Abstract
Study Objectives:
To study long-term effects of conditioned fear on REM sleep (REMS) parameters in albino rats.
Design:
We have investigated disturbances in sleep architecture, including muscle twitch density as REMS phasic activity, and freezing behavior in wakefulness, upon reexposure to a conditioned stimulus (CS) on Day 1 and Day 14 postconditioning.
Subjects:
Male Sprague-Dawley rats prepared for polysomnographic recordings.
Interventions:
After baseline sleep recording, the animals in the experimental group received five pairings of a 5-sec tone, co-terminating with a 1-sec, 1 mA footshock. The control rats received similar numbers of tones and shocks, but explicitly unpaired. On postconditioning days, after reexposure to tones alone, sleep and freezing behavior were recorded.
Measurements and Results:
Conditioned fear significantly altered REMS microarchitecture (characterized as sequential-REMS [seq-REMS: <3 min episode separation] and single-REMS [sin-REMS: >3 min episode separation]) on Day 14. The total amount and number of seq-REMS episodes decreased, while the total amount and number of sin-REMS episodes increased. Further, the CS induced significant increases in freezing and REMS myoclonic twitch density in the experimental group. Reexposure to the CS produced no alterations in controls.
Conclusions:
The results suggest that conditioned fear causes REMS alterations, including difficulty in initiating a REMS episode as indicated by the diminution in the number of seq-REMS episodes. Another finding, the increase in phasic activity, agrees with the inference from clinical investigations that retrieval of fearful memories can be associated with the long-term REMS disturbances characteristic of posttraumatic stress disorder.
Citation:
Madan V; Brennan FX; Mann GL; Horbal AA; Dunn GA; Ross RJ; Morrison AR. Long-term effect of cued fear conditioning on REM sleep microarchitecture in rats. SLEEP 2008;31(4):497-503.
Keywords: Anxiety, freezing, muscle twitches, fear conditioning, PTSD, REM sleep
LEARNING TO ASSOCIATE AVERSIVE EVENTS WITH ENVIRONMENTAL STIMULI THAT RELIABLY PREDICT THESE EVENTS IS INDISPENSABLE TO THE SURVIVAL of organisms. Pavlovian fear conditioning is an example of such learning in which an emotionally neutral conditioned stimulus (CS), such as a tone, is paired with an aversive, unconditioned stimulus (US), usually electrical footshock.1 The CS, by virtue of its relationship with the US, acquires aversive properties and elicits responses normally induced by threatening stimuli, such as freezing and ultrasonic vocalizations.2 This paradigm has been used in numerous studies to explore mechanisms that may underlie the psychophysiological alterations associated with posttraumatic stress disorder (PTSD).
Experiencing traumatic and aversive events in humans can induce several long-term behavioral and physiological disturbances, including changes in sleep.3,4 Sleep alterations also result from exposure to fear conditioning in both rats5–7 and mice.8,9 One observes changes in vigilance states characterized predominantly by decreased REM sleep (REMS), while NREM sleep (NREMS) remains unaffected.5,6,8
Although the effects of fear conditioning on REMS can be assessed by looking at REMS in toto, various stressors can also alter its microarchitectural pattern (i.e., parcellation of REMS into sequential and single episodes) in rats.10 REMS can be characterized as sequential-REMS (seq-REMS) (comprised of REMS episodes separated by ≤ 3 min intervals, which appear in clusters), and single-REMS (sin-REMS) (with episodes preceded and followed by >3 min intervals).10 This distinction is important because stressors such as low ambient temperature and immobilization have differential effects on sin-REMS and seq-REMS.10,11 For example, rats placed in a cold environment show a reduction in REMS amount. During the recovery period at a normal room temperature, REMS is increased via an increase in the number of seq-REMS bouts. Because temperature is not tightly regulated during REMS due to an absence of hypothalamic control, Amici et al10 suggest that the brief intervals of wakefulness or NREMS punctuating a REMS cluster allow sufficient REMS to be made up by ensuring short periods of homeostatic regulation of the body during a cluster, and that this could not occur, were the REMS deficit to be made up by extended periods of sin-REMS. Similarly, after immobilization stress, the increase in REMS is associated with only a modest increase in sin-REMS, but a considerable increase in seq-REMS, which may result from recovery from sleep deprivation during the immobilization procedure.11 A bimodal distribution of REMS intervals also exists in other species, including humans.10
Altered sleep quality and/or quantity can itself be stressful to an organism and contribute to the emotional and behavioral disturbances symptomatic of psychiatric disorders. We have reported that exposure to fear-inducing tones (the CS), previously paired with brief, mild electrical footshock, alters both REMS and its microarchitecture,5 but the persistence of these effects over time has received less research attention.8 Interestingly, there is often a progressive increase in memory strength over time following an aversive stimulus (measured as a change in the magnitude of the freezing response), without further exposure to the stimulus itself: this has been referred to as an incubation process.12 Therefore, in this study, we evaluated the impact of reexposure to fearful cues on the sleep architecture and REMS microarchitecture of rats, both 24 hr (short-term effect, Day 1) and two weeks (long-term effect, Day 14) after conditioning. In addition, we examined the effects of reexposure to the CS on a more common behavioral index of anxiety, freezing. We and others have reported earlier that military veterans with PTSD have increased phasic muscle activity during REMS13–15; hence, we also evaluated the frequency of myoclonic twitches as a measure of phasic REMS events.
