, despite standardization of the body posture, small changes in the position of the arms and legs can modify the effective skin surface area exchanging heat with the environment. This behavioral response cannot be taken into account in the calculation of the BHL. Despite this limitation, all of the relationships are highly significant and underline the importance of monitoring body heat exchanges when examining respiratory instability. The relationship between apneic events and BHL rather than with body temperatures may also explain why some authors have not found an increase in the number or duration of apneic episodes with increasing rectal temperature.48,49
The greater the body cooling, the lower the frequency and the mean and maximal durations of apneic episodes. This suggests that breathing instability is less intense in a cool environment, when extra metabolic heat production is required to maintain body homeothermia.50
The fall in the frequency and duration of respiratory pauses in the cool condition could favor the lung and blood oxygen stores that are required to fuel an increase of aerobic metabolism in response to a cool thermal challenge. Nonshivering thermogenesis is induced via sympathetic activation of the brown adipose tissue. This change in autonomic balance (in favor of the orthosympathetic system) could influence the frequency and length of apneic episodes. Indeed, several authors have evidenced a peak in muscle sympathetic nerve activity,51 abrupt tachycardia,52 or a steady increase in sympathetic activity (followed by a sudden reduction or cessation of this activity upon ventilation onset) during apnea.53 This peak may help end the apneic event.
In conclusion, the results of the present study show that breathing instability during mild thermal challenges in near-term neonates is unrelated to levels of body temperature changes but is controlled by processes involved in BHL. These findings, if confirmed for pathologic apnea, suggest that manipulating the thermal environment, (at least in the thermal range considered in the present study) with a view to increasing BHL may be an additional or even alternative means of clinical apnea treatment and/or may significantly hasten the resolution of apneas of prematurity. It is unknown whether this thermal effect exists in older infants and, if so, whether it could be used in prevention of sudden infant death syndrome. Indirectly, prevention of apnea of prematurity (especially when associated with hypoxemia) soon after birth may, in turn, limit predisposition to further apneic episodes and, as a result, may reduce the risk of sudden infant death syndrome.54 However, the occurrence of adverse side effects (such sleep alterations and a reduction in body mass gain) cannot be ruled out.21,26
ACKNOWLEDGMENTS
The authors thank S. Delanaud (for technical help), M.C. Godefroy (for valuable discussions), D. Fraser (for reviewing the English) and the staff of the Neonatal Department, the neonates & parents who participated in the study. This work was supported by the Picardy Regional Council and the French Ministry of Research.
Financial support: This work was funded by grants from the Regional Council of Picardy and the French Ministry of Research.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Curzi-Dascalova L, Peirano P, Christova E. Respiratory characteristics during sleep in healthy small-for- gestational age newborns. Pediatrics. 1996;974:554–9. [PubMed] [Google Scholar]
- 2.Waite SP, Thoman EB. Periodic apnea in the full-term infant: individual consistency, sex differences, and state specificity. Pediatrics. 1982;701:79–86. [PubMed] [Google Scholar]
- 3.Curzi-Dascalova L, Christova-Gueorguieva E. Respiratory pauses in normal prematurely born infants. A comparison with full-term newborns. Biol Neonate. 1983;446:325–32. doi: 10.1159/000241747. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Caces R, Kwiatkowski K, Cates D, Rigatto H. A developmental study on types and frequency distribution of short apneas (3 to 15 seconds) in term and preterm infants. Pediatr Res. 1987;223:344–9. doi: 10.1203/00006450-198709000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Don GW, Waters KA. Influence of sleep state on frequency of swallowing, apnea, and arousal in human infants. J Appl Physiol. 2003;946:2456–64. doi: 10.1152/japplphysiol.00361.2002. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel M, Albani M, Schulte FJ. Apneic spells and sleep states in preterm infants. Pediatrics. 1976;571:142–7. [PubMed] [Google Scholar]
- 7.Hoppenbrouwers T, Hodgman JE, Harper RM, Hofmann E, Sterman MB, McGinty DJ. Polygraphic studies of normal infants during the first six months of life: III. Incidence of apnea and periodic breathing. Pediatrics. 1977;604:418–25. [PubMed] [Google Scholar]