Several studies suggest that fear conditioning alters the amount and microarchitecture of REMS, but does not alter the sleep efficiency or NREMS amount.5,6,8,16 Hence, in this study we hypothesized that reexposure to cues associated with footshock should alter REMS microarchitecture, primarily by changing the sin-REMS, seq-REMS, and cluster amount. We also expected that fear conditioning would increase the number of myoclonic twitches during REMS. Finally, we hypothesized that changes in REMS microarchitecture and myoclonic twitches are also due to conditioned anxiety, and as such should correlate with freezing, a well-established indicator of conditioned anxiety.
METHODS
Male Sprague-Dawley rats (300–350 g, 9–11 weeks old during the course of the study), (n = 10), maintained under a 12:12 L:D cycle (lights on at 07:00), were studied. Subjects were divided into two groups: a Fear Conditioned (FC) group (n = 6) and an Unpaired (UP) control group (n = 4). Upon arrival at the facility, they were housed for one week in a temperature and humidity-controlled animal colony and given ad lib access to food and water. All experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Surgical Procedures
Under inhalation anesthesia and using sterile procedures, the rats were prepared for chronic electroencephalogram (EEG) and electromyogram (EMG) recording. The animal was anesthetized initially with a mixture of ketamine (85 mg/kg, i.m.) and xylazine (15 mg/kg, i.m.) and then maintained under anesthesia with isoflurane gas (0.25%), using a facemask. The animal was fixed in a standard rat stereotaxic device. A midline incision exposed the skull for electrode implantations. Two pairs of small, stainless-steel screw electrodes were affixed to the skull above the frontal and sensorimotor cortices to record a bipolar fronto-frontal and a bipolar parieto-parietal EEG. Two flexible, insulated (except at the tip) wires were attached bilaterally to the dorsal neck muscles for bipolar EMG recording. Another screw electrode was implanted on the midline to serve as a reference electrode. The free ends of the EEG, EMG, and reference electrodes were connected to a 9-pin miniature connector and cemented onto the skull with dental acrylic. Postoperative pain and potential infection were controlled with metacam (0.02 mg/100 g, s.c.) and triple antibiotic ointment. The drugs were given to the animal only during the initial days of recovery as observed from the animal's behavior: general vigor and normal eating. Animals were allowed to recover completely from surgery for at least one week prior to their habituation to the recording chamber.
Recording Procedures
After recovery from surgery, the rats were brought to the recording room in their home cage. The cage was placed in a sound-dampened and electrically insulated recording chamber (neutral chamber), and the animal was connected to an electrical cable, which was in turn connected to a commutator. The animals were habituated to these recording conditions for several hours a day for at least four days. A baseline sleep recording was then taken for 4 hours (11:00–15:00). Polysomnographic recordings were amplified with a Grass Model 7 polygraph (Grass-Telefactor, USA) amplifier system and recorded on a PC with commercial sleep acquisition software, Spike 2 (Cambridge Electronics, UK). EEG/EMG signals were processed with a high-pass (EEG: 0.3 Hz; EMG: 10 Hz) and a low-pass (100 Hz) filter and digitized by CED Power-1401 (Cambridge Electronics, UK). The animals' behavior was simultaneously videotaped using mini-video cameras permanently placed inside the recording chamber for offline visual scoring of freezing during the baseline and post-conditioning days.
Fear Conditioning Procedures
One day after the baseline sleep recording, rats in the FC group were trained, using an auditory CS (tone) paired with an aversive US (electric footshock). On the training day, an “unsafe” person, who had not handled the animal previously, performed the training. This individual placed the animal in a precleaned (Windex Original) shock chamber (Coulbourn Precision Regulated Animal Shocker Model E13–14) equipped with a grid floor for administering the electric shock. The animal was allowed to explore the cage freely for approximately 60 sec and was then presented with 5 tones (Coulbourn Tone/Noise Generator) (800 Hz, 90 dB, 5 sec), each co-terminating with a footshock (1 mA, 1 sec) (Coulbourn Habitest Model E10–8RF). The computer-automated, 5 tone-shock pairings (using Graphic State software) were administered at 30-sec intervals. The UP control animals also received 5 tones and 5 footshocks, but in an explicitly unpaired manner. For this group, a shock could occur anytime during the session, except during or within 5 sec of a tone. The length of the conditioning session was equivalent for both the groups. At the end of the session, all subjects were returned to their home cage in the animal colony.
The effects of CS exposure on sleep-wakefulness and freezing were studied on Days 1 and 14 following training. The same person who had conducted the baseline sleep recording (“safe person”) brought the rat, in its home cage, back to the neutral chamber for habituation on Day 13 and for sleep-wakefulness recording on Days 1 and 14. Animals were allowed 10–15 min to familiarize themselves to the neutral chamber on Days 1 and 14. Thereafter, they were reexposed to 5 computer-automated tone (CS) presentations alone, at a 30-sec intertone interval over a 145-sec period. Subjects were hooked up for sleep-wakefulness recording 5 minutes after the termination of the first CS to obtain exclusive and consistent freezing responses without interference from the sleep recording procedure, notably restriction of movement by the recording cable. During that 5-min period, the animal was videotaped for offline scoring of freezing behavior. Sleep-wakefulness was then recorded for 4 hours, after which the animals were returned to the colony. All subjects were studied at the same time on all days (sleep-wakefulness recording: 11:00–15:00; fear conditioning: 10:45–11:00).
Sleep Parameters
Computerized polysomnographic records were visually displayed, divided into 10-sec epochs and manually scored using commercial software (Somnologica) employing standard criteria for rats: Wakefulness—low voltage, high frequency EEG waves (alpha: 9–12 Hz; beta: 12–20 Hz; gamma: 20–40 Hz) associated with increased motor activity; NREMS—high voltage, low frequency EEG waves (0.5–4 Hz) and decreased motor activity; REMS—low voltage, high frequency EEG waves with a prominent theta peak (4–9 Hz) and nuchal muscle atonia. The total times in REMS and NREMS were calculated and REMS and NREMS amounts were also expressed as a percent of total sleep time (TST). Latencies to REMS and NREMS were calculated by measuring the time elapsed between the start of the recording and the first episode of each state. We also calculated the number and average duration of individual episodes of REMS, NREMS, and wakefulness. Finally, we evaluated the microarchitecture of REMS, by analyzing the amounts of sin-REMS and seq-REMS, as well as the number of sin-REMS and seq-REMS episodes. Additionally, the amount of seq-REMS clusters was calculated as the total amount of time within series of seq-REMS episodes, including intervening NREMS and/or periods of wakefulness.
Offline, we analyzed the freezing behavior of the rats for a period of 300 sec just after presentation of the first CS tone on the post-conditioning days. For comparison, freezing behavior was also recorded for 300 seconds before hook-up of the animal on the baseline recording day. The total time spent freezing at baseline and on postconditioning days was transformed into a percentage of the total observation period.
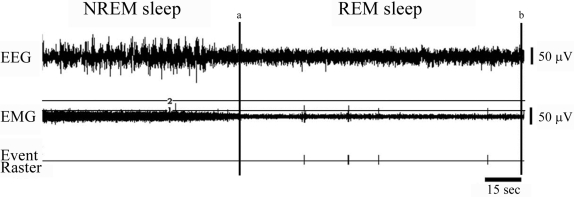
Myoclonic twitches of the nuchal muscles were analyzed for both the FC and UP groups at baseline and on the postconditioning days using Spike 2 software. Muscle twitches during each REMS episode were converted into event channels from the EMG data channel. An artifact-free window was made by placing 2 horizontal and 2 vertical cursors for each REMS episode (see Figure 1). The window was set so that it captured only the twitches from the EMG channel, which generated for each REMS episode an event raster indicating every twitch. The raster outputs were then analyzed for the total number of twitches, the mean number of twitches/REMS episode and the number of twitches/total REMS time.