- 8.Franco P, Szliwowski H, Dramaix M, Kahn A. Influence of ambient temperature on sleep characteristics and autonomic nervous control in healthy infants. Sleep. 2000;233:401–7. [PubMed] [Google Scholar]
- 9.Perlstein PH, Edwards NK, Sutherland JM. Apnea in premature infants and incubator-air-temperature changes. N Engl J Med. 1970;2829:461–6. doi: 10.1056/NEJM197002262820901. [DOI] [PubMed] [Google Scholar]
- 10.Steinschneider A, Weinstein S. Sleep respiratory instability in term neonates under hyperthermic conditions: age, sex, type of feeding, and rapid eye movements. Pediatr Res. 1983;171:35–41. doi: 10.1203/00006450-198301000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bader D, Tirosh E, Hodgins H, Abend M, Cohen A. Effect of increased environmental temperature on breathing patterns in preterm and term infants. J Perinatol. 1998;181:5–8. [PubMed] [Google Scholar]
- 12.Daily WJ, Klaus M, Meyer HB. Apnea in premature infants: monitoring, incidence, heart rate changes, and an effect of environmental temperature. Pediatrics. 1969;434:510–8. [PubMed] [Google Scholar]
- 13.Parmeggiani PL. Thermoregulation during sleep from the view point of homeostasis. In: Lydic RL, Biebuyk RL, editors. Clinical Physiology of Sleep. Vol. Vol. 3. American Physiology Society; 1998. pp. 159–169. [Google Scholar]
- 14.Libert JP, Bach V, Farges G. Neutral temperature range in incubators: performance of equipment in current use and new developments. Crit Rev Biomed Eng. 1997;254(-5):287–370. doi: 10.1615/critrevbiomedeng.v25.i4-5.10. [DOI] [PubMed] [Google Scholar]
- 15.Lenzi P, Libert JP, Franzini C, Cianci T, Guidalotti PL. Short-term thermoregulatory adjustments involving opposite regional temperature changes. J. Therm. Biol. 1986;113:151–156. [Google Scholar]
- 16.Curzi-Dascalova L, Mirmiran M, editors. Manual of methods for recording and analyzing sleep-wakefulness states in preterm and full-term infant. Paris: Les Editions INSERM; 1996. [Google Scholar]
- 17.Di Fiore JM. Neonatal cardiorespiratory monitoring techniques. Semin Neonatol. 2004;93:195–203. doi: 10.1016/j.siny.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Telliez F, Bach V, Delanaud S, Bouferrache B, Krim G, Libert JP. Skin derivative control of thermal environment in a closed incubator. Med Biol Eng Comput. 1997;355:521–7. doi: 10.1007/BF02525534. [DOI] [PubMed] [Google Scholar]
- 19.Sauer PJ, Dane HJ, Visser HK. New standards for neutral thermal environment of healthy very low birthweight infants in week one of life. Arch Dis Child. 1984;591:18–22. doi: 10.1136/adc.59.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glass L, Silverman WA, Sinclair JC. Effect of the thermal environment on cold resistance and growth of small infants after the first week of life. Pediatrics. 1968;416:1033–46. [PubMed] [Google Scholar]
- 21.Telliez F, Chardon K, Léké A, Cardot V, Tourneux P, Bach V. Thermal acclimation of neonates to prolonged cool exposure as regards sleep stages. J Sleep Res. 2004;134:337–43. doi: 10.1111/j.1365-2869.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 22.Bach V, Bouferrache B, Kremp O, Maingourd Y, Libert JP. Regulation of sleep and body temperature in response to exposure to cool and warm environments in neonates. Pediatrics. 1994;935:789–96. [PubMed] [Google Scholar]
- 23.Brück K, Parmelee AH. Neutral temperature range and range of “thermal comfort” in premature infants. Biol. Neonate. 1962;4:32–51. doi: 10.1159/000239824. [DOI] [PubMed] [Google Scholar]
- 24.Gould JB, Lee AF, James O, Sander L, Teager H, Fineberg N. The sleep state characteristics of apnea during infancy. Pediatrics. 1977;592:182–94. [PubMed] [Google Scholar]
- 25.Berterottière D, D'Allest AM, Dehan M, Gaultier C. Effects of increase in body temperature on the breathing pattern in premature infants. J Dev Physiol. 1990;136:303–8. [PubMed] [Google Scholar]
- 26.Bach V, Telliez F, Libert JP. The interaction between sleep and thermoregulation in adults and neonates. Sleep Med Rev. 2002;66:481–92. doi: 10.1053/smrv.2001.0177. [DOI] [PubMed] [Google Scholar]
- 27.Apédoh A, el Hajajji A, Telliez F, Bouferrache B, Libert JP, Rachid A. Mannequin-assessed dry-heat exchanges in the incubator-nursed newborn. Biomed Instrum Technol. 1999;335:446–54. [PubMed] [Google Scholar]
- 28.Boyd E. The growth of surface area of the human body. 1st ed. Mineapolis: University of Minnesota Press; 1935. [Google Scholar]
- 29.Museux N, Cardot V, Bach V, et al. A reproducible means of assessing the metabolic heat status of pretermneonates. Med Phys. 2007 doi: 10.1118/1.2815966. in press. [DOI] [PubMed] [Google Scholar]
- 30.Fanger PO. Thermal Comfort. 1st ed. Copenhagen: Danish Technical Press; 1970. [Google Scholar]
- 31.Elabbassi EB, Chardon K, Bach V, Telliez F, Delanaud S, Libert JP. Head insulation and heat loss in naked and clothed newborns using a thermal mannequin. Med Phys. 2002;296:1090–6. doi: 10.1118/1.1481518. [DOI] [PubMed] [Google Scholar]
- 32.Nishi Y, Gagge AP. Moisture permeation factor clothing - a factor governing thermal equilibrium and comfort. ASHRAE Trans. 1970;75:137–45. [Google Scholar]
- 33.ASHRAE. ASHRAE handbook of fundamentals. 1st ed. Vol. 1. U.S.A.: Atlanta; 1993. Physiological principles and thermal comfort; pp. 1–29. [Google Scholar]
- 34.Wheldon AE. Energy balance in the newborn baby: use of a manikin to estimate radiant and convective heat loss. Phys Med Biol. 1982;272:285–96. doi: 10.1088/0031-9155/27/2/009. [DOI] [PubMed] [Google Scholar]
- 35.Gagge AP. A new physiological variable associated with sensible and insensible perspiration. Am. J. Physiol. 1937;120:277–87. [Google Scholar]
- 36.Silverman WA, Agate FJ., Jr Variation In Cold Resistance Among Small Newborn Infants. Biol Neonat. 1964;6:113–27. doi: 10.1159/000239891. [DOI] [PubMed] [Google Scholar]
- 37.Adamson SK, Jr, Gandy GM, James LS. The Influence Of Thermal Factors Upon Oxygen Consumption Of The Newborn Human Infant. J Pediatr. 1965;66:495–508. doi: 10.1016/s0022-3476(65)80114-7. [DOI] [PubMed] [Google Scholar]
- 38.Pribylova H, Znamenacek K. The effect of body temperature on the level of carbohydrate metabolites and oxygen consumption in the newborn. Pediatrics. 1966;375:743–9. [PubMed] [Google Scholar]
- 39.Hill JR, Rahimtulla KA. Heat balance and the metabolic rate of new-born babies in relation to environmental temperature; and the effect of age and of weight on basal metabolic rate. J Physiol. 1965;1802:239–65. doi: 10.1113/jphysiol.1965.sp007701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messer J, Erny P, Isch-Treussard C, Terrade E, Bapst-Reiter J, Renaud S. [Sleep states and oxymetry in neonates (author's transl)] Arch Fr Pediatr. 1981;382:97–100. [PubMed] [Google Scholar]
- 41.Riesenfeld T, Hammarlund K, Norsted T, Sedin G. Irregular breathing in young lambs and newborn infants during heat stress. Acta Paediatr. 1996;854:467–70. doi: 10.1111/j.1651-2227.1996.tb14063.x. [DOI] [PubMed] [Google Scholar]
- 42.Fox GP, Matthews TG. Autonomic dysfunction at different ambient temperatures in infants at risk of sudden infant death syndrome. Lancet. 1989;28671:1065–7. doi: 10.1016/s0140-6736(89)91080-5. [DOI] [PubMed] [Google Scholar]
- 43.Grunstein MM, Fisk WM, Leiter LA, Milic-Emili J. Effect of body temperature on respiratory frequency in anesthetized cats. J Appl Physiol. 1973;342:154–9. doi: 10.1152/jappl.1973.34.2.154. [DOI] [PubMed] [Google Scholar]
- 44.Rigatto H, Kalapesi Z, Leahy FN, Durand M, Maccallum M, Cates D. Chemical control of respiratory frequency and tidal volume during sleep in preterm infants. Respir Physiol. 1980;411:117–25. doi: 10.1016/0034-5687(80)90027-4. [DOI] [PubMed] [Google Scholar]
- 45.Canet E, Praud JP, Bureau MA. Periodic breathing induced on demand in awake newborn lamb. J Appl Physiol. 1997;822:607–12. doi: 10.1152/jappl.1997.82.2.607. [DOI] [PubMed] [Google Scholar]
- 46.Merazzi D, Mortola JP. Hering-Breuer reflex in conscious newborn rats: effects of changes in ambient temperature during hypoxia. J Appl Physiol. 1999;875:1656–61. doi: 10.1152/jappl.1999.87.5.1656. [DOI] [PubMed] [Google Scholar]
- 47.Merazzi D, Mortola JP. Effects of changes in ambient temperature on the Hering-Breuer reflex of the conscious newborn rat. Pediatr Res. 1999;453:370–6. doi: 10.1203/00006450-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Rieger-Fackeldey E, Schaller-Bals S, Schulze A. Effect of body temperature on the pattern of spontaneous breathing in extremely low birth weight infants supported by proportional assist ventilation. Pediatr Res. 2003;543:332–6. doi: 10.1203/01.PDR.0000076664.65100.FF. [DOI] [PubMed] [Google Scholar]
- 49.Bohnhorst B, Gill D, Dordelmann M, Peter CS, Poets CF. Bradycardia and desaturation during skin-to-skin care: no relationship to hyperthermia. J Pediatr. 2004;1454:499–502. doi: 10.1016/j.jpeds.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Cameron YL, Merazzi D, Mortola JP. Variability of the breathing pattern in newborn rats: effects of ambient temperature in normoxia or hypoxia. Pediatr Res. 2000;476:813–8. doi: 10.1203/00006450-200006000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin Sci (Lond) 2003;1043:231–8. doi: 10.1042/CS20020157. [DOI] [PubMed] [Google Scholar]
- 52.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;18369:126–31. doi: 10.1016/s0140-6736(84)90062-x. [DOI] [PubMed] [Google Scholar]
- 53.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;64:S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 54.Cardot V, Chardon K, Tourneux P, et al. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res. 2007;625:591–6. doi: 10.1203/PDR.0b013e318155868e. [DOI] [PubMed] [Google Scholar]