Figure 1.
Example of muscle twitch scoring. Depicted is the EMG channel from a period of REM sleep (REMS) preceded by NREM sleep (NREMS). We created two vertical lines (a and b) to demarcate the REMS period, and two horizontal lines (1 and 2) to capture twitches. The lower horizontal line (1) was set above the EKG captured by the EMG, and the upper horizontal line (2) was set to exclude obvious artifacts. In the section of REMS depicted, five twitches, two occurring close together, are evident.
Data Analysis
Data were analyzed for statistical significance using commercial software SigmaStat (Systat Software, Inc). Sleep-wakefulness measures, time spent freezing and myoclonic twitch data during baseline and postconditioning days were compared using repeated measures, one-way analyses of variance (ANOVA) followed by Bonferroni post hoc tests (group x days interaction). Differences between groups on baseline days were statistically compared using one-way ANOVA followed by Bonferroni post hoc tests. This was done since there appeared to be small differences between groups at baseline attributable to random variation. At each time point, Spearman rank correlations were calculated between total amounts of seq-REMS and sin-REMS, total amount of seq-REMS and the percent time spent freezing, and total amount of sin-REMS and the percent time spent freezing. We also calculated correlations between freezing and the number of muscle twitches. Data were considered significant at P < 0.05.
RESULTS
Sleep Architecture Upon Reexposure To Fearful Cues
We observed no significant change in sleep macroarchitectural measures in either group upon CS reexposure on both Day 1 and Day 14 after conditioning. Sleep-wakefulness measures at baseline and after reexposure to fearful cues on Day 1 and Day 14 are presented in Table 1.
Table 1.
Changes in Sleep Measures During Baseline and Post Conditioning Days in the FC and UP Groups
| FC group (Mean ± S.E.M.) (n = 6) | UP group (Mean ± S.E.M.) (n = 4) | |
|---|---|---|
| Sleep efficiency | ||
| Baseline | 59.86 ± 2.37% | 70.85 ± 1.50% |
| Day 1 | 62.20 ± 1.51% | 61.91 ± 2.84% |
| Day 14 | 63.34 ± 4.35% | 65.59 ± 1.79% |
| NREMS (% TST) | ||
| Baseline | 79.34 ± 1.09% | 75.39 ± 2.12% |
| Day 1 | 79.71 ± 2.06% | 73.44 ± 2.86% |
| Day 14 | 78.78 ± 1.13% | 74.03 ± 1.04% |
| Total number of NREMS episodes | ||
| Baseline | 57.83 ± 3.77 | 78.75 ± 5.25 |
| Day 1 | 55.83 ± 5.38 | 70.25 ± 11.59 |
| Day 14 | 53.00 ± 3.43 | 77.50 ± 4.52 |
| REMS (% TST) | ||
| Baseline | 20.66 ± 1.10% | 24.61 ± 2.13% |
| Day 1 | 20.29 ± 2.07% | 26.56 ± 2.86% |
| Day 14 | 21.22 ± 1.14% | 25.97 ± 1.04% |
| Total number of REMS episodes | ||
| Baseline | 16.17 ± 1.25 | 20.50 ± 2.10 |
| Day 1 | 15.50 ± 1.61 | 19.75 ± 3.47 |
| Day 14 | 14.17 ± 1.80 | 21.75 ± 2.72 |
| Seq-REMS (% REMS) | ||
| Baseline | 45.71 ± 3.15% | 42.64 ± 5.90% |
| Day 1 | 32.90 ± 7.32% | 41.75 ± 7.28% |
| Day 14 | 14.72 ± 7.05% ** | 45.87 ± 11.86% |
| Sin-REMS (% REMS) | ||
| Baseline | 54.29 ± 3.15% | 57.36 ± 5.90% |
| Day 1 | 67.14 ± 7.32% | 58.25 ± 7.28% |
| Day 14 | 85.26 ± 7.05% ** | 54.13 ± 11.86% |
Significance level: **P < 0.01
REMS Microarchitectural Changes
[A] Effects of CS reexposure on seq-REMS:
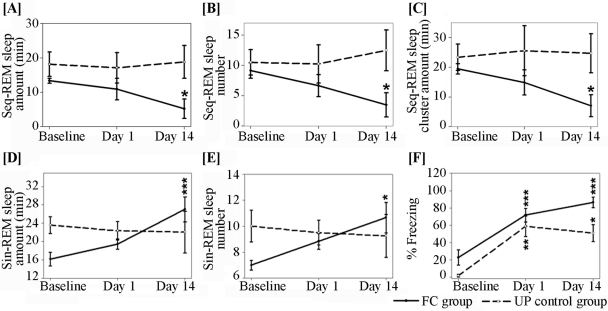
The changes in seq-REMS upon reexposure to fearful cues on Day 1 and Day 14 are shown in Figure 2. Both the total amount of seq-REMS (F 2, 17 = 4.15, P < 0.05) and the number of seq-REMS episodes (F 2, 17 = 4.27, P < 0.05) decreased in the FC group after 14 days (Figures 2A and 2B). In addition, the total amount of time in clusters (F 2, 17 = 4.88, P < 0.05; Figure 2C) also decreased on Day 14. On Day 1 the decreases in the above parameters did not reach significance. We also observed a significant decrease in seq-REMS amount as a percent of total REMS amount on Day 14 in the FC group (F2, 17 = 10.84, P < 0.01; Table 1). These measures did not change in the UP control group (Figure 2 and Table 1).
Figure 2.
Alterations in sequential REMS (seq-REMS) measures, single REMS (sin-REMS) measures and freezing (expressed as total time spent freezing/ total 5-min observation period x 100) upon reexposure to fearful stimuli on post-conditioning Day 1 and Day 14 in the FC and UP groups. Data is represented as mean + S.E.M. and shows a within group X days interaction. Significance level * P < 0.05, ** P < 0.01, *** P < 0.001
[B] Effects of CS reexposure on sin-REMS:
In contrast to its effect on seq-REMS measures, reexposure to fearful cues on Day 1 and Day 14 increased sin-REMS measures in the FC group on Day 14 (Figure 2). Presentation of fearful cues significantly increased the total amount of sin-REMS (F 2, 17 = 14.41, P < 0.001) and the number of sin-REMS episodes (F 2, 17 = 4.69, P < 0.05; Figures 2D and 2E). Animals in the UP group showed no difference in the total amount of sin-REMS and in the number of sin-REMS episodes on the test days when compared to baseline sleep (Figures 2D and 2E). Sin-REMS amount expressed as a percent of total REMS amount increased significantly on Day 14 in the FC group (F2, 17 = 10.84, P < 0.01) while there was no significant change in the UP control group (Table 1).
Freezing Behavior Upon Reexposure To Fearful Cues
We observed a significant increase in freezing behavior upon reexposure to the CS on both Day 1 and Day 14. During the baseline session, animals spent 22.50% + 8.79% (Mean + SEM) of the 5-min observation period freezing. Reexposure to the CS increased their freezing to 71.62% + 8.01% on Day 1 (F 1, 11 = 44.52, P < 0.001) and to 83.74% + 6.66% on Day 14 (F 1, 10 = 204.82, P < 0.001) (freezing data of one animal on Day 14 were lost due to a technical error) (Figure 2F). Although the UP animals had no changes in sleep, they also exhibited freezing to the tones (Day 1: F 1, 7 = 28.85, P < 0.05; Day 14: F1, 7 = 27.39, P < 0.05). While in the FC group there was a 16.92% increase in freezing from Day 1 to Day 14, in the UP group freezing decreased by 13.60% from Day 1 to Day 14.
Relationship between REMS Microarchitecture and Freezing Behavior
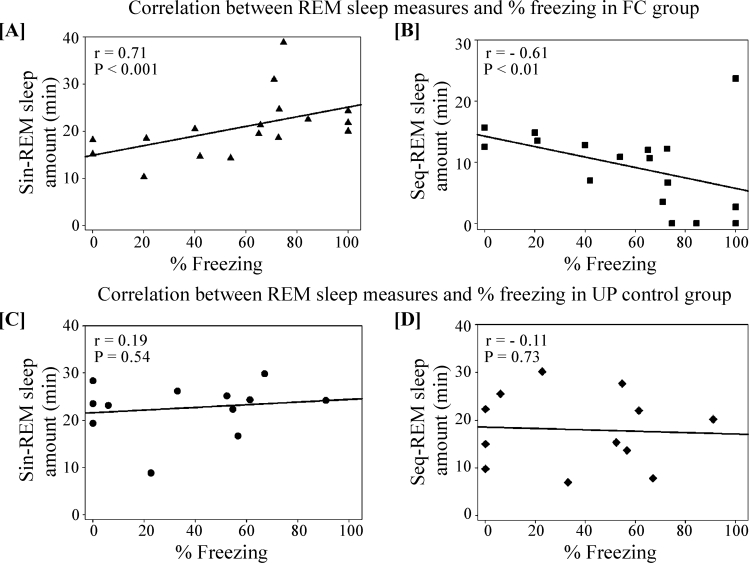
Various stressful and fearful conditions alter the pattern of seq-REMS and sin-REMS10 and also induce freezing behavior.17 We observed a significant positive correlation between the amount of sin-REMS and the percent of time spent freezing (r = 0.71, P < 0.001; Figure 3A) and a significant negative correlation between seq-REMS amount and percent of time spent freezing at all time points in the FC group (r = −0.61, P < 0.01; Figure 3B). However, in the UP group, these measures were not correlated (sin-REMS amount vs. percent of time spent freezing: r = 0.19, P = 0.54; seq-REMS amount vs. percent of time spent freezing: r = −0.11, P = 0.73; Figures 3C and 3D).
Figure 3.
Significant correlations between [A] sin-REMS amount and % time spent freezing, [B] seq-REMS amount and % time spent freezing, at all time points, in the FC group. These measures were not correlated in the UP group [C and D].
Changes in Myoclonic Twitches during REMS after Fear Conditioning
Changes in the total number of myoclonic twitches during REMS and the mean number of twitches per REMS episode upon reexposure to fearful cues on Day 1 and Day 14 are shown in Figure 4. Significant changes were observed in the total number of muscle twitches (F2, 17 = 4.34, P < 0.05; Figure 4A) and the mean number of twitches/REMS episode (F2, 17 = 6.36, P < 0.05; Figure 4B) in the rats in the FC group. The total number of myoclonic twitches/ total REMS time did not change significantly, although we observed a trend towards an increase (Baseline: 0.1 + 0.02; Day 1: 0.13 + 0.02; Day 14: 0.16 + 0.03) [F 2, 17 = 3.62, P = 0.07]. Similar to changes in sleep measures, muscle twitch parameters changed significantly only at Day 14 compared to baseline, P's < 0.05. There were no changes in the total number of muscle twitches (F2, 11 = 0.71, P = 0.53) and the mean number of twitches/REMS episode (F2, 11 = 2.08, P = 0.21) in the UP group (Figure 4).
Figure 4.
Changes in myoclonic twitch measures during REMS and its correlation with % time spent freezing in the FC and UP groups. Total number of muscle twitches during REMS [A]; and number of muscle twitches/REMS episode [B] increased significantly (compared to baseline) in the FC group. There was a significant positive correlation between the total number of muscle twitches during REMS and % time spent freezing, in the FC group [C] but not in the UP group [D]. Significance level * P < 0.05
Relationship between Muscle Twitches during REMS and Freezing Behavior
We observed a significant positive correlation between the total number of muscle twitches during REMS and the percent of time spent freezing in the FC group [r = 0.57, P < 0.05] (Pearson correlation) (Figure 4C). The UP group did not exhibit a significant correlation between the total number of muscle twitches and the freezing response (r = −0.52, P = 0.08; Figure 4D).
DISCUSSION
In this study, we characterized the detailed alterations in REMS and freezing observed upon reexposure to fearful cues on Day 1 and Day 14 after fear conditioning. On the postconditioning days, rats exhibited significantly more freezing, indicating that they had been conditioned to fear the CS. Interestingly, more freezing on Day 14 compared to Day 1 suggests that not only are rats able to retain fearful memories for at least two weeks, but these memories may actually strengthen over time.
Presentation of the stimuli one day after fear conditioning and also on Day 14 did not alter either total sleep or REMS amounts. However, fear conditioning did elicit significant REMS microarchitectural changes in the FC group. Total seq-REMS amount was significantly reduced, while total sin-REMS amount was significantly increased upon reexposure to the CS on Day 14 with a trend observed in the same direction on Day 1. These changes were due to differences in the number of episodes; seq-REMS and sin-REMS episode durations remained unchanged. None of these changes were observed in the UP group. The significant increase in sin-REMS by Day 14 suggests that the rats had more difficulty entering REMS, hence the shift to longer intervals between REMS episodes.
We have previously reported that reexposure to fearful cues did not alter NREMS but significantly decreased total REMS amount, primarily by decreasing the amount of sin-REMS at Day 1.5 Mice also showed significantly decreased REMS after presentation of fearful cues.8 The absence of an effect on total REMS and the increase in sin-REMS at Day 14 in the current study are most likely due to the different conditioning protocol that we used. In contrast to our previous investigation,5 in which animals were trained in a different room, in the current study fear conditioning was carried out in the same room used for sleep recording, although in a different chamber. The animals were brought into the room used for both training and sleep recording in a cloth-concealed cage, and two distinct persons (“safe” and “unsafe”) handled the animals on recording and training days, respectively. This enabled us to minimize potential confounding effects of contextual cues. It is well known that “surprising” or novel conditions produce greater responses than well-predicted outcomes.18 In the present study, compared to the protocol in Jha et al.,5 recording sleep in a familiar room on the postconditioning days may have contributed to a reduction in surprise by the tones and a failure to modify sleep macroarchitecture. This result emphasizes the keen sensitivity of rats to their surroundings. Furthermore, the animals in the Jha et al.5 study received 5 tone-shock pairings over 30 min, spending much more time in the shock chamber than they did in the present study, which likely intensified their negative experience on the training day.
The current study, however, has some limitations. The use of 5 relatively mild tone-shock pairings, although standard in fear-conditioning work using freezing as a measure of successful training, is apparently less intense than required to affect the more complex behavior of sleep consistently. More tone-shock pairings or more intense shock intensities studied with a larger sample size may reveal even stronger effects on sleep, characteristic of anxiety disorders in humans. Further, since we could not eliminate contextual effects absolutely, the presence of some contextual cues might explain the modest freezing response observed in the control group of rats.
The strength of the study is that we report significant effects when the CS is presented 14 days after the initial training. The effect on sleep might have been larger were the stimulus presented even later. It is well known that alterations in sleep in PTSD, some of which likely are conditioned, are chronic, and may persist for decades after the initial trauma.15 Future experiments will likely resolve these issues.
REMS phasic events, such as rapid eye movements and leg muscle twitches, have been reported to increase as a result of emotional stress and anxiety in humans.13–15 In this study, we evaluated neck muscle twitches as a measure of the effect of fear conditioning on REMS phasic events. Presentation of fear-inducing cues on Day 14 significantly increased myoclonic twitches during REMS. Discharge profiles of neurons in the nucleus subcoeruleus, the nucleus pontis oralis and the dorsolateral pontine tegmentum (DLPT) suggest the involvement of these brainstem areas in the modulation of muscle tone and the production of myoclonic twitches.19 Lesions of the DLPT also decrease twitching.19 Liu et al.20 have shown that after aversive CS (tone) presentation, neurons in the DLPT remain activated for up to 2 hr. Therefore, it is likely that presentation of the CS activates neurons in the DLPT that are involved in producing myoclonic twitches. Further, such twitches are correlated with bursts of eye movements in REMS,21 and we and others have reported that rapid eye movements during REMS are increased in individuals with PTSD,13,15 who characteristically reexperience fearful events during sleep as well as wakefulness. Therefore, our observation of increased myoclonic twitches during REMS in fear-conditioned rats is consistent with several human studies.
The significant positive correlation between the total number of muscle twitches and freezing in the FC group also suggests that conditioned fear can increase the generation of muscle twitches during REMS. Not unexpectedly, the UP animals exhibited no correlation between these two measures, suggesting that any sensitization mechanism initiated by footshock presentations was not alone sufficient to increase REMS phasic event generation.
Long-term Effect of Fear Conditioning on REMS Microarchitecture
On both postconditioning days, we observed significant freezing with presentations of the CS in the FC group. Interestingly, freezing was greater on Day 14 compared to Day 1. Consistent with this observation, alterations in REMS microarchitecture were significant only on Day 14, when we observed a reduction in seq-REMS amount and an increase in sin-REMS amount; there were only trends in this direction on Day 1. The significant effects at Day 14 could have been the result of memory “incubation,” defined as a progressive increase in the strength of a memory with time following an aversive stimulus.12 For example, freezing, the suppression of movement that rats show when a fearful CS is presented in a novel context, increases over time.12
A key component of the circuitry involved in cued fear conditioning is the lateral nucleus of the amygdala (LA). Cued fear conditioning induces long-term potentiation (LTP) in LA neurons. LTP is an experience-dependent form of neural plasticity believed to involve mechanisms that underlie memory formation.22 With the passage of time, reexposure to a fearful CS might result in a decrease in the threshold and/or latency for the induction of LTP in LA neurons, and this might lead to a more robust fear response. The amygdala is involved in the modulation of REMS23,24 and changes in amygdalar neurocircuitry that result from fear conditioning could lead to alterations in REMS microarchitecture. Further, stimulating the major source of amygdalar outputs, the central nucleus of the amygdala, during REMS, increased the number of ponto-geniculo-occipital (PGO) waves, another REMS phasic event.25
Factors Influencing Cued Fear Conditioning and seq-REMS
Serotonin (5-HT) plays an important role in both fear conditioning and in seq-REMS control.26,27 The 5-HT2 agonist DOI administered into the lateral dorsal tegmentum (LDT) inhibits seq-REMS generation.26 It has been demonstrated that fearful cues increase cellular activity in the serotonergic dorsal raphe nucleus and in the LDT,20 two regions important in REMS control. Thus, changes in brain 5-HT level could be involved in the changes in REMS microarchitecture produced by cued fear conditioning.
The second messenger cyclic AMP (cAMP) also modulates the acquisition of fear responses28 and the expression of seq-REMS.10 Nonspecific physiological stimuli induce a transient increase in cAMP level in the brain, which in turn affects the activity of protein kinase A, involved in the formation of fearful memories.28 Conditioning to a discrete cue such as a tone depends on the amygdala29 and the phosphorylation of cAMP response element-binding protein (CREB) in this area is important for memory formation.28 Further, the level of cAMP in the anterior hypothalamus has been shown to be related to the occurrence of seq-REMS.10 Thus, a change in the level of cAMP after fear conditioning may be related to the reduction in seq-REMS amount upon reexposure to the CS.
In this study, we have shown that fear conditioning has some long-term effects on sleep. Based on the current promising results, it will be important to investigate, in the future, the time course of these effects and the neuroanatomical sites and neurophysiological and molecular mechanisms collectively involved in fear-related learning and sleep. Investigating the possible roles of neurotransmitters, such as serotonin and norepinephrine, as well as biomolecules such as CREB and cAMP in the amygdala and brainstem, could provide important insights into the mechanism of sleep disturbances induced by cued fear learning. This could potentially generate new treatments for the multitude of patients with sleep difficulties associated with the presence of an anxiety disorder.
ACKNOWLEDGMENTS
This study was supported by NIH grant RO1-MH072897.
Institution at which the work was performed: Department of Animal Biology, School of Veterinary Medicine, University of Pennsylvania
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 2.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition (DSM-IV) Arlington, VA: American Psychiatric Publishing, Inc.; 1994. [Google Scholar]
- 4.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 5.Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–80. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 6.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 8.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 9.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 10.Amici R, Jones C, Perez E, Zamboni G. A physiological view of REM sleep structure. In: Parmeggiani PL, Velluti RA, editors. The physiologic nature of sleep. London: Imperial College Press; 2005. pp. 161–85. [Google Scholar]
- 11.Dewasmes G, Loos N, Delanaud S, Dewasmes D, Ramadan W. Pattern of rapid-eye movement sleep episode occurrence after an immobilization stress in the rat. Neurosci Lett. 2004;355:17–20. doi: 10.1016/j.neulet.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- 14.Ross RJ, Ball WA, Dinges DF, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17:723–32. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 15.Ross RJ, Ball WA, Dinges DF, et al. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/6J mice. Physiol Behav. 2005;85:419–29. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Jhou T. Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol. 2005;493:111–4. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J Neurosci. 2006;26:6077–81. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:0891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Tang X, Sanford LD. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991:1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–84. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 22.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 23.Morrison AR, Sanford LD, Ross RJ. The amygdala: a critical modulator of sensory influence on sleep. Biol Signals Recept. 2000;9:283–96. doi: 10.1159/000014652. [DOI] [PubMed] [Google Scholar]
- 24.Jha SK, Ross RJ, Morrison AR. Sleep-related neurons in the central nucleus of the amygdala of rats and their modulation by the dorsal raphe nucleus. Physiol Behav. 2005;86:415–26. doi: 10.1016/j.physbeh.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Deboer T, Sanford LD, Ross RJ, Morrison AR. Effects of electrical stimulation in the amygdala on ponto-geniculo-occipital waves in rats. Brain Res. 1998;793:305–10. doi: 10.1016/s0006-8993(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 26.Amici R, Sanford LD, Kearney K, et al. A serotonergic (5-HT2) receptor mechanism in the laterodorsal tegmental nucleus participates in regulating the pattern of rapid-eye-movement sleep occurrence in the rat. Brain Res. 2004;996:9–18. doi: 10.1016/j.brainres.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–78. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]